Abstract
Objective: Central nervous system (CNS) injury can increased the risk of secondary mortality because of its late inflammatory complications. Alcohol intake increases the risk of damage and complications subsequent to a (CNS) injury. How about the risk of pneumonia after CNS injury under the effect of alcoholic drink? Though animal trails of material prosperity and studies for human have been investigated in recent decades, the outcome maintains poor understanding. Pneumonia is one of the serious complication at the time of hospitalization and it should be known as more as possible for steadying patient conditions in intensive care unit and shortening length of stay. Thus, we conducted a meta-analysis of published materials to assess the association between alcohol intake and pneumonia in CNS injury. Methods: Two authors searched the PUBMED, EMBASE, Cochrane Library, and web of science up to September, 2014 for published literatures without any limitations. Reference lists from identified studies were also screened carefully by us for additional data. The summary relative risks (RRs) and 95% confidence intervals (CI) were calculated by statistical analysis software (Stata 12.0) with fixed-effects models to estimate the risk. Result: The results indicated that a higher incidence of pneumonia was found in CNS injury under the influence of alcohol (RR = 1.32, 95% CI = 1.21-1.43), and the risk has no relation to blood alcohol concentration (BAC) (BAC ≥ 80 mg/dl vs < 80 mg/dl, BAC ≥ 100 mg/dl vs < 100 mg/dl). Conclusion: Traumatic brain injury (TBI) and spinal cord injury patients who are under the influence of alcoholic drink have a higher risk of pneumonia.
Keywords: Central nervous system, pneumonia, alcohol, meta-analysis
Introduction
Alcohol intake is prevalent in trauma patients and a positive serum of alcohol is detected in 66% traumatic brain injury (TBI) patients in USA [1]. The outcome is worse with an incremental length of stay in intensive care unit or hospital [2,3]. We are informed that alcohol positive trauma patients are more susceptible to have a higher incidence of complications [3-5]. Infection develops a major cause of morbidity following head injury and multiple traumatic [6]. Incidences of pneumonia of TBI and spinal cord injury with alcohol intake are 10.6% and 15.7% respectively. Additionally, intoxicated patients are more likely to be intubated for opening the airway in the field or emergency room, or develop pneumonia [7].
However, opponents have had found no specific association between worse complications or more serious injury and alcohol drinking [8-10]. Besides, several studies shown improved outcome are obtained from people who are acute alcohol intoxicated rather than patients who does not drink alcohol at all [11-13]. Hadjibashi and his cooperator indicated a significant lower pneumonia incidence in isolated moderate to severe TBI patients with a positive serum alcohol level [1]. We get a contradictory conclusion resulting from inconsistent results from different studies. Therefore, we are eager for the association between alcohol intake and the risk of pneumonia in TBI and spinal cord injury patients. Then a meta-analysis of published observational studies was performed to clarify conclusion.
Material and methods
Inclusion criteria
The following selection criterions were defined according the PICOS: (1) Participants (P): All the patients were TBI or spinal cord injury. (2) Interventions (I) and comparisons (C): Alcohol or alcoholic drink were regarded as interventions; Comparing the effect of alcohol versus no alcohol; Comparing the effect of different serum alcohol levels. (3) Outcomes (O): Pneumonia in comparison of alcohol versus no alcohol, or different serum alcohol levels was reviewed. (4) Study design (S): A case-control study.
We excluded the following publications: (1) The studies had on complete data (RRs with 95% corresponding or sufficient data to estimate them). (2) For repeat published articles or data from the same study, the largest sample size or the most recent studies were included. (3) The chronic alcohol ingestion studies. (4) No full-text articles and failed to retrieve the data from authors.
Search strategy
Two reviews identified literatures by searching four databases, PUBMED, EMBASE, Cochrane Library, and web of science up to September, 2014, for published studies with no limitations. For adjusting in four databases correctly, reviewers use free terms combined with MeSH as research strategy: (“Spinal Cord Injuries” [Mesh] OR “Brain Injuries” [Mesh] OR “spinal cord shock” OR “spinal cord contusion” OR “brain trauma” OR “brain injury” OR “head injury” OR “traumatic brain injury”) AND (“Ethanol” [Mesh] OR “Alcohols” [Mesh] OR alcohol OR ethanol) OR “grain alcohol” OR “ethyl alcohol” OR “alcoholic beverages” OR beer OR wine) AND (“Pneumonia” [Mesh] OR “Pneumonia, Bacterial” [Mesh] OR pneumonitis OR pneumonia). No language limitations were imposed. We contacted the authors for more complete information if we needed. A manual search of identified articles’ references was conducted for more studies.
Date extraction
We (CMS and LS) extracted the following date independently from the eligible studies: author, country, year of publication, ages, case/control numbers, Glasgow Coma Scale (GCS), head abbreviated injury scale (AIS), detection of blood alcohol concentration (BAC). RRs and 95% corresponding or sufficient data can be used to calculate them. Two reviews (LS and CMS) performed an eligibility assessment independently in a standardized manner and resolved the disagreement by discussion or in consultation with the third author.
Quality analysis
Authors assessed the quality of included studies independently using the Newcastle-Ottawa Scale (NOS) criteria [14]. Three steps in the NOS criteria in total: (1) subject selection, 0~4; (2) comparability of subject, 0~2; (3) clinical outcome, 0~3. The NOS scores ranged from zero to nine. The study was indicated a good quality if it got more than 6 scores; Otherwise the study was deemed to have relatively lower quality.
Data analysis
The STATA software (Version 12.0, Stata Corporation, College Station, TX, USA) was used for data analysis. For comparison of pneumonia results, the relative risks (RR) and its 95% confidence intervals (CI) were used as estimation of relationship between alcohol intake and the risk of pneumonia for CNS injury. The RR and 95% CI values were pooled directory from the studies or were obtained from the available data. Heterogeneity among studies was assessed by the Q statistic and I2 statistic [15,16]. For Q statistic, heterogeneity was considered significant when P < 0.1 [16]. I2 statistic is a quantitative measure of inconsistency across studies. I2 < 50% is considered to be indicative of acceptable heterogeneity (I2 = 0-25%, no heterogeneity; I2 = 25-50%, moderate heterogeneity; I2 = 50-75%, large heterogeneity; I2 = 75-100%, extreme heterogeneity) [15,16]. The random-effects model was used when heterogeneity was observed significantly [17]. When significant heterogeneity was still observed, subgroup analysis was performed to explore potential factors. For a stable assessment, sensitivity and publication bias analysis were conducted as previously [18,19]. Egger’s regression test was chosen for potential publication bias estimating and P < 0.05 was considered indicative of significant publication bias [20]. Besides, sensitivity analysis was conducted to evaluate the impact of each study to overall assessment.
Results
Literature search and study characteristics
Figure 1 shows the flow diagram of literature selection progress. The literature searches yielded 228 articles, of which 34 were selected as relevant topic based on the title and abstract. Five studies [4,21-24] with no available data were excluded finally. Data of six included studies [1,7,12,25-27] with 10726 cases and 29262 controls were suitable for meat-analysis. Two of the studies [7,25] attached importance to analyzing link between different BACs and the risk of pneumonia. six moderate or high quality studies, of which got a NOS score > 6 were performed in USA. More characteristics of included studies were shown in Table 1. A large heterogeneity coming from the study [1] conducted by Hadjibashi et al by excluding each study in turn was found. The heterogeneity occurring in the analysis probably dues to its study designs, selection criterions or other potential factors.
Figure 1.
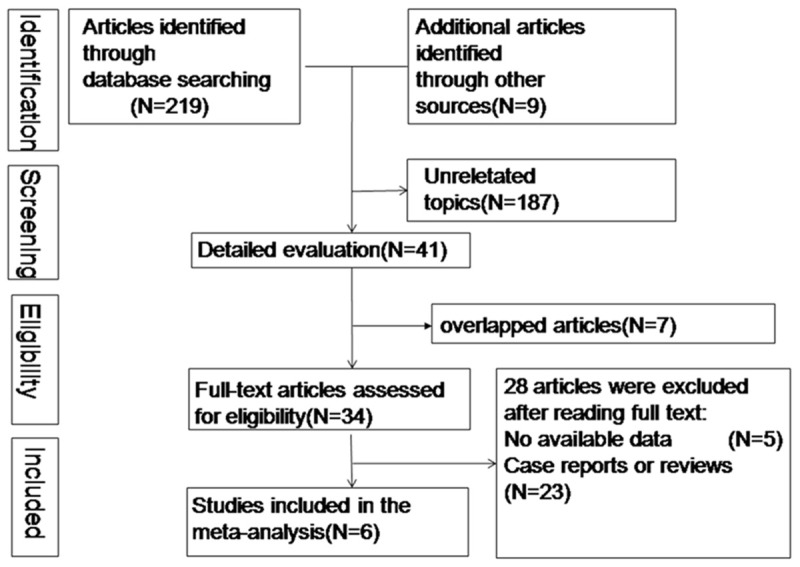
Flow diagram of literature selection progress.
Table 1.
Characteristics of studies included in the meta-analysis
| Study | Country | Ages (years) | Case/Control | GCS ≤ 8 | Head AIS | BAC Detection | Study Quality |
|---|---|---|---|---|---|---|---|
| Gurney 1992 | USA | ≥ 18 | 191/329 | NR | NR | Serum alcohol | 8 |
| Salim 2009 | USA | 38.6 ± 16.9a | 179/303 | 109a | AIS ≥ 4 116a | Serum alcohol | 8 |
| 37.7 ± 23.4b | 124b | 208b | |||||
| Talving 2010 | USA | 14-64 | 347/468 | NR | AIS ≥ 3 58a | NR | 7 |
| 56b | |||||||
| Hadjibashi 2012 | USA | 42.7 ± 18.7a | 2345/1202 | 585a | 3.73 ± 0.76a | Serum alcohol | 8 |
| 44.7 ± 21.5b | 246b | 3.71 ± 0.77b | |||||
| Crutcher 2014 | USA | 39 ± 24 | 2203/8408 | 1169 | NR | Serum alcohol | 8 |
| Pandit 2014 | USA | 43.4 ± 16.8a | 5461/18552 | 1240a | 4-5a | Serum alcohol | 8 |
| 50.2 ± 21.4b | 2278b | 4-5b |
GCS: Glasgow Coma Scale; AIS: Abbreviated Injury Scale; BAC: blood alcohol concentration;
case group;
control group.
Alcohol intake and pneumonia
Figure 2 shows the RR and 95% CI of pneumonia of alcohol intake from six studies. Among four studies, the pooled RR for alcohol intake versus no alcohol intake of CNS injured was 1.32 (95% CI = 1.21-1.43; I2 = 83.1%, P for heterogeneity = 0). And the combined risk estimate was 1.24 (95% CI = 1.10-1.39; I2 = 87.1%, P for heterogeneity = 0) for TBI with alcohol intake. The RR for BCA ≥ 80 mg/dl versus < 80 mg/dl was 0.70 (95% CI = 0.38-1.28), and the RR for BCA ≥ 100 mg/dl versus < 100 mg/dl was 1.41 (95% CI = 0.91-2.18).
Figure 2.
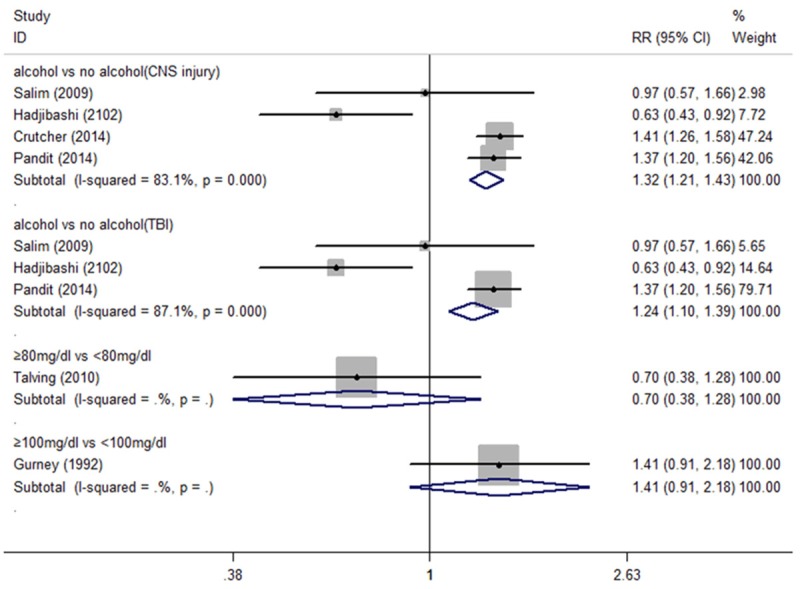
Forest plot of alcohol intake and risk of pneumonia in CNS injury patients.
Sensitivity analysis and publication bias
In our sensitivity analysis, we leaved out each study in turn for investigating the influence of a single study on the overall risk estimate. In the Figure 3, the corresponding RRs for alcohol versus no alcohol in CNS injury patients were not significant altered. And the result of Egger’s test suggested that no pronounced publication bias was found in our meta-analysis of CNS injury and TBI (P for Egger’s test were 0.432, 0.839 respectively).
Figure 3.
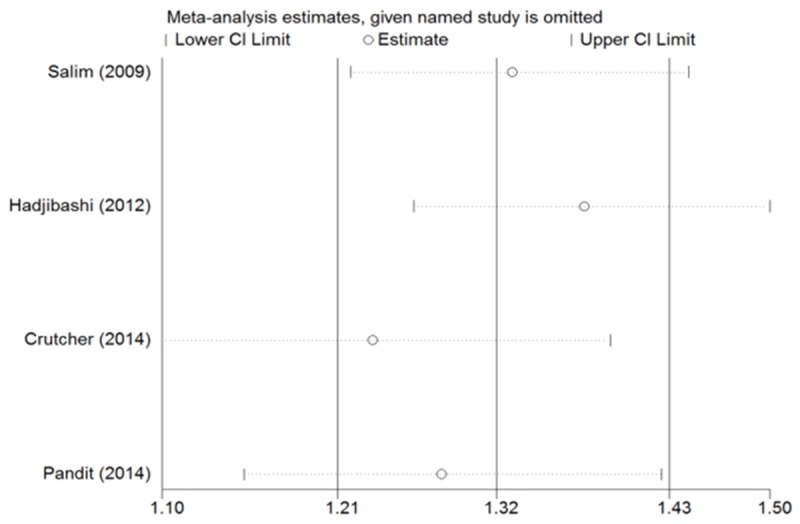
Sensitivity analyses of alcohol intake and no alcohol.
Discussion
It is well known that alcohol intake is highly prevalent in traffic-related fatalities. And 64% of post-traumatic deaths were attributable to a finite list of post-injury complications [28], for example deep vein thrombosis, the requirement for mechanical ventilation, pneumonia and so on. However, we were lack of convictive evidence about the relationship between the effect of alcohol and risk of pneumonia of patients with TBI and spinal cord injury. Then, a meta-analysis of relative studies was conducted to clear up confusion to some extent.
A systematic review and meta-analysis performed by Samokhvalov indicated that alcohol-use disorders got stuck in an eightfold increased risk of community-acquired pneumonia [29]. Our study finds that alcohol intake increases the risk of pneumonia in acute TBI and spinal cord injury patients. Two recent studies [26,27] published in 2014 come to an agreement towards to the effect of alcohol intake and are consistent with our consequences. An increased risk of pneumonia probably could be explained by that alcohol alters CNS sensitivity to glutamate and γ-aminobutyric acid and is regard as one kind of CNS depressant. As a result, depressant acts mainly on CNS by depressing the cough reflex which prevents aspiration of different gastric content. Hence, a close relationship is observed between alcohol intoxication and aspiration pneumonia. On the micro level, the damage of normally tight intercellular junction between adjacent epithelial cells created conditions for developing pneumonia. At the same time, alcohol abuse probably alters the host immune response by several mechanisms and predisposes to pneumonia [30-34]. Subsequently, researchers found in invasive pneumococcal disease patients who were alcohol abusers had a higher mortality [35]. These hindsight guided doctors and told them more attention should be paid in pneumonia of CNS injury with alcohol intake. However, Hadjibashi and colleagues [1] confirmed that a significant lower pneumonia rate was found in TBI patients with a positive serum alcohol level. Their result was of interest. But only one study coming up with this conclusion was unconvincing.
Previous study confirmed that individuals consuming 24, 60, and 120 g of pure alcohol daily demonstrated RRs for incident community-acquired pneumonia of 1.12 (95% CI = 1.02-1.23), 1.33 (95% CI = 1.06-1.67) and 1.76 (95% CI = 1.13-2.77), respectively, versus non-drinkers. However, as shown in Figure 2, the results have no statistical significant (RR = 0.70, 95% CI = 0.38-1.28 for BCA ≥ 80 mg/dl versus < 80 mg/dl, RR = 1.41, 95% CI = 0.91-2.18 for BCA ≥ 100 mg/dl versus < 100 mg/dl, respectively). The former study focuses on the relationship between alcohol intake and community-acquired pneumonia rather than hospital acquired pneumonia. It seems that it is probably the main reason for non-uniform between the studies. On the other hand, the results coming from two case-control studies with 1335 patients, may lack of powerful persuasion. We need more studies with more efficient evidence to support or overthrow the view.
Awareness of several potential limitations of our meta-analysis should be heightened. First, all of our studies are case-control studies. Thus, some influenced factors are unavoidable, and randomized controlled trails are always wanted if it is possible. Second, there is not enough literature on the subject to get a stable conclusion, especially in estimated risk of different BACs. The current results could be corrected if more studies are obtained. Third, CNS injury is a wide range of topics. Therefore, the data may not be precise. Fourth, the period of investigation are not reported in current included studies. Patients with a long hospitial stay are more likely to suffer from pneumonia. Thus, extra risk factors were imposed to affect the results at different levels. Finally, all the studies were conducted in USA. So the results might have region limitations.
Acknowledgements
We would like to acknowledge the researchers for their published studies and the reviewers for their helpful comments on this paper.
Disclosure of conflict of interest
None.
References
- 1.Hadjibashi AA, Berry C, Ley EJ, Bukur M, Mirocha J, Stolpner D, Salim A. Alcohol is associated with a lower pneumonia rate after traumatic brain injury. J Surg Res. 2012;173:212–215. doi: 10.1016/j.jss.2011.05.029. [DOI] [PubMed] [Google Scholar]
- 2.Levy RS, Hebert CK, Munn BG, Barrack RL. Drug and alcohol use in orthopedic trauma patients: a prospective study. J Orthop Trauma. 1996;10:21–27. doi: 10.1097/00005131-199601000-00004. [DOI] [PubMed] [Google Scholar]
- 3.Spies CD, Kissner M, Neumann T, Blum S, Voigt C, Funk T, Runkel N, Pragst F. Elevated carbohydrate-deficient transferrin predicts prolonged intensive care unit stay in traumatized men. Alcohol Alcohol. 1998;33:661–669. doi: 10.1093/alcalc/33.6.661. [DOI] [PubMed] [Google Scholar]
- 4.Rootman DB, Mustard R, Kalia V, Ahmed N. Increased incidence of complications in trauma patients cointoxicated with alcohol and other drugs. J Trauma. 2007;62:755–758. doi: 10.1097/TA.0b013e318031aa7f. [DOI] [PubMed] [Google Scholar]
- 5.Vaaramo K, Puljula J, Tetri S, Juvela S, Hillbom M. Mortality of subjects with alcohol-related seizures increased after alcohol cheapening. Acta Neurol Scand. 2014;129:56–60. doi: 10.1111/ane.12150. [DOI] [PubMed] [Google Scholar]
- 6.Quattrocchi KB, Frank EH, Miller CH, MacDermott JP, Hein L, Frey L, Wagner FC Jr. Suppression of cellular immune activity following severe head injury. J Neurotrauma. 1990;7:77–87. doi: 10.1089/neu.1990.7.77. [DOI] [PubMed] [Google Scholar]
- 7.Gurney JG, Rivara FP, Mueller BA, Newell DW, Copass MK, Jurkovich GJ. The effects of alcohol intoxication on the initial treatment and hospital course of patients with acute brain injury. J Trauma. 1992;33:709–713. doi: 10.1097/00005373-199211000-00020. [DOI] [PubMed] [Google Scholar]
- 8.Andersen JA, McLellan BA, Pagliarello G, Nelson WR. The relative influence of alcohol and seatbelt usage on severity of injury from motor vehicle crashes. J Trauma. 1990;30:415–417. [PubMed] [Google Scholar]
- 9.Porter RS. Alcohol and injury in adolescents. Pediatr Emerg Care. 2000;16:316–320. doi: 10.1097/00006565-200010000-00003. [DOI] [PubMed] [Google Scholar]
- 10.Shih HC, Hu SC, Yang CC, Ko TJ, Wu JK, Lee CH. Alcohol intoxication increases morbidity in drivers involved in motor vehicle accidents. Am J Emerg Med. 2003;21:91–94. doi: 10.1053/ajem.2003.50025. [DOI] [PubMed] [Google Scholar]
- 11.Yaghoubian A, Kaji A, Putnam B, De Virgilio N, De Virgilio C. Elevated blood alcohol level may be protective of trauma patient mortality. Am Surg. 2009;75:950–953. [PubMed] [Google Scholar]
- 12.Salim A, Teixeira P, Ley EJ, DuBose J, Inaba K, Margulies DR. Serum ethanol levels: predictor of survival after severe traumatic brain injury. J Trauma. 2009;67:697–703. doi: 10.1097/TA.0b013e3181b5dcf2. [DOI] [PubMed] [Google Scholar]
- 13.Blondell RD, Looney SW, Krieg CL, Spain DA. A comparison of alcohol-positive and alcohol-negative trauma patients. J Stud Alcohol. 2002;63:380–383. doi: 10.15288/jsa.2002.63.380. [DOI] [PubMed] [Google Scholar]
- 14.Wells GA, Shea B, O’Connell D, Peterson J, Welch V, Losos M, Tugwell P. The Newcastle-Ottawa Scale (NOS) for Assessing the Quality of Nonrandomised Studies in Meta-Analyses. Ottawa Hospital Research Institute. 2012 [Google Scholar]
- 15.Higgins JP, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta-analyses. BMJ. 2003;327:557–560. doi: 10.1136/bmj.327.7414.557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Higgins JP, Thompson SG. Quantifying heterogeneity in a meta-analysis. Stat Med. 2002;21:1539–1558. doi: 10.1002/sim.1186. [DOI] [PubMed] [Google Scholar]
- 17.Zintzaras E, Ioannidis JP. Heterogeneity testing in meta-analysis of genome searches. Genet Epidemiol. 2005;28:123–137. doi: 10.1002/gepi.20048. [DOI] [PubMed] [Google Scholar]
- 18.Gandini S, Iodice S, Koomen E, Di Pietro A, Sera F, Caini S. Hormonal and reproductive factors in relation to melanoma in women: current review and meta-analysis. Eur J Cancer. 2011;47:2607–2617. doi: 10.1016/j.ejca.2011.04.023. [DOI] [PubMed] [Google Scholar]
- 19.Camargo MC, Goto Y, Zabaleta J, Morgan DR, Correa P, Rabkin CS. Sex hormones, hormonal interventions, and gastric cancer risk: a meta-analysis. Cancer Epidemiol Biomarkers Prev. 2012;21:20–38. doi: 10.1158/1055-9965.EPI-11-0834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Egger M, Davey Smith G, Schneider M, Minder C. Bias in meta-analysis detected by a simple, graphical test. BMJ. 1997;315:629–634. doi: 10.1136/bmj.315.7109.629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Corrigan JD. Substance abuse as a mediating factor in outcome from traumatic brain injury. Arch Phys Med Rehabil. 1995;76:302–309. doi: 10.1016/s0003-9993(95)80654-7. [DOI] [PubMed] [Google Scholar]
- 22.Christensen MA, Janson S, Seago JA. Alcohol, head injury, and pulmonary complications. J Neurosci Nurs. 2001;33:184–189. doi: 10.1097/01376517-200108000-00003. [DOI] [PubMed] [Google Scholar]
- 23.Wang HE, Balasubramani GK, Cook LJ, Yealy DM, Lave JR. Medical conditions associated with out-of-hospital endotracheal intubation. Prehosp Emerg Care. 2011;15:338–346. doi: 10.3109/10903127.2011.569850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Friedman LS. Complications associated with blood alcohol concentration following injury. Alcohol. 2014;48:391–400. doi: 10.1016/j.alcohol.2014.01.008. [DOI] [PubMed] [Google Scholar]
- 25.Talving P, Plurad D, Barmparas G, DuBose J, Inaba K, Lam L, Chan L, Demetriades D. Isolated Severe Traumatic Brain Injuries: Association of Blood Alcohol Levels With the Severity of Injuries and Outcomes. J Trauma. 2010;68:357–362. doi: 10.1097/TA.0b013e3181bb80bf. [DOI] [PubMed] [Google Scholar]
- 26.Crutcher CL 2nd, Ugiliweneza B, Hodes JE, Kong M, Boakye M. Alcohol intoxication and its effects on traumatic spinal cord injury outcomes. J Neurotrauma. 2014;31:798–802. doi: 10.1089/neu.2014.3329. [DOI] [PubMed] [Google Scholar]
- 27.Pandit V, Patel N, Rhee P, Kulvatunyou N, Aziz H, Green DJ, O’Keeffe T, Zangbar B, Tang A, Gries L, Friese RS, Joseph B. Effect of alcohol in traumatic brain injury: Is it really protective? J Surg Res. 2014;190:634–639. doi: 10.1016/j.jss.2014.04.039. [DOI] [PubMed] [Google Scholar]
- 28.Osler T, Glance LG, Hosmer DW. Complication-associated mortality following trauma: a population-based observational study. Arch Surg. 2012;147:152–158. doi: 10.1001/archsurg.2011.888. [DOI] [PubMed] [Google Scholar]
- 29.Samokhvalov AV, Irving HM, Rehm J. Alcohol consumption as a risk factor for pneumonia: a systematic review and meta-analysis. Epidemiol Infect. 2010;138:1789–1795. doi: 10.1017/S0950268810000774. [DOI] [PubMed] [Google Scholar]
- 30.Siggins RW, Melvan JN, Welsh DA, Bagby GJ, Nelson S, Zhang P. Alcohol suppresses the granulopoietic response to pulmonary Streptococcus pneumoniae infection with enhancement of STAT3 signaling. J Immunol. 2011;186:4306–4313. doi: 10.4049/jimmunol.1002885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Burnham EL, Gaydos J, Hess E, House R, Cooper J. Alcohol use disorders affect antimicrobial proteins and anti-pneumococcal activity in epithelial lining fluid obtained via bronchoalveolar lavage. Alcohol Alcohol. 2010;45:414–421. doi: 10.1093/alcalc/agq045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Raasch CE, Zhang P, Siggins RW 2nd, LaMotte LR, Nelson S, Bagby GJ. Acute alcohol intoxication impairs the hematopoietic precursor cell response to pneumococcal pneumonia. Alcohol Clin Exp Res. 2010;34:2035–2043. doi: 10.1111/j.1530-0277.2010.01291.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Boe DM, Nelson S, Zhang P, Quinton L, Bagby GJ. Alcohol-induced suppression of lung chemokine production and the host defense response to Streptococcus pneumoniae. Alcohol Clin Exp Res. 2003;27:1838–1845. doi: 10.1097/01.ALC.0000095634.82310.53. [DOI] [PubMed] [Google Scholar]
- 34.Mehta AJ, Guidot DM. Alcohol abuse, the alveolar macrophage and pneumonia. Am J Med Sci. 2012;343:244–247. doi: 10.1097/MAJ.0b013e31823ede77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Jawa RS, Stothert JC, Shostrom VK, Yetter DL, Templin HR, Cemaj SK, Lander L, Forse AR, Young DH. Alcohol withdrawal syndrome in admitted trauma patients. Am J Surg. 2014;208:781–787. doi: 10.1016/j.amjsurg.2014.04.007. [DOI] [PubMed] [Google Scholar]


