Abstract
The aim of this study is to develop a high fat diet and over nutrition induced nonalcoholic fatty liver disease(NAFLD) in rat, and to investigate the effect of 4-(Methylthio)-3-butenyl isothiocyanate (MTBITC) on ameliorating the NAFLD. Twenty Sprague-Dawley (SD) rats were equally divided into 4 groups (C, M, E1 & E2). Control group (C) were treated with standard restricted diet; Model group (M) were given high fat liquid diet ad libitum; Experimental group (E1 & E2) were treated with high fat liquid diet ad libitum and MTBITC by gavage. The experiment last 9 weeks, and serum chemistry and liver histology were assessed. The rats of M group showed severe lipid deposition and peroxidation in liver. When compared with group C, group M also showed significantly higher serum concentration of low-density lipoprotein, tumor necrosis factor-α and glucose. Histopathologic sections demonstrated lipid accumulation and macrovascular steatosis with ballooning degeneration in the livers of M. Group E2 presented significantly better conditions when assessed based on the parameters of NAFLD. The data suggested that MTBITC might significantly attenuate fat liquid diet induced NAFLD.
Keywords: Nonalcoholic fatty liver disease, Chinese white radish, 4-(Methylthio)-3-butenyl isothiocyanate, phytochemical, antioxidative stress
Introduction
Nonalcoholic fatty liver disease (NAFLD) is one of the most common forms of chronic liver disease in the world. It is characterized by fatty infiltration of the liver without significant consumption of alcohol. NAFLD is considered as the hepatic manifestation of metabolic syndrome mainly caused by obesity-associated insulin resistance, and is also closely associated with hepatic lipid accumulation and oxidative stress. NAFLD can be separated into three stages: simple steatosis (fatty liver), non-alcoholic steatohepatitis (NASH) and cirrhosis [1]. Simple steatosis is usually benign, however NASH, which is characterized by steatosis, lobular inflammation and progressive pericellular fibrosis, is recognized as a precursor of more severe liver disease such as cirrhosis, hepatocellular carcinoma and liver failure [2,3]. Recent studies indicate that the prevalence of NAFLD in the general population of the United States is over 20% and that of NASH is 3.5%-5%. A study conducted by Fan in 2009 showed the number of NAFLD patients is also growing rapidly in China (with 15% of the urban population being affected) [4].
The pathogenesis of NAFLD is still not fully understood, mainly because the lack of a suitable experimental animal model which can fully mimic the pathogenesis in human, both reflecting the liver pathology and the metabolic context correctly [5]. The genetic rodent model, such as mice having a missense mutation on the leptin receptor gene (db/db) [6], and the nutritional manipulations model, such as the choline and methionine deficiency model (MCD) [7,8] can successfully induce NAFLD. However, the main disadvantage of these models is the different etiological factors when compared to common patients. Therefore, using the caloric overload model is preferred as an NAFLD inducing method, because it has conditions similar to that of humans. Lieber used a liquid high-fat diet to develop a NAFLD rat model within three weeks [9]. But the metabolic biochemical markers and bodyweight of this model are similar to control because feeding the rats high fat diet ad libitum cannot make them over-intake calories. By virtue of the surgery and special equipment, several intragastric diet infusion techniques have been applied to over-feed the rats [10,11]. Though these animal models have basically fulfilled the requirements above, the technique is however not flawless as the experimental animals may suffer from the negative post-surgery stress, and the technique is difficult to expand to most laboratories due to the requirement of special facilities and experienced operators.
Asian white radish (Raphanus sativus L.), with an edible strong taproot, is a popular cruciferous vegetable throughout Asia, especially in China, Japan, Korea and Southeast Asia and it has been thought to show anti-tumor and anti-inflammation activities [12]. Recently studies have demonstrated that the extract of radish’s taproot have potential in inhibiting the abnormal growth of vascular smooth muscle cells [13], protecting the cell membranes against lipid peroxidation and intervening in dysfunction of gastrointestinal motility [14]. 4-(Methylthio)-3-butenyl isothiocyanate (MTBITC), the pungent principle and bioactive chemical of radish which is produced via myrosinase-catalyzed hydrolysis of 4-(Methylthio)-3-butenyl glucosinolates, has been studied as a potential phytochemical that has the effect of inducing the apoptosis and reducing the growth of tumour cells by affecting it’s signaling pathways [15,16]. Also, MTBITC alone was proved to be safe and have the chemoprotective effects against the toxicity of Zearalenone [17,18]. However, although there are considerable evidence to suggest that the radish and its bioactive compounds, especially MTBITC, are closely related with the improvement of the metabolic system of digestion, only limited research has been conducted on the effect of MTBITC in the intervention of NAFLD in vivo.
Therefore, the present study aimed to evaluate whether dietary intake of MTBITC can attenuate fat liquid diet induced NAFLD. Thus, a moderate calories over-intake rat based NAFLD model was established to address this problem.
Materials and methods
Isolation and detection of MTBITC
Fresh taproots of eight varieties of Asian white radishes (Raphanus sativus L) were obtained from the suburb of Beijing (Tianan Agriculture Development Co., Ltd. China) and the one which maintained the highest level of MTBITC was used in the next-step experiment. They were stored in a 4°C fridge and processed within 15 days to ensure the quality. Large quantities of MTBITC were extracted from radishes. In brief, every 500 g radish roots was grated using a food processing machine (Royalstar Co., Ltd., China) and extracted twice with 500 mL of n-hexane for 30 min at 25°C [19]. Then, 5 μL of 6 M HCl was added to avoid the degradation of the MTBITC. After combination and centrifuging the mixture, the solvent fraction was collected and sterilized by filtration using a 0.22 μm membrane and 5 mL of the filtered mixture was taken for the detection of MTBITC. The left over n-hexane extract was dried in a rotary evaporator (Ya Rong Co., Ltd., China) at 35°C. As a dietary solvent, soybean oil was added to the extract and the mixture was stored at -80°C until use.
Agilent 7890 Gas Chromatography (GC) attached to an Agilent 5975C Mass Selective Detector (MSD) with a HP-5MS capillaries column (30 m × 0.25 mm × 0.25 μm, J & W Scientific, CA) was used to identify the structure and detect the concentration of MTBITC in the hexane extract. The operating conditions were: 1:20 split injection mode, injector temperature 250°C, helium flow rate 30 mL/min, oven temperature programmed from 80°C to 270°C at 20°C/min with a 5 min initial and a 5 min final temperature hold. Mass spectra were obtained by electron impact ionization over the range of 35-500 amu at a rate of 2 scans/s. The ion source temperature was 200°C and the electronic impact energy was 70 eV. MTBITC had a retention time of 6.56 min and was identified via NIST08 database (Agilent, CA). Purified erucin (LKT Laboratories; St. Paul, MN) was used as the external standard to calculate the concentrations of MTBITC as previously reported [20].
Animals and diets
Three-week-old male Sprague-Dawley (SD) rats (Beijing Vital River Laboratory Animal Tech. Co., Ltd., China) were housed in a room at a temperature of 23 ± 1°C with a 12-h light-dark cycle with free access to water. All the protocols and procedures were carried out in accordance with “The guidelines of Beijing Community for care and use of laboratory animals”.
After 1 week of acclimatization, the animals were randomly divided into four groups (five rats per group) and fed the experimental diets for 9 weeks. The different diets is listed as follows: group C was normal control group treated with standard liquid diet (SLD) and soybean oil; group M was the model group treated with high fat liquid diet and soybean oil; group E1 was the first experimental group that was treated with High Fat Liquid Diet (HFLD) and MTBITC (9 mg/kg body weight per day); group E2 was the second experimental group treated with HFLD and MTBITC (18 mg/kg body weight per day). The soybean oil with MTBITC and the normal soybean oil were administered orally by means of gastric tube. It was given to the animals in the ratio of 35 g per 1 L diet, and its aim was maintaining the balance of nutrition and acting as vehicle simultaneously. The intake volume of soybean oil was adjusted every week after estimating the diet consumption of each animal group by weighing the remaining food. To protect the hepatic health, group C was treated with SLD in an amount restricted to the three-fourths of group M [21]. Groups M, E1 and E2 were provided ad libitum with HFLD that induced a slightly calorie overload (about 15% excess compared with ad libitum) due to the force feeding of soybean oil. The composition of the experimental diets (Table 1) was designed calorically equivalent in order to attenuate the volume’s impact on different diets. The SLD was designed with 65% energy derived from carbohydrate, 15.9% from fat and 19.1% from protein. The HFLD was designed with 17.7% energy derived from carbohydrate, 63.3% from fat and 19.1% from protein. Each diet contained about 2000 kcal/L, including the separate soybean oil for gavage. All the ingredients of diets were provided by HFK Bio-Technology. Co., Ltd. (Beijing, China).
Table 1.
Composition of diet
| Component | Standard Diet (g/L) | High Fat Diet (g/L) |
|---|---|---|
| Corn starch | 50 | 15 |
| Maltodextrin | 218.695 | 48.84 |
| Sucrose | 50 | 15 |
| Casein | 100 | 100 |
| L-cystine | 1.5 | 1.5 |
| DL-methionine | 1 | 1 |
| Soybean oil* | 35 | 35 |
| Corn oil | 0 | 104.8 |
| AIN-93G Mineral mix | 17.5 | 17.5 |
| AIN-93G Vitamine mix | 5 | 5 |
| Choline bitartrate | 1.25 | 1.25 |
| Fiber | 25 | 25 |
| Xanthan gum | 2.5 | 2.5 |
| Tertiary-butylhydroquinone | 0.007 | 0.007 |
Soybean oil was administered by gavage as vehicle.
Collection and process of samples
At the end of the experiment period, all rats were fasted overnight. After being deeply anesthetized, with diethylether, they were exsanguinated from the orbital venous plexus and the blood samples were collected in plastic tubes and incubated at 37°C for 30 min. The serum samples were separated by centrifugation for 7 min at 4000 rpm and stored at -80°C until analysis. The rats were sacrificed by cervical dislocation and the livers were immediately excised, weighed and divided into smaller pieces for storage at -80°C (for biochemical analysis) or in 4% paraformaldehyde (for histological analysis).
Histopathologic evaluation
Liver samples were examined histologically after embedding in paraffin, cutting into 5-μm-thick sections, and staining with hematoxylin and eosin. For hepatic steatosis analysis the frozen livers were cut into 14-μm-thick slices and stained with Oil-Red-O.
Biochemical analyses
Serum alanine and aspartate aminotransferases (ALT, AST), total cholesterol (TC), triglyceride (TG), glucose (GLU), high-density lipoprotein (HDL) and low-density lipoprotein (LDL) were assayed using commercial kits (Biosino bio-technology and science Inc., China) on Hitachi 7020 automatic biochemistry analyzer (Hitachi, Ltd., Japan). Serum insulin, tumor necrosis factor-alpha (TNF-α) and adiponectin were measured using enzyme-linked immunosorbent assay (ELISA) kits (R & D Systems, Inc., USA). HOMA-IR is calculated using the followed formula: Serum glucose (mmol/L) × Serum insulin (uU/mL)/22.5. Malondialdehyde (MDA), superoxide dismutase (SOD), and glutathione-peroxidase (GSH-Px) levels in liver homogenate were detected using commercial kits (Nanjing Jiancheng bioengineering Inc., China). Hepatic concentrations of TC and TG were measured after the extraction of liver homogenate using chloroform-methanol mixture [22].
Statistical analysis
All data were expressed as means ± SD. Statistical analysis was performed using one-way analysis of variance (ANOVA) followed by Duncan’s multiple range tests. P < 0.05 was considered to be statistically significant. Data were analyzed with SPSS 17.0 for Windows (SPSS Inc., Chicago IL. U.S.A.).
Result
Identification and quantitation of MTBITC
When compared to the other seven varieties, the Changchun Daikon was detected to contain the highest level of MTBITC (Data not listed). The purity of the MTBITC was assessed to be 91.6% in the hexane extract of white radish and the concentration of MTBITC was detected to be 52.4 μg/g fresh roots.
Body weight, liver weight and liver index (the ratio of liver weight to body weight)
As shown in Figure 1A, the body weight of rats of group M was significantly greater (P < 0.05) than the control group after three weeks of experimental diets. The administration of high dosage MTBITC produced a relatively slow increase of body weight before week six. However, having no different between them, the body weight of groups M, E1 and E2 increased at a greater (P < 0.05) rate than group C towards the end of the experiment. Also, the liver weight of M, E1 and E2 and the liver index of M and E1 were significantly higher (P < 0.05) than C (Figure 1B and 1C). However, the liver index of E2 showed no significant difference with that of C.
Figure 1.
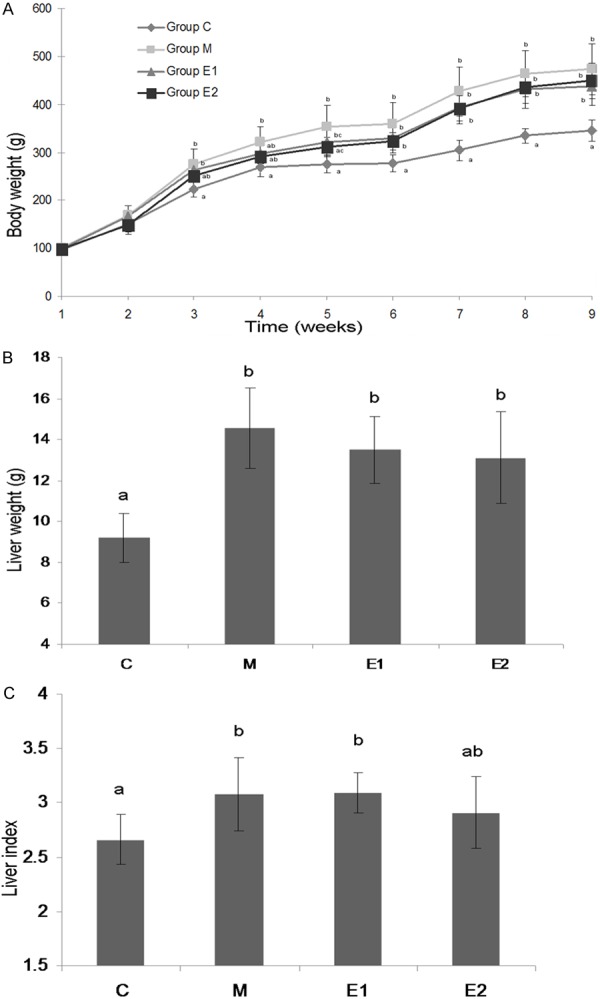
Body weight (A), liver weight (B) and liver index (C) the ratio of liver weight to body weight) of different experimental groups. Group C were treated with standard restricted diet (3/4 amount compared with ad libitum, n = 5); Group M were treated with high fat liquid diet ad libitum (n = 5); Group E1 were treated with high fat liquid diet ad libitum and MTBITC by gavage (9 mg/kg body weight per day, n = 5); Group E2 were treated with high fat liquid diet ad libitum and MTBITC by gavage (18 mg/kg body weight per day, n = 5). All the rats were forced to intake soybean oil (35 g/L diet) as vehicle. MTBITC, 4-(Methylthio)-3-butenyl isothiocyanate. Values are expressed as mean ± SD (n = 5). Values in a line with a different letter are significantly different at P < 0.05.
Histopathologic evaluation
There were no accumulations of lipid droplets in the hepatocytes of group C in the microscopic examination (Figure 2A, 2B). The liver sections of group M showed several macrovascular displaced nucleus in the periphery of hepatocytes (Figure 2C) and a severe deposition of small lipid droplets (Figure 2D). The hematoxylin and eosin staining of liver sections indicated that E1 (Figure 2E) and E2 (Figure 2G) had a normal liver structure when compared with M. However, liver sections which stained with Oil red O showed there were still some lipid droplets in the MTBITC treated group (E1, E2). The high dosage treatment group (E2) presented more less accumulation of lipid at the first glance (Figure 2F, 2H).
Figure 2.
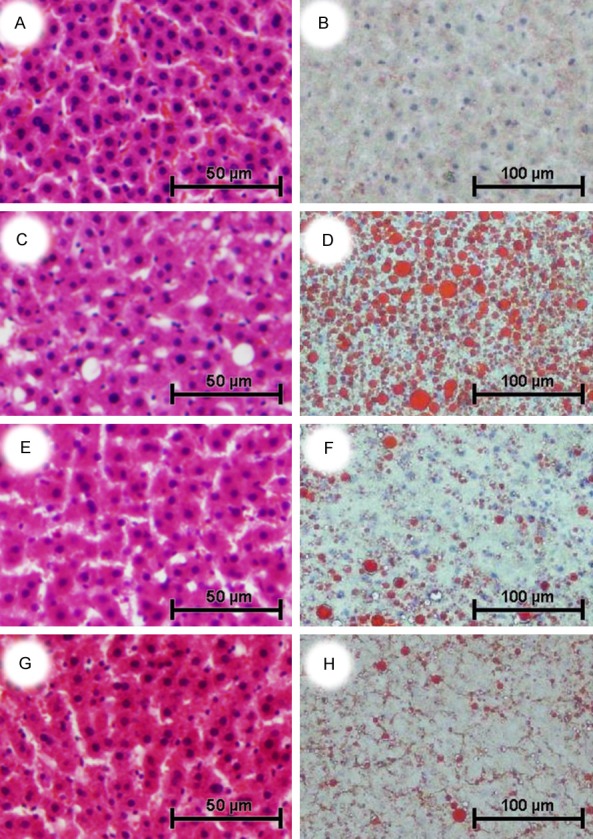
Effect of treatment with MTBITC on high fat-induced NAFLD rats (n = 5). Liver sections were stained with hematoxylin and eosin (A, C, E, G, original magnification × 200) and oil Red O (B, D, F, H, original magnification × 100). Group C were treated with standard restricted diet and showed a normal hepatic structure with no accumulation of fat droplets (A & B). Group M, which were given high fat liquid diet ad libitum, displayed hepatocytes macrovesicular steatosis with ballooning degeneration, and numerous lipid droplets (C & D). Group E1 were treated with high fat diet ad libitum and MTBITC by gavage (9 mg/kg body weight per day). It showed normal hepatic structure with less lipid accumulation than group M (E & F). Group E2 were treated with high fat diet ad libitum and MTBITC by gavage(18 mg/kg body weight per day). It displayed normal hepatic structure with minimal fat deposition in hepatocytes (G & H). All the rats were orally treated with soybean oil (35 g/L diet) as vehicle. MTBITC, 4-(Methylthio)-3-butenyl isothiocyanate.
Hepatic biochemical parameters
As shown in Table 2, after 9 weeks of treatment, the amount of hepatic TG and TC in M, E1 and E2 was significantly increased compared to those in C group. The hepatic TG of E2 was markedly lower than M (P < 0.05). The high dosage feeding of MTBITC made the hepatic MDA of E2 greatly lower than the other groups while the activity of hepatic SOD and GSH-Px in E2 were greater than those in M and E1 (P < 0.05). However, hepatic GSH-Px of M, E1 and E2 was markedly lower than C (P < 0.05). All hepatic parameters were similar between M and E1.
Table 2.
Hepatic lipid indices, concentrations of SOD, MDA and GSH-Px in 10% liver homogenate
| Parameter | Group C | Group M | Group E1 | Group E2 |
|---|---|---|---|---|
| TG (mmol/L) | 0.62 ± 0.27a | 2.24 ± 0.45b | 1.84 ± 0.45b,c | 1.46 ± 0.67c |
| TC (mmol/L) | 0.70 ± 0.18a | 2.41 ± 0.51b | 2.60 ± 1.02b | 1.94 ± 0.82b |
| MDA (nmol/mgprot) | 2.18 ± 0.56a | 2.34 ± 0.31a | 1.86 ± 0.22a | 0.99 ± 0.30b |
| SOD (U/mgprot) | 32.99 ± 4.88a | 31.11 ± 2.86a | 33.57 ± 5.03a | 44.28 ± 7.40b |
| GSH-Px (U/mgprot) | 642 ± 108a | 330 ± 68b | 341 ± 41b | 448 ± 68c |
The concentration of liver homogenate was diluted to one-tenth with normal saline. Group C were treated with standard restricted diet (3/4 amount compared with ad libitum, n = 5); Group M were given high fat liquid diet ad libitum (n = 5); Group E1 were treated with high fat liquid diet ad libitum and MTBITC by gavage (9 mg/kg body weight per day, n = 5); Group E2 were treated with high fat liquid diet ad libitum and MTBITC by gavage (18 mg/kg body weight per day, n = 5); All the rats were orally treated with soybean oil (35 g/L diet) as vehicle. TG, triglyceride; TC, total cholesterol; MDA, malondialdehyde; SOD, superoxide dismutase; GSH-Px, glutathione-peroxidase; MTBITC, 4-(Methylthio)-3-butenyl isothiocyanate. Values are expressed as mean ± SD (n = 5).
Values in a line with a different letter are significantly different at P < 0.05.
Values in a line with a different letter are significantly different at P < 0.05.
Values in a line with a different letter are significantly different at P < 0.05.
Serum biochemistry
The levels of lipid profiles, enzymes, glucose, insulin, TNF-α and adiponectin in serum of experimental rats were listed in Table 3. There were no differences in the serum TG, TC, HDL, AST and adiponectin levels during the feeding period in all of groups. Compared to C, the serum from group M had a significantly higher level of GLU, HOMA-IR and TNF-α. When compared to the model group, the low dosage treatment of MTBITC presented lower serum GLU and HOMA-IR, while high dosage of MTBITC brought about a markedly decrease in the level of LDL, Insulin and HOMA-IR, and a greatly improved AST/ALT ratio. There were no significant difference between C and E1 in the values of serum LDL, AST/ALT ratio, Insulin and TNF-α. Compared to group C, E2 presented no difference in serum ALT, AST/ALT ratio, GLU, HOMA-IR and TNF-α.
Table 3.
Results of serum chemistry level
| Parameter | Group C | Group M | Group E1 | Group E2 |
|---|---|---|---|---|
| TG (mmol/L) | 0.61 ± 0.19 | 0.57 ± 0.21 | 0.55 ± 0.16 | 0.44 ± 0.12 |
| TC (mmol/L) | 1.54 ± 0.12 | 1.46 ± 0.26 | 1.22 ± 0.41 | 1.44 ± 0.29 |
| HDL (mmol/L) | 1.1 ± 0.09 | 1.09 ± 0.16 | 0.92 ± 0.45 | 1.17 ± 0.27 |
| LDL (mmol/L) | 0.29 ± 0.09a | 0.3 ± 0.10a | 0.22 ± 0.04a,b | 0.17 ± 0.03b |
| ALT (U/L) | 45.0 ± 3.5a | 52.4 ± 2.1a,b | 54.8 ± 9.9b | 47.2 ± 5.0a,b |
| AST (U/L) | 195.8 ± 13.4 | 195.6 ± 15.79 | 216.6 ± 34.2 | 214.2 ± 23.09 |
| AST/ALT ratio | 4.39 ± 0.63a,b | 3.74 ± 0.38a | 4.02 ± 0.70a,b | 4.55 ± 0.28b |
| GLU (mmol/L) | 6.90 ± 1.5a | 8.30 ± 0.14b | 5.10 ± 0.39c | 7.72 ± 0.25a,b |
| Insulin (mU/L) | 27.17 ± 0.87a | 29.3 ± 1.76a | 26.23 ± 2.60a,b | 23.08 ± 4.21b |
| HOMA-IR | 8.33 ± 1.63a | 10.8 ± 0.54b | 5.98 ± 1.01c | 7.92 ± 1.44a |
| TNF-α (ng/L) | 243.0 ± 35.9a | 320.1 ± 30.4b | 256.8 ± 19.5a,b | 269.8 ± 86.4a,b |
| Adiponectin (μg/L) | 105.4 ± 7.1 | 100.6 ± 22.0 | 88.5± 17.1 | 80.3 ± 16.7 |
Group C were treated with standard restricted diet (3/4 amount compared with ad- libitum, n = 5); Group M were given high fat liquid diet ad libitum (n = 5); Group E1 were treated with high fat liquid diet ad libitum and MTBITC by gavage (9 mg/kg body weight per day, n = 5); Group E2 were treated with high fat liquid diet ad libitum and MTBITC by gavage (18 mg/kg body weight per day, n = 5); All the rats were orally treated with soybean oil (35 g/L diet) as vehicle. TG, triglyceride; TC, total cholesterol; HDL, high-density lipoprotein; LDL, low-density lipoprotein; ALT, alanine aminotransferases; AST, aspartate aminotransferases; GLU, glucose; TNF-α, tumor necrosis factor-α; ADP, adiponectin; MTBITC, 4-(Methylthio)-3-butenyl isothiocyanate. Values are expressed as mean ± SD (n = 5).
Values in a line with a different letter are significantly different at P < 0.05.
Values in a line with a different letter are significantly different at P < 0.05.
Values in a line with a different letter are significantly different at P < 0.05.
Discussion
Though NAFLD is known as one of the most prevalent chronic liver diseases worldwide, the studies of its pathogenesis are still hampered by the lack of suitable animal models, which can fully mimic the conditions of it. Most of the previous data are gained from genetic (db/db or fa/fa) [23] or MCD (choline and methionine deficiency) models [7,8]. However, the main drawback of these models is that they don’t share the same pathogenesis with human patients. Therefore, the over-nutrition model has been preferred in recent studies [24].
In this study, we used a self-designed diet which based on AIN-93G21 to fulfill the basic nutritional demand of animals and used the calorically equivalent liquid formula to attenuate the volume’s impact on different diets. However, it was difficult to measure the dietary calorie by calculating the volume due to the evaporation of liquid diet. To induce a modest caloric overload and to act as the vehicle of MTBITC, soybean oil of the formula was given separately by gavage. Because soybean oil is one of the necessary ingredients of the control diet, there is no need for a group of separate vehicles of delivery, which is necessary in other similar studies. It also benefits the experimental animals and saves reagents. SD rats were chosen because in which NAFLD can be easily induced, while the Wistar specie has been proved to be unsuitable [25,26]. The experimental time course and the dosage of dietary MTBITC were set to meet experimental requirements, and were based on the studies of other functions of this chemical, and our preliminary experiment in vitro (Data not listed).
Approximately 90% of NAFLD patients have one or more characteristics of metabolic syndrome and about 33% have the complete diagnosis [26]. Thus, a suitable rat model of NAFLD should process both the over accumulation of hepatic lipid and the characteristics of metabolic syndrome. In the present study, we succeeded in constructing a rat NAFLD model. The animals in the model group (M) had a significant increase in body weight, liver weight as well as liver index, when compared to the control group (C). Also, significantly higher hepatic lipid profile and obviously abnormal histological photographs indicated the presence of lipid accumulation and hepatic steatosis in the livers of the M group. Evidence suggested that high fat diet-induced NAFLD was associated with free radical injury and oxidative stress, which were characterized by increased lipid peroxidation in liver [27,28]. In the model rats, decreased SOD and GSH-Px, and increased MDA levels indicated hepatic lipid peroxidation and a negatively altered antioxidant system. Moreover, an enhancement of TNF-α, HOMA-IR and LDL levels further shows the presence of a metabolic syndrome.
Insulin resistance has been proved to be the first pathologic step in the development of NAFLD and HOMA-IR is a common parameter to evaluate its severity. In the present study, HOMA-IR of group M was significantly higher than group C. However, it is a moderate change compared with the published data [2,3]. Adiponectin is a kind of adipokine which can inhibit lipogenesis in tissues. In the silent stage of NAFLD, the ratio of AST/ALT or ALT/AST is considered as an index reflecting the degree of hepatic health. In addition, most studies have shown alterations of serum biomarkers, such as, TG, TC and HDL levels in NAFLD rats. However, no statistically significant changes were shown in the above parameters between the M and C group in our result. Besides, the histopathology examination of liver sections indicated that though there were pronounced deposition of lipid droplets and macrovascular steatosis in the livers of M, severe inflammation and fibrosis was not presented. Therefore, based on the evaluation standard of NAFLD, we only successfully induced the early stage of NAFLD. Liquid diet could hardly induce a severe NAFLD, but induce a mild one first, which could be attributed to the lack of experimental time because the over deposition of hepatic lipid has been emerged.
MTBITC, a kind of isothiocyanate, is mainly extracted from radish (Raphanus sativus L.). In our study, the dietary MTBITC has proved to be effective in the treatment of NAFLD in SD rats. The results indicated MTBITC could alleviate high fat & over nutrition-induced hepatic injury. Though there was no change in the weight of body or organ, the high dosage of MTBITC (18 mg/kg body weight per day) significantly decreased hepatic lipid profile, LDL level and the severity of insulin resistance. It also presented an excellent effect on the hepatic pathology conditions. Moreover, due to the amelioration of hepatic lipid peroxidation, the liver enzymatic antioxidant defense system recovered considerably. Low concentration (9 mg/kg body weight per day) administration of MTBITC also attenuated NAFLD, but the effect was limited comparatively.
Liver has been proved to be one of the target organs of MTBITC in this study. The chemo-protective effect of MTBITC can be attributed to the efficient antioxidant activity, which is higher than that of vitamin C & E [18]. The high fat diet and the stress of over nutrition can readily induced an over accumulation of lipid in the liver. Lipid deposition in the liver causes an excessive generation of free radicals and reactive oxygen species, and liver is the main organ suffering from free radical reactions [29]. In addition, oxidative stress exerts a negative impact on the regulation of glucose metabolism [30]. Hence, the effect of MTBITC on relieving insulin resistance might attribute to the antioxidant activity. Oxidative stress also induces a serious damage of biological membranes containing polyunsaturated fatty acids, and the extract of radish maintained the integrity of membrane [31]. By attenuating the increase of hepatic TG and serum LDL, MTBITC may affect the activity of LDL receptor and influencing the metabolism of lipids, especially hepatic lipid metabolism. A recent study demonstrated that an overgrowth of small intestinal bacteria may be an important pathogenesis of NAFLD [32]. Thus, another reason of our positive results may attribute to the excellent anti-bacteria ability of MTBITC [33]. In addition, the extract of radish stimulate stomach and intestinal vermicular motion, this shortens the retention time of dietary lipid in the digestion system. This may help avoid the accumulation of lipid in liver. However, whether the MTBITC is the bioactive chemical in this study should be clarified in future research.
In summary, based on the previous methods, we successfully developed a new rat based NAFLD model. The data showed that high fat diet (over 60% of calories from fat) and modest caloric overload induced an early stage of NAFLD, both reflecting the liver pathology and the metabolic context of human disease. The results also indicated that, administration of MTBITC in a relatively high dosage (18 mg/kg body weight per day) ameliorated the severity of NAFLD in an early stage, possibly by alleviating insulin resistance and hepatic oxidative stress.
Acknowledgements
This study was supported by the National Natural Science Foundation of China (31101263). The authors appreciate Dr. Jalila Ben Salah-Abbès and Prof. Ridha Oueslati of Faculty of Sciences Bizerte in Tunisia for supporting the identification of MTBITC. The authors would also like to thank Prof. Fan J.G. from the Department of Gastroenterology in Shanghai Xinhua Hospital for his advice on the rodent model of NAFLD.
Disclosure of conflict of interest
None.
References
- 1.Farrell GC, Larter CZ. Nonalcoholic fatty liver disease: from steatosis tocirrhosis. Hepatology. 2006;43:S99–S112. doi: 10.1002/hep.20973. [DOI] [PubMed] [Google Scholar]
- 2.Fan JG, Qiao L. Commonly used animal models of non-alcoholic steatohepatitis. Hepatobiliary Pancreat Dis Int. 2009;8:233–240. [PubMed] [Google Scholar]
- 3.Safwat GM, Pisano S, D’Amore E, Borioni G, Napolitano M, Kamal AA, Ballanti P, Botham KM, Bravo E. Induction of non-alcoholic fatty liver disease and insulin resistance by feeding a high-fat diet in rats: does coenzyme Q monomethyl ether have a modulatory effect? Nutrition. 2009;25:1157–1168. doi: 10.1016/j.nut.2009.02.009. [DOI] [PubMed] [Google Scholar]
- 4.Fan JG, Farrell GC. Epidemiology of non-alcoholic fatty liver disease in China. J Hepatol. 2009;50:204–210. doi: 10.1016/j.jhep.2008.10.010. [DOI] [PubMed] [Google Scholar]
- 5.Larter CZ, Yeh MM. Animal models of NASH: getting both pathology and metabolic context right. J Gastroenterol Hepatol. 2008;23:1635–1648. doi: 10.1111/j.1440-1746.2008.05543.x. [DOI] [PubMed] [Google Scholar]
- 6.Nagao K, Inoue N, Inafuku M, Shirouchi B, Morooka T, Nomura S, Nagamori N, Yanagita T. Mukitake mushroom (Panellus serotinus) alleviates nonalcoholic fatty liver disease through the suppression of monocyte chemoattractant protein 1 production in db/db mice. J Nutr Biochem. 2010;21:418–423. doi: 10.1016/j.jnutbio.2009.01.021. [DOI] [PubMed] [Google Scholar]
- 7.Ustundag B, Bahcecioglu IH, Sahin K, Duzgun S, Koca S, Gulcu F, Ozercan IH. Protective effect of soy isoflavones and activity levels of plasma paraoxonase and arylesterase in the experimental nonalcoholic steatohepatitis model. Dig Dis Sci. 2007;52:2006–2014. doi: 10.1007/s10620-006-9251-9. [DOI] [PubMed] [Google Scholar]
- 8.Larter CZ, Yeh MM, Haigh WG, Williams J, Brown S, Bell-Anderson KS, Lee SP, Farrell GC. Hepatic free fatty acids accumulate in experimental steatohepatitis: role of adaptive pathways. J Hepatol. 2008;48:638–647. doi: 10.1016/j.jhep.2007.12.011. [DOI] [PubMed] [Google Scholar]
- 9.Lieber CS, Leo MA, Mak KM, Xu Y, Cao Q, Ren C, Ponomarenko A, DeCarli LM. Model of nonalcoholic steatohepatitis. Am J Clin Nutr. 2004;79:502–509. doi: 10.1093/ajcn/79.3.502. [DOI] [PubMed] [Google Scholar]
- 10.Baumgardner JN, Shankar K, Hennings L, Badger TM, Ronis MJ. A new model for nonalcoholic steatohepatitis in the rat utilizing total enteral nutrition to overfeed a high-polyunsaturated fat diet. Am J Physiol Gastrointest Liver Physiol. 2008;294:G27–38. doi: 10.1152/ajpgi.00296.2007. [DOI] [PubMed] [Google Scholar]
- 11.Deng QG, She H, Cheng JH, French SW, Koop DR, Xiong S, Tsukamoto H. Steatohepatitis induced by intragastric overfeeding in mice. Hepatology. 2005;42:905–914. doi: 10.1002/hep.20877. [DOI] [PubMed] [Google Scholar]
- 12.Kim KH, Moon E, Kim SY, Choi SU, Lee JH, Lee KR. 4-Methylthio-butanyl derivatives from the seeds of Raphanus sativus and their biological evaluation on anti-inflammatory and antitumor activities. J Ethnopharmacol. 2014;151:503–508. doi: 10.1016/j.jep.2013.11.003. [DOI] [PubMed] [Google Scholar]
- 13.Suh SJ, Moon SK, Kim CH. Raphanus sativus and its isothiocyanates inhibit vascular smooth muscle cells proliferation and induce G (1) cell cycle arrest. Int Immunopharmacol. 2006;6:854–861. doi: 10.1016/j.intimp.2005.11.014. [DOI] [PubMed] [Google Scholar]
- 14.Jeong SI, Lee S, Kim KJ, Keum KS, Choo YK, Choi BK, Jung KY. Methylisogermabullone isolated from radish roots stimulates small bowel motility via activation of acetylcholinergic receptors. J Pharm Pharmacol. 2005;57:1653–1659. doi: 10.1211/jpp.57.12.0016. [DOI] [PubMed] [Google Scholar]
- 15.Barillari J, Iori R, Papi A, Orlandi M, Bartolini G, Gabbanini S, Pedulli GF, Valgimigli L. Kaiware Daikon (Raphanus sativus L. ) extract: a naturally multipotent chemopreventive agent. J Agric Food Chem. 2008;56:7823–7830. doi: 10.1021/jf8011213. [DOI] [PubMed] [Google Scholar]
- 16.Lamy E, Mersch-Sundermann V. MTBITC mediates cell cycle arrest and apoptosis induction in human HepG2 cells despite its rapid degradation kinetics in the in vitro model. Environ Mol Mutagen. 2009;50:190–200. doi: 10.1002/em.20448. [DOI] [PubMed] [Google Scholar]
- 17.Ben Salah-Abbes J, Abbes S, Abdel-Wahhab MA, Oueslati R. Raphanus sativus extract protects against Zearalenone induced reproductive toxicity, oxidative stress and mutagenic alterations in male Balb/c mice. Toxicon. 2009;53:525–533. doi: 10.1016/j.toxicon.2009.01.013. [DOI] [PubMed] [Google Scholar]
- 18.Ben Salah-Abbes J, Abbes S, Ouanes Z, Abdel-Wahhab MA, Bacha H, Oueslati R. Isothiocyanate from the Tunisian radish (Raphanus sativus) prevents genotoxicity of Zearalenone in vivo and in vitro. Mutat Res. 2009;677:59–65. doi: 10.1016/j.mrgentox.2009.05.017. [DOI] [PubMed] [Google Scholar]
- 19.Nakamura Y, Iwahashi T, Tanaka A, Koutani J, Matsuo T, Okamoto S, Sato K, Ohtsuki K. 4-(Methylthio)-3-butenyl isothiocyanate, a principal antimutagen in daikon (Raphanus sativus; Japanese white radish) J Agric Food Chem. 2001;49:5755–5760. doi: 10.1021/jf0108415. [DOI] [PubMed] [Google Scholar]
- 20.Hanlon PR, Webber DM, Barnes DM. Aqueous extract from Spanish black radish (Raphanus sativus L. Var. niger) induces detoxification enzymes in the HepG2 human hepatoma cell line. J Agric Food Chem. 2007;55:6439–6446. doi: 10.1021/jf070530f. [DOI] [PubMed] [Google Scholar]
- 21.Ahmed U, Redgrave TG, Oates PS. Effect of dietary fat to produce non-alcoholic fatty liver in the rat. J Gastroenterol Hepatol. 2009;24:1463–1471. doi: 10.1111/j.1440-1746.2009.05870.x. [DOI] [PubMed] [Google Scholar]
- 22.Folch J, Lees M, Sloane Stanley GH. A simple method for the isolation and purification of total lipides from animal tissues. J Biol Chem. 1957;226:497–509. [PubMed] [Google Scholar]
- 23.Carmiel-Haggai M, Cederbaum AI, Nieto N. A high-fat diet leads to the progression of non-alcoholic fatty liver disease in obese rats. FASEB J. 2005;19:136–138. doi: 10.1096/fj.04-2291fje. [DOI] [PubMed] [Google Scholar]
- 24.Buettner R, Scholmerich J, Bollheimer LC. High-fat diets: modeling the metabolic disorders of human obesity in rodents. Obesity (Silver Spring) 2007;15:798–808. doi: 10.1038/oby.2007.608. [DOI] [PubMed] [Google Scholar]
- 25.Zhou GD, Li MR, Zhang J, Pan D, Zhao SX, Yang JF, Yu J, Zhao JM. Chitosan ameliorates the severity of steatohepatitis induced by high fat diet in rats. Scand J Gastroenterol. 2008;43:1371–1377. doi: 10.1080/00365520802240230. [DOI] [PubMed] [Google Scholar]
- 26.Zou Y, Li J, Lu C, Wang J, Ge J, Huang Y, Zhang L, Wang Y. High-fat emulsion-induced rat model of nonalcoholic steatohepatitis. Life Sci. 2006;79:1100–1107. doi: 10.1016/j.lfs.2006.03.021. [DOI] [PubMed] [Google Scholar]
- 27.Matsuzawa N, Takamura T, Kurita S, Misu H, Ota T, Ando H, Yokoyama M, Honda M, Zen Y, Nakanuma Y, Miyamoto K, Kaneko S. Lipid-induced oxidative stress causes steatohepatitis in mice fed an atherogenic diet. Hepatology. 2007;46:1392–1403. doi: 10.1002/hep.21874. [DOI] [PubMed] [Google Scholar]
- 28.Chen SW, Chen YX, Shi J, Lin Y, Xie WF. The restorative effect of taurine on experimental nonalcoholic steatohepatitis. Dig Dis Sci. 2006;51:2225–2234. doi: 10.1007/s10620-006-9359-y. [DOI] [PubMed] [Google Scholar]
- 29.Lugasi A, Blazovics A, Hagymasi K, Kocsis I, Kery A. Antioxidant effect of squeezed juice from black radish (Raphanus sativus L. var niger) in alimentary hyperlipidaemia in rats. Phytother Res. 2005;19:587–591. doi: 10.1002/ptr.1655. [DOI] [PubMed] [Google Scholar]
- 30.Dalen KT, Ulven SM, Arntsen BM, Solaas K, Nebb HI. PPARalpha activators and fasting induce the expression of adipose differentiation-related protein in liver. J Lipid Res. 2006;47:931–943. doi: 10.1194/jlr.M500459-JLR200. [DOI] [PubMed] [Google Scholar]
- 31.Sipos P, Hagymasi K, Lugasi A, Feher E, Blazovics A. Effects of black radish root (Raphanus sativus L. var niger) on the colon mucosa in rats fed a fat rich diet. Phytother Res. 2002;16:677–679. doi: 10.1002/ptr.950. [DOI] [PubMed] [Google Scholar]
- 32.Wu WC, Zhao W, Li S. Small intestinal bacteria overgrowth decreases small intestinal motility in the NASH rats. World J Gastroenterol. 2008;14:313–317. doi: 10.3748/wjg.14.313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Beevi SS, Mangamoori LN, Dhand V, Ramakrishna DS. Isothiocyanate profile and selective antibacterial activity of root, stem, and leaf extracts derived from Raphanus sativus L. Foodborne Pathog Dis. 2009;6:129–136. doi: 10.1089/fpd.2008.0166. [DOI] [PubMed] [Google Scholar]


