Abstract
Background: Bilirubin is a potent antioxidant and previous studies have reported the relationship between low serum bilirubin concentration and atherosclerosis. Objective: To evaluate the prognostic value of serum total bilirubin (STB) in patients with angina pectoris undergoing percutaneous coronary intervention (PCI). Methods: In total of 1419 patients (931 men, mean age 60.9±10.5 years) with angina pectoris who had undergone successfully percutaneous coronary intervention (PCI) were included in this study. Patients were divided into 2 groups according to the median baseline STB (0.49 mg/dL in this cohort), which was measured before the PCI. Patients with a STB ≥0.49 mg/dL were classified into the high STB group and those with a STB <0.49 mg/dL were classified into the low STB group. Results: The incidence of in-hospital mortality and myocardial infraction was similar in the two groups. After a mean follow-up of 29.0±7.6 months, the incidence of death/myocardial infarction/stroke was significantly higher in low STB group compared with high STB group. Multivariate Cox regression analysis showed that low STB was an independent predictor of death/myocardial infarction/stroke (hazard ratio (HR) = 1.59, 95% confidence interval (CI) = 1.04-2.41, P = 0.031). The cumulative survival rate free from death/myocardial infarction/stroke was lower in low STB group than in high STB group (P = 0.002). Conclusion: Low STB levels before PCI is an independent predictor of long-term adverse clinical outcomes in patients with angina pectoris.
Keywords: Bilirubin, percutaneous coronary intervention, angina pectoris
Introduction
Cardiovascular disease is the most common cause of mortality in industrialized countries and accounts for up to one-third of all deaths worldwide [1]. Although the pathogenesis of atherosclerosis has not been thoroughly investigated, oxidative stress and DNA damage induced by oxidized low-density lipoprotein (LDL) cholesterol and by diet induced hypercholesterolemia contribute to the progression of atherosclerosis [2]. Bilirubin, an endogenous product of hemoglobin catabolism, has antioxidant and anti-inflammatory properties that attenuate endothelial activation and dysfunction in response to pro-inflammatory stress [3]. It has been shown to prevent oxidation of low-density lipoproteins and to inhibit vascular cell adhesion molecule-1 (sVCAM-1)-dependent migration of leukocytes into the endothelium [4]. Many studies have shown that bilirubin may protect against atherosclerosis in this way [5-7]. In addition, Gilbert’s syndrome is caused by a mutation that increases the level of bilirubin. The mutation carriers show a strong association with a lower risk of cardiovascular disease [8]. Recent studies have shown that serum total bilirubin (STB) levels are independently associated with short-term outcomes but not long-term outcome in patients with ST elevation myocardial infarction (STEMI) [9,10]. The association between STB and angina pectoris remains unclear. The aim of this study was to evaluate the prognostic value of STB in patients with angina Pectoris undergoing percutaneous coronary intervention (PCI).
Material and methods
Subjects
This study recruited consecutive patients with angina pectoris who had undergone successful PCI from July 2009 to August 2011 at a single large-volume PCI center. Qualitative and quantitative coronary angiographic analyses were carried out according to standard methods. PCI was performed using standard techniques. All patients were given loading doses of aspirin (300 mg) and clopidogrel (300 mg) before the coronary intervention, unless they had already received these antiplatelet medications. The treatment strategy, stenting techniques, selection of stent type, as well as use of glycoprotein IIb/IIIa receptor inhibitors were all left to the operator’s discretion. Daily aspirin (100 mg) and clopidogrel (75 mg) were prescribed for at least the first 12 months after the procedure. Patients presented with acute myocardial infarction (AMI), with known heart failure, autoimmune disease, neoplastic disease, chronic kidney disease, chronic hepatic disease, chronic or current infections, and any systemic disease that could cause elevated bilirubin concentrations were excluded from analysis. The study was approved by the First Affiliated Hospital of Zhengzhou University Research Ethics Committee. The data were anonymized and therefore no additional informed consent was required.
Definitions
Cardiovascular risk factors were assessed at the time of hospital admission. Patients who ≥65 years old were defined as being elderly. A history of smoking was assumed if the patient had smoked within the last 10 years. Patients were classed as having diabetes mellitus if their fasting plasma glucose concentration was >6.1 mmol/L, their hemoglobin A1c (HbA1c) was >6.5%, or if they were currently being treated with insulin or oral hypoglycemic agents. Patients were defined as having hypertension if their systolic blood pressure was ≥140 mmHg, their diastolic blood pressure was ≥90 mmHg, or if antihypertensive drugs were prescribed. Dyslipidemia was defined as total cholesterol or triglyceride levels were >200 mg/dL, low-density lipoprotein cholesterol >140 mg/dL, or lipid-lowering drugs were prescribed. Anemia was defined as a hemoglobin level <12.0 g/dL in women and <13.0 g/dL in men, based on the World Health Organization definition [11]. Glomerular filtration rate was estimated by the Cockcroft-Gault formula [12]. Target vessel revascularization (TVR) was defined as a repeat procedure, either PCI or coronary artery bypass grafting (CABG) in the target vessel.
Measurement of laboratory data
In the morning, after an overnight fasting for at least 12 hour, venous blood was collected for the measurement of total serum bilirubin concentrations, gamma-glutamyl transpeptidase (γ-GTP), aspartate aminotransferase (AST), alanine aminotransferase (ALT), hemoglobin, serum creatinine, total cholesterol, high-density lipoprotein-cholesterol (HDL-C), low-density lipoprotein-cholesterol (LDL-C), triglyceride and fasting plasma glucose. Serum total bilirubin levels were measured by the bilirubin oxidase method.
Classification of STB
To evaluate associations between STB and clinical outcome, patients were divided into 2 groups according to the median baseline STB (0.49 mg/dL in this cohort). Patients with a STB ≥0.49 mg/dL were classified into the high STB group and those with a STB <0.49 mg/dL were classified into the low STB group.
Determination of clinical outcomes and data
Prospective data were entered into a database that contained demographic, clinical, angiographic, and procedural information. Primary endpoints included all-cause death, occurrence of myocardial infarction (MI), and in-stent restenosis. The composite endpoint was defined as major adverse cardiovascular event (MACE), namely death/MI/stroke. Clinical follow-up was carried out through patient visits, telephone interview, and medical record review. Data were entered by independent research personnel and clinical events were adjudicated by physicians who were not involved in the procedures themselves. All deaths were considered to be cardiac unless an unequivocal noncardiac cause could be established. Cardiac deaths included all events related to a cardiac diagnosis, any complication of a procedure and treatment thereof, or any unexplained cause. Unexpected death, even in patients with a coexisting and potentially fatal noncardiac disease (e.g., cancer or infection), was classified as cardiac unless their history relating to the noncardiac diagnosis suggested death was imminent.
Statistics analysis
Categorical variables were expressed as percentages. Continuous variables were expressed as mean ± standard deviation (SD). Normally distributed continuous variables were analyzed using the Student’s t test and/or Mann-Whitney U test. Variables whose distribution could not be assumed to be normal were analyzed using the nonparametric Wilcoxon Rank-Sum tests. The Chi-square or Fisher’s Exact test were used for categorical variables. Cumulative survival curves were constructed using the Kaplan-Meier method and comparisons made using log-rank tests. Cox regression analysis was performed to identify independent predictors of death and MACE. All baseline, demographic, clinical, and angiographic variables were entered into the model. Results were reported as hazard ratios (HRs) and 95% confidence intervals (CIs). All statistical tests were 2-tailed, and p values were statistically significant at <0.05. All data were analyzed with SPSS 17.0 (SPSS Inc., Chicago, Illinois, USA).
Results
Baseline clinical characteristics
Between July 2009 and August 2011, there were 1545 patients who fulfilled all the inclusion and exclusion criteria were registered. Data were collected from 1419 patients (91.8%) over a follow-up period of 29.0±7.6 months. Table 1 presents the baseline clinical characteristics of patients. The mean age was 60.9±10.5 years and 66.3% of the patients were men. The level of STB ranged from 0.06 to 3.54 mg/dL (mean, 0.55±0.31 mg/dL).
Table 1.
Baseline characteristics of study population and medication
| Low STB N = 698 | High STB N = 721 | P | |
|---|---|---|---|
| Age (years) | 60.32±10.9 | 61.45±10.2 | 0.126 |
| Elderly (n, %) | 263 (37.7) | 297 (41.2) | 0.18 |
| Male gender (n, %) | 420 (60.2.5) | 511 (70.9) | <0.001 |
| Body mass index (kg/m2) | 24.04±4.0 | 24.11±3.92 | 0.74 |
| Prior PCI (n, %) | 47 (6.7) | 60 (8.3) | 0.26 |
| Prior CABG (n, %) | 6 (0.9) | 8 (1.1) | 0.63 |
| Prior myocardial infarction (n, %) | 75 (10.7) | 97 (13.5) | 0.103 |
| Peripheral vessel disease (n, %) | 1 (0.1) | 4 (0.6) | 0.19 |
| Prior stroke (n, %) | 36 (5.2) | 26 (3.6) | 0.15 |
| Anemia (n/%) | 191 (28.2) | 139 (19.5) | <0.001 |
| Systolic blood pressure (mmHg) | 122.6±19.8 | 126.6±17.5 | <0.001 |
| Diastolic blood pressure (mmHg) | 75.3±11.7 | 77.8±11.7 | <0.001 |
| Risk factors (n, %) | |||
| Arterial hypertension | 381 (54.7) | 375 (52) | 0.32 |
| Dyslipidemia | 322 (46.2) | 373 (51.8) | 0.035 |
| Diabetes mellitus | 142 (20.4) | 153 (21.3) | 0.68 |
| GFR (ml·min-1·1.73 m-2) | 74.5±14.1 | 74.7±14.3 | 0.79 |
| Current smoker | 217 (31.1) | 232 (32.2) | 0.67 |
| Clinical presentation (n, %) | |||
| Stable angina | 108 (15.5) | 115 (16) | 0.81 |
| Unstable angina | 588 (84.2) | 602 (83.5) | 0.70 |
| Total cholesterol (mmol/L) | 4.37±1.15 | 4.24±1.06 | 0.027 |
| Triglyceride (mmol/L) | 2.06±1.15 | 1.88±1.19 | 0.004 |
| LDL-C (mmol/L) | 2.75±1.05 | 2.68±0.91 | 0.18 |
| HDL-C (mmol/L) | 1.09±0.32 | 1.08±0.34 | 0.57 |
| Glycemia (mmol/L) | 5.83±2.15 | 5.71±1.95 | 0.27 |
| Haemoglobin (g/L) | 131.4±17.6 | 136.9±15.9 | <0.001 |
| AST (IU/L) | 18.4±5.1 | 18.35±5.4 | 0.86 |
| ALT (IU/L) | 19.8±6.2 | 19.7±6.5 | 0.77 |
| GGT (IU/L) | 22.6±9.4 | 23.1±11.2 | 0.36 |
| Medications at discharge (n, %) | |||
| Aspirin | 688 (98.6) | 712 (98.9) | 0.59 |
| Clopidogrel | 661 (94.7) | 689 (95.6) | 0.54 |
| ACEI/ARB | 371 (53.2) | 379 (52.6) | 0.85 |
| Beta-blocker | 494 (70.8) | 506 (70.2) | 0.81 |
| Statins | 651 (93.3) | 673 (93.3) | 0.95 |
PCI: percutaneous coronary intervention; CABG: coronary artery bypass graft; LDL-C: Low density lipoprotein cholesterol; HDL-C: High density lipoprotein cholesterol; AST: aspartate aminotransferase; ALT: alanine aminotransferase; GGT: gamma-glutamyl transferase; ACEI: Angiotensin-converting enzyme inhibitor; ARB: angiotensin receptor blocker.
The high STB group included 721 patients (mean age (61.45±10.2) years and 70.9 % males). There were more male patients in the high STB group than in the low STB group. There were no significant differences between the two groups with respect to age, body mass index, clinical presentation, medications at discharge, and the history of hypertension and diabetes mellitus. The percentage of prior revascularization, peripheral vessel disease, and current smoker was similar in the two groups. Aspartate aminotransferase and alanine aminotransferase, which reflect liver function, were not different between the groups. However, the total blood cholesterol and triglyceride levels in the high STB group were lower than in the low STB (P<0.05). The systolic blood pressure and diastolic blood pressure in the high STB group were higher than in the low STB group (P<0.001). The prevalence of anemia was lower in the high STB group than in the low STB group (P<0.001). Furthermore, the level of haemoglobin was lower in low STB group than in the high STB group (P<0.001). Other factors that were independently associated with baseline STB level are shown in Table 1.
Angiographic and procedural characteristics
Over 97% of PCI were performed through radial access. The percentage of patients with left ventricular ejection fraction (LVEF) ≤40% was similar in the two groups. There were no significant differences between the two groups with regard to the location and characteristics of target lesion, number of diseased vessels, treated vessels, stents, and the length of stents implanted per patient (Table 2).
Table 2.
Baseline angiographic and procedural characteristics
| Low STB N = 698 | High STB N = 721 | P | |
|---|---|---|---|
| Radial artery access (n, %) | 678 (97.1) | 706 (97.9) | 0.34 |
| Number of diseased (n, %) | |||
| 1-vessel disease | 268 (38.4) | 272 (37.7) | 0.80 |
| 2-vessel disease | 256 (36.7) | 282 (39.1) | 0.34 |
| 3-vessel disease | 170 (24.4) | 157 (21.8) | 0.25 |
| LVEF ≤40% (n/N, %) | 6/458 (1.3) | 6/468 (1.3) | 0.97 |
| Complex lesions* (n, %) | 741 (59) | 758 (58.8) | 0.903 |
| Chronic total occlusions (n, %) | 64 (9.2) | 62 (8.6) | 0.71 |
| Ostial lesions (n, %) | 62 (8.9) | 77 (10.7) | 0.25 |
| Number of treat vessels | 1.53±0.68 | 1.47±0.63 | 0.207 |
| Location of target lesion (n, %) | |||
| Left main coronary | 20 (2.9) | 14 (1.9) | 0.255 |
| Left anterior descending artery | 477 (68.3) | 503 (69.8) | 0.561 |
| Left circumflex artery | 283 (40.5) | 259 (35.9) | 0.073 |
| Rright coronary artery | 289 (41.4) | 284 (39.4) | 0.439 |
| Number of stents per patient | 2.18±1.34 | 2.06±1.16 | 0.409 |
| Length of stents implanted (sum of length per patient) | 51.1±35.34 | 46.98±29.52 | 0.193 |
| Diameter of stents implanted | 3.06±0.44 | 3.08±0.43 | 0.257 |
LVEF: Left ventricular ejection fraction;
Complex lesions was defined as lesion type B2 and C, according the American Heart Association/American College of Cardiology.
Clinical outcomes
The incidence of in-hospital death and myocardial infarction was similar in the two groups. (0.4% vs 0.1% and 0.3% vs. 0.4%, respectively). During the mean follow-up of 29 months, the incidence of MACE was significantly higher in the low STB group compared with high STB group (10.6% vs. 6.8%, P = 0.011). The incidence of all cause death, cardiac death, nonfatal myocardial infarction, nonfatal stroke, any revascularization (PCI/CABG), TVR and in-stent restenosis were slightly higher in the low STB group than in the high STB group, but there were no statistical differences (Table 3). Kaplan-Meier analyses revealed a significant difference between two groups in MACE (P = 0.002) (Figure 1), but not in all-cause death (P = 0.112) (Figure 2).
Table 3.
Clinical events from PCI until discharge and end of follow up
| Low STB N = 698 | High STB N = 721 | P | |
|---|---|---|---|
| In-hospital events | |||
| Death | 3 (0.4) | 1 (0.1) | 0.367 |
| Any myocardial infarction | 2 (0.3) | 3 (0.4) | 1.0 |
| Follow-up (cumulated events) | |||
| All cause death | 32 (4.6) | 22 (3.1) | 0.131 |
| Cardiac death | 22 (3.2) | 14 (1.9) | 0.147 |
| Nonfatal myocardial infarction | 14 (2.0) | 10 (1.4) | 0.366 |
| Nonfatal stroke | 21 (3.0) | 14 (1.9) | 0.195 |
| MACCE (dearh/myocardial infarction/stroke) | 74 (10.6) | 49 (6.8) | 0.011 |
| Any revascularization (PCI/CABG) | 44 (6.3) | 29 (4.0) | 0.052 |
| TVR | 22 (3.2) | 17 (2.4) | 0.36 |
| In-stent restenosis | 24 (4.2) | 25 (2.8) | 0.154 |
PCI: percutaneous coronary intervention; CABG: coronary artery bypass graft; MACE: major cardiovascular adverse events; TVR: target vessel revascularization.
Figure 1.
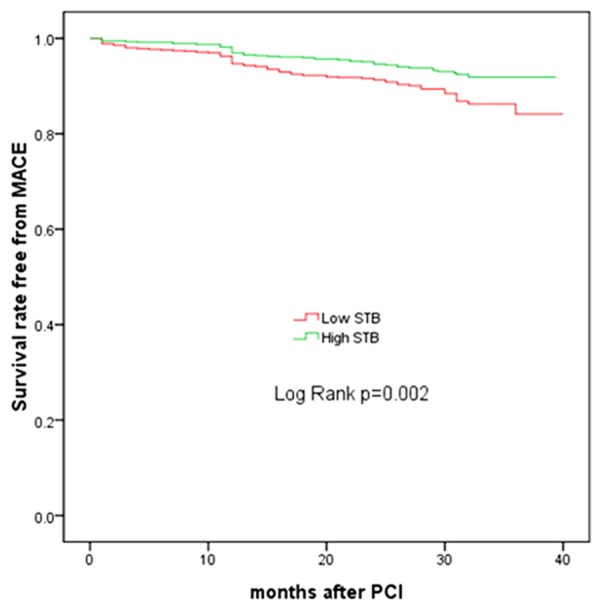
Kaplan-Meier estimates of survival for MACE. STB, serum total bilirubin.
Figure 2.
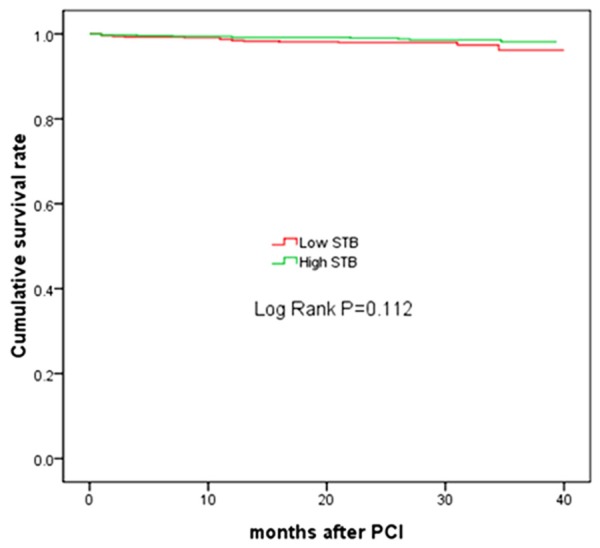
Kaplan-Meier estimates of survival for all-cause mortality. STB, serum total bilirubin.
Univariate and multivariate analysis results
Results of univariate analyses for all-cause death and MACE are shown in Table 4. After adjusting for age, gender, diabetes mellitus, peripheral vascular disease, history of revascularization, multivessel disease, LVEF, chronic total occlusion, left main coronary lesion, number of stents per patient, smoking status, length of stents implanted (sum of length per patient), and triglycerides, low STB was significantly associated with an increased incidence of MACE (hazard ratio [HR] 1.59, 95% confidence interval [CI] 1.04 to 2.41, P = 0.031), but not with all-cause death. Other independent predictors of long term MACE were left main coronary lesion (HR 3.03, 95% CI 1.32 to 6.95, P = 0.009), multivessel disease (HR 1.27, 95% CI 1.12 to 4.23, P = 0.021), and chronic total occlusion (HR 2.11, 95% CI 1.8 to 5.21, P = 0.033) (Table 5).
Table 4.
Univariate analysis for All-cause death and MACE
| All-cause death | MACE | |||||
|---|---|---|---|---|---|---|
|
|
|
|||||
| HR | 95% CI | P | HR | 95% CI | P | |
| Low STB | 1.89 | 0.85-4.22 | 0.116 | 1.75 | 1.22-2.51 | 0.003 |
| Elderly | 2.46 | 1.11-5.41 | 0.026 | 1.05 | 0.73-1.50 | 0.809 |
| Gender (male) | 0.61 | 0.28-1.31 | 0.203 | 1.51 | 1.01-2.26 | 0.045 |
| Hypertension | 1.67 | 0.74-3.75 | 0.214 | 1.13 | 0.79-1.61 | 0.52 |
| Diabetes mellitus | 2.39 | 1.08-5.27 | 0.031 | 1.24 | 0.82-1.87 | 0.30 |
| Dyslipidemia | 1.09 | 0.51-2.38 | 0.812 | 1.13 | 0.79-1.61 | 0.51 |
| GFR (ml·min-1·1.73 m-2) | 0.94 | 0.87-1.86 | 0.732 | 0.96 | 0.83-2.11 | 0.87 |
| Total cholesterol | 0.91 | 0.62-1.33 | 0.626 | 1.09 | 0.94-1.28 | 0.24 |
| HDL-C | 0.61 | 0.16-2.37 | 0.478 | 0.91 | 0.52-1.59 | 0.73 |
| LDL-C | 0.99 | 0.66-1.50 | 0.99 | 1.12 | 0.94-1.32 | 0.21 |
| Triglyceride | 0.82 | 0.54-1.27 | 0.82 | 1.11 | 1.02-1.21 | 0.02 |
| Peripheral vascular disease | 9.46 | 1.27-70.38 | 0.028 | 1.98 | 0.28-14.2 | 0.29 |
| Prior myocardial infarction | 1.28 | 0.44-3.72 | 0.648 | 0.92 | 0.53-1.59 | 0.76 |
| Cerebral vascular disease | 1.26 | 0.82-3.38 | 0.62 | 0.89 | 0.37-2.19 | 0.81 |
| History of revascularization | 1.18 | 0.76-4.82 | 0.32 | 1.83 | 1.07-3.14 | 0.028 |
| Anemia | 1.78 | 0.79-3.99 | 0.163 | 0.98 | 0.64-1.49 | 0.91 |
| Multivessel disease | 3.29 | 1.52-7.09 | 0.002 | 1.56 | 1.07-2.28 | 0.22 |
| LVEF ≤40 | 2.12 | 1.45-4.82 | 0.008 | 0.89 | 0.45-1.79 | 0.75 |
| Chronic total occlusions | 3.29 | 1.32-8.21 | 0.011 | 2.04 | 1.25-3.32 | 0.004 |
| Left main coronary lesion | 3.64 | 0.86-15.4 | 0.079 | 2.84 | 1.32-6.09 | 0.007 |
| Number of treated vessels | 1.64 | 0.97-2.77 | 0.063 | 1.24 | 0.96-1.59 | 0.104 |
| Number of stents per patient | 1.35 | 1.05-1.74 | 0.027 | 1.17 | 1.03-1.33 | 0.013 |
| Length of stents implanted (sum of length per patient) | 1.01 | 1.00-1.02 | 0.01 | 1.01 | 1.00-1.01 | 0.015 |
STB: serum total bilirubin; LDL-C: Low density lipoprotein cholesterol; HDL-C: High density lipoprotein cholesterol; LVEF: left ventricle ejection fraction.
Table 5.
Multivariate analysis for All-cause mortality and MACE
| HR | 95% CI | P | |
|---|---|---|---|
| All-cause death | |||
| Chronic total occlusion | 4.57 | 1.77-11.81 | 0.002 |
| Elderly | 3.39 | 1.31-8.82 | 0.012 |
| Diabetes mellitus | 2.87 | 1.21-6.79 | 0.017 |
| MACCE | |||
| Low STB | 1.59 | 1.04-2.41 | 0.031 |
| Left main coronary lesion | 3.03 | 1.32-6.95 | 0.009 |
| Multivessel disease | 1.27 | 1.12-4.23 | 0.021 |
| Chronic total occlusion | 2.11 | 1.8-5.21 | 0.033 |
MACE: major cardiovascular adverse events; STB: serum total bilirubin.
Discussion
In this study, we aimed to evaluate the prognostic value of STB in patients with angina pectoris who had undergone PCI. Our findings demonstrated that low STB levels before PCI serve as an independent predictor of long term MACE. However, no association was found with in hospital mortality and myocardial infarction. To our knowledge, our study is the first to demonstrate that low STB levels before PCI are an independent predictor of long term MACE in patients with angina pectoris.
The development of atherosclerosis in vasculature includes some oxidative reactions such as the formation of oxygen and peroxyl radicals, and especially oxidation of low-density lipoprotein cholesterol (LDL) [13,14]. The uptake of oxidized LDL by intimal macrophages leads to the accumulation of lipid-rich foam cells in vascular intima. Inflammatory cells further potentiate the formation of oxygen and peroxyl radicals. Those radicals play an important role in the inflammatory atherosclerotic process, especially due to disruption of the function of various cells including endothelial cells [15]. Therefore, antioxidants may play a protective role from atherosclerotic vascular involvement preventing oxidative modification of LDL [16,17].
Bilirubin is the final metabolite of heme catabolism, and has been found to be an effective antioxidant [18], it has been found to be a marker of cardiovascular risk. Schwertner et al. [19] found that serum bilirubin is an inverse and independent risk factor for CAD. A meta-analysis also showed a similar result [7]. Ghem et al. [20] have shown that reduced serum bilirubin levels were shown to be associated with a higher prevalence of CAD. In addition, Gilbert’s syndrome is caused by a mutation that increases the level of bilirubin. The mutation carriers show a strong association with a lower risk of cardiovascular disease [8]. One exception to these previous findings may be the PRIME study, which has described the relationship of serum bilirubin levels and cardiovascular risk as a U-shaped curve, implying that bilirubin exerts a protective effect, yet excessive concentrations may have a detrimental effect [21]. But the PRIME study included both patients with and without elevated liver enzyme. In another study [22], participants with bilirubin in the highest fifth, with a concentration greater than or equal to 17 mmol/L, had liver enzymes measured. Participants with high bilirubin and elevated alanine transaminase and aspartate amino transferase had an even greater risk of CHD than those with high bilirubin only, suggesting an interaction between bilirubin, elevated liver enzymes and CHD risk.
The status of bilirubin as a protective agent against atherosclerosis can be attributed to different mechanisms. Neuzil et al. [23] found that bilirubin inhibits oxidation of LDL lipids initiated within the lipoprotein core. Heme oxygenase (HO) is an important enzyme of bilirubin production. Increased activity of this enzyme may account for the anti-atherogenic through increased elimination of heme and reducing tissue iron [24]. Increased tissue iron because of decreased HO activity can trigger inflammation. This may explain the association of low serum bilirubin levels in the atherosclerotic process. In addition, another study has reported a beneficial effect on the endothelial function of bilirubin [25].
Heme oxygenase (HO) is a rate-limiting enzyme in the degradation of heme into biliverdin, which is rapidly converted to bilirubin. HO has commonly two kinds of isoenzyme: HO-1 and HO-2. Its inducible isoform, HO-1, is induced by stress but is not expressed under normal conditions. HO-2 is constitutively expressed, which has low activity (<10% of that of HO-1) [26]. In previous in vitro studies, it was demonstrated that HO-1 protein and activity were upregulated in the myocardium in response to MI [27]. Okuhara et al. [28] reported that serum bilirubin levels were elevated in patients with acute MI but not in non-acute MI. In addition, the serum HO-1 protein level was elevated, and the correlation was positive between HO-1 and bilirubin. In another study, Gul et al. [10] found that bilirubin levels were high in patients with acute STEMI, and high STB is independently associated with in-hospital adverse outcomes in patients who underwent primary PCI. However, no association was found with long-term mortality. In patients with AMI, elevated bilirubin levels might reflect the production of humoral mediators. In acute STEMI, infarction-related inflammatory cytokines, neurohumoral mediators, and HO-1 enzyme are activated. The degree of HO-1 enzyme activation was associated with the intensity of the inflammatory response to myocardial damage. This might be the reason of high in-hospital mortality rate in patients with high STB.
The present study only included patients with angina pectoris. The STB level, in theory, should not be affected by the activation of HO-1, and patients with liver dysfunction were excluded from the study. So STB should reflect the “baseline” STB level in these patients. It is known that serum bilirubin levels are modified by genetic factors. Serum bilirubin concentrations are affected by a major locus at the chromosome 2q telomere. A gene in this locus encodes hepatic bilirubin uridine diphosphate-glucuronosyltransferase (UGT1A1). An allele of this gene, designated UGT1A1*28, decreases transcription of the gene. Individuals homozygous for UGT1A1*28 have higher serum bilirubin concentrations. In an analysis of 1,780 unrelated individuals from the Framingham Offspring cohort who had been followed up for 24 years, homozygote UGT1A1*28 allele carriers with higher serum bilirubin concentrations had significantly lower risk of cardiovascular disease [29].
In our study, we showed that low STB was an independent predictor of long term MACE in patients with angina pectoris after PCI, but was not associated with in hospital mortality and myocardial infarction. All cause death, cardiac death, nonfatal myocardial infarction, nonfatal stroke and any revascularization were higher in the low STB group but did not reach significance. It might be due to the lower incidence of events in our study. Therefore, longer follow up was required to make sure the issue.
Previous studies [30,31] demonstrated that anemia was associated with increased adverse clinical outcomes in patients after PCI. In our study, the percentage of anemia was significant higher in the low STB group than in the high STB group, and the level of haemoglobin was significant lower in the low STB group than in the high STB group. We could not make clear whether low STB was contribute to the adverse clinical outcomes in patients with animia. Further studies are required to make clear of the question.
Kuwano et al. [32] reported that there was a significant negative correlation between the STB level and the in-stent restenosis. In our study, the rate of in-stent stenosis was also higher in the low STB group but did not reach significance. The possible explanation for such difference might be due to the following reasons: First, in our study, almost all of the patients (over 98% patients, data not shown) were implanted drug-eluting stent, which was associated with a significantly reduced incidence of in-stent restenosis and need for revascularization of the target lesion. Second, the percentage of follow up angiography was low (22%, data not shown), which might render an underestimation of the incidence of in-stent restenosis.
The present study has the well-known limitations of retrospective design, and it was a single-center study. However, our population contains homogeneous consecutive patients with angina pectoris, therefore mirroring a real-world scenario. We did not examine HO-1 activity, high-sensitivity C-reactive protein, markers of oxidative stress, and inflammation. In addition, bilirubin was assessed only once. We have no data on any changes in bilirubin levels during the course of the hospital stay and follow-up.
Conclusion
To the best of our knowledge, our study is the first to demonstrate that low STB levels before PCI is an independent predictor of long term adverse clinical outcomes in patients with angina pectoris. However, no association was found with in hospital mortality and myocardial infarction. STB has the potential for use as a tool for risk stratification.
Disclosure of conflict of interest
None.
References
- 1.Ross R. The pathogenesis of atherosclerosis: A perspective for the 1990s. Nature. 1993;362:801–809. doi: 10.1038/362801a0. [DOI] [PubMed] [Google Scholar]
- 2.Ceaser EK, Ramachandran A, Levonen AL, Darley-Usmar VM. Oxidized low-density lipoprotein and 15-deoxy-delta 12, 14-PGJ2 increase mitochondrial complex I activity in endothelial cells. Am J Physiol Heart Circ Physiol. 2003;285:H2298–2308. doi: 10.1152/ajpheart.00508.2003. [DOI] [PubMed] [Google Scholar]
- 3.Kawamura K, Ishikawa K, Wada Y, Kimura S, Matsumoto H, Kohro T, Itabe H, Kodama T, Maruyama Y. Bilirubin from heme oxygenase-1 attenuates vascular endothelial activation and dysfunction. Arterioscler Thromb Vasc Biol. 2005;25:155–160. doi: 10.1161/01.ATV.0000148405.18071.6a. [DOI] [PubMed] [Google Scholar]
- 4.Keshavan P, Deem TL, Schwemberger SJ, Babcock GF, Cook-Mills JM, Zucker SD. Unconjugated bilirubin inhibits VCAM-1-mediated transendothelial leukocyte migration. J Immunol. 2005;174:3709–3718. doi: 10.4049/jimmunol.174.6.3709. [DOI] [PubMed] [Google Scholar]
- 5.Kimm H, Yun JE, Jo J, Jee SH. Low serum bilirubin level as an independent predictor of stroke incidence: A prospective study in Korean men and women. Stroke. 2009;40:3422–3427. doi: 10.1161/STROKEAHA.109.560649. [DOI] [PubMed] [Google Scholar]
- 6.Zhang ZY, Bian LQ, Kim SJ, Zhou CC, Choi YH. Inverse relation of total serum bilirubin to coronary artery calcification score detected by multidetector computed tomography in males. Clin Cardiol. 2012;35:301–306. doi: 10.1002/clc.21964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Novotny L, Vitek L. Inverse relationship between serum bilirubin and atherosclerosis in men: A meta-analysis of published studies. Exp Biol Med (Maywood) 2003;228:568–571. doi: 10.1177/15353702-0322805-29. [DOI] [PubMed] [Google Scholar]
- 8.Lin JP, O’Donnell CJ, Schwaiger JP, Cupples LA, Lingenhel A, Hunt SC, Yang S, Kronenberg F. Association between the UGT1A1*28 allele, bilirubin levels, and coronary heart disease in the framingham heart study. Circulation. 2006;114:1476–1481. doi: 10.1161/CIRCULATIONAHA.106.633206. [DOI] [PubMed] [Google Scholar]
- 9.Celik T, Kaya MG, Akpek M, Yarlioglues M, Sarli B, Topsakal R, Gibson CM. Does serum bilirubin level on admission predict TIMI flow grade and in-hospital MACE in patients with STEMI undergoing primary PCI. Angiology. 2014;65:198–204. doi: 10.1177/0003319712474948. [DOI] [PubMed] [Google Scholar]
- 10.Gul M, Uyarel H, Ergelen M, Akgul O, Karaca G, Turen S, Ugur M, Ertürk M, Kul S, Surgit O, Bozbay M, Uslu N. Prognostic value of total bilirubin in patients with ST-segment elevation acute myocardial infarction undergoing primary coronary intervention. Am J Cardiol. 2013;111:166–171. doi: 10.1016/j.amjcard.2012.09.011. [DOI] [PubMed] [Google Scholar]
- 11.Nutritional anaemias. Report of a WHO scientific group. World Health Organ Tech Rep Ser. 1968;405:5–37. [PubMed] [Google Scholar]
- 12.Cockcroft DW, Gault MH. Prediction of creatinine clearance from serum creatinine. Nephron. 1976;16:31–41. doi: 10.1159/000180580. [DOI] [PubMed] [Google Scholar]
- 13.Ross R. Atherosclerosis-an inflamatory disease. N Engl J Med. 1999;340:115–126. doi: 10.1056/NEJM199901143400207. [DOI] [PubMed] [Google Scholar]
- 14.Mylonas C, Kouretas D. Lipid peroxidation and tissue damage. In Vivo. 1999;13:295–309. [PubMed] [Google Scholar]
- 15.Vítek L, Jirsa M, Brodanová M, Kalab M, Marecek Z, Danzig V, Novotný L, Kotal P. Gilbert syndrome and ischemic heart disease: a protective effect of elevated bilirubin levels. Atherosclerosis. 2002;160:449–456. doi: 10.1016/s0021-9150(01)00601-3. [DOI] [PubMed] [Google Scholar]
- 16.Hertog MG, Feskens EJ, Hollman PC, Katan MB, Kromhout D. Dietary antioxidant flavonoids and risk of coronary heart disease: the Zutphen Elderly Study. Lancet. 1993;342:1007–1011. doi: 10.1016/0140-6736(93)92876-u. [DOI] [PubMed] [Google Scholar]
- 17.Frankel EN, Kanner J, German JB, Parks E, Kinsella JE. Inhibition of oxidation of human low-density lipoprotein by phenolic substances in red wine. Lancet. 1993;341:454–457. doi: 10.1016/0140-6736(93)90206-v. [DOI] [PubMed] [Google Scholar]
- 18.Stocker R, Yamamoto Y, McDonagh AF, Glazer AN, Ames BN. Bilirubin is an anti-oxidant of possible physiological importance. Science. 1987;235:1043–1046. doi: 10.1126/science.3029864. [DOI] [PubMed] [Google Scholar]
- 19.Schwertner HA, Jackson WG, Tolan G. Association of low serum concentration of bilirubin with increased risk of coronary artery disease. Clin Chem. 1994;40:18–23. [PubMed] [Google Scholar]
- 20.Ghem C, Sarmento-Leite RE, de Quadros AS, Rossetto S, Gottschall CA. Serum bilirubin concentration in patients with an established coronary artery disease. Int Heart J. 2010;51:86–91. doi: 10.1536/ihj.51.86. [DOI] [PubMed] [Google Scholar]
- 21.Troughton JA, Woodside JV, Young IS, Arveiler D, Amouyel P, Ferrières J, Ducimetière P, Patterson CC, Kee F, Yarnell JW, Evans A PRIME Study Group. Bilirubin and coronary heart disease risk in the Prospective Epidemiological Study of Myocardial Infarction (PRIME) Eur J Cardiovasc Prev Rehabil. 2007;14:79–84. doi: 10.1097/01.hjr.0000230097.81202.9f. [DOI] [PubMed] [Google Scholar]
- 22.Breimer LH, Wannamethee G, Ebrahim S, Shaper AG. Serum bilirubin and risk of ischemic heart disease in middle-aged British men. Clin Chem. 1995;41:1504–1508. [PubMed] [Google Scholar]
- 23.Neuzil J, Stocker R. Free and albumin-bound bilirubin are efficient co-antioxidants for alpha-tocopherol, inhibiting plasma and low density lipoprotein lipid peroxidation. J Biol Chem. 1994;269:16712–16719. [PubMed] [Google Scholar]
- 24.Mayer M. Association of serum bilirubin concentration with risk of coronary artery disease. Clin Chem. 2000;46:1723–1727. [PubMed] [Google Scholar]
- 25.Yoshino S, Hamasaki S, Ishida S, Kataoka T, Yoshikawa A, Oketani N, Saihara K, Ichiki H, Kuwahata S, Fujita S, Takumi T, Yoshimoto I, Nakazaki M, Tei C. Characterization of the effect of serum bilirubin concentrations on coronary endothelial function via measurement of high-sensitivity C-reactive protein and high-density lipoprotein cholesterol. Heart Vessels. 2013;28:157–165. doi: 10.1007/s00380-011-0228-z. [DOI] [PubMed] [Google Scholar]
- 26.Abraham NG, Kappas A. Pharmacological and clinical aspects of heme oxygenase. Pharmacol Rev. 2008;60:79–127. doi: 10.1124/pr.107.07104. [DOI] [PubMed] [Google Scholar]
- 27.Lakkisto P, Palojoki E, Bäcklund T, Saraste A, Tikkanen I, Voipio-Pulkki LM, Pulkki K. Expression of heme oxygenase-1 in response to myocardial infarction in rats. J Mol Cell Cardiol. 2002;34:1357–1365. doi: 10.1006/jmcc.2002.2094. [DOI] [PubMed] [Google Scholar]
- 28.Okuhara K, Kisaka T, Ozono R, Kurisu S, Inoue I, Soga J, Yano Y, Oshima T, Kihara Y, Yoshizumi M. Change in bilirubin level following acute myocardial infarction is an index for heme oxygenase activation. South Med J. 2010;103:876–881. doi: 10.1097/SMJ.0b013e3181eac06a. [DOI] [PubMed] [Google Scholar]
- 29.Lin JP, O’Donnell CJ, Schwaiger JP, Cupples LA, Lingenhel A, Hunt SC, Yang S, Kronenberg F. Association between the UGT1A1*28 allele, bilirubin levels, and coronary heart disease in the Framingham Heart Study. Circulation. 2006;114:1476–1481. doi: 10.1161/CIRCULATIONAHA.106.633206. [DOI] [PubMed] [Google Scholar]
- 30.Pilgrim T, Vetterli F, Kalesan B, Stefanini GG, Räber L, Stortecky S, Gloekler S, Binder RK, Wenaweser P, Moschovitis A, Khattab AA, Buellesfeld L, Zwahlen M, Meier B, Jüni P, Windecker S. The impact of anemia on long-term clinical outcome in patients undergoing revascularization with the unrestricted use of drug-eluting stents. Circ Cardiovasc Interv. 2012;5:202–210. doi: 10.1161/CIRCINTERVENTIONS.111.965749. [DOI] [PubMed] [Google Scholar]
- 31.Nikolsky E, Mehran R, Aymong ED, Mintz GS, Lansky AJ, Lasic Z, Negoita M, Fahy M, Pocock SJ, Na Y, Krieger S, Moses JW, Stone GW, Leon MB, Dangas G. Impact of anemia on outcomes of patients undergoing percutaneous coronary interventions. Am J Cardiol. 2004;94:1023–1027. doi: 10.1016/j.amjcard.2004.06.058. [DOI] [PubMed] [Google Scholar]
- 32.Kuwano T, Miura S, Shirai K, Ike A, Mori K, Shimizu T, Zhang B, Iwata A, Nishikawa H, Kawamura A, Saku K. Serum levels of bilirubin as an independent predictor of coronary in-stent restinosis: a new look at an old molecule. J Atheroscler Thromb. 2011;18:574–583. doi: 10.5551/jat.6643. [DOI] [PubMed] [Google Scholar]


