Abstract
Several clinical research studies have demonstrated that chronic cutaneous wounds can be treated with hair follicle grafts. However, the clinical outcomes of hair follicle grafting compared to split-thickness skin grafting have not been examined. This study sought to compare the clinical outcomes of patients with chronic wounds following hair follicle therapy and split-thickness skin graft therapy in a relatively large cohort of patients. Forty patients were enrolled in the study, a retrospective analysis of all patients underwent therapy with hair follicles (cohort A) and split-thickness skin grafts (cohort B) was performed. Safety, healing duration, skin quality (recipient site), scar formation (donor site) and overall postoperative outcome were analyzed. The wound sites were examined using photography at weeks 2, 8, and 12 after surgery. Five non-biased reviewers estimated the above-mentioned clinical outcomes using a five-point Likert scale. The ages and wound areas were similar between cohorts A (n=20) and B (n=20). Total wound closure was observed and adverse events were rare and controllable in both cohorts. The skin and scar quality were rated significantly higher in the hair follicle cohort than the split-thickness skin graft cohort (4.40 vs 3.45, P<0.05 and 4.65 vs 3.20, P<0.05; respectively). Hair follicle therapy resulted in a significantly higher overall score than split-thickness skin graft treatment (4.45 vs 3.40, P<0.05). This study demonstrated that hair follicles can achieve better skin/scar quality and overall clinical outcomes than split-thickness skin grafts. Hair follicles should be considered an effective surgical technique for the treatment of chronic cutaneous wounds.
Keywords: Hair follicle, re-epithelialization, wound healing, chronic wound
Introduction
Chronic cutaneous wounds are among the most difficult-to-treat pathophysiological processes in the routine practice of dermatologic and plastic surgery. Split-thickness skin grafting is the gold standard for covering chronic wounds with a well-vascularized wound bed [1]. Although some headway has been made in developing biological agents to accelerate healing, there is still no treatment that has sufficiently replaced skin grafts. Over the past decade, numerous studies have led to an abundant body of evidence showing a direct association between hair follicles and the wound healing process [2]. Thus, the use of hair follicles might be a feasible approach to developing topical wound applications for more rapid wound healing.
Cutaneous wound healing consists of a series of cascades that involves epithelial, dermal and mesenchymal tissues, blood vessels, nerves, and immune cells, which all respond during wound healing within a complex network of signals [3] and behaviors that are necessary for wound healing [4]. Re-establishing epithelial integrity is essential, and it is the first step in maintaining a regenerative response. The scalp has been considered an excellent donor site for thin skin grafts [5] due to its rapid healing capacity. Clinical and histological evidence has shown that the rapid healing of scalp dermal grafts can be attributed to the differential potentialities of cells in hair follicles, and re-epithelialization also occurs in grafted hair follicles [6]. Numerous types of multi-differential potential cells have been identified in the hair follicles, and their relative contributions to re-epithelialization were recently determined [7]. Several small single-center research studies postulated that transplanted hair follicles would activate progenitor cells and promote re-epithelialization of a wound. Given the abundant body of evidence suggesting the crucial function of the hair follicle as a promoter of re-epithelialization, the idea of grafting hair follicles into chronic cutaneous wounds has been considered.
Thus far, no studies have directly compared clinical outcomes using objective measurements and multiple blinded raters. Thus, the aim of this two-center clinical study was to compare the clinical outcomes in a relatively large cohort of patients with chronic cutaneous wounds following hair follicle therapy and skin graft therapy.
Materials and methods
Patients
We performed a two-center, institutional review board-approved, retrospective analysis of patients who underwent chronic wound reconstruction performed by the senior author from 2006 to 2011. Medical charts were reviewed to identify all of the patients who underwent reconstruction utilizing hair follicles or split-thickness skin graft therapy. The eligible patients had traumatic or surgical defect wounds that persisted for at least 6 weeks, were nonresponsive to non-surgical approaches, had signs of delayed healing, and were ≥5 cm2 and full thickness in depth. Patients with alopecia, coagulopathy or any other contraindications for the hair transplant technique, as well as diabetics and elderly patients (older than 80 years old), were excluded. Medical records were reviewed, and demographic, operative and postoperative data were collected. Digital photographs of the donor and recipient sites were obtained at weeks 2, 8 and 12 post-intervention. This study was conducted in accordance with the declaration of Helsinki. This study was conducted with approval from the Ethics Committee of Fudan University. Written informed consent was obtained from all participants.
Evaluation of clinical outcomes
A clinical outcomes evaluation of the two procedures was synchronously performed. To avoid misclassification bias, five independent, remote plastic surgeons assessed outcome parameters, including safety, healing duration, skin quality (recipient site), scar formation (donor site) and overall postoperative outcomes, using a five-point Likert scale (Table 1).
Table 1.
Five-point Likert Scale for outcome parameters
| Variable | Very poor | Poor | Average | Good | Very good |
|---|---|---|---|---|---|
| Procedure safety | 1 | 2 | 3 | 4 | 5 |
| Healing duration | 1 | 2 | 3 | 4 | 5 |
| Skin quality (recipient site) | 1 | 2 | 3 | 4 | 5 |
| Scar quality (donate site) | 1 | 2 | 3 | 4 | 5 |
| Overall outcome | 1 | 2 | 3 | 4 | 5 |
Statistical analysis
The data were pooled for each cohort to compare the outcomes between the two treatment arms. Scores were linked to each corresponding patient following the evaluation. Statistical Package for the Social Sciences (SPSS) software, version 20 for Windows (SPSS Inc., Chicago, IL, USA), was used to conduct Student’s t test, the Wilcoxon-Mann-Whitney test, the Kruskal-Wallis test and Spearman’s test. P<0.05 were considered statistically significant.
Results
Patients
In total, forty eligible patients were enrolled in the study. Twenty patients underwent hair follicle transplants (cohort A), and twenty patients underwent split-thickness skin grafting (cohort B). The patient demographics were not significantly different between cohorts A and B (Table 2). The mean age was 58.75 years old in cohort A and 49.00 years old in cohort B (P>0.05). The mean body mass index (BMI) was 28.44 kg/m2 in cohort A and 28.37 kg/m2 in cohort B (P>0.05). The wound area was similar between cohorts A and B (7.33 cm2 vs 7.16 cm2, P>0.05).
Table 2.
Patient demographics
| Variable | Hair follicles | Split-thickness skin | P-value |
|---|---|---|---|
| Age (year) | 57.1±14.8 | 49.0±14.4 | 0.09 |
| BMI (kg/cm2) | 28.44±1.37 | 28.37±1.41 | 0.86 |
| Wound square (cm2) | 7.33±1.43 | 7.16±1.53 | 0.71 |
Clinical outcomes
Hair follicle treatment seemed to be a safe and effective surgical procedure, as surveyed by the five non-biased reviewers (Table 3). Based on the safety evaluation, cohort A scored 4.40, and cohort B scored 4.50 (P>0.05). Adverse events were rare and controllable in both cohorts. Three patients had minor unrelated complications throughout their hospitalizations (two had atrial fibrillation, and one had recurrent edema in the right lower extremity), and these complications were thought to be related to the patient population rather than to the surgical operation. Based on the evaluation of healing duration, cohort A was not superior to cohort B (4.40 vs 4.30, P>0.05), thus demonstrating that hair follicle therapy did not lead to an extension in the length of postoperative hospital stay.
Table 3.
Clinical outcomes score
| Variable | Cohort A | Cohort B | P-value |
|---|---|---|---|
| Procedure safety | 4.40±0.50 | 4.50±0.51 | 0.54 |
| Healing duration | 4.40±0.50 | 4.30±0.80 | 0.64 |
| Skin quality (recipient site) | 4.40±0.50 | 3.45±0.95 | 0.00 |
| Scar quality (donate site) | 4.60±0.49 | 3.20±1.20 | 0.00 |
| Overall outcome | 4.45±0.51 | 3.40±0.88 | 0.00 |
Cohort A was statistically superior to cohort B in terms of the recipient site skin, donor site scarring and overall outcomes, as measured by the Likert scale (Table 3). Specific differences included skin and scar quality, which were rated significantly higher in cohort A (4.40 vs 3.45, P<0.05 and 4.65 vs 3.20, P<0.05; respectively). In cohort A, the scalp donor area was preoperatively shaved, and hair follicles were extracted from the scalp (Figure 1A). At week 2, the evaluation of the scalp-donor sites revealed no evidence of hair thinning, hair loss, or alopecia, and all of the donor sites healed with no signs of aesthetic compromise (Figure 1B). Hair follicle therapy had a significantly higher overall outcome score compared to split-thickness skin graft treatment (4.45 vs 3.40, P<0.05). In cohort A, clinical progression of epithelialization was explicitly observed at week 2 (Figure 2A), wound reduction was significant over the first 8 weeks postoperatively (Figure 2B), and total healing was observed for all of the patients who underwent hair follicle therapy at week 12, and unlike with hair transplantation, hair shafts were rarely observed at the recipient site (Figure 2C). Complex wounds that were not appropriate for split-thickness skin grafts had to receive hair follicle therapy, and they achieved satisfactory outcomes: patient No. 7 underwent breast reconstruction surgery and suffered from skin flap necrosis, with a concave wound on the right lateral chest wall (Figure 3A). Previously, this wound was considered to be at a relatively high failure risk with split-thickness skin grafting. We applied hair follicles to the wound bed (Figure 3B) and achieved total wound closure (Figure 3C). The disproportionate rarity of hair, in contrast to the viability of hair follicles, was also interestingly observed in all of the patients in cohort A.
Figure 1.
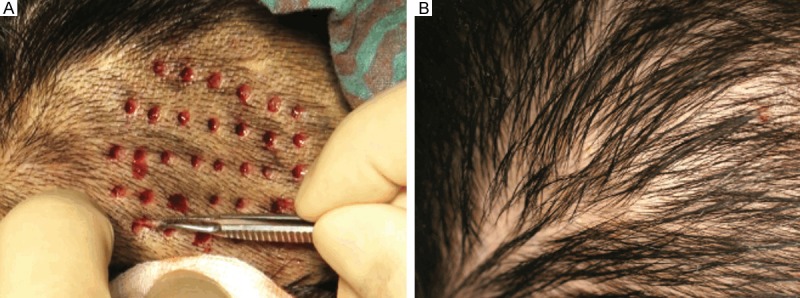
Hair follicles therapy achieved no scar healing in donor site. A. The scalp donor area was preoperatively shaved. After infiltration of the scalp with adrenalized saline, hair follicles were extracted from the scalp. B. At week 2, the donor site was completely healed, with no sign of aesthetic compromise, and it was available to be utilized repeatedly.
Figure 2.
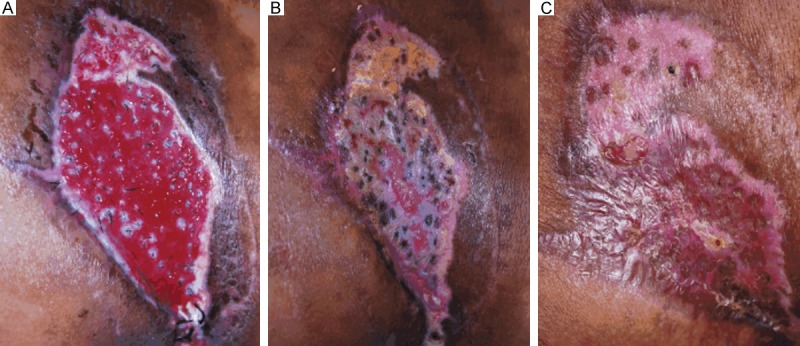
Hair follicles promoted epithelialization in wound bed. A. At week 2, non-viable hair follicles were mostly absent, and fabric scarring around the hair follicles and clinical epithelialization were observed. Consequently, wound reduction had been initiated. B. Clinical epithelialization was explicitly observed, and wound reduction was significant at week 8 postoperatively. C. Total healing was achieved. Unlike with hair transplantation, hair shafts were rarely observed at the recipient site.
Figure 3.
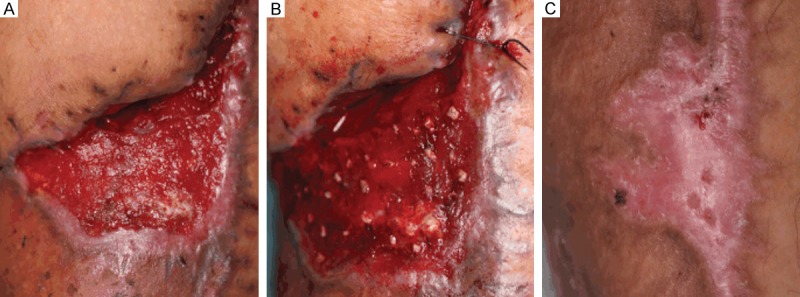
Hair follicles enhanced quality of healing in chronic wound. A. Debridement of necrotic tissue was performed preoperatively. B. The hair follicles were transplanted into the wound bed at a density of 4 units/cm2, which we considered to be the minimum density required to guarantee tissue regeneration and re-epithelialization. C. Total healing was achieved, and the re-epithelialized wound was elastic and less contracted than with a split-thickness skin graft, contributing to improved healing quality. This patient, who had a wounded leg, experienced enhanced ambulation.
Age, sex and BMI were found to have no statistically significant relationships with the survey scores in either cohort, indicating indicates that these variables had no statistically significant effects on the final outcomes of this study.
Discussion
Chronic cutaneous wounds, regardless of pathogenesis, can be devastating to patients: they have a high prevalence, and they have been significantly associated with a high cost burden on the healthcare system. The complete closure of the wound is particularly important for chronic wound patients. Despite decades of therapeutic advancement, autologous split-thickness skin grafting remains the gold standard used in routine practice. Although adequate to resurface a chronic wound, the resultant skin is chronically abnormal [8], and additionally, autologous split-thickness skin grafts are limited by the donor sites and by the inevitable secondary injury. Thus far, technological improvements, including cellularized engineering of biological skin [9], biofilm dressing [10] and synthetic growth factors [11], have recently been used in the treatment of wounds. However, biofilm products are costly to produce, and further, the single topical growth factor yielded insignificant results [12]. In a previous study, we dissected intact hair follicles as individual units and then transplanted them into the wound bed, the results demonstrated that hair follicles can function as promoters of re-epithelialization. Then, we performed retrospective study on those patients to compare the clinical outcomes between hair follicles and split-thickness grafts in chronic wound repair.
In this study, we utilized objective measurements and multiple blinded raters. Hair follicle therapy achieved statistically superior clinical outcomes to split-thickness skin grafts in terms of recipient skin sites, donor site scarring and overall outcomes, as measured by non-biased reviewers utilizing a Likert scale. At the endpoint of 12 weeks, as far as we could determine from the photographs, the hair follicle-treated wound areas were elastic, unlike the scar tissue, and this elasticity contributed to improved skin quality scores. Age did not have a statistically significant effect on the final outcomes, supporting the idea that patient age should not be a contraindication to using hair follicles in the chronic wounds of elderly patients [13]. Interestingly, in contrast to the viability of hair follicles in surgery, we noted a disproportionate rarity of hair shafts postoperatively, but this mechanism remains unknown.
Impaired wound healing is the consequence of the interactions among patient-related factors, wound-related factors, and the knowledge of the healthcare professional, as well as resources, surgical skill, and treatment-related factors [14]. However, the basic biology underlying chronic wounds is poorly understood. Recent advances in epithelial stem cell biology research have improved our understanding of the important role of the hair follicle in wound healing mechanisms, providing a basis for the development of novel therapies [15]. Various follicular cells contribute to wound healing through different pathways. Bulge stem cells produce transiently amplifying epidermal progeny, and non-hair-follicle-fated isthmus stem cells generate long-term populations in the wound epidermis [16]. Several studies have unambiguously identified the hair follicle as a major reservoir of adult stem cells [17]. The epithelium can be restored by the migration of epithelial cells from the epithelium adjoining a wound or by centrifugal migration from any hair follicle that remains within a wound [18]. Dermal grafts from the scalp and early implantation of microdissected hair follicles through the silicone epidermis and hair breaking grafts [19] are currently used for cutaneous wound repair. Clinical and experimental data have suggested that the involvement of follicle-derived dermal cells results in qualitatively improved wound repair [20].
Due to the limitations of the experimental methods, we did not find evidence supporting the notion that the stem cells contained in hair follicles can directly improve wound-healing outcomes. The results did not demonstrate that improved skin or scar quality resulted from follicle-derived dermal cells. However, we still observed superior clinical outcomes with hair follicles applied on the chronic wound bed, and this observation might indirectly support the idea that the wound healing-promoting effects of the hair follicle can be translated to clinical practice. Although the current state of knowledge suggests that stem cell therapy continues to march from bench to bedside, more specific research is needed.
Our results indicated that the procedure of hair follicle therapy was safe and effective. After the exclusion of alopecic or insufficiently hairy individuals, this method could be chosen as a new surgical option to avoid other complicated, costly and time-consuming procedures. In combining hair transplantation techniques and plastic reconstruction strategies, hair follicles could be considered a novel surgical protocol that is likely to provide another option in cases of chronic wounds. However, this method might require a surgeon with good training in hair follicle harvesting. In addition, the objective healing rate should be based on more substantial data and stronger evidence. Thus, a larger and more extensive range of samples should be utilized in future research.
This study shows the capacity of hair follicles to repair chronic cutaneous wounds. In particular, hair follicles can achieve better skin/scar quality and overall clinical outcomes than split-thickness skin grafting. Our observations supported the hypothesis that hair follicles perform an important function in the repair of cutaneous wounds, and they provide hope for high-quality clinical outcomes.
Acknowledgements
We are indebted to the patients who participated in this study. This work was supported by grants from the Major State Basic Research Development Program of China (973 Program), 2013CB932502, and the National High Technology Research and Development Program of China (863 Program), 2014AA020705.
Disclosure of conflict of interest
None.
References
- 1.Shareef M, Hussain W. ‘Skimming the surface’: a review of split-thickness skin grafting practices and preferences among U. K. dermatological surgeons. Br J Dermatol. 2013;169:943–944. doi: 10.1111/bjd.12447. [DOI] [PubMed] [Google Scholar]
- 2.Jimenez F, Poblet E, Izeta A. Reflections on how wound healing-promoting effects of the hair follicle can be translated into clinical practice. Exp Dermatol. 2015;24:91–94. doi: 10.1111/exd.12521. [DOI] [PubMed] [Google Scholar]
- 3.Jahoda CA, Christiano AM. Niche crosstalk: intercellular signals at the hair follicle. Cell. 2011;146:678–681. doi: 10.1016/j.cell.2011.08.020. [DOI] [PubMed] [Google Scholar]
- 4.Murawala P, Tanaka EM, Currie JD. Regeneration: the ultimate example of wound healing. Semin Cell Dev Biol. 2012;23:954–962. doi: 10.1016/j.semcdb.2012.09.013. [DOI] [PubMed] [Google Scholar]
- 5.Mimoun M, Chaouat M, Picovski D, Serroussi D, Smarrito S. The scalp is an advantageous donor site for thin-skin grafts: a report on 945 harvested samples. Plast Reconstr Surg. 2006;118:369–373. doi: 10.1097/01.prs.0000227739.23850.4a. [DOI] [PubMed] [Google Scholar]
- 6.Navsaria HA, Ojeh NO, Moiemen N, Griffiths MA, Frame JD. Reepithelialization of a full-thickness burn from stem cells of hair follicles micrografted into a tissue-engineered dermal template (Integra) Plast Reconstr Surg. 2004;113:978–981. doi: 10.1097/01.prs.0000105632.86651.ef. [DOI] [PubMed] [Google Scholar]
- 7.Plikus MV, Gay DL, Treffeisen E, Wang A, Supapannachart RJ, Cotsarelis G. Epithelial stem cells and implications for wound repair. Semin Cell Dev Biol. 2012;23:946–953. doi: 10.1016/j.semcdb.2012.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Burnett LN, Carr E, Tapp D, Raffin Bouchal S, Horch JD, Biernaskie J, Gabriel V. Patient experiences living with split thickness skin grafts. Burns. 2014;40:1097–1105. doi: 10.1016/j.burns.2014.03.005. [DOI] [PubMed] [Google Scholar]
- 9.Skardal A, Mack D, Kapetanovic E, Atala A, Jackson JD, Yoo J, Soker S. Bioprinted amniotic fluid-derived stem cells accelerate healing of large skin wounds. Stem Cells Transl Med. 2012;1:792–802. doi: 10.5966/sctm.2012-0088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ramasamy P, Shanmugam A. Characterization and wound healing property of collagen-chitosan film from Sepia kobiensis (Hoyle, 1885) Int J Biol Macromol. 2015;74:93–102. doi: 10.1016/j.ijbiomac.2014.11.034. [DOI] [PubMed] [Google Scholar]
- 11.Goldman R. Growth factors and chronic wound healing: past, present, and future. Adv Skin Wound Care. 2004;17:24–35. doi: 10.1097/00129334-200401000-00012. [DOI] [PubMed] [Google Scholar]
- 12.Bolukbasi N, Balcioglu HA, Ozkan BT, Tekkesin MS, Ustek D. Topical Single-dose Vascular Endothelial Growth Factor has No Effect on Soft Tissue Healing. N Am J Med Sci. 2014;6:505–509. doi: 10.4103/1947-2714.143281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Baquerizo Nole KL, Kirsner RS. Hair follicles and their potential in wound healing. Exp Dermatol. 2015;24:95–96. doi: 10.1111/exd.12607. [DOI] [PubMed] [Google Scholar]
- 14.Montfrans CV, Stok M, Geerkens M. Biology of chronic wounds and new treatment strategies. Phlebology. 2014;29:165–167. doi: 10.1177/0268355514528844. [DOI] [PubMed] [Google Scholar]
- 15.Hill RP, Gardner A, Crawford HC, Richer R, Dodds A, Owens WA, Lawrence C, Rao S, Kara B, James SE, Jahoda CA. Human hair follicle dermal sheath and papilla cells support keratinocyte growth in monolayer coculture. Exp Dermatol. 2013;22:236–238. doi: 10.1111/exd.12107. [DOI] [PubMed] [Google Scholar]
- 16.Jensen KB, Collins CA, Nascimento E, Tan DW, Frye M, Itami S, Watt FM. Lrig1 expression defines a distinct multipotent stem cell population in mammalian epidermis. Cell Stem Cell. 2009;4:427–439. doi: 10.1016/j.stem.2009.04.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Jaks V, Kasper M, Toftgard R. The hair follicle-a stem cell zoo. Exp Cell Res. 2010;316:1422–1428. doi: 10.1016/j.yexcr.2010.03.014. [DOI] [PubMed] [Google Scholar]
- 18.Brown JB, McDowell F. Epithelial healing and the transplantation of skin. Ann Surg. 1942;115:1166–1181. doi: 10.1097/00000658-194206000-00027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Jimenez F, Garde C, Poblet E, Jimeno B, Ortiz J, Martinez ML, Gutierrez-Rivera A, Perez-Lopez V, Etxaniz U, Naveda C, Higuera JL, Egües N, Escario E, Izeta A. A pilot clinical study of hair grafting in chronic leg ulcers. Wound Repair Regen. 2012;20:806–814. doi: 10.1111/j.1524-475X.2012.00846.x. [DOI] [PubMed] [Google Scholar]
- 20.Jahoda CA, Reynolds AJ. Hair follicle dermal sheath cells: unsung participants in wound healing. Lancet. 2001;358:1445–1448. doi: 10.1016/S0140-6736(01)06532-1. [DOI] [PubMed] [Google Scholar]


