Abstract
This study was to investigate the effects of Rosa laevigata Michx. (RLM) extract on reactive oxygen species (ROS) production and mitochondrial membrane potential (MMP) in lens epithelial cells (LECs) cultured under high glucose. SRA01/04 cell models of diabetic cataract were established by high glucose culture, and these cells were administrated with RLM extract. Levels of ROS and MMP in SRA01/04 cells were detected with fluorescent probes. HO-1 expression level, Akt phosphorylation, and Nrf2 translocation were detected by Western blot. RLM treatment could exert protective effects on SRA01/04 cells under high glucose condition, decreasing the ROS production and elevating the MMP in these cells. Western blot analysis indicated that the expression level of HO-1 was significantly elevated in SRA01/04 cells after the RLM treatment. Moreover, when HO-1 was interfered with siRNA, the effects of RLM on the levels of ROS and MMP were diminished, indicating that HO-1 induction was necessary for the function of RLM. Furthermore, HO-1 inducing effects of RLM were mediated by the PI3K/Akt and Nrf2/ARE pathways. The effects would be abolished when either pathway was inhibited by pharmacological manipulation or siRNA silencing. RLM inhibits ROS production and elevates MMP, through the induction of HO-1 expression, in LECs under high glucose condition. The protective effects of RLM are mediated by the PI3K/Akt and Nrf2/ARE pathways. Our findings provide theoretic basis and experimental evidence for the application of RLM in the treatment of diabetic cataract.
Keywords: Rosa laevigata Michx., reactive oxygen species, mitochondrial membrane potential, heme oxygenase-1
Introduction
Diabetic cataract is the most serious complication of diabetes mellitus [1]. The pathogenesis of diabetic cataract is complicated, which could probably be induced by osmotic changes, oxidative stress, and altered crystallin glycosylation [2,3]. More and more evidence indicates that metabolic abnormality in mitochondrial production of reactive oxygen species (ROS) might be an important factor in diabetic cataract. ROS can not only interfere with the activities of Na+, K+-ATPase, but also induce the lens epithelial cells (LECs) to differentiate into lens fiber cells [4,5]. Apoptosis of LECs is actually the final step in the pathogenesis of diabetic cataract [6]. Therefore, early intervention targeting the oxidative stress may be beneficial in the management of diabetic cataract, preventing or delaying the disease onset.
Rosa laevigata Michx. (RLM) is one of the well-known medicinal plants in China, belonging to the Rosaceae family. Studies have shown that RLM extract can exert potent anti-oxidant and free radical-scavenging effects [7]. It has been shown that RLM significantly reduces the levels of ROS and MDA, enhances the activities of SOD and T-AOC, decreases the oxidative stress level, and down-regulates the expression levels of NF-κB, MCP-1 and TNF-α, in kidney tissues in rat models of diabetes [8]. In addition, the anti-oxidant effects of RLM ingredients have also been confirmed in vitro [9]. However, the therapeutic effects of RLM extract on diabetic cataract, especially concerning its anti-oxidant activities, have not yet been fully elucidated.
In the present study, LEC model of diabetic cataract was established by high glucose culture, and the effects of RLM extract on the ROS production and the mitochondrial membrane potential (MMP) were investigated. Possible molecular mechanisms were also explored and discussed. Our findings provide theoretic basis for the application of RLM in the treatment of diabetic cataract in clinic.
Materials and methods
Rosa laevigata Michx. (RLM) extraction
RLM was provided by the Shiyan Chinese Herbal Medicine Company (Shiyan, Hubei, China). After the seeds were removed, the medicinal herbs were boiled in an extracting machine. RLM extract with a final concentration of 2.0 g/mL was used as the store solution for the following experiments.
Cell culture and drug treatment
Immortalized human lens epithelial cell (LEC) lines (SRA01/04) were obtained from the American Type Culture Collection (ATCC), and cultured with low glucose DMEM medium (Gibco, Grand Island, NY, USA), containing 20% fetal bovine serum (Gibco), in a 37°C, 5% CO2 incubator. In the normal control group, cells were cultured with 5.5 mmol/L glucose and 2% serum in the DMEM medium. In the model group, cells were treated with high concentration of glucose (30.5 mmol/L). In the RLM-treated groups, cells were first cultured with high glucose, and then subjected to the incubation with RLM extract at indicated concentrations, i.e., 0.1, 5 and 10 g/L, for 24 h before subsequent assessments.
RNA silencing analysis
RNA interference of Heme Oxygenase-1 (HO-1) and Nrf2 was performed with the kits from Ambion (Austin, TX, USA), according to the manufacturer’s instructions. Small interfering RNA (siRNA) sequences specific for HO-1 and Nrf2 were produced by Invitrogen (Shanghai, China): HO-1, forward 5’-AACUUUCAGAAGGGCCAGGUGTT-3’ and reverse 5’-CACCUGGCCCUUCUGAAAGUUTT-3’; Nrf2, 5’-GCCGCUUAGAGGCUCAUCUtt-3’. SRA01/04 cells were cultured in 100-mm dishes. After washing, 0.5 mL transfection solution (containing 8 μL siPORT Amine and 20 nM siRNA) was added to incubate the cells at 37°C, 5% CO2 for 5 h. Then 1.5 mL complete medium was added for a further incubation for 48 h. The expression levels of the target genes were confirmed by Western blot.
Western blot analysis
Total proteins were collected with the kits from Pierce (Rockford, IL, USA), according to the manufacturer’s instructions. Protein samples were subjected to SDS-PAGE, and then transferred onto PVDF membranes (Bio-Rad, Hercules, CA, USA) by the semi-dry transfer method. After blocking and washing, the membranes were incubated with the primary antibodies against HO-1 (Santa Cruz, Santa Cruz, CA, USA), Nrf2 (Santa Cruz), phospho-Akt (Cell Signaling, Beverly, MA, USA), and total Akt (Cell Signaling), respectively, at 4°C for 12 h. Then HRP-labeled goat anti-mouse secondary antibody was added for a further incubation. The protein bands were visualized after ECL development.
ROS level determination
SRA01/04 cells were incubated with 5 μmol/L H2DCFDA staining solution, at 37°C in dark, for 30 min. After washing, these cells were collected and re-suspended in PBS. The fluorescence intensity was detected using a fluorescent plate reader, at excitation wavelength of 485 nm and emission wavelength of 530 nm.
Mitochondrial membrane potential (MMP) detection
MMP was detected with rhodamine 123 staining. Cells in each group were collected, and 0.25 μmol/L rhodamine 123 was used to incubate the cells at 37°C in dark for 20 min. After centrifugation at 1000 rpm for 5 min, cells were washed and then re-suspended in PBS. The fluorescence intensity was detected with a microplate reader, at excitation wavelength of 490 nm and emission wavelength of 520 nm.
Statistical analysis
Data were expressed as mean ± SD. SPSS 15.0 software was used for statistical analysis. One-way analysis of variance and Student’s t-test were applied for the comparisons. P < 0.05 was considered statistically significant.
Results
RLM extract reduces ROS production and elevates MMP in SRA01/04 cells cultured under high glucose
To investigate the protective effects of RLM extract on SRA01/04 cells cultured under high glucose conditions, the levels of ROS and the MMP in these cells were measured after RLM treatment. The cells were cultured with 30.5 mmol/L glucose, and then treated with RLM extract at indicated concentrations, i.e., 0.1, 5 and 10 g/L, for 24 h. The detection of the oxidative stress level indicated that, compared with the normal control cells, high glucose culture in the high glucose model group induced significantly higher levels of ROS (136%) in SRA01/04 cells (P < 0.05) (Figure 1A). However, after the treatments with RLM extract, ROS levels in these cells under high glucose were dramatically decreased (105%), to a comparable level to those in the control cells with the highest treatment concentration (100%) (Figure 1A).
Figure 1.
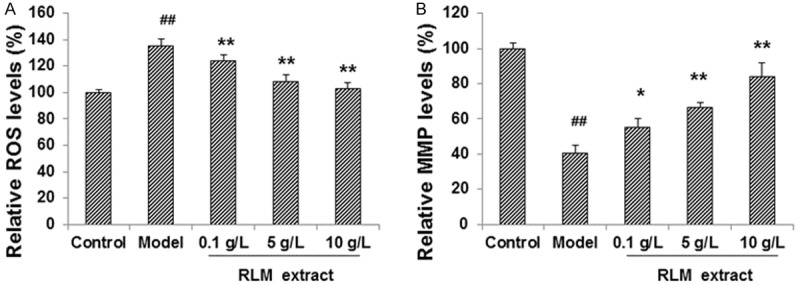
RLM decreased ROS production and increases MMP in SRA01/04 LECs under high glucose. SRA01/04 cells were cultured under high glucose condition, and treated with RLM extract, at indicated concentrations (0.1, 5 and 10 g/L), for 24 h. Then the levels of ROS (A) and MMP (B) in SRA01/04 cells were detected, respectively, with fluorescent probes. Compared with the normal control group, ##P < 0.01; compare with the model group, *P < 0.05, **P < 0.01.
On the other hand, as shown in Figure 1B, MMP was significantly declined to 42% in the high glucose model group, compared with the control group (100%) (P < 0.05). In the RLM-treated groups, MMP was increased along with the increasing concentrations of RLM. The MMP in the model cells treated with 10 g/L RLM was restored to 86.4% of the control level. Taken together, these results suggest that RLM treatment could decrease the ROS production and elevate the MMP in these cells, thus exerting protective effects on SRA01/04 cells under high glucose condition.
Protective effects of RLM extract depend on HO-1 induction in SRA01/04 cells cultured under high glucose
HO-1 expression levels have been found to be associated with the oxidative stress and the apoptotic process in cells under pathophysiological conditions. So we next investigate whether the expression level of HO-1 was affected in SRA01/04 cells cultured under high glucose, and whether its expression could be changed by the treatment of RLM. Our results indicated that the expression level of HO-1 was relatively low in the control group, and the high glucose culture condition caused a slightly increased expression of HO-1 in these cells (Figure 2A). Surprisingly, the expression levels of HO-1 in LECs under high glucose were drastically elevated after RLM extract treatments, in a dose-dependent manner, indicating that RLM extract could induce HO-1 up-regulation in SRA01/04 cells under pathological conditions.
Figure 2.
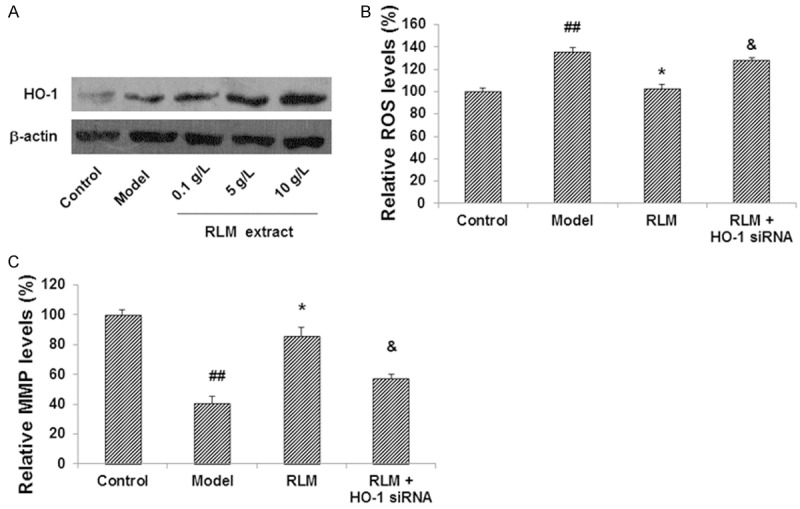
Effects of RLM depended on HO-1 induction in SRA01/04 LECs under high glucose. (A) Expression level of HO-1 was up-regulated in SRA01/04 cells treated with RLM extract. (B, C) Interference of HO-1 blocked the effects of RLM on the levels of ROS (B) and MMP (C) in SRA01/04 cells. HO-1 was interfered with siRNA, and then the effects of 10 g/L RLM extract on levels of ROS and MMP in SRA01/04 cells were assessed. Compared with the normal control group, ##P < 0.01; compared with the model group, *P < 0.05; compared with the RLM-treated group, &P < 0.05.
To investigate the role of over-expressed HO-1 in the protective effects of RLM, HO-1 RNA interference was conducted in these model cells. As shown in Figure 2B, when treated with HO-1 siRNA for 24 h, the inhibiting effects of RLM on ROS production was significantly weakened (P < 0.05). Similar results were observed for MMP. HO-1 siRNA interfered with the MMP-increasing effects of RLM extract (P < 0.05) (Figure 2C). These results indicate that RLM extract could significantly induce HO-1 expression in SRA01/04 cells under high glucose, which is necessary for the protective effects of RLM in these cells.
RLM extract induces HO-1 expression through PI3K/Akt and Nrf2/ARE pathways
HO-1 is a heat shock protein, whose expression and function have been closely linked with several cell signaling pathways, including the PI3K/Akt and Nrf2/ARE pathways. To find out if these signaling pathways were involved in the HO-1-increasing effects of RLM in SRA01/04 cells under high glucose, the phosphorylation levels of Akt in these cells, with or without RLM treatments, were detected with Western blot. As shown in Figure 3A, the phosphorylation level of Akt was very low in the control SRA01/04 cells, which was marginally increased under high glucose condition. After the treatments with RLM extract, the levels of phosphorylated Akt were significantly up-regulated (P < 0.05), in a dose-dependent manner, while the Akt levels did not change among these groups. To investigate whether the activated Akt was associated with the HO-1-inducing effects of RLM, these cells were pre-treated with PI3K inhibitor LY294002. Our results showed that after the treatment with LY294002, the over-expression of HO-1 in the RLM-treated group was obviously suppressed (Figure 3B), indicating that the PI3K/Alt pathway might be involved in the inducing effects of RLM on HO-1 expression.
Figure 3.
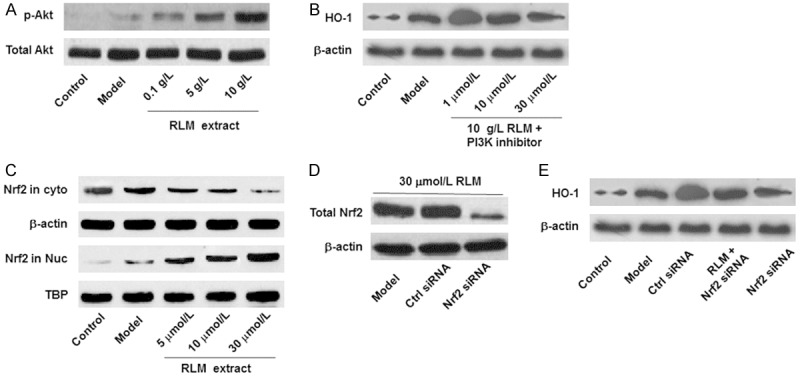
RLM induced HO-1 expression through the PI3K/Akt and Nrf2/ARE pathways in SRA01/04 LECs. A, B. The PI3/Akt pathway was involved in the function of RLM in LECs cultured under high glucose condition. A. RLM significantly elevated the levels of phosphorylated Akt (p-Akt) in SRA01/04 cells under high glucose. B. PI3K inhibitor down-regulated the expression levels of HO-1 in SRA01/04 cells under high glucose. C-E. The Nrf2/ARE pathway was involved in the function of RLM in LECs cultured under high glucose condition. C. RLM stimulated the translocation of Nrf2 from cytoplasm into nucleus in SRA01/04 cells. D. RNA silencing of Nrf2. Cells were treated with 30 μmol/L RLM, 30 μmol/L RLM + control siRNA, and 30 μmol/L RLM + Nrf2 siRNA, respectively. E. RNS silencing of Hrf2 canceled the inducing effects of RLM on HO-1 expression in SRA01/04 cells. RLM extract was administrated at the concentration of 30 μmol/L.
In addition, our results showed that Nrf2, the transcription factor of HO-1, was mainly located in the cytoplasm in the control and high glucose groups, with extremely low abundance in the nucleus. However, when treated with RLM extract (0.1-10 g/L), the Nrf2 levels in the cytoplasm were gradually decreased, while the Nrf2 contents were increased in the nucleus (Figure 3C). Moreover, after Nrf2 was interfered with siRNA, the expression levels of HO-1 in the RLM-treated groups were dramatically declined (Figure 3D and 3E). These results suggest that the HO-1 up-regulation induced by RLM are mediated by the PI3K/Akt and Nrf2/ARE pathways, which would also be necessary for the beneficial effects of RLM extract on SRA01/04 LECs under high glucose condition.
Discussion
In patients with diabetes mellitus, the levels of ATP and GSH in lens are declined, and the transport function of active cations like Rb+ is damaged, vulnerable to ROS insult [10,11]. Excess ROS production can inhibit the activity of GAPDH, and accelerate the development of diabetic cataract [12,13]. In addition, ROS can also lead to increased dihydroxyacetone phosphate and fructose 6-phosphate levels, which in turn activate PKC and the hexosamine pathway [14-16]. Rosa laevigata Michx. (RLM) (Rosaceae) is a commonly distributed evergreen climbing shrub in South China, and its fruits are rich in saponins, flavonoids, polysaccharides, and other physiologically active ingredients. Pharmacological studies have shown that RLM could exert potent antioxidant effects, protecting against the cell membrane damages and the lipid peroxidation induced by excessive free radicals [17,18]. Based on these findings, we investigated the role of RLM extract in the treatment of diabetic cataract. Our results indicated that, when treated with RLM extract, the ROS level in SRA01/04 cells cultured under high glucose condition was significantly decreased, indicating that RLM could exert protective effects in LECs under high glucose by inhibiting ROS. On the other hand, another important function of ROS is inducing apoptosis in LECs, in which the change in MMP has been considered as an indicator for early apoptotic processes [19]. Our results showed that RLM extract could dramatically elevated MMP in SRA01/04 cells, which might antagonize the toxic effects of high glucose on LECs.
HO-1 is an inducible enzyme located on chromosome 22q12, which is also known as heat shock protein 32 (HSP32). HO-1 is expressed in almost all tissues, specifically with the highest expression level in the endoplasmic reticulum. The expression could be stimulated by a variety of injuries. HO-1 can catalyze the degradation of hemocrystallin into CO, ferrous ion, and biliverdin, which could subsequently be converted to bilirubin by biliverdin reductase [20,21]. Studies have shown that HO-1 and its metabolites have anti-inflammatory, anti-proliferative, anti-oxidant, and anti-apoptotic effects in maintaining cellular homeostasis [7,12]. For examples, the down-regulated expression or absence of HO-1 (pharmacological inhibition or knockout) could reduce the body’s tolerance to the oxidative stress, leading to extensive oxidative damage or organ failure [14]. In this study, although the high glucose condition could induce the expression of HO-1 in SRA01/04 cells, this feedback response is not strong enough to fight against the oxidative stress. Our results showed that RLM could efficiently up-regulate the expression of HO-1. Furthermore, after RNA interference of HO-1, the ROS level was increased, while MMP was decreased, indicating that HO-1 could inhibit the production of ROS and restore the declined MMP. This might be one of the mechanisms underlying the antioxidant effects of RLM. However, further studies are still needed to investigate the way HO-1 protects against the oxidative stress.
We have also found that RLM could activate the PI3K/Akt pathway, and induce the nuclear translocation of Nrf2. Nrf2 can bind with antioxidant response elements (ARE) to facilitate the expression of a variety of endogenous antioxidant enzymes (including HO-1), achieving in vivo rebalancing without causing excessive antioxidant responses [22,23]. These results suggest that, the anti-oxidant effects of RLM in LECs under high glucose are mediated by the PI3K/Akt and the Nrf2/ARE pathways. Our results also showed that, when the PI3K/Akt pathway was pharmacologically inhibited, or the expression of Nrf2 was interfered with siRNA, the inducing effects of RLM on HO-1 expression could be eliminated. In addition, under normal conditions, there were no obviously elevated expression of HO-1 and Nrf2 in LECs, indicating that the modulation of antioxidant cell signaling pathways might be necessary for the inducing effects of RLM on HO-1 expression. Of course, further studies are still needed to investigate the detailed mechanism.
In conclusion, our results showed that RLM extract could up-regulate the expression of HO-1, inhibit ROS production and elevate MMP, in LECs cultured under high glucose condition. The effects of RLM on HO-1 expression are mediated by the PI3K/Akt and Nrf2/ARE pathways. Our findings provide the theoretic basis and experimental evidence for the application of RLM in the treatment of diabetic cataract.
Acknowledgements
This work was supported by the Science and Technology Program from Shiyan, Hubei (No. 2010st30).
Disclosure of conflict of interest
None.
References
- 1.Bourne RR, Jonas JB, Flaxman SR, Keeffe J, Leasher J, Naidoo K, Parodi MB, Pesudovs K, Price H, White RA, Wong TY, Resnikoff S, Taylor HR Vision Loss Expert Group of the Global Burden of Disease Study. Prevalence and causes of vision loss in high-income countries and in Eastern and Central Europe: 1990-2010. Br J Ophthalmol. 2014;98:629–638. doi: 10.1136/bjophthalmol-2013-304033. [DOI] [PubMed] [Google Scholar]
- 2.Njie-Mbye YF, Kulkarni-Chitnis M, Opere CA, Barrett A, Ohia SE. Lipid peroxidation: pathophysiological and pharmacological implications in the eye. Front Physiol. 2013;4:366. doi: 10.3389/fphys.2013.00366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Samanta A, Kumar P, Machhua S, Rao GN, Pal A. Incidence of cystoid macular oedema in diabetic patients after phacoemulsification and free radical link to its pathogenesis. Br J Ophthalmol. 2014;98:1266–1272. doi: 10.1136/bjophthalmol-2013-304438. [DOI] [PubMed] [Google Scholar]
- 4.Zheng Y, Liu Y, Ge J, Wang X, Liu L, Bu Z, Liu P. Resveratrol protects human lens epithelial cells against H2O2-induced oxidative stress by increasing catalase, SOD-1, and HO-1 expression. Mol Vis. 2010;16:1467–1474. [PMC free article] [PubMed] [Google Scholar]
- 5.Lupachyk S, Stavniichuk R, Komissarenko JI, Drel VR, Obrosov AA, El-Remessy AB, Pacher P, Obrosova IG. Na+/H+-exchanger-1 inhibition counteracts diabetic cataract formation and retinal oxidative-nitrative stress and apoptosis. Int J Mol Med. 2012;29:989–998. doi: 10.3892/ijmm.2012.933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kim J, Kim CS, Sohn E, Kim H, Jeong IH, Kim JS. Lens epithelial cell apoptosis initiates diabetic cataractogenesis in the Zucker diabetic fatty rat. Graefes Arch Clin Exp Ophthalmol. 2010;248:811–818. doi: 10.1007/s00417-010-1313-1. [DOI] [PubMed] [Google Scholar]
- 7.Zhou Y, Liao Q, Luo Y, Qing Z, Zhang Q, He G. Renal protective effect of Rosa laevigata Michx. by the inhibition of oxidative stress in streptozotocin-induced diabetic rats. Mol Med Rep. 2012;5:1548–1554. doi: 10.3892/mmr.2012.855. [DOI] [PubMed] [Google Scholar]
- 8.Zhou TJ, Liao QJ, Luo YP, Qing ZQ, Zhang QJ, He GP. Renal protective effect of Rosa laevigata Michx. by the inhibition of oxidative stress in streptozotocin-induced diabetic rats. Mol Med Rep. 2012;5:1548–1554. doi: 10.3892/mmr.2012.855. [DOI] [PubMed] [Google Scholar]
- 9.Zhao YT. Antioxidant activity of polysaccharide. J Biol. 2003;2:3739. [Google Scholar]
- 10.Choi AJ, Ryter SW. Inflammasomes: molecular regulation and implications for metabolic and cognitive diseases. Mol Cells. 2014;37:441–448. doi: 10.14348/molcells.2014.0104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bondeva T, Wolf G. Reactive oxygen species in diabetic nephropathy: friend or foe? Nephrol Dial Transplant. 2014;29:1998–2003. doi: 10.1093/ndt/gfu037. [DOI] [PubMed] [Google Scholar]
- 12.Wang JL, Kang GJ. Basic and clinical research of advanced glycation end products and diabetic cataract. Recent Advances in Ophthalmology. 2011;31:893–896. [Google Scholar]
- 13.Fulgêncio Cunha AA, Bosco AA, Veloso CA, Volpe CM, Chaves MM, Nogueira-Machado JA. Suppressive effect of aqueous humor from person with type 2 diabetes with or without retinopathy on reactive oxygen species generation. Diabetes Res Clin Pract. 2013;100:69–73. doi: 10.1016/j.diabres.2013.01.018. [DOI] [PubMed] [Google Scholar]
- 14.Zhang M, Shoeb M, Liu P, Xiao T, Hogan D, Wong IG, Campbell GA, Ansari NH. Topical metal chelation therapy ameliorates oxidation-induced toxicity in diabetic cataract. J Toxicol Environ Health A. 2011;74:380–391. doi: 10.1080/15287394.2011.538835. [DOI] [PubMed] [Google Scholar]
- 15.Kanth VR, Lavanya K, Srinivas J, Raju TN. Elevated Expression of indoleamine 2,3-dioxygenase (IDO) and accumulation of kynurenic acid in the pathogenesis of STZ-induced diabetic cataract in Wistar rats. Curr Eye Res. 2009;34:274–281. doi: 10.1080/02713680902725954. [DOI] [PubMed] [Google Scholar]
- 16.Qin D, Kang GJ. Polyol pathway and diabetic cataract. Recent Advances in Ophthalmology. 2010;30:698–700. [Google Scholar]
- 17.Zhou Y, Liao Q, Luo Y, Qing Z, Zhang Q, He G. Renal protective effect of Rosa laevigata Michx. by the inhibition of oxidative stress in streptozotocin-induced diabetic rats. Mol Med Rep. 2012;5:1548–1554. doi: 10.3892/mmr.2012.855. [DOI] [PubMed] [Google Scholar]
- 18.Zhang S, Zheng L, Dong D, Xu L, Yin L, Qi Y, Han X, Lin Y, Liu K, Peng J. Effects of flavonoids from Rosa laevigata Michx fruit against high-fat diet-induced non-alcoholic fatty liver disease in rats. Food Chem. 2013;141:2108–2116. doi: 10.1016/j.foodchem.2013.05.019. [DOI] [PubMed] [Google Scholar]
- 19.Kanth VR, Lavanya K, Srinivas J, Raju TN. Elevated Expression of indoleamine 2,3-dioxygenase (IDO) and accumulation of kynurenic acid in the pathogenesis of STZ-induced diabetic cataract in Wistar rats. Curr Eye Res. 2009;34:274–281. doi: 10.1080/02713680902725954. [DOI] [PubMed] [Google Scholar]
- 20.Kyselova Z, Stefek M, Bauer V. Pharmacological prevention of diabetic cataract. J Diabetes Complications. 2004;18:129–140. doi: 10.1016/S1056-8727(03)00009-6. [DOI] [PubMed] [Google Scholar]
- 21.Chen J. Heme oxygenase in neuroprotection: from mechanisms to therapeutic implications. Rev Neurosci. 2014;25:269–280. doi: 10.1515/revneuro-2013-0046. [DOI] [PubMed] [Google Scholar]
- 22.Ishii T, Mann GE. Redox status in mammalian cells and stem cells during culture in vitro: critical roles of Nrf2 and cystine transporter activity in the maintenance of redox balance. Redox Biol. 2014;2:786–794. doi: 10.1016/j.redox.2014.04.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ma JQ, Ding J, Xiao ZH, Liu CM. Puerarin ameliorates carbon tetrachloride-induced oxidative DNA damage and inflammation in mouse kidney through ERK/Nrf2/ARE pathway. Food Chem Toxicol. 2014;71:264–271. doi: 10.1016/j.fct.2014.06.017. [DOI] [PubMed] [Google Scholar]


