Abstract
Objective: This study explored a community nursing service mode in which respiratory nurse specialists cared for patients with chronic obstructive pulmonary disease (COPD) in a 12-week period after hospital discharge, with the aim of better preventing acute exacerbations, improving health-related quality of life (HRQOL) and reducing medical expenses in these patients. Methods: We carried out a prospective randomized controlled study in which 68 COPD patients discharged were recruited from a general hospital in Guangzhou, China, were randomized divided into two groups. The control group underwent conventional nursing care, and the intervention group received community continuing care by respiratory nurse specialists. The observation period was 12 weeks. The results of intervention were evaluated using the Seattle Obstructive Lung Disease Questionnaire (SOLDQ) and the COPD Self-Efficacy Scale (CSES). In addition, the frequency of acute exacerbations, emergency treatments or hospitalizations, and medical expenses were recorded in the 12-week observation period. Results: After six weeks, the total and subscale scores (P < 0.05) of SOLDQ and CSES significantly improved compared to the baseline ones in the intervention group. The control group had significantly higher scores in the treatment satisfaction (TS) of SOLDQ, the total score, and the weather/environment and behavioral risk factors of CSES. After 12 weeks, the total and subscale scores of SOLDQ and CSES showed a sustained and significant growth in the intervention group (P < 0.05). The control group had significantly higher scores only in the weather/environment risk factor of CSES. During the 12-week observation, the intervention group had significantly fewer acute exacerbations, emergency treatments or re-hospitalizations and significantly lower average medical expenses than the control group (P < 0.05). Conclusions: Community continuing care by respiratory nurse specialists may improve HRQOL, increase self-efficacy, reduce incidence of acute exacerbation, and lower medical expenses in patients with COPD after hospital discharge.
Keywords: Community continuing care, chronic obstructive pulmonary disease, respiratory nurse specialists, health-related quality of life (HRQOL)
Introduction
Chronic obstructive pulmonary disease (COPD) is a common chronic respiratory disease that progresses gradually and manifests late. In China where COPD has been increasing in prevalence over the past decade and affecting over 9% of the population aged above 40 [1], a large-scale population-based survey has identified high prevalence of asymptomatic COPD [2]. High mortality and huge medical expenses related to this disorder have also been well recognized [3,4]. In addition, COPD as a leading cause of debilitation and work loss results in heavy financial burden upon patients and their family members, and immensely interferes with their health-related quality of life (HRQOL) [5,6].
Since COPD is not fully reversible and recurrent exacerbations may expedite decline in lung function, the major goals of clinical treatment, as proposed by Global Strategy for the Diagnosis, Management, and Prevention of COPD (GOLD) [7], are to improve overall health, increase exercise tolerance and reduce frequency of COPD exacerbations in the patients [8]. In many countries, post-hospital continuing care in the community for stable COPD patients has become an important step towards achieving these goals [9,10], shown to have improved the disease severity and increased patient satisfaction for care-giving services [9,11]. However, Hood et al. noted that community workers faced difficulties in providing high-quality services and home follow-up visits [12]. Lewis and his colleagues found that follow-up management with telemonitors in the community did not reduce hospital visits or specialist team intervention in optimized COPD patients [10]. Therefore, the British Thoracic Society Guidelines on Intermediate Care for COPD proposes that continuing community care for patients with COPD can be properly implemented only when hospitals follow an integrated assessment protocol for nursing [13].
In China, community nursing is yet a new field of care-giving services to be developed, where primary healthcare institutions are usually not actively involved in the rehabilitation management, follow-up visit or home caring, compared to the largely unmet needs of stable COPD patients when they have been discharged from general hospitals [14], We hypothesized that continued involvement of a team of respiratory nurse specialists, who have previously taken care of the patients during their hospital stay and hence are familiar with their medical history, in the post-hospital continuing care, would show promising benefits for stable COPD patients in the community compared with current situation, in terms of patients’ life quality, exacerbation of COPD and overall medical costs.
Patients and methods
This was a 12-week randomized controlled study among a cohort of stable COPD patients in the community. These patients had been treated for confirmed COPD between April 2012 and October 2013 in the respiratory medicine ward of the First Affiliated Hospital, Guangzhou Medical University, China, and were recruited prior to their discharge from the hospital. The inclusion criteria were: 1) post-bronchodilator ratio of FEV1 to FVC ratio less than 70%; 2) no change in COPD medication dosage and symptoms in the preceding 4 weeks; 3) ability to communicate fluently in Mandarin or Cantonese; (4) ability to use telephones or mobile phones; and (5) residence in Guangzhou after discharge. The exclusion criteria were: 1) serious cardiovascular co-morbidities, musculoskeletal disease, or any other disease affecting convalescence; 2) presence of any mental disorder; (3) refusal to participate in the study or comply with the study intervention.
Study groups and interventions
The patients were randomized into the intervention and control groups (n = 34 each) by using the “envelope method”. Sequentially numbered, opaque, sealed envelopes were prepared by a designated research nurse. After determining a patient’s eligibility, the physician responsible for treatment opened the next envelope in the sequence to determine the patient’s group assignment. An independent team of outpatient nurses, blinded to the group assignment throughout the study, were responsible for assessment of these patients.
Control group
The control group underwent conventional nursing care, including health education before hospital discharge and after transfer to a community healthcare institution. Nurses at the latter provided advices on COPD medications, oxygen therapy, and pulmonary rehabilitation exercise, as well as other medical services according to the patients’ needs. In case of an acute exacerbation, the patient was referred to a higher level hospital by the recommendation of a registered community physician. Patients could also contact the respiratory nurse specialists any time they felt necessary so that they could solve any nursing problems in due time.
Intervention group
The outpatient nurse was responsible for data collection. Each respiratory nurse specialist had at least five years of experience, was not the same as outpatient nurse. Three days prior to discharge, each patient was assessed for essential knowledge and skills for self-management (see Table 1) [15] by the respiratory nurse specialist in charge of his/her care. The respiratory nurse specialist offered prioritized guidance and intensive practice on the situations associated with patients’ health, for example, inappropriate use of diskus inhalers, huge stock of overdue medicines, rejecting rehabilitation exercises due to fear of anhelation induced by exercises, etc.
Table 1.
Essential Self-Management Knowledge and Skills for Discharged COPD Patients
| Essential Knowledge | Essential Skills |
|---|---|
| 1. Name and symptoms of the disease | 1. Walking |
| 2. Main cause of illness | 2. Upper limb exercises |
| 3. Typical signs and symptoms | 3. Effective coughing and expectoration techniques |
| 4. Importance of self-care and common sense in illness | 4. Ways to relieve asthma |
| 5. Importance and methods of home rehabilitation | 5. Self-monitoring of body temperature |
| 6. Drug treatment | 6. Use of aerosols (if needed) |
| 7. Exacerbations and how to cope | 7. Use of easy inhalants (if needed) |
| 8. Use of the “Handbook of COPD Self-management Plan” | 8. Use of diskus inhaler (if needed) |
| 9. Ways to quit smoking (if needed) | 9. Use of Turbuhaler (if needed) |
| 10. Food and nutrition | 10. Cleaning and maintenance of inhalers (if needed) |
| 11. Arrangement of daily life and ways to conserve physical strength | 11. Safe use of home oxygen therapy (if needed) |
| 12. Outdoor and recreational activities | 12. Pursed-lip breathing |
| 13. Seeking medical help and use of health service resources | 13. Breathing during daily activities |
| 14. Physical and mental relaxation | 14. Muscle relaxation exercises |
The respiratory nurse specialists instructed the patients to conduct self-management after discharge, including (1) walking without pause at the highest speed for 30 minutes every day; (2) doing upper limb movement exercise three times every day; (3) taking prescribed post-discharge medicines or prescription medicines at reexamination; (4) Adjusting diet according to COPD and principles of diabetes; (5) taking measures to avoid passive smoking or cooking fumes; (6) responding to acute exacerbation with self-detecting signs and symptoms. A rehabilitation plan was drawn up, specifying one home follow-up visit within three days and one telephone follow-up every week after discharge from the hospital (12 times altogether). The timing was based on previous research on the appropriate frequency of telephone follow-ups [15]. Patients could also contact the respiratory nurse specialists for health consultation or other health-related issues. During the home follow-up, the nurse designated for the case assessed the living environment, physiological functional status, health-related behavior and other variables of the patient. She recorded the information and provided necessary health education. Post-discharge telephone follow-ups were conducted once a week by following the protocol developed by Picariello et al. [16]. During each telephone follow-up, the patient’s basic condition, relevant medical behavior and drug use were assessed according to the patient’s self-report. Other elements discussed during the telephone conversation were also recorded, and appropriate health education was provided. To eliminate possible risks associated with telephone follow-ups [17], such as neglecting important information or problem related to the patients’ health. And each telephone conversation was audio recorded by nurse, which served as a basis for further analysis of the patient’s condition and better understanding of the patient’s needs.
Outcome measures
Recorded were acute exacerbations, emergency treatments or hospitalizations for acute exacerbations, and the amount of medical expenses incurred during the 12 weeks after discharge, along with measurements of HRQOL and self-efficacy, using internationally recognized assessment tools. Figure 1 is a diagram of the study protocol.
Figure 1.
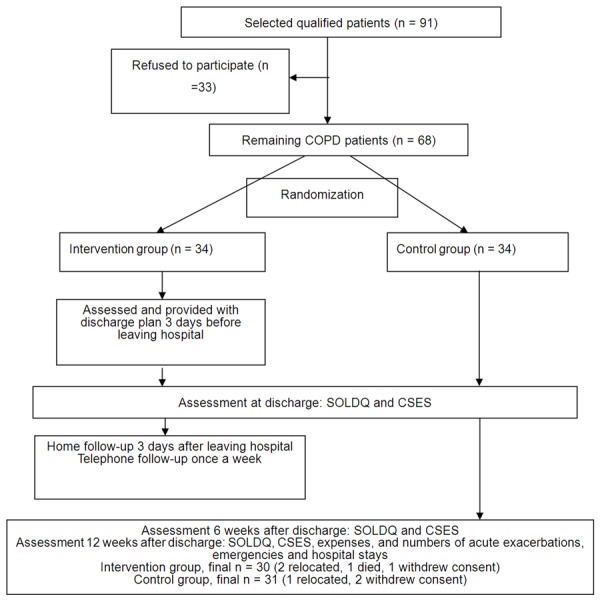
The flow diagram for this study SOLDQ, Seattle Obstructive Lung Disease Questionnaire CSES, COPD Self-Efficacy Scale.
SOLDQ
The Seattle Obstructive Lung Disease Questionnaire (SOLDQ) is a quantitative analysis tool widely used to measure the COPD patients’ HRQOL. SOLDQ contains 29 questions in four subscales including physical function, emotional function, coping skills, and treatment satisfaction. Individual items of the SOLDQ are scored on a simple linear scale with a response of 1 representing the lowest function. Responses to the question of a scale are summed into a raw score, and then transformed to a normalized score ranging from 0 to 100, with a score of 100 indicating the highest HRQOL and a score of 0 indicating the lowest HRQOL [18]. Extensive research has shown that SOLDQ has good test-retest reliability, content validity, construct validity and a high response rate and can provide identification for patients to benefit from preventive interventions [19].
CSES
The COPD Self-Efficacy Scale (CSES), developed by Wigal et al. [20], is used to assess the self-efficacy of patients with COPD in their ability to manage and avoid breathing difficulty. Several studies have shown that increasing patients’ self-efficacy, or their confidence in their ability to manage their COPD symptoms, can be beneficial in improving quality of life and other measures [21-23]. CSES comprises 31 questions in five dimensions including negative effect, emotional arousal, physical exertion, weather/environment, and behavioral risk factors. The items are scored on a 5-point Likert scale ranging from 1 (not at all confident) to 5 (very confident). The more confident the patients are in their ability to manage and avoid breathing difficulty, the higher scores they get. We used a Chinese version of the scale with proven reliability and validity [24].
Data collection
The SOLDQ and CSES questionnaires were administered by nurses other than those participating in the present study for each patient in week 6 and week 12 after discharge from hospital. The patients were instructed to return to the hospital to fill in them. The questionnaire nurses explained to the patients in detail how to fill in the two forms and answered all the questions about the questionnaires before the patients filled in the forms independently. All of the information was audited by the questionnaire nurses and unclear points were clarified with the patients immediately. After information collection, the scores were calculated and input into the computer by two research nurses. If the information records showed any discrepancy, the original document was checked.
Ethical issues
This study was approved by the ethics committee of First Affiliated Hospital of Guangzhou Medical University. The researchers explained in detail the objectives and significance of the study to the patients who chose to participate before they signed a document of informed consent. Anonymity and confidentiality of the study were assured, and the patients were informed that they were free to withdraw from the study any time they wanted.
Estimation of sample size
Estimation of sample size for this study was made on the basis of the primary outcome measure, the SOLDQ score. In the sample size assessment, n = 2 [u (uα + uβ) σ/δ]2 comparing two samples was adopted. At an early stage of this study, the SOLDQ questionnaire survey was conducted in 20 patients. The average score of the patients was 281.91 ± 29.2. It was expected that the total average SOLDQ score of the intervention group would increase by μa = 53.0 and that of the control group by μβ = 7.7, σ = 53.0-7.7 = 45.3 [25]. With α = 0.05 and β = 0.05, it was calculated that the total sample size was 63 participants. Considering the expected dropout rate was 7% [25], 68 participants were needed to complete this study.
Statistical analysis
Descriptive statistics were employed to analyze patients’ general information, and Chi-squared and Fisher’s exact tests were employed to test demographic differences between the two groups. Repeated measures ANOVA were used for the analysis of patients’ SOLDQ and CSES scores. Multivariate analysis was used for pairwise comparison within each group. The Mann-Whitney U-test was used to test differences between the two groups regarding frequency of acute exacerbations and amount of medical expenses after discharge. For all tests, the significance level was set at P < 0.05.
Results
Demographic information
A total of 68 patients were included in this study. Of them, 7 were lost to follow-up (intervention group: 2 migrated, 1 died and 1 withdrew consent; control group: 1 migrated, and 2 withdrew consent). 61 patients persisted to the end of the study (31 in the intervention group and 30 in the control group). Most of them were males (n = 51, 83.6%) with an average age of 64.4 years (SD = 7.7, range: 51-80 years). The average duration of COPD was 9.8 years. Thirteen patients (21.3%) had received non-formal education, nine (14.8%) primary education, eighteen (29.5%) secondary education, and twenty-one (34.4%) higher education. Fifty-six patients (91.8%) lived with their families, and forty-four (72.1%) received care from their families. In addition, forty patients (65.6%) needed home oxygen therapy, and twenty (32.8%) had a history of smoking. Forty-four patients (80.3%) were married, three (4.9%) single, and nine (14.8%) widowed. Forty-four patients (72.1%) had an income between 325 and 813 Dollar per month, and fifty-one patients (83.6%) had medical insurance for their medical expenses. The intervention and control groups did not differ significantly from each other regarding all the above indicators (P > 0.05; Table 2).
Table 2.
Demographic Characteristics of the Two Patient Groups
| Characteristics | Group | Statistical tests | |||
|---|---|---|---|---|---|
|
| |||||
| Total (n = 61) | Intervention (n = 30) | Control (n = 31) | Statistics | P-value | |
| Sex | |||||
| Male | 51 (83.6) | 27 (90.0) | 24 (77.4) | 1.76 | 0.30a |
| Female | 10 (16.4) | 3 (10.0) | 7 (22.6) | ||
| Age (years), mean (SD) | 64.4 (7.7) | 65.0 (7.9) | 63.8 (7.5) | 425.00 | 0.56b |
| Year of COPD, mean (SD) | 9.8 (6.4) | 9.9 (6.4) | 9.6 (6.4) | 460.50 | 0.95b |
| Education level | |||||
| No formal education | 13 (21.3) | 7 (23.3) | 6 (19.3) | 0.44 | 0.93c |
| Primary | 9 (14.8) | 5 (16.7) | 4 (12.9) | ||
| Secondary | 18 (29.5) | 8 (26.7) | 10 (32.3) | ||
| Tertiary or above | 21 (34.4) | 10 (33.3) | 11 (35.5) | ||
| Living alone | |||||
| No | 56 (91.8) | 26 (86.7) | 30 (96.7) | 2.07 | 0.20a |
| Yes | 5 (8.2) | 4 (12.9) | 1 (3.3) | ||
| Caregivers | |||||
| Own | 8 (13.1) | 5 (16.7) | 3 (9.7) | 0.69 | 0.71c |
| Families | 44 (72.1) | 21 (70.0) | 23 (74.2) | ||
| Other | 9 (14.8) | 4 (13.3) | 5 (16.1) | ||
| Oxygen | |||||
| No | 21 (34.4) | 12 (40.0) | 9 (29.0) | 0.81 | 0.37c |
| Yes | 40 (65.6) | 18 (60.0) | 22 (71.0) | ||
| Smoker | |||||
| No | 40 (65.6) | 21 (70.0) | 20 (64.5) | 0.21 | 0.65c |
| Yes | 20 (34.4) | 9 (30.0) | 11 (35.5) | ||
| Marital status | |||||
| Single | 3 (4.9) | 2 (6.7) | 1 (3.2) | 1.34 | 0.51c |
| Married | 49 (80.3) | 25 (83.3) | 24 (77.4) | ||
| Widowed | 9 (14.8) | 3 (10.0) | 6 (19.4) | ||
| Income per month (Dollar) | |||||
| < 325/month | 8 (13.1) | 5 (16.7) | 3 (9.7) | 0.69 | 0.71c |
| 325~813/month | 44 (72.1) | 21 (70.0) | 23 (74.2) | ||
| > 813/month | 9 (14.8) | 4 (13.3) | 5 (16.1) | ||
| Medical payment forms | |||||
| Own expense | 3 (4.9) | 1 (3.3) | 2 (6.5) | 0.48 | 0.79c |
| Medical insurance | 51 (83.6) | 26 (86.7) | 25 (80.6) | ||
| Other | 7 (11.5) | 3 (10.0) | 4 (12.9) | ||
Values shown are n (%), except for age and years since COPD diagnosis, which are shown as mean (SD); Age (years), control group: median = 62.0, range = 51-80; intervention group: median = 65.0, range = 51-80; Year since COPD diagnosis, control group: median = 7.5, range = 2-26; intervention group: median = 9, range = 1-25.
Fisher’s exact test.
Mann-Whitney U-test.
Chi-squared test.
Health-related quality of life
As shown in Table 3, the changes in the two groups were compared. After 6 weeks, the SOLDQ total score, physical function, coping skills and treatment satisfaction for patients in the intervention group had increased significantly (Mean Difference, MD = 5.32-29.81, P < 0.05). The scores of the control group were significantly lower in the treatment satisfaction and coping skills subscale (MD = -3.24-6.86, P < 0.05). After 12 weeks, the SOLDQ total score had increased significantly compared to baseline (MD = 50.96, P < 0.001), representing on scores of each subscale (MD = 6.51-19.17, P < 0.05). The scores of the control group were significantly higher in the total score and physical function (MD = 3.93-8.82, P < 0.05) (Table 3).
Table 3.
Mean difference of total and subscale scores in SOLDQ and CSES in the two groups at three time point
| Variable | GROUP | Change 1a | Change 2b | Change 3c | |||
|---|---|---|---|---|---|---|---|
|
| |||||||
| MD ± SD | P Value | MD ± SD | P Value | MD ± SD | P Value | ||
| The Seattle Obstructive Lung Disease Questionnaire | |||||||
| TOTAL | Intervention | 29.81 (4.71) | < 0.001 | 21.15 (5.64) | 0.001 | 50.96 (6.18) | < 0.001 |
| Control | -3.18 (3.36) | 0.352 | 11.99 (4.63) | 0.015 | 8.82 (4.02) | 0.036 | |
| PF | Intervention | 9.16 (1.99) | < 0.001 | 6.16 (2.37) | 0.015 | 15.22 (1.84) | < 0.001 |
| Control | -3.24 (1.19) | 0.011 | 0.68 (1.82) | 0.709 | 3.93 (1.45) | 0.011 | |
| EF | Intervention | 2.25 (1.70) | 0.197 | 4.26 (1.65) | 0.015 | 6.51 (1.71) | 0.001 |
| Control | -0.16 (1.85) | 0.931 | 0.46 (0.83) | 0.935 | 0.29 (1.83) | 0.874 | |
| CS | Intervention | 5.32 (1.92) | 0.010 | 5.32 (1.92) | 0.090 | 10.63 (3.32) | 0.003 |
| Control | -0.60 (1.88) | 0.753 | -0.77 (2.50) | 0.759 | -1.37 (2.00) | 0.499 | |
| TS | Intervention | 13.75 (3.41) | < 0.001 | 5.42 (3.27) | 0.108 | 19.17 (3.63) | < 0.001 |
| Control | -6.86 (3.11) | 0.035 | 10.08 (3.59) | 0.009 | 3.23 (3.38) | 0.347 | |
| The COPD Self-Efficacy Scale | |||||||
| TOTAL | Intervention | -0.30 (0.04) | < 0.001 | 0.01 (0.04) | 0.689 | -0.31 (0.04) | < 0.001 |
| Control | -0.07 (0.04) | 0.052 | -0.01 (0.04) | 0.862 | 0.07 (0.04) | 0.111 | |
| NA | Intervention | 0.35 (0.07) | < 0.001 | -0.02 (0.08) | 0.856 | 0.34 (0.09) | < 0.001 |
| Control | 0.01 (0.07) | 0.883 | 0.05 (0.08) | 0.514 | 0.07 (0.08) | 0.443 | |
| EA | Intervention | 2.25 (1.70) | 0.197 | 4.26 (1.65) | 0.015 | 6.51 (1.71) | 0.001 |
| Control | 0.05 (0.05) | 0.342 | -0.06 (0.05) | 0.257 | 0.01 (0.06) | 0.869 | |
| PE | Intervention | 0.34 (0.07) | < 0.001 | 0.00 (0.08) | 1.000 | 0.34 (0.08) | < 0.001 |
| Control | -0.12 (0.07) | 0.086 | -0.08 (0.08) | 0.289 | -0.04 (0.08) | 0.628 | |
| WE | Intervention | 0.37 (0.07) | < 0.001 | 0.05 (0.06) | 0.437 | 0.41 (0.06) | < 0.001 |
| Control | 0.14 (0.07) | 0.042 | 0.01 (0.06) | 0.925 | 0.15 (0.62) | 0.022 | |
| BF | Intervention | 0.35 (0.11) | 0.001 | 0.10 (0.10) | 0.322 | 0.45 (0.11) | < 0.001 |
| Control | 0.24 (0.10) | 0.02 | -0.08 (0.10) | 0.416 | 0.16 (0.10) | 0.127 | |
PF physical function, EF emotional function, CS coping skills, TS treatment satisfaction. NA negative effect, EA emotional arousal, PF physical exertion, WE weather/environment, BF behavioral risk factors.
Mean change 6 week-Mean change pretest = change 1.
Mean change 12 weeks-Mean change 6 weeks = change 2.
Mean change 12 weeks-Mean change baseline = change 3.
Repeated-measures analysis of variance showed a significant effect of time on the SOLDQ total and all subscale scores (F = 4.56-26.73, P < 0.05). In addition, there were significant effects of time × group on the total score and all subscale scores (F = 3.45-21.10, P < 0.05). The effect of group was significant on the total score, the physical function, emotional function and treatment satisfaction subscale scores (F = 4.32-18.47, P < 0.05). (See results in Figure 2A and 2B).
Figure 2.

A: Comparing the mean total score of SOLDQ pretest, 6 weeks and 12 weeks after discharge. B: Comparing the mean dimension score of SOLDQ pretest, 6 weeks and 12 weeks after discharge.
Self-efficacy
As shown in Table 4, after six weeks, the CSES total and all subscale scores in the intervention group had increased significantly compared to the pre-intervention ones (MD = 0.14-0.35, P < 0.05). The control group showed significant increases (P < 0.05) in the CSES total score (MD = 0.24, P = 0.03) and in the subscales of weather or environment (MD = 0.14, P = 0.04) and behavioral risk factors (MD = 0.24, P = 0.03). After 12 weeks, the total CSES score and scores for each subscale in the intervention group had increased significantly compared to the pre-intervention levels (MD = 0.18-0.45, P < 0.05).
Table 4.
Total and subscale scores of SOLDQ and CSES at discharge and 6 and 12 weeks after discharge
| Variable | Pretest | 6 weeks point | 12 weeks point | F | F | |||
|---|---|---|---|---|---|---|---|---|
|
| ||||||||
| Intervention | Control | Intervention | Control | Intervention | Control | |||
| Seattle Obstructive Lung Disease Questionnaire | ||||||||
| TOTAL | 206.85 (29.37) | 210.32 (29.24) | 236.66 (22.83) | 207.15 (27.35) | 257.81 (25.14) | 219.14 (23.48) | 33.82a | 23.14b |
| PF | 49.88 (7.35) | 50.36 (6.72) | 59.04 (11.31) | 53.61 (7.41) | 65.20 (9.82) | 54.29 (8.71) | 41.19a | 13.16b |
| EF | 49.51 (9.57) | 49.59 (10.11) | 51.77 (5.63) | 49.44 (4.41) | 56.02 (8.05) | 49.89 (4.37) | 5.08a | 3.83b |
| CS | 44.96 (17.97) | 45.04 (17.61) | 50.28 (13.89) | 45.64 (16.17) | 55.59 (9.08) | 46.41 (15.55) | 5.58a | 3.39b |
| TS | 62.50 (14.68) | 65.32 (19.01) | 75.58 (12.78) | 58.47 (18.08) | 81.00 (15.12) | 68.55 (13.64) | 9.93a | 10.14b |
| The COPD Self-Efficacy Scale | ||||||||
| TOTAL | 2.74 (0.28) | 2.78 (0.33) | 3.08 (0.24) | 2.81 (0.31) | 3.09 (0.20) | 2.80 (0.25) | 32.96a | 13.35b |
| NA | 3.16 (0.55) | 3.09 (0.52) | 3.49 (0.48) | 3.08 (0.47) | 3.48 (0.30) | 3.13 (0.49) | 7.79a | 5.20b |
| EA | 2.80 (0.45) | 2.79 (0.46) | 2.94 (0.33) | 2.84 (0.40) | 2.98 (0.28) | 2.78 (0.31) | 3.66a | 2.93 |
| PE | 2.66 (0.52) | 2.56 (0.48) | 3.00 (0.37) | 2.68 (0.48) | 3.00 (0.34) | 2.60 (0.26) | 10.29a | 4.10b |
| WE | 2.68 (0.55) | 2.67 (0.37) | 3.04 (0.48) | 2.80 (0.39) | 3.09 (0.48) | 2.81 (0.29) | 23.76a | 5.11b |
| BF | 3.12 (0.64) | 3.13 (0.63) | 3.18 (0.55) | 3.02 (0.66) | 3.28 (0.55) | 2.94 (0.36) | 11.41a | 2.01 |
PF physical function, EF emotional function, CS coping skills, TS treatment satisfaction; NA negative effect, EA emotional arousal, PF physical exertion, WE weather/environment, BF behavioral risk factors; Values shown are mean (SD).
Test of within-Subjects Effects (Time), P < 0.05;
Test of within-Subjects Effects (Time × Group), P < 0.05.
Repeated-measures analysis of variance revealed a significant effect of time on the CSES total and subscale scores (F = 3.66-32.96, P < 0.05). There was also a significant effect of time × group on the CSES total score (F = 13.35, P < 0.001) and the subscale scores of negative effect, physical exertion, and weather or environment (F = 4.10-15.35, P < 0.05). The effect of group was significant on the CSES total score and the subscale scores of negative affect and physical exertion (F = 7.30-9.85, P < 0.05).
Exacerbations and medical expenses
The Mann-Whitney U-test was used to compare numbers of acute exacerbations and medical expenses between the two groups of patients. During the 12-week observation period, the number of acute exacerbations in the intervention group was significantly smaller than that in the control group (Z = -2.42, P = 0.015). Patients in the intervention group also had a significantly lower frequency of hospitalizations and emergency treatments due to exacerbation (Z= -2.24, P = 0.025). Twelve weeks after discharge, patients in the intervention group had significantly lower medical expenses than the control group (Z = -1.97, P = 0.049) (Table 5).
Table 5.
Acute exacerbations and treatments and health care costs during the 12-week observation period
| Health use | Group | Statistical tests | |||
|---|---|---|---|---|---|
|
|
|||||
| Total (n = 61) | Intervention (n = 30) | Control (n = 31) | Statistics | P-value | |
| Frequency of Acute exacerbation | |||||
| Mean (SD) | 1.05 (0.78) | 0.80 (0.66) | 1.29 (0.82) | -2.42 | 0.015 |
| Median [range] | 1 [0-3] | 1 [0-2] | 1 [0-3] | ||
| Frequency of Emergency treatment or hospitalization | |||||
| Mean (SD) | 0.55 (0.53) | 0.40 (0.50) | 0.71 (0.53) | -2.24 | 0.025 |
| Median [range] | 1 [0-2] | 0 [0-1] | 1 [0-2] | ||
| The cost of medical treatment | |||||
| Mean (SD) | 6163.85 (5771.08) | 4551.1 (5334.8) | 7724.6 (5829.4) | -1.97 | 0.049 |
| Median [range] | 7957 [0-25107.0] | 1044.5 [0-180003] | 8900 [0-25107] | ||
Statistics are Z-scores for Mann-Whitney U-tests for differences between the control and intervention groups.
Discussion
In this study, we investigated the effects of community continuing care by respiratory nurse specialists on reducing the risk of COPD exacerbation, improving the quality of life and self-efficacy of the COPD patients after discharge. The Asia Pacific COPD Roundtable Group pointed out that effective self-care was a key healthcare to delay progressive decline of lung function in COPD patients [26]. The availability of continuous treatment and nursing care throughout the disease course directly affects the quality of life and self-efficacy of the discharged COPD patients [3]. The necessary continuing care is commonly provided by caregivers from community or family members, but not the hospitals they have been hospitalized. However, the community and family members of the patients often lack medical expertise for respiratory care [27], particularly in China. This study shows that community continuing care by respiratory nurse specialists when there is a high recurrence risk of COPD is a nursing care mode that can improve the current community health services in Chinese cities. It can improve COPD patients’ life quality after discharge. This mode has several advantages. First, as respiratory nurse specialists are familiar with the patients’ condition, they can conveniently provide individualized nursing care and other types of professional support. Second, the professional expertise of respiratory nurse specialists enables them to discover problems timely and provide appropriate solutions or instructions. Third, a home follow-up within three days after discharge helps specialists find potential problems timely that the patients cannot express on the phone. Problems found at the home visit can be further addressed in the next telephone follow-up. This mode can help COPD patients recover steadily, though it requires much time and devotion of the respiratory nurses, as well as employment of extra respiratory nurses by hospitals to make the mode feasible.
The respiratory nurse specialists had made a comprehensive assessment according to patients’ needs, and given health education, guidance and counseling, treatment and procedure, case management and monitoring individually, the patients could actively cooperate to complete the care plan after discharge. The Intervention group had increased significantly compared to baseline in SOLDQ total score, physiological function, coping skills, emotional function and satisfaction with nursing services 12 weeks after hospital discharge. Repeated measures ANOVA showed that there were significant effects of time × group on the total score and all subscale scores. It demonstrated that continuing community care provided by respiratory nurse specialists could lead to significantly higher HRQOL of COPD patients. Then, the implementation of individualized care plan was the key to success.
Anyhow, this mode can help patients and their family members and caregivers better understand appropriate ways of care and ways of obtaining health and medical services from the community after discharge from hospital which is helpful to improve the patients’ self-efficacy levels. This finding is consistent with that of Scherer and Shimmel, who also aimed to improve patients’ self-efficacy through health education [28]. Studies by Zimmerman et al. [29] and Scherer et al. [30] also demonstrate that health education of rehabilitation knowledge and skills can increase patients’ self-efficacy, which in turn allows the patients to control symptoms of difficulty breathing. The improvements in quality of life and self-efficacy can be attributed to the fact that the patients experienced a significantly lower frequency of acute exacerbations during the observation period. Consequently, decreased acute exacerbations reduced emergency treatments or hospitalizations, and the amount of medical expenses as well.
This study is a pre-feasibility study. Although patients benefit from continuing care service, in practice this service need to invest a lot of manpower and materials. The demand and supply should operate simultaneously to achieve a relative balance under the regulation of the medical service, allowing the fair and effective allocation and use of limited resources and satisfaction of patients’ demands. In recent years, global aging, urbanization, increase of COPD numbers, and independence of older adults from their children have led to formation of great demands for community nursing [31]. A potential solution may be to establish a COPD continuing management center within hospitals as a component of clinical nursing care. These centers will be managed by professional respiratory nurse specialists. In addition, it is necessary to formulate regulations on appropriate service processes and service standards.
Several aspects of limitations in this study should be acknowledged. First, we discovered that some patients were not entirely satisfied with the home visits and telephone follow-ups. As our information collection lacked a systematic protocol, and the respiratory nurse specialists lacked knowledge of nutrition, rehabilitation, psychology and other multi-disciplinary skills, the nursing intervention was provided only in response to limited problems. Second, our study was conducted only in one center for respiratory specialist treatment in Guangzhou. Large-sample multi-center studies should be carried out in the future. Third, our observation was limited to the 12-week period after discharge from hospital. Longer observation on the respiratory nurse specialist mode should be conducted.
In summary, the standardized nursing service in the present study would benefit communities by flexibly meeting the continuing care needs of patients, in combination with local healthcare programs and medical insurance systems. Our findings need further validation in future, large-sample studies, and if they are confirmed, would be noteworthy to the management of a much wider range of chronic diseases.
Acknowledgements
Thanks for all author, there is no funding sources supported to this paper.
Disclosure of conflict of interest
None.
References
- 1.Zhong N, Wang C, Yao W, Chen P, Kang J, Huang S, Chen B, Wang C, Ni D, Zhou Y, Liu S, Wang X, Wang D, Lu J, Zheng J, Ran P. Prevalence of chronic obstructive pulmonary disease in China: A large population-based survey. Am J Respir Crit Care Med. 2007;167:753–760. doi: 10.1164/rccm.200612-1749OC. [DOI] [PubMed] [Google Scholar]
- 2.Lu M, Yao WZ, Zhong NS, Zhou YM, Wang C, Chen P, Kang J, Huang SG, Chen BY, Wang CZ, Ni DT, Wang XP, Wang DL, Liu SM, Lü JC, Shen N, Ding YL, Ran PX. Asymptomatic patients of chronic obstructive pulmonary disease in China. Chin Med J (Engl) 2010;123:1494–1499. [PubMed] [Google Scholar]
- 3.Yao WZ, Zhu H, Shen N, Han X, Liang YJ, Zhang LQ, Sun YC, Hao ZT, Zhao MW. Epidemiological data of chronic obstructive pulmonary disease in Yanqing County in Beijing. Bei Jing Da Xue Xue Bao (Yi Xue Ban) 2005;37:121–125. [PubMed] [Google Scholar]
- 4.Zhong N. Nipping it in the bud: An inspiring mission for prevention and management of COPD. J Thorac Dis. 2012;4:102–105. doi: 10.3978/j.issn.2072-1439.2012.03.16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Jones PW. Activity limitation and quality of life in COPD. COPD. 2007;4:273–278. doi: 10.1080/15412550701480265. [DOI] [PubMed] [Google Scholar]
- 6.Bhome AB. COPD in India: Iceberg or volcano? J Thorac Dis. 2012;4:298–309. doi: 10.3978/j.issn.2072-1439.2012.03.15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. http://www.goldcopd.org/guidelines-global-strategy-for-diagnosis-management.html (assessed November 3, 2013)
- 8.Rabe KF, Hurd S, Anzueto A, Barnes PJ, Buist SA, Calverley P, Fukuchi Y, Jenkins C, Rodriguez-Roisin R, van Weel C, Zielinski J. Global strategy for the diagnosis, management, and prevention of chronic obstructive pulmonary disease: GOLD Executive Summary. Am J Respir Crit Care Med. 2007;176:532–555. doi: 10.1164/rccm.200703-456SO. [DOI] [PubMed] [Google Scholar]
- 9.Sindhu S, Pholpet C, Puttapitukpol S. Meeting the challenges of chronic illness: A nurse-led collaborative community care program in Thailand. Collegian. 2010;17:93–99. doi: 10.1016/j.colegn.2010.05.003. [DOI] [PubMed] [Google Scholar]
- 10.Lewis KE, Annandale JA, Warm DL, Rees SE, Hurlin C, Blyth H, Syed Y, Lewis L. Does home telemonitoring after pulmonary rehabilitation reduce healthcare use in optimized COPD? A pilot randomized trial. COPD. 2010;7:44–50. doi: 10.3109/15412550903499555. [DOI] [PubMed] [Google Scholar]
- 11.Braman SS, Lee DW. Primary care management of chronic obstructive pulmonary disease: an integrated goal-directed approach. Curr Opin Pulm Med. 2010;16:83–88. doi: 10.1097/MCP.0b013e3283354981. [DOI] [PubMed] [Google Scholar]
- 12.Hood S, Parsons S, Fulop NJ. Shifting care: GP opinions of hospital at home. Br J Gen Pract. 1999;49:221–222. [PMC free article] [PubMed] [Google Scholar]
- 13.British Thoracic Society Guideline Development Group. Intermediate care--Hospital-at-Home in chronic obstructive pulmonary disease: British Thoracic Society guideline. Thorax. 2007;62:200–210. doi: 10.1136/thx.2006.064931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Cao MJ, Guo XL, Yu H, McDonald TA. Chinese community-dwelling elders’ needs: promoting ageing in place. Int Nurs Rev. 2014;61:327–335. doi: 10.1111/inr.12119. [DOI] [PubMed] [Google Scholar]
- 15.Wang SL, Huang JY, Zhou JW. Development of evidence-based transitional care practice for chronic obstructive pulmonary disease. Zhonghua Hu Li Za Zhi [in Chinese] 2009;44:431–434. [Google Scholar]
- 16.Picariello G, Hanson C, Futterman R, Hill J, Anselm E. Impact of a geriatric case management program on health plan costs. Popul Health Manag. 2008;11:209–215. doi: 10.1089/pop.2007.0023. [DOI] [PubMed] [Google Scholar]
- 17.Wong FKY, Chow S, Wan V. Nurse telephone follow-up for A&E patients: Implications for health intervention using telephone intervention. Xianggang Hu Li Za Zhi. 2001;37:15. [Google Scholar]
- 18.Belza B, Steele BG, Cain K, Coppersmith J, Howard J, Lakshminarayan S. Seattle Obstructive Lung Disease Questionnaire: Sensitivity to outcomes in pulmonary rehabilitation in severe pulmonary illness. J Cardiopulm Rehabil. 2005;25:107–114. doi: 10.1097/00008483-200503000-00011. [DOI] [PubMed] [Google Scholar]
- 19.Fan VS, Curtis JR, Tu SP, McDonell MB, Fihn SD. Using quality of life to predict hospitalization and mortality in patients with obstructive lung diseases. Chest. 2002;122:429–436. doi: 10.1378/chest.122.2.429. [DOI] [PubMed] [Google Scholar]
- 20.Wigal JK, Creer TL, Kotses H. The COPD Self-Efficacy Scale. Chest. 1991;99:1193–1196. doi: 10.1378/chest.99.5.1193. [DOI] [PubMed] [Google Scholar]
- 21.Bandura A, O’Leary A, Taylor CB, Gauthier J, Gossard D. Perceived self-efficacy and pain control: Opioid and nonopioid mechanisms. J Pers Soc Psychol. 1987;53:563–571. doi: 10.1037//0022-3514.53.3.563. [DOI] [PubMed] [Google Scholar]
- 22.Jackson BE, Coultas DB, Ashmore J, Russo R, Peoples J, Uhm M, Singh KP, Bae S. Domain-specific self-efficacy is associated with measures of functional capacity and quality of life among patients with moderate to severe chronic obstructive pulmonary disease. Ann Am Thorac Soc. 2014;11:310–315. doi: 10.1513/AnnalsATS.201308-273BC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Vincent E, Sewell L, Wagg K, Deacon S, Williams J, Singh S. Measuring a change in self-efficacy following pulmonary rehabilitation: an evaluation of the PRAISE tool. Chest. 2011;140:1534–1539. doi: 10.1378/chest.10-2649. [DOI] [PubMed] [Google Scholar]
- 24.Wong KW, Wong FK, Chan MF. Effects of nurse-initiated telephone follow-up on self-efficacy among patients with chronic obstructive pulmonary disease. J Adv Nurs. 2005;49:210–222. doi: 10.1111/j.1365-2648.2004.03280.x. [DOI] [PubMed] [Google Scholar]
- 25.Chen Q, Jiang XY, Zhong QL, Fang XH, Li XH. Effect of discharge planning services in patients with chronic obstructive pulmonary disease. Zhonghua Hu Li Za Zhi [in Chinese] 2012;47:790–794. [Google Scholar]
- 26.Asia Pacific COPD Roundtable Group. Global Initiative for Chronic Obstructive Lung Disease strategy for the diagnosis, management and prevention of chronic obstructive pulmonary disease: An Asia-Pacific perspective. Respirology. 2005;10:9–17. doi: 10.1111/j.1440-1843.2005.00692.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Halpin DM. Community care for COPD: The good, the bad and the ugly. Thorax. 2008;63:187–189. doi: 10.1136/thx.2007.087783. [DOI] [PubMed] [Google Scholar]
- 28.Scherer YK, Shimmel S. Using self-efficacy theory to educate patients with chronic obstructive pulmonary disease. Rehabil Nurs. 1996;21:262–266. doi: 10.1002/j.2048-7940.1996.tb00840.x. [DOI] [PubMed] [Google Scholar]
- 29.Zimmerman BW, Brown ST, Bowman JM. A self-management program for chronic obstructive pulmonary disease: Relationship to dyspnea and self-efficacy. Rehabil Nurs. 1996;21:253–257. doi: 10.1002/j.2048-7940.1996.tb00838.x. [DOI] [PubMed] [Google Scholar]
- 30.Scherer YK, Schmieder LE, Shimmel S. The effects of education alone and in combination with pulmonary rehabilitation on self-efficacy in patients with COPD. Rehabil Nurs. 1998;23:71–77. doi: 10.1002/j.2048-7940.1998.tb02133.x. [DOI] [PubMed] [Google Scholar]
- 31.Algera M, Francke AL, Kerkstra A, van der Zee J. Home care needs of patients with long-term conditions: Literature review. J Adv Nurs. 2004;46:417–429. doi: 10.1111/j.1365-2648.2004.03031.x. [DOI] [PubMed] [Google Scholar]


