Abstract
Previous studies suggested a close association between the thymic stromal lymphopoietin (TSLP) genetic variants and allergic diseases. Here, we explored the correlation between the TSLP polymorphisms and allergic rhinitis susceptibility using meta-analysis. We searched PubMed, Ovid, Cochrane libraries, CNKI, Wanfang, and CQVIP databases until Apr 19, 2015. Quality assessment was conducted for each article according to the Strengthening the Reporting of Genetic Association studies (STREGA). The pooled measure was calculated as the inverse variance-weighted mean of the logarithm of the OR with 95% CI to assess the strength of association between TSLP polymorphisms and the risk of allergic rhinitis. Five case-control studies with 6351 cases and 11472 controls were included in this study. TSLP rs1898671 polymorphism was significantly associated with an increased risk of allergic rhinitis (OR=1.13; 95% CI, 1.07-1.20; P<0.0001). In the subgroup analysis by race, TSLP rs1898671 polymorphism was significantly associated with an increased risk of allergic rhinitis in Caucasian (OR=1.14; 95% CI, 1.08-1.21; P<0.00001). In the subgroup analysis by age group, adult individuals with TSLP rs1898671 polymorphism showed increased risk of allergic rhinitis (OR=1.13; 95% CI, 1.07-1.21; P<0.0001). Because only one study investigated the association between other SNPs in TSLP and allergic rhinitis risk, the meta-analyses were not performed. In summary, we demonstrated that TSLP rs1898671 polymorphism was associated with a higher risk for allergic rhinitis.
Keywords: Allergic rhinitis, thymic stromal lymphopoietin, meta-analysis, genetic
Introduction
Allergic rhinitis is a global health problem affecting at least 10 to 25% of the population. In the treatment of allergic disorders, specific immunotherapy occupies a central role as the only currently available causal method of treatment [1]. Severe allergic rhinitis has been associated with significant impairments in quality of life, sleep and work performance [2].
Thymic stromal lymphopoietin (TSLP) has been shown to play an important role in the initiation and maintenance of the allergic immune response. TSLP is an IL-7 like cytokine that induces type 2 inflammation. Several animal studies indicated that TSLP may contribute to both innate and adaptive type 2 inflammation [3]. The production of TSLP is known to be controlled by both innate and adaptive immune signaling including via activation of toll like receptors and cytokine receptors [4]. Mou et al. suggested that TSLP was increased significantly in allergic rhinitis nasal mucosa compared with normal control [5]. In addition, TSLP-mediated activation of human nasal mucosal CD1c (+) DCs triggered CCR7-dependent migration to the draining lymph nodes and enhanced their capacity to initiate TH2 responses [6].
Extensive research in humans and mice support a role of TSLP in allergic rhinitis development. Several genetic studies (genome-wide and single polymorphism studies) have shown multiple SNPs at the TSLP genomic locus associated with increased allergic rhinitis susceptibility. However, other studies did not confirm this result [7-11]. Thus, we did a meta-analysis to assess the association between TSLP polymorphisms and the risk of allergic rhinitis.
Methods
Publication search
We searched PubMed, Ovid, Cochrane libraries, CNKI, Wanfang, and CQVIP databases until Apr 19, 2015. The following medical subject headings were used: “allergic rhinitis”, “Thymic stromal lymphopoietin”, and “TSLP”. Electronic searches were supplemented with manual searches of reference lists of all retrieved review articles, primary studies, and abstracts from meetings to identify other studies not found in the electronic searches. Literature was searched by two authors independently.
Inclusion and exclusion criteria
Two authors independently selected trials and discussed with each other when inconsistencies were found. Articles that meet the following criteria were included: (1) Regarding study types, they should be case-control or cohort studies; (2) Regarding participants, they should be allergic rhinitis patients and normal population; (3) There should investigate TSLP polymorphisms and the risk of allergic rhinitis; (4) Regarding outcome measure, studies should have sufficient data to examine an odds ratio (OR) with 95% confidence interval (CI); and (5) Full texts should be available. Studies with the following situations were excluded: (1) Not case-control or cohort studies; (2) Case reports, letters, reviews, editorial articles, and animal studies; (3) Duplicate or insufficient data; (4) Family-based design; (5) Controls were not in Hardy-Weinberg equilibrium (HWE).
Data extraction
Data from published studies were extracted carefully. For each study, we collected the following information: first author, year of publication, ethnicity, age, sex, numbers of cases and controls, and SNPs in TSLP.
Quality assessment
Quality assessment was conducted for each article according to Strengthening the Reporting of Genetic Association studies (STREGA) [12] containing eleven items associated with valid data reported in the study. For each item, there are three degrees, “yes” (scored 2), “can’t tell” (scored 1) or “no” (scored 0), after evaluating each item, a total score from 0 to 22 was reported for each article. Studies would be divided into 3 grades: Grade A (scored 15-22, high quality), Grade B (scored 8-14, medium quality), or Grade C (scored 0-7, inferior quality). Only the studies of Grade A or B would be included in the final analysis.
Statistical analysis
The pooled measure was calculated as the inverse variance-weighted mean of the logarithm of the OR with 95% CI to assess the strength of association between TSLP polymorphisms and the risk of allergic rhinitis. The significance of the pooled ORs was determined by the Z test with a P value less than 0.05 considering statistically significant. The per-allele model was examined to assess this association. Heterogeneity among studies was assessed using the Q test and the I2 statistic, which describes the proportion of total variation attributable to between-study heterogeneity as opposed to random error or chance. In the presence of substantial heterogeneity (I2>50%), the DerSimonian and Laird random effect model was applied as the pooling method; otherwise, the fixed effect model was adopted. Statistical analyses were conducted in STATA version 11.0 (Stata Corporation, College station, TX, USA). All the tests were two-sided.
Results
Study characteristics
As shown in Figure 1, a total of 62 abstracts were reviewed; among these articles, 44 were retrieved that are closely related to the current subject. However, 39 studies were excluded. Finally, 5 case-control studies met our inclusion criteria (Table 1). In total, 6351 cases and 11472 controls were included in this systematic review. The baseline characteristics of each study included in this meta-analysis are described in Table 1.
Figure 1.
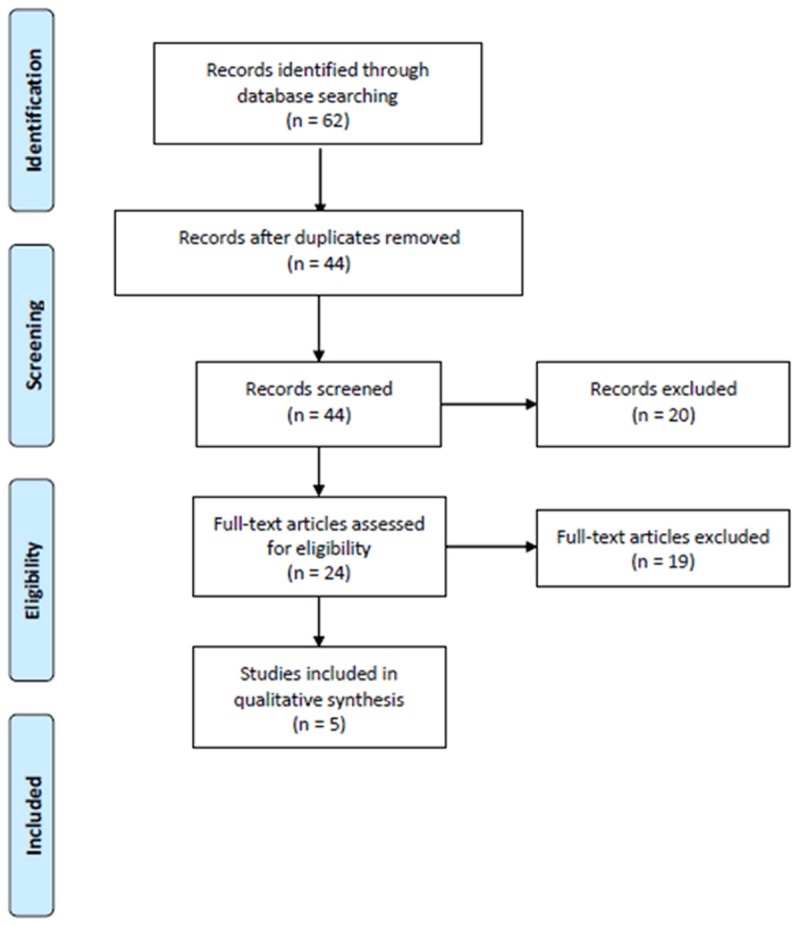
Flow chart of the literature search.
Table 1.
Characteristics of the studies included in this meta-analysis
| Study | Year | Ethnicity | Age group | Gender | No. of Case | No. of Control | Quality grade | Polymorphism |
|---|---|---|---|---|---|---|---|---|
| Ramasamy | 2011 | Caucasian | Adult | Both | 3393 | 8965 | A (scored 20) | rs1898671 |
| Andiappan | 2012 | Asian | Adult | Both | 1858 | 668 | A (scored 21) | rs1898671 |
| Zhang | 2012 | Asian | Adult | Both | 368 | 325 | A (scored 17) | rs12653736, rs1837253, rs12654933, rs10455025, rs11466741, rs13156086, rs6886755, rs252706, rs2416259 |
| Birben | 2014 | Caucasian | Children | Both | 138 | 157 | A (scored 16) | rs3806933, rs2289276, rs10073816, rs11466749 |
| Nilsson | 2014 | Caucasian | Children | Both | 594 | 1357 | A (scored 16) | rs1898671 |
Results of meta-analysis
Table 2 presents the results of meta-analysis and the heterogeneity test. TSLP rs1898671 polymorphism was significantly associated with an increased risk of allergic rhinitis (OR=1.13; 95% CI, 1.07-1.20; P<0.0001; Figure 2). In the subgroup analysis by race, TSLP rs1898671 polymorphism was significantly associated with an increased risk of allergic rhinitis in Caucasian (OR=1.14; 95% CI, 1.08-1.21; P<0.00001). In the subgroup analysis by age group, adult individuals with TSLP rs1898671 polymorphism showed increased risk of allergic rhinitis (OR=1.13; 95% CI, 1.07-1.21; P<0.0001). Because only one study investigated the association between other SNPs in TSLP and allergic rhinitis risk, the meta-analyses were not performed.
Table 2.
Result of this meta-analysis
| Polymorphism | No. of studies | Test of association | Heterogeneity | ||
|---|---|---|---|---|---|
|
|
|
||||
| OR (95% CI) | P Value | I 2 (%) | P Value | ||
| rs1898671 | 3 | 1.13 (1.07-1.20) | <0.0001 | 46 | 0.16 |
| Caucasian | 2 | 1.14 (1.08-1.21) | <0.00001 | 0 | 0.58 |
| Adult | 2 | 1.13 (1.07-1.21) | <0.0001 | 72 | 0.06 |
Figure 2.
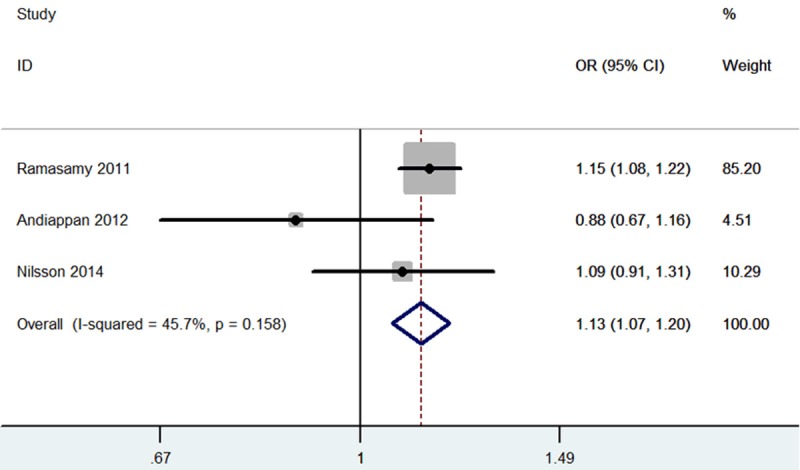
Meta-analysis for the association between TSLP rs1898671 polymorphism and allergic rhinitis.
A funnel plot was symmetrical and didn’t suggest a possibility of publication bias (Figure 3). The P-value of the Egger’s test is 0.274, suggesting no evidence of publication bias.
Figure 3.
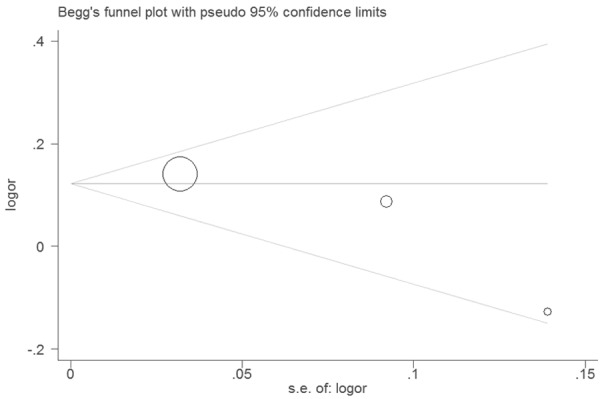
Funnel plot of for the association between TSLP rs1898671 polymorphism and allergic rhinitis.
Discussion
To the best of our knowledge, this is the first systematic meta-analysis covering available English literature to date demonstrating that carriers of TSLP rs1898671 polymorphism had a higher risk for allergic rhinitis.
Previous study found that the mRNA level of TSLP was significantly increased in nasal epithelial cells (NECs) of patients with allergic rhinitis compared with the non-allergic control group. In addition, in situ hybridization (ISH) results showed that expression of TSLP in NECs from patients with allergic rhinitis was up-regulated which was correlated with Visual Analog Scale (VAS) score and nasal eosinophils count [13]. Kamekura et al. found that TSLP thus not only activates dendritic cell (DC) but also preserves the epithelial barrier via the up-regulation of tight-junction proteins, thereby regulating antigen sensitization during the early stage of allergic rhinitis [14]. TSLP polymorphisms were also associated with other diseases. For example, Miyake et al. suggested that TSLP rs1837253 polymorphism may be significantly positively associated with eczema [15]. Rs2289278 in TSLP gene had relevance to breast cancer prognosis among Korean women [16]. Tsai and colleagues suggested that polymorphisms in TSLP may be used as genetic markers for the diagnosis and prognosis of Graves’s disease [17].
We should also pay attention to the limitations in this study, which may affect the result. Firstly, we only included published English articles available from online databases. Relevant articles published in other languages, in other databases and unpublished studies may have been missed, which might bias the results. Secondly, only five studies (two for Asian and three for Caucasian) were included in this meta-analysis. Thus, data on Asian and Caucasian subjects are inadequate. Considering this fact, the results from these two ethnicities should be interpreted with caution. Therefore, additional research in these two ethnicities and in other ethnicities is needed to generalize the findings. Thirdly, some studies had small sample sizes, which may affect the statistical power of the publication bias. Although publication bias in the meta-analysis by Egger’s test was not significant, there is also relatively little bias in the summary effect size estimate, so the results of these tests must be interpreted with caution in small-sample meta-analyses. Finally, considering the complex genetic network within, the potential role of the TSLP rs1898671 polymorphism might be diluted or masked by other gene-gene or gene-environment interactions.
In summary, we demonstrated that TSLP rs1898671 polymorphism was associated with a higher risk for allergic rhinitis. For practical reasons, we hope this study will enrich our understandings of TSLP polymorphisms in allergic rhinitis development. Future investigations to elucidate the specific role of TSLP polymorphisms in allergic rhinitis are warranted.
Acknowledgements
This work was funded by Jiangxi Provincial natural science foundation (No. 20151BAB205028).
Disclosure of conflict of interest
None.
References
- 1.Kägi MK, Wüthrich B. Different methods of local allergen-specific immunotherapy. Allergy. 2002;57:379–88. doi: 10.1034/j.1398-9995.2002.01153.x. [DOI] [PubMed] [Google Scholar]
- 2.Dykewicz MS, Hamilos DL. Rhinitis and sinusitis. J Allergy Clin Immunol. 2010;125:S103–15. doi: 10.1016/j.jaci.2009.12.989. [DOI] [PubMed] [Google Scholar]
- 3.Zhou B, Headley MB, Aye T, Tocker J, Comeau MR, Ziegler SF. Reversal of thymic stromal lymphopoietin-induced airway inflammation through inhibition of Th2 responses. J Immunol. 2008;181:6557–62. doi: 10.4049/jimmunol.181.9.6557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kato A, Favoreto S Jr, Avila PC, Schleimer RP. TLR3- and Th2 cytokine-dependent production of thymic stromal lymphopoietin in human airway epithelial cells. J Immunol. 2007;179:1080–7. doi: 10.4049/jimmunol.179.2.1080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Mou Z, Xia J, Tan Y, Wang X, Zhang Y, Zhou B, Li H, Han D. Overexpression of thymic stromal lymphopoietin in allergic rhinitis. Acta Otolaryngol. 2009;129:297–301. doi: 10.1080/00016480802225884. [DOI] [PubMed] [Google Scholar]
- 6.Melum GR, Farkas L, Scheel C, Van Dieren B, Gran E, Liu YJ, Johansen FE, Jahnsen FL, Baekkevold ES. A thymic stromal lymphopoietin-responsive dendritic cell subset mediates allergic responses in the upper airway mucosa. J Immunol. 2013;191:4903–7. doi: 10.1016/j.jaci.2014.05.010. [DOI] [PubMed] [Google Scholar]
- 7.Ramasamy A, Curjuric I, Coin LJ, Kumar A, McArdle WL, Imboden M, Leynaert B, Kogevinas M, Schmid-Grendelmeier P, Pekkanen J, Wjst M, Bircher AJ, Sovio U, Rochat T, Hartikainen AL, Balding DJ, Jarvelin MR, Probst-Hensch N, Strachan DP, Jarvis DL. A genome-wide meta-analysis of genetic variants associated with allergic rhinitis and grass sensitization and their interaction with birth order. J Allergy Clin Immunol. 2011;128:996–1005. doi: 10.1016/j.jaci.2011.08.030. [DOI] [PubMed] [Google Scholar]
- 8.Andiappan AK, Wang de Y, Anantharaman R, Suri BK, Lee BT, Rotzschke O, Liu J, Chew FT. Replication of genome-wide association study loci for allergic rhinitis and house dust mite sensitization in an Asian population of ethnic Chinese in Singapore. J Allergy Clin Immunol. 2013;131:1431–3. doi: 10.1016/j.jaci.2012.11.001. [DOI] [PubMed] [Google Scholar]
- 9.Zhang Y, Song X, Zhao Y, Zhang L, Bachert C. Single nucleotide polymorphisms in thymic stromal lymphopoietin gene are not associated with allergic rhinitis susceptibility in Chinese subjects. BMC Med Genet. 2012;13:79. doi: 10.1186/1471-2350-13-79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Birben E, Sahiner UM, Karaaslan C, Yavuz TS, Cosgun E, Kalayci O, Sackesen C. The genetic variants of thymic stromal lymphopoietin protein in children with asthma and allergic rhinitis. Int Arch Allergy Immunol. 2014;163:185–92. doi: 10.1159/000358488. [DOI] [PubMed] [Google Scholar]
- 11.Nilsson D, Henmyr V, Halldén C, Säll T, Kull I, Wickman M, Melén E, Cardell LO. Replication of genomewide associations with allergic sensitization and allergic rhinitis. Allergy. 2014;69:1506–14. doi: 10.1111/all.12495. [DOI] [PubMed] [Google Scholar]
- 12.Little J, Bradley L, Bray MS, Clyne M, Dorman J, Ellsworth DL, Hanson J, Khoury M, Lau J, O’Brien TR, Rothman N, Stroup D, Taioli E, Thomas D, Vainio H, Wacholder S, Weinberg C. Reporting, appraising, and integrating data on genotype prevalence and gene-disease associations. Am J Epidemiol. 2002;156:300–10. doi: 10.1093/oxfordjournals.aje.a000179. [DOI] [PubMed] [Google Scholar]
- 13.Zhu DD, Zhu XW, Jiang XD, Dong Z. Thymic stromal lymphopoietin expression is increased in nasal epithelial cells of patients with mugwort pollen sensitive-seasonal allergic rhinitis. Chin Med J (Engl) 2009;122:2303–7. [PubMed] [Google Scholar]
- 14.Kamekura R, Kojima T, Koizumi J, Ogasawara N, Kurose M, Go M, Harimaya A, Murata M, Tanaka S, Chiba H, Himi T, Sawada N. Thymic stromal lymphopoietin enhances tight-junction barrier function of human nasal epithelial cells. Cell Tissue Res. 2009;338:283–93. doi: 10.1007/s00441-009-0855-1. [DOI] [PubMed] [Google Scholar]
- 15.Miyake Y, Hitsumoto S, Tanaka K, Arakawa M. Association Between TSLP Polymorphisms and Eczema in Japanese Women: the Kyushu Okinawa Maternal and Child Health Study. Inflammation. 2015;38:1663–8. doi: 10.1007/s10753-015-0143-z. [DOI] [PubMed] [Google Scholar]
- 16.Choi J, Song N, Han S, Chung S, Sung H, Lee JY, Jung S, Park SK, Yoo KY, Han W, Lee JW, Noh DY, Kang D, Choi JY. The associations between immunity-related genes and breast cancer prognosis in Korean women. PLoS One. 2014;9:e103593. doi: 10.1371/journal.pone.0103593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Tsai KH, Tsai FJ, Lin HJ, Lin HJ, Liu YH, Liao WL, Wan L. Thymic stromal lymphopoietin gene promoter polymorphisms and expression levels in Graves’ disease and Graves’ ophthalmopathy. BMC Med Genet. 2012;13:116. doi: 10.1186/1471-2350-13-116. [DOI] [PMC free article] [PubMed] [Google Scholar]


