Abstract
We aimed to investigate the role of serum levels of polyunsaturated fatty acid (PUFA) in the development of colorectal cancer (CRC). Serum levels of n-3 and n-6 PUFA in 69 healthy control (Ctrl), 62 benign colorectal polys (CRP) and 100 CRC patients were detected by gas chromatograph. The adjusted odds ratio (OR) by quartiles of n-3 and n-6 PUFA were analyzed. During the process of Ctrl to CRP, total n-3 PUFA (OR=0.159, P<0.001), total n-6 PUFA (OR=0.190, P<0.001), C20:5 n-3 (OR=0.263, P=0.030), C22:6 n-3 (OR=0.125, P<0.001), and C18:2 n-6 (OR=0.299, P=0.025) were inversely associated with CRP risk. The ratio of total n-6 PUFA and total n-3 PUFA (OR=4.667, P=0.002), and the ratio of C20:4 n-6 and (C20:5 n-3+C22:6 n-3) (OR=6.000, P<0.001) were positively associated with CRP risk. During the process of CRP to CRC, total n-3 PUFA (OR=4.059, P=0.007), total n-6 PUFA (OR=8.146, P<0.001), C22:6 n-3 (OR=3.789, P=0.048), and C18:2 n-6 (OR=3.667, P=0.045) were positively associated with CRC risk. The ratio of C20:4 n-6 and (C20:5 n-3+C22:6 n-3) (OR=0.588, P=0.001) was inversely associated with CRC. In conclusion, our results found that the total n-3 PUFA, C22:6 n-3, the total n-6 PUFA, C18:2 n-6, and the ratio of C20:4 n-6 and (C20:5 n-3 +C22:6 n-3) played controversy role in the process of CRP and the process of CRC, and may provide nutritional intervention suggestions for the clinical practice.
Keywords: Polyunsaturated fatty acids, colorectal polys, colorectal cancer, odds ratio
Introduction
Colorectal cancer (CRC) is one of the most common cancers the world. The etiology of CRC is complex. It may evolve from genetic alterations in oncogenes or tumor suppressor genes. However, about 50%-80% of CRC patients are considered due to environmental factors, such as dietary habits which play important role in the development and progression of CRC [1,2]. In some studies, the level of dietary fat had been demonstrated to be positively associated with CRC, however, there were also some studies indicated that the incidence of CRC is low in populations consuming large amounts of fish. The controversy effects depend mainly on the type of dietary fat.
n-3 polyunsaturated fatty acid (PUFA) is demonstrated to have an inverse association with the risk of CRC [3-5]. However, the results of the association are inconsistent. Some other studies found that the n-3 showed null or positive association [6-8]. n-6 PUFA is demonstrated to have a positive association with the risk of CRC [5,9], but also some studies found null or positive association [10-13]. The association of n-3 PUFA, n-6 PUFA with the risk of CRC is inconsistent. In addition, most of the studies focused on the risk of healthy control (Ctrl) and CRC patients [5,14,15]. As we known, the natural history of CRC is long in humans, it was improper to use of CRC incidence as the end point in clinical intervention studies, and colorectal polys (CRP) should be required for the analysis.
In our study, we aimed to investigate the role of serum levels of PUFAs in the process of Ctrl to CRP, and CRP to CRC. Our study may identify the association of serum levels of PUFAs and the risk of CRP and CRP, and may be helpful for the nutritional intervention in clinical practice.
Materials and methods
Study population
The study was approved by the Ethics Committee of the Chinese People’s Liberation Army General Hospital (Beijing, China). All patients provided informed written consent for the study sample collection, as well as permission for their use in research.
231 serum samples included 69 Ctrl people, 62 benign CRP patients, 100 CRC patients were collected for detection. Serum samples were collected before any treatment, such as surgery, chemotherapy or radiation therapy. Ctrl people were detected based on based on their negative results including blood biomarker test, X-ray, ultrasound, CT examination, fecal occult-blood testing, and colonoscopy. CRP and CRC patients were diagnosed according to combined clinical criteria, including imaging data, serum tumor markers, and further confirmed by histopathological analysis. All the patients had no history of CRC. All study populations are Han Chinese in origin and lived in northern inland cities, and without extra PUFAs intake. Body mass index (BMI) was calculated as weight (kilograms)/height (square meters). Smoke and alcohol drinking are reported as current (C), former (F), and never (N) status. Clinical characteristics were shown in Table 1.
Table 1.
Clinical characteristic of the samples in our study
| Characteristic | Ctrl (n=69) | CRP (n=62) | CRC (n=100) |
|---|---|---|---|
| Age | 49.7±0.9 | 54.5±2.0 | 59.0±1.1 |
| Sex (Male/Female) | (38, 31) | (37, 25) | (64, 36) |
| Smoke (C/F/N, %) | (4.35, 18.84, 76.81) | (24.19, 37.10, 38.71) | (28.00, 21.00, 51.00) |
| Alcohol (C/F/N, %) | (27.54, 18.84, 53.62) | (54.84, 19.35, 25.81) | (42.00, 14.00, 44.00) |
| Body mass index | 25.7±3.1 | 24.9±3.7 | 26.2±4.1 |
| Total cholesterol | 4.52 (4.03, 4.92) | 4.57 (4.08, 5.02) | 4.37 (3.81, 5.09) |
| LDL cholesterol | 2.56 (1.87, 3.34) | 2.82 (2.19, 3.58) | 2.84 (2.61, 3.09) |
| HDL cholesterol | 1.36 (1.18, 1.59) | 1.19 (0.97, 1.31) | 1.02 (0.90, 1.19) |
| Triglycerides | 1.00 (0.81, 1.32) | 1.36 (0.95, 1.78) | 1.31 (0.98, 1.64) |
| Total energy intake (Kcal/d) | 2039 (1521, 2588) | 1982 (1376, 2607) | 1996 (1262, 2733) |
| Total protein intake (g/d) | 76 (60, 93) | 73 (61, 88) | 77 (57, 95) |
| Total fat intake (g/d) | 42 (34, 47) | 45 (38, 49) | 51 (32, 68) |
| Total carbohydrate intake (g/d) | 313 (197, 428) | 327 (219, 446) | 319 (201, 442) |
Abbreviation: Ctrl: healthy controls; CRP: colorectal polys; CRC: colorectal cancer.
Serum collection
10 mL of peripheral blood samples were collected in tubes containing separating gel and clot activator in the morning after 12 hours fast. After centrifuging at 3400 rpm for 7 minutes, the supernatant was transferred into new tubes, and the serum was aliquoted and stored at -80°C until detection. No freeze thawing was allowed prior to polyunsaturated fatty acids and cytokine detection.
Measurement of serum PUFA
The procedure of measuring the serum levels of PUFAs After thawing, 200 μL fasting serum sample was collected and transferred to a glass methylation tube. 5 μg C23:0 which served as intern control, 1 mL of hexane and 1 mL of 14% BF3/MeOH reagent were added into the methylation tube. Then the mixture was blanketed with nitrogen, and heated to 100°C for 45 minutes. After cooled to room temperature, 1 mL H2O was added to the tube. After centrifugation at 1200 r/min for 5 minutes, the upper hexane layer was transferred to a new tube and concentrated by nitrogen. Total Fatty acid methyl esters were carried out on GC-2010 Plus Gas Chromatograph (Chiyoda-ku, Tokyo, Japan) with a OmegawaxTM 250 column (Supelco, Belletonte, PA) 30 m ×0.25 mm ×0.25 um film thickness. Column temperature Program was 210°C and held 45 min. The concentrations of polyunsaturated fatty acids were expressed as a percentage [16]. The total n-3 PUFA included C18:3 n-3 (α-linolenic acid), C20:5 n-3 (eicosapentaenoic acid), C22:5 n-3 (docosapentaenoic acid) and C22:6 n-3 (docosahexaenoic acid). The total n-6 PUFA included C18:2 n-6 (linoleic acid), C18:3 n-6 (γ-linolenic acid), C20:3 n-6 (Dihomo-γ-linolenic acid), C20:4 n-6 (arachidonic acid) and C22:5 n-6 (docosapentaenoic acid).
Statistical analysis
The serum levels of n-3 and n-6 PUFAs between groups were compared by one-way analysis of variance with the Bonferroni correction. Conditional logistic regression models were used to calculate the odds ratios (OR) and 95% confidence interval (CI) for the incidence of CRP-Ctrl and CRC-CRP study design for the serum levels of PUFAs. The models were adjusted for age, sex, smoke, alcohol drinking, body mass index (BMI), total cholesterol, LDL cholesterol, HDL cholesterol, triglycerides, total energy, protein, fat and carbohydrate intake. OR were calculated for the second quartile (Q2), third quartile (Q3), and highest quartile (Q4) versus the lowest quartile (Q1). To test for linear trends in odds ratios over quartiles, we coded each quartile as 0, 1, 2, or 3 and incorporated these data into the logistic model as a single variable. P values for the trend were estimated by creating a continuous variable using the median value within quartiles. All statistical analyses were performed on SAS 9.2 statistical package (SAS Institute, Inc. Cary, USA) with a statistical significance level set at P<0.05.
Results
Comparison of n-3 PUFA and n-6 PUFA in the different groups
The percentage of n-3 PUFA ( including C18:3 n-3, C20:5 n-3, C22:5 n-3 and C22:6 n-3) and n-6 PUFA (including C18:2 n-6, C18:3 n-6, C20:3 n-6, C20:4 n-6 and C22:5 n-6) in serum of Ctrl, CRP and CRC group were compared. Compared to the Ctrl group, n-3 PUFA (P<0.001), C20:5 n-3 (P=0.016), C22:6 n-3 (P<0.001), n-6 PUFA (P<0.001) and C18:2 n-6 (P=0.002) in the CRP group showed significantly reduced as shown in Figure 1. The other kinds of PUFAs showed no significant difference in the CRP group when compared to the Ctrl group. Compared to the CRP group, n-3 PUFA (P=0.001), C22:6 n-3 (P=0.003), n-6 PUFA (P<0.001), C18:2 n-6 (P=0.002) and C20:4 n-6 (P=0.016) showed significantly increased in the CRC group, as also shown in Figure 1. The other kinds of PUFAs showed no significant difference in the CRC group when compared to the CRP group.
Figure 1.
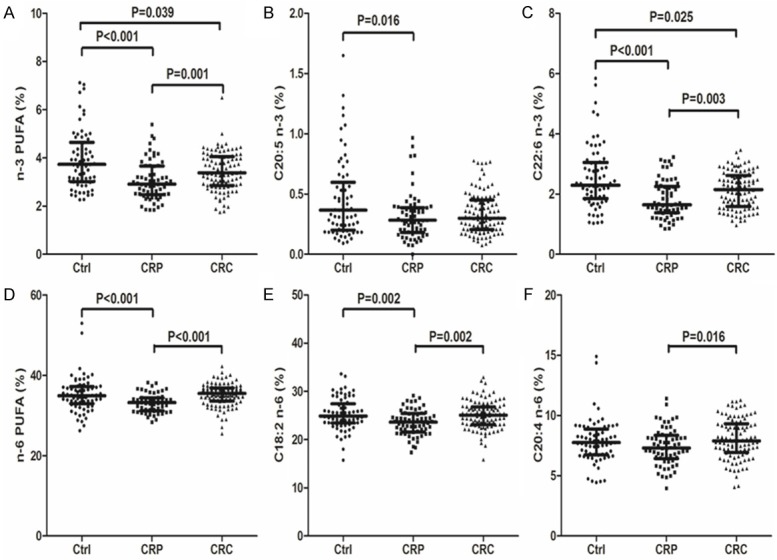
Comparison of the serum levels of n-3 PUFA (including total n-3 PUFA, C18:3 n-3, C20:5 n-3, C22:5 n-3 and C22:6 n-3) and n-6 PUFA (including total n-6 PUFA, C18:2 n-6, C18:3 n-6, C20:3 n-6, C20:4 n-6 and C22:5 n-6) in the Ctrl, CRP and CRC group. Abbreviation: Ctrl: Healthy controls; CRP: Colorectal polys; CRC: Colorectal cancer.
The ratio of total n-6 PUFA and total n-3 PUFA, ratio of 20:4 n-6 and C20:5 n-3, ratio of C20:4 n-6 and (C20:5 n-3+C22:6 n-3) and the ratio of C20:4 n-6 and (total n-6 PUFA+ total n-3 PUFA) in the Ctrl, CRP and CRC groups were also compared, as shown in Figure 2. Compared to the Ctrl group, the ratio of n-6 PUFA and n-3 PUFA (P=0.001) and ratio of C20:4 n-6 and (C20:5 n-3+C22:6 n-3) (P<0.001) and showed significantly increased in the CRP group, the other kinds of PUFAs indicators showed no significant difference in the CRP group when compared to the Ctrl group. Compared to the CRP group, the ratio of C20:4 n-6 and (C20:5 n-3+C22:6 n-3) (P=0.048) showed significantly reduced in the CRC group, the other kinds of PUFAs indicators showed no significant difference in the CRC group when compared to the CRP group.
Figure 2.
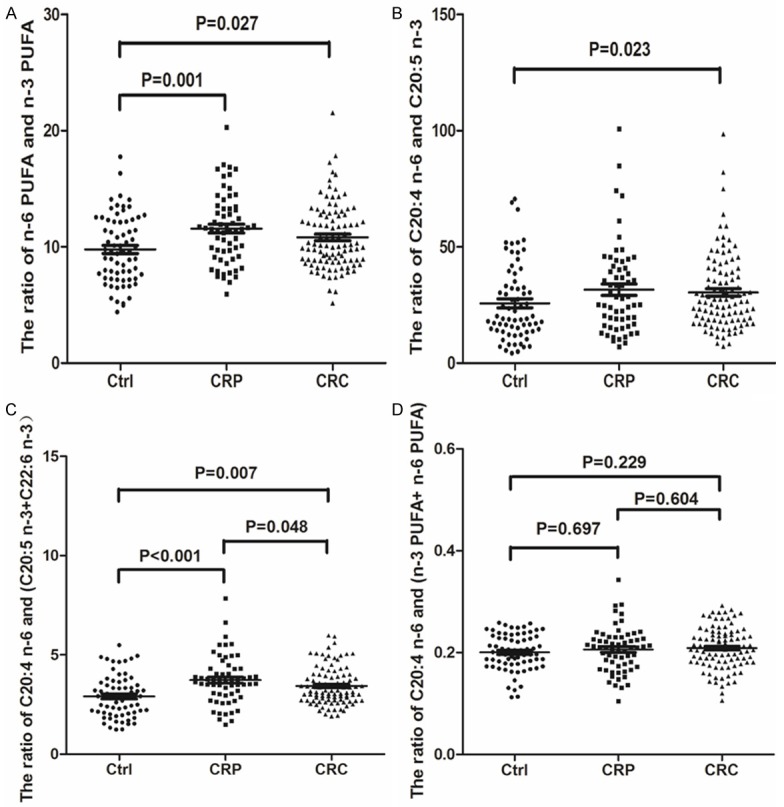
Comparison of the ratio of total n-6 PUFA and total n-3 PUFA, the ratio of C20:4 n-6 and C20:5 n-3, the ratio of C20:4 n-6 and (C20:5 n-3+C22:6 n-3) and the ratio of C20:4 n-6 and (total n-6 PUFA+ total n-3 PUFA) in the Ctrl, CRP and CRC groups. Abbreviation: Ctrl: Healthy controls; CRP: Colorectal polys; CRC: Colorectal cancer.
Association of n-3 PUFAs with the development of CRC
As shown in Table 2, during the process of Ctrl to CRP, total n-3 PUFA was inversely associated with CRP risk, showing a 84.1 percent risk reduction when Q4 and Q1 were compared (OR=0.159, 95% CI: 0.054-0.476; P for trend <0.001). C20:5 n-3 and C22:6 n-3 were also inversely associated with CRP risk, showing separate 73.7 and 87.5 percent risk reduction (OR=0.263 and 0.125, 95% CI: 0.088-0.785 and 0.041-0.379; P for trend=0.030 and <0.001). C18:3 n-3 and C22:5 n-3 showed no significant association with the CRP risk. These results indicated that the total n-3 PUFA, C20:5 n-3 and C22:6 n-3 were protective factors for CRP.
Table 2.
Association of n-3 PUFAs with the development of CRC
| Nutrient | Q* | Value | CRP (no.) | Ctrl (no.) | OR*,† | 95% CI* | P for trend | Q | Value | CRC (no.) | CRP (no.) | OR*,† | 95% CI* | P for trend |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Total n-3 PUFA | Q1 | <2.66 | 23 | 11 | 1.000 | <0.001 | Q1 | <2.70 | 17 | 23 | 1.000 | 0.007 | ||
| Q2 | 2.66-3.18 | 18 | 13 | 0.662 | 0.241, 1.823 | Q2 | 2.70-3.19 | 23 | 18 | 1.729 | 0.717, 4.166 | |||
| Q3 | 3.18-4.14 | 13 | 21 | 0.296 | 0.109, 0.803 | Q3 | 3.19-3.95 | 30 | 11 | 3.690 | 1.452, 9.379 | |||
| Q4 | >4.14 | 8 | 24 | 0.159 | 0.054, 0.476 | Q4 | >3.95 | 30 | 10 | 4.059 | 1.568, 10.510 | |||
| C18:3 n-3 | Q1 | <0.33 | 21 | 16 | 1.000 | 0.803 | Q1 | <0.32 | 23 | 17 | 1.000 | 0.431 | ||
| Q2 | 0.33-0.43 | 9 | 19 | 0.586 | 0.220, 1.562 | Q2 | 0.32-0.49 | 22 | 18 | 0.903 | 0.373, 2.196 | |||
| Q3 | 0.43-0.57 | 12 | 21 | 0.707 | 0.284, 1.763 | Q3 | 0.49-0.64 | 30 | 12 | 1.848 | 0.738, 4.624 | |||
| Q4 | >0.57 | 20 | 13 | 1.905 | 0.771, 4.706 | Q4 | >0.64 | 25 | 15 | 1.232 | 0.503, 3.018 | |||
| C20:5 n-3 | Q1 | <0.19 | 16 | 15 | 1.000 | 0.030 | Q1 | <0.20 | 24 | 17 | 1.000 | 0.293 | ||
| Q2 | 0.19-0.31 | 18 | 14 | 1.205 | 0.447, 3.250 | Q2 | 0.20-0.29 | 24 | 16 | 1.063 | 0.438, 2.579 | |||
| Q3 | 0.31-0.47 | 21 | 15 | 1.313 | 0.499, 3.452 | Q3 | 0.29-0.42 | 23 | 19 | 0.857 | 0.360, 2.045 | |||
| Q4 | >0.47 | 7 | 25 | 0.263 | 0.088, 0.785 | Q4 | >0.42 | 29 | 10 | 2.054 | 0.794, 5.312 | |||
| C22:5 n-3 | Q1 | <0.32 | 15 | 17 | 1.000 | 0.965 | Q1 | <0.32 | 23 | 15 | 1.000 | 0.843 | ||
| Q2 | 0.32-0.41 | 17 | 19 | 1.014 | 0.391, 2.633 | Q2 | 0.32-0.40 | 29 | 14 | 1.351 | 0.543, 3.360 | |||
| Q3 | 0.41-0.49 | 13 | 14 | 1.052 | 0.377, 2.935 | Q3 | 0.40-0.49 | 23 | 16 | 0.938 | 0.377, 2.332 | |||
| Q4 | >0.49 | 17 | 19 | 1.014 | 0.391, 2.633 | Q4 | >0.49 | 25 | 17 | 0.959 | 0.392, 2.349 | |||
| C22:6 n-3 | Q1 | <1.53 | 23 | 9 | 1.000 | <0.001 | Q1 | <1.51 | 20 | 22 | 1.000 | 0.048 | ||
| Q2 | 1.53-2.08 | 19 | 15 | 0.496 | 0.178, 1.382 | Q2 | 1.51-1.93 | 23 | 16 | 1.581 | 0.656, 3.811 | |||
| Q3 | 2.08-2.62 | 12 | 20 | 0.235 | 0.082, 0.672 | Q3 | 1.93-2.50 | 26 | 15 | 1.907 | 0.793, 4.587 | |||
| Q4 | >2.62 | 8 | 25 | 0.125 | 0.041, 0.379 | Q4 | >2.50 | 31 | 9 | 3.789 | 1.454, 9.874 |
Q: quartiles; OR: odds ratio; CI: confidence interval.
Odds ratio were derived from a conditional logistic analysis model adjusted for potential confounding factors, including age, sex, smoke, alcohol drinking, body mass index (BMI), total cholesterol, LDL cholesterol, HDL cholesterol, triglycerides, total energy, protein, fat and carbohydrate intake.
Abbreviation: Ctrl: healthy controls; CRP: colorectal polys; CRC: colorectal cancer.
During the process of CRP to CRC, total n-3 PUFA was positively associated with CRC risk, with a 4.059-fold increased risk of CRC when Q4 and Q1 were compared (OR=4.059, 95% CI: 1.568-10.510; P for trend=0.007). C22:6 n-3 were also positively associated with CRC risk, with a 3.789-fold increased risk of CRC (OR=3.789, 95% CI: 1.454-9.874; P for trend=0.048). C18:3 n-3, C20:5 n-3 and C22:5 n-3 showed no significant association with the CRC risk. These results indicated that the total n-3 PUFA and C22:6 n-3 were risk factors for CRC.
Our results found that the total n-3 PUFA and C22:6 n-3 played converse role in the process of CRP and the process of CRC. During the process of Ctrl to CRP, they were protective factors, but during the process of CRP to CRC, they were risk factors.
Association of n-6 PUFAs with the development of CRC
As shown in Table 3, during the process of Ctrl to CRP, total n-6 PUFA was inversely associated with CRP risk, showing a 81.0 percent risk reduction when Q4 and Q1 were compared (OR=0.190, 95% CI: 0.065-0.555; P for trend <0.001). C18:2 n-6 were also inversely associated with CRP risk, showing a 70.1 percent risk reduction (OR=0.299, 95% CI: 0.108-0.828; P for trend=0.025). C18:3 n-6, C20:3 n-6, C20:4 n-6 and C22:5 n-6 showed no significant association with the CRP risk. These results indicated that the total n-6 PUFA and C18:2 n-6 were protective factors for CRP.
Table 3.
Association of n-6 PUFAs with the development of CRC
| Nutrient | Q* | Value | CRP (no.) | Ctrl (no.) | OR*,† | 95% CI* | P for trend | Q | Value | CRC (no.) | CRP (no.) | OR*,† | 95% CI* | P for trend |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Total n-6 PUFA | Q1 | <31.85 | 21 | 12 | 1.000 | <0.001 | Q1 | <32.34 | 16 | 23 | 1.000 | <0.001 | ||
| Q2 | 31.85-33.86 | 20 | 12 | 0.952 | 0.348, 2.609 | Q2 | 32.34-34.46 | 16 | 26 | 0.885 | 0.363, 2.158 | |||
| Q3 | 33.86-36.24 | 13 | 21 | 0.354 | 0.131, 0.953 | Q3 | 34.46-36.45 | 34 | 7 | 6.982 | 2.483, 19.633 | |||
| Q4 | >36.24 | 8 | 24 | 0.190 | 0.065, 0.555 | Q4 | >36.45 | 34 | 6 | 8.146 | 2.774, 23.920 | |||
| C18:2 n-6 | Q1 | <22.52 | 21 | 11 | 1.000 | 0.025 | Q1 | <22.60 | 18 | 22 | 1.000 | 0.045 | ||
| Q2 | 22.52-24.28 | 15 | 19 | 0.414 | 0.153, 1.119 | Q2 | 22.60-24.38 | 25 | 16 | 1.910 | 0.789, 4.623 | |||
| Q3 | 24.28-26.07 | 14 | 18 | 0.407 | 0.148, 1.118 | Q3 | 24.38-26.32 | 27 | 14 | 2.357 | 0.961, 5.781 | |||
| Q4 | >26.07 | 12 | 21 | 0.299 | 0.108, 0.828 | Q4 | >26.32 | 30 | 10 | 3.667 | 1.420, 9.470 | |||
| C18:3 n-6 | Q1 | <0.22 | 17 | 15 | 1.000 | 0.075 | Q1 | <0.20 | 30 | 13 | 1.000 | 0.487 | ||
| Q2 | 0.22-0.28 | 13 | 21 | 0.546 | 0.205, 1.455 | Q2 | 0.20-0.28 | 22 | 17 | 0.567 | 0.226, 1.390 | |||
| Q3 | 0.28-0.34 | 13 | 21 | 0.546 | 0.205, 1.455 | Q3 | 0.28-0.34 | 24 | 13 | 0.800 | 0.313, 2.043 | |||
| Q4 | >0.34 | 19 | 12 | 1.397 | 0.513, 3.806 | Q4 | >0.34 | 24 | 19 | 0.547 | 0.226, 1.328 | |||
| C20:3 n-6 | Q1 | <1.30 | 16 | 25 | 1.000 | 0.411 | Q1 | <1.28 | 27 | 13 | 1.000 | 0.698 | ||
| Q2 | 1.30-1.58 | 12 | 12 | 1.563 | 0.565, 4.320 | Q2 | 1.28-1.60 | 22 | 16 | 0.662 | 0.263, 1.667 | |||
| Q3 | 1.58-1.91 | 20 | 15 | 2.083 | 0.832, 5.215 | Q3 | 1.60-1.92 | 25 | 19 | 0.634 | 0.260, 1.544 | |||
| Q4 | >1.91 | 14 | 17 | 1.287 | 0.500, 3.313 | Q4 | >1.92 | 26 | 14 | 0.894 | 0.354, 2.260 | |||
| C20:4 n-6 | Q1 | <6.64 | 18 | 15 | 1.000 | 0.108 | Q1 | <6.70 | 21 | 19 | 1.000 | 0.203 | ||
| Q2 | 6.64-7.64 | 18 | 15 | 1.000 | 0.379, 2.653 | Q2 | 6.70-7.73 | 24 | 17 | 1.277 | 0.531, 3.074 | |||
| Q3 | 7.64-8.57 | 14 | 19 | 0.614 | 0.232, 1.624 | Q3 | 7.73-9.03 | 25 | 16 | 1.414 | 0.585, 3.417 | |||
| Q4 | >8.57 | 12 | 20 | 0.500 | 0.186, 1.347 | Q4 | >9.03 | 30 | 10 | 2.714 | 1.053, 6.999 | |||
| C22:5 n-6 | Q1 | <0.13 | 15 | 17 | 1.000 | 0.064 | Q1 | <0.14 | 27 | 16 | 1.000 | 0.542 | ||
| Q2 | 0.13-0.18 | 9 | 23 | 0.443 | 0.157, 1.251 | Q2 | 0.14-0.20 | 26 | 11 | 1.401 | 0.548, 3.578 | |||
| Q3 | 0.18-0.25 | 17 | 17 | 1.133 | 0.431, 2.979 | Q3 | 0.20-0.26 | 23 | 15 | 0.909 | 0.370, 2.229 | |||
| Q4 | >0.25 | 21 | 12 | 1.983 | 0.735, 5.351 | Q4 | >0.26 | 24 | 20 | 0.711 | 0.302, 1.675 |
Q: quartiles; OR: odds ratio; CI: confidence interval.
Odds ratio were derived from a conditional logistic analysis model adjusted for age, sex, smoke, alcohol drinking, body mass index (BMI), total cholesterol, LDL cholesterol, HDL cholesterol, triglycerides, total energy, protein, fat and carbohydrate intake.
Abbreviation: Ctrl: healthy controls; CRP: colorectal polys; CRC: colorectal cancer.
During the process of CRP to CRC, total n-6 PUFA was positively associated with CRC risk, with a 8.146-fold increased risk of CRC when Q4 and Q1 were compared (OR=8.146, 95% CI: 2.774-23.920; P for trend <0.001). C18:2 n-6 were also positively associated with CRC risk, with a 3.667-fold increased risk of CRC (OR=3.667, 95% CI: 1.420-9.470; P for trend=0.045). C18:3 n-6, C20:3 n-6, C20:4 n-6 and C22:5 n-6 showed no significant association with the CRC risk. These results indicated that the total n-6 PUFA and C18:2 n-6 were risk factors for the CRC.
Our results found that the total n-6 PUFA and C18:2 n-6 played converse role in the process of CRP and the process of CRC. During the process of Ctrl to CRP, they were protective factors, but during the process of CRP to CRC, they were risk factors.
Association of n-6 PUFA and n-3 PUFA indicators with the development of CRC
As shown in Table 4, during the process of Ctrl to CRP, the ratio of total n-6 PUFA and total n-3 PUFA was positively associated with CRP risk, with a 4.667-fold increased risk of CRP risk when Q4 and Q1 were compared (OR=4.667, 95% CI: 1.643-13.256; P for trend=0.002). The ratio of C20:4 n-6 and (C20:5 n-3+C22:6 n-3) also was positively associated with CRP risk, with a 6.000-fold increased risk of CRP risk (OR=6.000, 95% CI: 2.040-17.649; P for trend <0.001). The other n-6 PUFA and n-3 PUFA indicators showed no significant association with CRP risk.
Table 4.
Association of n-6 and n-3 PUFA indicators with the development of CRC
| Indicators | Q* | Value | CRP (no.) | Ctrl (no.) | OR*,† | 95% CI* | P for trend | Q | Value | CRC (no.) | CRP (no.) | OR*,† | 95% CI* | P for trend |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Total n-6 PUFA/total n-3 PUFA | Q1 | <7.95 | 9 | 24 | 1.000 | 0.002 | Q1 | <8.88 | 28 | 12 | 1.000 | 0.284 | ||
| Q2 | 7.95-10.61 | 14 | 18 | 2.074 | 0.736, 5.849 | Q2 | 8.88-10.76 | 28 | 13 | 0.923 | 0.359, 2.371 | |||
| Q3 | 10.61-12.62 | 18 | 15 | 3.200 | 1.145, 8.944 | Q3 | 10.76-12.93 | 22 | 19 | 0.496 | 0.199, 1.237 | |||
| Q4 | >12.62 | 21 | 12 | 4.667 | 1.643, 13.256 | Q4 | >12.93 | 22 | 18 | 0.524 | 0.209, 1.314 | |||
| C20:4 n-6/(C20:5 n-3+C22:6 n-3) | Q1 | <2.35 | 8 | 24 | 1.000 | <0.001 | Q1 | <2.80 | 27 | 13 | 1.000 | 0.001 | ||
| Q2 | 2.35-3.32 | 11 | 23 | 1.435 | 0.489, 4.206 | Q2 | 2.80-3.47 | 34 | 7 | 2.339 | 0.820, 6.674 | |||
| Q3 | 3.32-3.90 | 21 | 11 | 5.727 | 1.940, 16.912 | Q3 | 3.47-4.02 | 17 | 24 | 0.341 | 0.138, 0.845 | |||
| Q4 | >3.90 | 22 | 11 | 6.000 | 2.040, 17.649 | Q4 | >4.02 | 22 | 18 | 0.588 | 0.237, 1.460 | |||
| C20:4 n-6/C20:5 n-3 | Q1 | <15.01 | 11 | 20 | 1.000 | 0.070 | Q1 | <18.22 | 23 | 16 | 1.000 | 0.886 | ||
| Q2 | 15.01-24.10 | 15 | 19 | 1.435 | 0.528, 3.901 | Q2 | 18.22-26.50 | 27 | 14 | 1.342 | 0.541, 3.325 | |||
| Q3 | 24.10-39.33 | 17 | 15 | 2.061 | 0.749, 5.667 | Q3 | 26.50-40.32 | 26 | 15 | 1.206 | 0.490, 2.967 | |||
| Q4 | >39.33 | 19 | 15 | 2.303 | 0.847, 6.259 | Q4 | >40.32 | 24 | 17 | 0.982 | 0.403, 2.393 | |||
| C20:4 n-6/(total n-6 PUFA+total n-3 PUFA) | Q1 | <0.18 | 17 | 19 | 1.000 | 0.671 | Q1 | <0.18 | 22 | 16 | 1.000 | 0.804 | ||
| Q2 | 0.18-0.20 | 9 | 16 | 0.629 | 0.221, 1.790 | Q2 | 0.18-0.21 | 31 | 15 | 1.503 | 0.616, 3.665 | |||
| Q3 | 0.20-0.23 | 20 | 16 | 1.397 | 0.553, 3.532 | Q3 | 0.21-0.23 | 21 | 13 | 1.175 | 0.457, 3.023 | |||
| Q4 | >0.23 | 16 | 18 | 0.993 | 0.388, 2.541 | Q4 | >0.23 | 26 | 18 | 1.051 | 0.435, 2.535 |
Q: quartiles; OR: odds ratio; CI: confidence interval.
Odds ratio were derived from a conditional logistic analysis model adjusted for potential confounding factors, including age, sex, smoke, alcohol drinking, body mass index (BMI), total cholesterol, LDL cholesterol, HDL cholesterol, triglycerides, total energy, protein, fat and carbohydrate intake.
Abbreviation: Ctrl: healthy controls; CRP: colorectal polys; CRC: colorectal cancer.
During the process of CRP to CRC, the ratio of C20:4 n-6 and (C20:5 n-3+C22:6 n-3) was inversely associated with CRC, showing a 41.2 percent risk reduction (OR=0.588, 95% CI: 0.209-1.314; P for trend=0.001). The other n-6 PUFA and n-3 PUFA indicators showed no significant association with CRC risk.
Our results found that the ratio of C20:4 n-6 and (C20:5 n-3+C22:6 n-3) played converse role in the process of CRP and the process of CRC. During the process of Ctrl to CRP, they were risk factors, but during the process of CRP to CRC, they were protective factors.
Discussion
In our study, n-3 PUFA and C22:6 n-3 were positively associated with CRC risk. The results were opposite to some of the previous studies. In review of eight studies about n-3 PUFA supplementation in patients with previous sporadic colorectal adenomas, six of eight studies showed a 13-70% reduction in mucosal epithelial cell proliferation index compared to the placebo group, the other two studies showed no change in proliferation index [17]. The increased consumption of dietary C22:6 n-3 may result in increased incorporation in immune cell membranes [18], compete with arachidonic acid as a substrate for cyclooxygenase (COX) to result in inhibit the production of prostaglandin E2 and leukotriene B4 [19]. They can also influence the lipid raft composition and signaling properties of immune cells [20]. Although n-3 PUFA were demonstrated to be protective factor for gastrointestinal inflammation, however, recent studies provide controversial results [21]. It seemed that n-3 PUFA supplementation may depress immune environment through alterations in cytokine production, T-cell proliferation, and T-cell-mediated cytotoxicity. In addition, C22:6 n-3 can also exclusively suppress T regulatory function [22]. Other authors also found that the exaggerated inflammation and carcinogenesis induced by dietary C22:6 n-3 was associated with altered CD8+ T-cell populations, CD69+ activation, FoxP3 expression, and the frequency of FoxP3+ CD25+ CD4+ Treg cells expressing L-selectin. These findings implicated that high doses of DHA consumed may promote impaired immune function [23]. In our study, the n-3 PUFA and C22:6 n-3 were demonstrated to be risk factor for the CRC when compared to the CRP. An analysis including 5 studies about Chinese people, one study of the 5 found that n-3 PUFA showed no significant association with the CRC [24], three studies showed significantly positive association with the CRC [6-8]. One showed significantly reverse association with the CRC [25]. The positive association between high intake of marine n-3 PUFA and rectal cancer risk may be related to at least one PARP codon 762 Ala allele [6].
In contrast to n-3 PUFA, n-6 PUFA was generally accepted as an increased risk of CRC. Animal studies showed that n-6 PUFA may enhance the risk of colorectal carcinogenesis [26]. However, in other studies, the results were not consistent. Some studies found there were positive association with the CRC [5,27], some studies showed no association [15,28], or inverse association [10,29]. In our study, total n-6 PUFA and C18:2 n-6 were inversely associated with CRP risk. Our results indicated that the n-6 PUFA and C18:2 n-6 were protective factors for CRP risk. But n-6 PUFA and C18:2 n-6 were positively associated with CRC risk. Our results implied that the n-6 PUFA and C18:2 n-6 may play converse role in the process of CRP and CRC. In the previous studies, some authors found that n-6 PUFA can prevent or reduce the severity of autoimmune disease, and the desaturated/elongated metabolites are protective. n-6 PUFA are clinically useful in human autoimmune-inflammatory disorders [30]. C20:4 n-6 which had methylene interrupted double bonds may inhibit growth and perform cytotoxic effects because of peroxidation products that are generated during lipid peroxidation and COX activity [10].
The controversy role of n-3 PUFA and n-6 PUFA in CRP and CRC may be because the membrane phospholipids. When the n-6 PUFA concentration was low, it can serve as parts of the membrane phospholipids of the immune system to be protective factor, however, when the concentration was high, its derived eicosanoids such as PGE2 may be immunosuppressive. Studies had shown that n-3 PUFA in membrane can compete with n-6 PUFA as the substrates of cyclooxygenase and lipoxygenase enzymes. It can also decrease the production of n-6 PUFA derived eicosanoids such as PGE2 which is required for normal T cell function, however, when high concentration, it was immunosuppressive. In addition, when the n-3 PUFA concentration was low, it can bind with the PPAR-γ to regulate the IL-8, iNOS and MMP-1 to inhibit the cell proliferation, it can also increase the ROS to increase the cell apoptosis. When the concentration was high, it can incorporate into the member phospholipids to alter their fluidity to inhibit the T-cell proliferation. The lipid rafts are crucial for T-cell activation, as are fences and pickets and protein-protein interactions that take part in the formation of the immunological synapse as a highly organized structure at the T-cell contact site to the antigen-presenting cell. n-3 PUFA treatment alters lipid rafts in altering the protein composition of the inner membrane lipid leaflet and inhibits T-cell responses. In addition, ROS which are the cellular consequences of oxidative stress may cause DNA oxidation, resulting in damage to all four bases and in the deoxy-ribose-molecule triggering the appearance of genetic mutations and initiating colorectal carcinogenesis [31].
In conclusion, our results demonstrated that the total n-3 PUFA, C22:6 n-3, the total n-6 PUFA, C18:2 n-6, and the ratio of C20:4 n-6 and (C20:5 n-3+C22:6 n-3) played controversy role in the process of CRP and the process of CRC, and may provide nutritional intervention suggestions for the clinical practice.
Acknowledgements
This work was supported by the National High Technology Research and Development Program 863 (2011AA02A111), the Capital Health Development Special Scientific Research Projects (2014-2-2154), China Postdoctoral Science Special Foundation Funded Project (2014T70963), China Postdoctoral Science Foundation Funded Project (2013M532110), National Science and Technology Infrastructure (2009BAI86B05).
Disclosure of conflict of interest
None.
References
- 1.Augenlicht LH, Heerdt BG, Mariadason JM, Yang W, Wilson AJ, Fragale A, Velcich A. Environment-gene interactions in intestinal cancer. Eur J Cancer Prev. 2002;11(Suppl 2):S12–17. [PubMed] [Google Scholar]
- 2.Roynette CE, Calder PC, Dupertuis YM, Pichard C. n-3 polyunsaturated fatty acids and colon cancer prevention. Clin Nutr. 2004;23:139–151. doi: 10.1016/j.clnu.2003.07.005. [DOI] [PubMed] [Google Scholar]
- 3.Hall MN, Chavarro JE, Lee IM, Willett WC, Ma J. A 22-year prospective study of fish, n-3 fatty acid intake, and colorectal cancer risk in men. Cancer Epidemiol Biomarkers Prev. 2008;17:1136–1143. doi: 10.1158/1055-9965.EPI-07-2803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sasazuki S, Inoue M, Iwasaki M, Sawada N, Shimazu T, Yamaji T, Takachi R, Tsugane S. Intake of n-3 and n-6 polyunsaturated fatty acids and development of colorectal cancer by subsite: Japan Public Health Center-based prospective study. Int J Cancer. 2011;129:1718–1729. doi: 10.1002/ijc.25802. [DOI] [PubMed] [Google Scholar]
- 5.Pot GK, Geelen A, van Heijningen EM, Siezen CL, van Kranen HJ, Kampman E. Opposing associations of serum n-3 and n-6 polyunsaturated fatty acids with colorectal adenoma risk: an endoscopy-based case-control study. Int J Cancer. 2008;123:1974–1977. doi: 10.1002/ijc.23729. [DOI] [PubMed] [Google Scholar]
- 6.Stern MC, Butler LM, Corral R, Joshi AD, Yuan JM, Koh WP, Yu MC. Polyunsaturated fatty acids, DNA repair single nucleotide polymorphisms and colorectal cancer in the Singapore Chinese Health Study. J Nutrigenet Nutrigenomics. 2009;2:273–279. doi: 10.1159/000308467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Butler LM, Wang R, Koh WP, Stern MC, Yuan JM, Yu MC. Marine n-3 and saturated fatty acids in relation to risk of colorectal cancer in Singapore Chinese: a prospective study. Int J Cancer. 2009;124:678–686. doi: 10.1002/ijc.23950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Takezaki T, Gao CM, Wu JZ, Ding JH, Liu YT, Zhang Y, Li SP, Su P, Liu TK, Tajima K. Dietary protective and risk factors for esophageal and stomach cancers in a low-epidemic area for stomach cancer in Jiangsu Province, China: comparison with those in a high-epidemic area. Jpn J Cancer Res. 2001;92:1157–1165. doi: 10.1111/j.1349-7006.2001.tb02135.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bartsch H, Nair J, Owen RW. Dietary polyunsaturated fatty acids and cancers of the breast and colorectum: emerging evidence for their role as risk modifiers. Carcinogenesis. 1999;20:2209–2218. doi: 10.1093/carcin/20.12.2209. [DOI] [PubMed] [Google Scholar]
- 10.Dommels YE, Haring MM, Keestra NG, Alink GM, van Bladeren PJ, van Ommen B. The role of cyclooxygenase in n-6 and n-3 polyunsaturated fatty acid mediated effects on cell proliferation, PGE(2) synthesis and cytotoxicity in human colorectal carcinoma cell lines. Carcinogenesis. 2003;24:385–392. doi: 10.1093/carcin/24.3.385. [DOI] [PubMed] [Google Scholar]
- 11.Nkondjock A, Shatenstein B, Maisonneuve P, Ghadirian P. Assessment of risk associated with specific fatty acids and colorectal cancer among French-Canadians in Montreal: a case-control study. Int J Epidemiol. 2003;32:200–209. doi: 10.1093/ije/dyg048. [DOI] [PubMed] [Google Scholar]
- 12.Terry P, Bergkvist L, Holmberg L, Wolk A. No association between fat and fatty acids intake and risk of colorectal cancer. Cancer Epidemiol Biomarkers Prev. 2001;10:913–914. [PubMed] [Google Scholar]
- 13.Patterson E, Wall R, Fitzgerald GF, Ross RP, Stanton C. Health implications of high dietary omega-6 polyunsaturated Fatty acids. J Nutr Metab. 2012;2012:539426. doi: 10.1155/2012/539426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Theodoratou E, McNeill G, Cetnarskyj R, Farrington SM, Tenesa A, Barnetson R, Porteous M, Dunlop M, Campbell H. Dietary fatty acids and colorectal cancer: a case-control study. Am J Epidemiol. 2007;166:181–195. doi: 10.1093/aje/kwm063. [DOI] [PubMed] [Google Scholar]
- 15.Kojima M, Wakai K, Tokudome S, Suzuki K, Tamakoshi K, Watanabe Y, Kawado M, Hashimoto S, Hayakawa N, Ozasa K, Toyoshima H, Suzuki S, Ito Y, Tamakoshi A. Serum levels of polyunsaturated fatty acids and risk of colorectal cancer: a prospective study. Am J Epidemiol. 2005;161:462–471. doi: 10.1093/aje/kwi066. [DOI] [PubMed] [Google Scholar]
- 16.Kang JX, Wang J. A simplified method for analysis of polyunsaturated fatty acids. BMC Biochem. 2005;6:5. doi: 10.1186/1471-2091-6-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Cockbain AJ, Toogood GJ, Hull MA. Omega-3 polyunsaturated fatty acids for the treatment and prevention of colorectal cancer. Gut. 2012;61:135–149. doi: 10.1136/gut.2010.233718. [DOI] [PubMed] [Google Scholar]
- 18.Gibney MJ, Hunter B. The effects of short- and long-term supplementation with fish oil on the incorporation of n-3 polyunsaturated fatty acids into cells of the immune system in healthy volunteers. Eur J Clin Nutr. 1993;47:255–259. [PubMed] [Google Scholar]
- 19.Caughey GE, Mantzioris E, Gibson RA, Cleland LG, James MJ. The effect on human tumor necrosis factor alpha and interleukin 1 beta production of diets enriched in n-3 fatty acids from vegetable oil or fish oil. Am J Clin Nutr. 1996;63:116–122. doi: 10.1093/ajcn/63.1.116. [DOI] [PubMed] [Google Scholar]
- 20.Chapkin RS, Kim W, Lupton JR, McMurray DN. Dietary docosahexaenoic and eicosapentaenoic acid: emerging mediators of inflammation. Prostaglandins Leukot Essent Fatty Acids. 2009;81:187–191. doi: 10.1016/j.plefa.2009.05.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sijben JW, Calder PC. Differential immunomodulation with long-chain n-3 PUFA in health and chronic disease. Proc Nutr Soc. 2007;66:237–259. doi: 10.1017/S0029665107005472. [DOI] [PubMed] [Google Scholar]
- 22.Yessoufou A, Ple A, Moutairou K, Hichami A, Khan NA. Docosahexaenoic acid reduces suppressive and migratory functions of CD4+ CD25+ regulatory T-cells. J Lipid Res. 2009;50:2377–2388. doi: 10.1194/jlr.M900101-JLR200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Woodworth HL, McCaskey SJ, Duriancik DM, Clinthorne JF, Langohr IM, Gardner EM, Fenton JI. Dietary fish oil alters T lymphocyte cell populations and exacerbates disease in a mouse model of inflammatory colitis. Cancer Res. 2010;70:7960–7969. doi: 10.1158/0008-5472.CAN-10-1396. [DOI] [PubMed] [Google Scholar]
- 24.Murff HJ, Shu XO, Li H, Dai Q, Kallianpur A, Yang G, Cai H, Wen W, Gao YT, Zheng W. A prospective study of dietary polyunsaturated fatty acids and colorectal cancer risk in Chinese women. Cancer Epidemiol Biomarkers Prev. 2009;18:2283–2291. doi: 10.1158/1055-9965.EPI-08-1196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Chen H, Ward MH, Graubard BI, Heineman EF, Markin RM, Potischman NA, Russell RM, Weisenburger DD, Tucker KL. Dietary patterns and adenocarcinoma of the esophagus and distal stomach. Am J Clin Nutr. 2002;75:137–144. doi: 10.1093/ajcn/75.1.137. [DOI] [PubMed] [Google Scholar]
- 26.Whelan J, McEntee MF. Dietary (n-6) PUFA and intestinal tumorigenesis. J Nutr. 2004;134:3421S–3426S. doi: 10.1093/jn/134.12.3421S. [DOI] [PubMed] [Google Scholar]
- 27.Koh WP, Yuan JM, van den Berg D, Lee HP, Yu MC. Interaction between cyclooxygenase-2 gene polymorphism and dietary n-6 polyunsaturated fatty acids on colon cancer risk: the Singapore Chinese Health Study. Br J Cancer. 2004;90:1760–1764. doi: 10.1038/sj.bjc.6601797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hall MN, Campos H, Li H, Sesso HD, Stampfer MJ, Willett WC, Ma J. Blood levels of long-chain polyunsaturated fatty acids, aspirin, and the risk of colorectal cancer. Cancer Epidemiol Biomarkers Prev. 2007;16:314–321. doi: 10.1158/1055-9965.EPI-06-0346. [DOI] [PubMed] [Google Scholar]
- 29.Kuriki K, Wakai K, Hirose K, Matsuo K, Ito H, Suzuki T, Saito T, Kanemitsu Y, Hirai T, Kato T, Tatematsu M, Tajima K. Risk of colorectal cancer is linked to erythrocyte compositions of fatty acids as biomarkers for dietary intakes of fish, fat, and fatty acids. Cancer Epidemiol Biomarkers Prev. 2006;15:1791–1798. doi: 10.1158/1055-9965.EPI-06-0180. [DOI] [PubMed] [Google Scholar]
- 30.Harbige LS. Fatty acids, the immune response, and autoimmunity: a question of n-6 essentiality and the balance between n-6 and n-3. Lipids. 2003;38:323–341. doi: 10.1007/s11745-003-1067-z. [DOI] [PubMed] [Google Scholar]
- 31.Chapkin RS, Davidson LA, Ly L, Weeks BR, Lupton JR, McMurray DN. Immunomodulatory effects of (n-3) fatty acids: putative link to inflammation and colon cancer. J Nutr. 2007;137:200S–204S. doi: 10.1093/jn/137.1.200S. [DOI] [PubMed] [Google Scholar]


