Abstract
Background: This study was aimed at estimating the associations between coffee intake and osteoporosis (OP) in Chinese postmenopausal women. Methods: We conducted a large-scale, community-based, cross-sectional study to investigate the associations by using self-report questionnaire to access frequency of coffee intake. The total of 1817 participants was available to data analysis in this study. Multiple regression models controlling for confounding factors to include frequency of coffee intake variable were performed to investigate the relationships for OP. Results: Positive correlations between frequency of meat food intake and T-score were reported (β = 0.216, P value < 0.001). Multiple regression analysis indicated that the frequency of meat food intake was significantly associated with OP (P < 0.05 for model 1 and model 2). The postmenopausal women with high frequency of meat food intake had a lower prevalence of OP. Conclusion: The findings indicated that frequency of coffee intake was independently and significantly associated with OP. The prevalence of OP was more frequent in Chinese postmenopausal women not preferring coffee habits.
Keywords: Frequency, coffee intake, osteoporosis, Chinese postmenopausal women, association
Introduction
Osteoporosis (OP) is a bone disease in which the amount of bone decreases and the structural integrity of trabecular bone is impaired [1]. Cortical bone becomes more porous and thinner, which makes the bone weaker and more likely to fracture. The T-score is the most common method for measuring the level of OP with a bone density detector device. The residual lifetime risk of fracture was estimated to be around 50% in elderly women and 30% in elderly men [2]. OP has become a major health problem with increased morbidity and mortality in postmenopausal women all over the world.
Although OP is associated with multiple factors, lifestyle plays a key role in the prevention of OP [3]. Therapeutic lifestyle modification is mainly recommended, including regular exercise, a well-balanced diet, smoking cessation, and reduced alcohol consumption [3]. A dietary adjustment that might show promise for the prevention of OP is increasing consumption of products that correlate positively with bone health. Coffee is widely consumed in Western countries, and increasingly, Chinese are also consuming coffee. Coffee consumption has potential benefits, such as prevention of type 2 diabetes and cardiovascular disease [4-7]; however, coffee consumption can also have harmful effects on human health. Generally, the average individual daily coffee consumption in China is equivalent to a moderate level in Western countries.
Recently, studies revealed possible associations between coffee consumption and OP in postmenopausal women [8,9]. However, no associations between coffee consumption and outcome in postmenopausal women have been reported [10-13]. No consistent conclusion has been drawn from studies that investigated the associations. Thus, we conducted a large-scale study that evaluated risk factors for common diseases using a self-report questionnaire. The data showed that a subjective self-report questionnaire on the frequency of coffee consumption can reflect actual caffeine intake. The purpose of this study was to estimate the associations between coffee consumption and OP in Chinese postmenopausal women in a large-community sample by using a self-report questionnaire.
Methods
Study population
We performed a risk-factor study for OP using a random sample of the Chinese population. Participants were recruited from rural and urban communities in Shanghai. Participants aged 30-90 years were included in this study. More than 2,000 postmenopausal women were invited to a screening visit between 2011 and 2014. A total of 1817 participants (90.26%) with complete information was available to data analysis in this study. Written consent was obtained from all patients before the study, which was performed in accordance with the ethical standards in the Declaration of Helsinki, and approved by the Medicine Ethical Committee of the Changfeng Healthcare Center.
Some participants with chronic diseases and conditions that might potentially affect bone mass, structure, or metabolism were excluded. Briefly, the exclusion criteria were as follows: a history of 1) serious residual effects of cerebral vascular disease; 2) serious chronic renal disease (Glomerular filtration rate-GFR < 30 mL/min/1.73 m2); 3) serious chronic liver disease or alcoholism; 4) significant chronic lung disease; 5) corticosteroid therapy at pharmacologic levels; 6) evidence of other metabolic or inherited bone disease, such as hyper-or hypoparathyroidism, Paget disease, osteomalacia, or osteogenesis imperfecta; 7) recent (within the past year) major gastrointestinal disease, such as peptic ulcer, malabsorption, chronic ulcerative colitis, regional enteritis, or significant chronic diarrhea; 8) Cushing syndrome; 9) hyperthyroidism; and 10) any neurologic or musculoskeletal condition that would be a non-genetic cause of low bone mass.
Data collection
All study subjects underwent complete clinical baseline characteristics evaluation, which included a physical examination and response to a structured, nurse-assisted, self-administrated questionnaire to collect information on age, gender, residential region, visit date, family history, lifestyle, dietary habits, physical activity level during leisure time, use of vitamins and medications, smoking, alcohol consumption, and self-reported medical history. Body weight and height were measured according to a standard protocol. Smoking and alcohol consumption were categorized as never, current (smoking or consuming alcohol regularly in the past 6 months), or ever (cessation of smoking or alcohol consumption for more than 6 months).
Regular exercise was defined as any kind of physical activity 3 or more times per week. Education was commonly divided into four stages: preschool, primary school, secondary school, and college. Self-reported medical and therapy history was categorized as “no” or “yes.” HTN was defined as blood pressure ≥ 140/90 mmHg, or a history of hypertension medication. Diabetes mellitus (DM) was defined by oral glucose tolerance test (OGTT) and either HBALC ≥ 6.5% or the use of insulin or hypoglycemic medications.
Dietary habits, including consumption of coffee was evaluated by a semi-quantitative food frequency questionnaire (group 1: seldom, group 2: sometimes, group 3: always). To determine frequency of coffee preference, the participants were asked, “How often you drink coffee?” The possible answers were: “seldom,” “sometimes,” or “always,” and the answers were taken as a subjective assessment. To answer the question, the participants were required to decide two issues based on their impressions: 1) whether or not the consumed drink were actually coffee; and 2) the frequency with which they consumed coffee.
The study outcomes
The bone mineral density (BMD g/cm2) was measured at calcaneus by standardized quantitative ultrasound (QUS, Hologic Inc., Bedford, MA, USA) utilizing T-scores based on WHO criteria [14], which were obtained from the automated equipment. T-score refers to the ratio between patient’s BMD and that of young adult population of same sex and ethnicity. T-score of > -1 was taken as normal, between -1 and -2.5 osteopenic and < -2.5 as osteoporotic.
Daily calibration was performed during the entire study period by a trained technician. The coefficients of variation of the accuracy of the QUS measurement were 0.9%. The QUS technology is less expensive, portable and also has the advantage of not using ionising radiation, so it is safer than dual energy X-ray absorptiometry (DEXA).
Statistical analysis
Continuous variables were analyzed to determine whether they followed normal distributions, using the Kolmogorov-Smirnov Test. Variables that were not normally distributed were log-transformed to approximate a normal distribution for analysis. Results are described as mean ± SD or median, unless stated otherwise. Differences in variables among subjects grouped by frequency of coffee intake were determined by one way analysis of variance. Among groups, differences in properties were detected by χ2 analysis.
For the associations analysis, there model have been developed. In model 1, frequency of coffee intake were categorized by group 1: seldom, group 2: sometimes and group 3: always. In model 2: frequency of coffee intake were categorized by low frequency and high frequency groups. Univariate regression analysis was performed to determine variables associated with outcomes (T-score or OP), and to estimate confounding factors possibly disturbing the relation of frequency of coffee intake to outcomes (T-score or OP). Multivariable regression (MR) was performed to control potential confounding factors and determine the independent contribution of variables to outcomes (T-score or OP).
Results were analyzed using the Statistical Package for Social Sciences for Windows, version 16.0 (SPSS, Chicago, IL, USA). Tests were two-sided, and a p-value of < 0.05 was considered significant. Odds ratios (OR) with 95% confidence intervals (CI) were calculated for the relative risk of frequency of coffee intake with the outcome of OP.
Results
Clinical characteristics of subjects
The clinical baseline characteristics of the 1817 Chinese postmenopausal women are listed in Table 1. In the total sample, the mean age was 62.51 years. The proportions of subjects having current smoking and alcohol habits were 0.83% and 1.98%, respectively. The prevalence of HTN, coronary artery disease (CAD), DM and Rheumatoid arthritis (RA) were 44.89%, 9.60%, 11.47%, and 5.99%, respectively. There were significant differences in age, education, alcohol intake and prevalence of DM among groups according to frequency of coffee intake (P value < 0.05 for all). An average T-score of -1.87 was reported and the prevalence of OP was 28.84% in our study sample. Significant differences in T-Score and the prevalence of OP among the three groups were reported (P value < 0.001 for T-score and the prevalence of OP).
Table 1.
Baseline characteristics of subjects
| Variable | Total sample | Frequency of coffee intake | P-value | ||
|---|---|---|---|---|---|
|
| |||||
| Seldom | Sometimes | Always | |||
| Demographical information | |||||
| N | 1817 | 1614 | 108 | 95 | - |
| Age | 62.51 ± 9.02 | 62.95 ± 9.02 | 58.45 ± 8.11 | 59.66 ± 8.44 | < 0.001 |
| Education | 251 (13.81%) | 214 (13.26%) | 21 (19.44%) | 16 (16.84%) | < 0.001 |
| Exercise | 1294 (71.22%) | 1148 (71.13%) | 82 (75.93%) | 64 (67.37%) | 0.394 |
| Smoking | 15 (0.83%) | 11 (0.68%) | 1 (0.93%) | 3 (3.16%) | 0.125 |
| Alcohol intake | 36 (1.98%) | 26 (1.61%) | 6 (5.56%) | 4 (4.21%) | 0.028 |
| Oil | 18.92 ± 9.02 | 18.97 ± 8.97 | 18.9 ± 9.98 | 18.21 ± 8.77 | 0.726 |
| Medical history | |||||
| HTN | 804 (44.89%) | 730 (45.83%) | 37 (34.58%) | 37 (40.66%) | 0.054 |
| CAD | 167 (9.60%) | 153 (9.88%) | 8 (7.92%) | 6 (6.67%) | 0.506 |
| DM | 204 (11.47%) | 195 (12.34%) | 5 (4.72%) | 4 (4.35%) | 0.005 |
| RA | 105 (5.99%) | 99 (6.35%) | 3 (2.91%) | 3 (3.3%) | 0.195 |
| Therapy history | |||||
| Vitamin C | 234 (12.88%) | 203 (12.58%) | 14 (12.96%) | 17 (17.89%) | 0.323 |
| Vitamin D | 76 (4.18%) | 67 (4.15%) | 5 (4.63%) | 4 (4.21%) | 0.971 |
| Outcome | |||||
| T-score | -1.87 ± 0.74 | -1.91 ± 0.73 | -1.62 ± 0.69 | -1.52 ± 0.8 | < 0.001 |
| OP | 524 (28.84%) | 495 (30.67%) | 13 (12.04%) | 16 (16.84%) | < 0.001 |
Note: HTN - hypertension, CAD - coronary artery disease, DM - diabetes mellitus, RA - Rheumatoid arthritis, OP - Osteoporosis.
Association analysis for T-score
Univariate linear regression analyses were developed to include demographical information, lifestyle, medical and therapy history to estimate the association of various clinical factors and T-score (Table 2). The variables age, education, Vitamin C supplement and coffee preference were significantly associated with the T-score (P < 0.05 for all).
Table 2.
Univariate linear regression analysis for associations among variables and T-score
| Variable | β | SE | P value | 95% CI for β |
|---|---|---|---|---|
| Age | -0.032 | 0.002 | < 0.001 | -0.037 - -0.029 |
| Education | 0.089 | 0.014 | < 0.001 | 0.061-0.116 |
| Exercise | 0.064 | 0.037 | 0.095 | -0.012-0.136 |
| Smoking | -0.091 | 0.091 | 0.334 | -0.271-0.092 |
| Drink | 0.032 | 0.050 | 0.591 | -0.084-0.147 |
| HTN | -0.045 | 0.035 | 0.191 | -0.113-0.0213 |
| CAD | -0.115 | 0.058 | 0.048 | -0.231 - -0.001 |
| DM | 0.039 | 0.057 | 0.467 | -0.067-0.146 |
| RA | -0.134 | 0.075 | 0.075 | -0.278-0.013 |
| Vitamin C | -0.114 | 0.051 | 0.024 | -0.213 - -0.015 |
| Vitamin D | -0.058 | 0.085 | 0.503 | -0.225-0.111 |
| Frequency of coffee intake | 0.213 | 0.035 | < 0.001 | 0.144-0.282 |
Note: HTN - hypertension, CAD - coronary artery disease, DM - diabetes mellitus, RA - Rheumatoid arthritis.
The comparison of T-scores among groups according to Model 1 discovered that the mean T-score was -1.91, -1.62 and -1.52 in the three groups, respectively (Figure 1A). There were significant differences among the three groups (P value < 0.001). Additionally, there were significant differences among groups according to model 2 (Figure 1B, P value < 0.001), Univariate analysis demonstrated a positive correlation between frequency of coffee intake and T-score.
Figure 1.
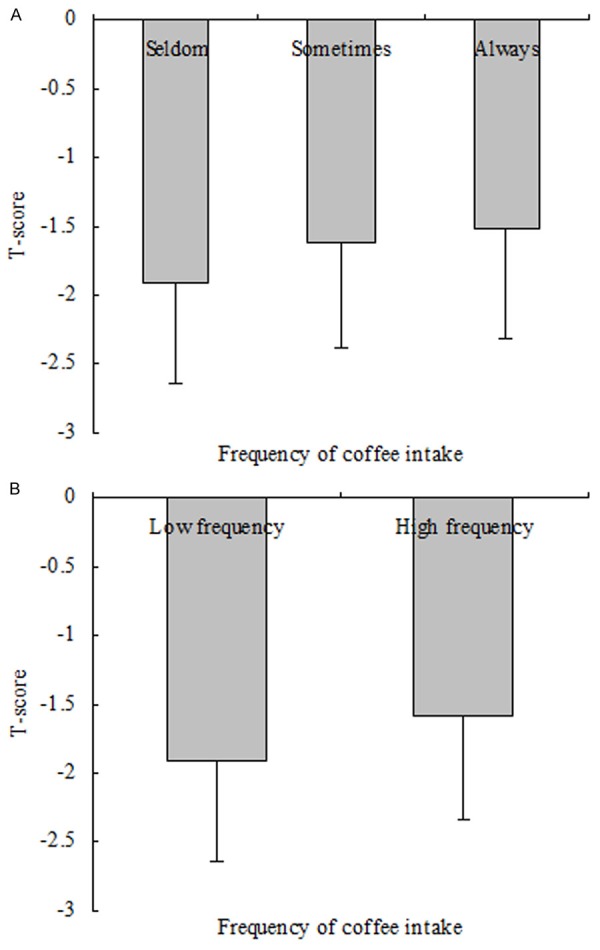
Comparison of T score among groups according to frequency of coffee intake. A. The results of comparison of T-score among groups according to Model 1 (Model 1: frequency of coffee intake were categorized by group 1: seldom, group 2: sometimes, group 3: always). The mean T-score was -1.91, -1.62 and -1.52 in the three groups, respectively. There were significantly differences among the three groups (P value < 0.001). B. The results of comparison of T-score between groups according to Model 2 (Model 2: frequency of coffee intake were categorized by low frequency and high frequency groups). The mean T-score was -1.91 and -1.57 in the two groups, respectively. There were no significantly differences between the two groups (P value < 0.001).
Multivariate linear regression analyses were developed to include frequency of coffee intake and the outcome of T-score. After adjustment for relevant potential confounding factors, the multivariate linear regression analyses detected significant associations (β = 0.147, p-value < 0.001, 95% CI for β: 0.078-0.215 for model 1; and β = 0.216, p-value < 0.001, 95% CI for β: 0.108-0.324 for model 2, Table 4).
Table 4.
Multiple variables linear regression analysis for the associations between frequency of coffee intake and T score
| Model | Variable | β | SE | P value | 95% CI for β |
|---|---|---|---|---|---|
| Model 1 | Frequency of coffee intake | 0.147 | 0.035 | < 0.001 | 0.078-0.215 |
| Model 2 | Frequency of coffee intake | 0.216 | 0.055 | < 0.001 | 0.108-0.324 |
Note: Model 1: frequency of coffee intake were categorized by group 1: seldom, group 2: sometimes, group 3: always; Model 2: frequency of coffee food intake were categorized by low frequency and high frequency groups; and all models adjusted for age, smoking, alcohol intake, education, exercise, medical and therapy history.
Association analysis for OP
Univariate logistic analyses were performed to evaluate associations with OP. The results indicate that age, education, HTN, CAD, RA, Vitamin C, Vitamin D and frequency of coffee intake were significantly associated with OP (P value < 0.05 for all, Table 3).
Table 3.
Univariate logistic regression analysis for associations among variables and osteoporosis
| Variable | β | S.E. | P value | OR | 95.0% CI |
|---|---|---|---|---|---|
| Age | 0.098 | 0.007 | < 0.001 | 1.105 | 1.091-1.119 |
| Education | -0.241 | 0.043 | < 0.001 | 0.788 | 0.722-0.857 |
| Exercise | -0.236 | 0.112 | 0.033 | 0.792 | 0.635-0.982 |
| Smoking | 0.032 | 0.276 | 0.913 | 1.032 | 0.602-1.769 |
| Drink | -0.193 | 0.192 | 0.315 | 0.825 | 0.564-1.202 |
| HTN | 0.312 | 0.103 | 0.003 | 1.365 | 1.114-1.669 |
| CAD | 0.498 | 0.162 | 0.002 | 1.647 | 1.197-2.261 |
| DM | 0.178 | 0.158 | 0.255 | 1.194 | 0.879-1.626 |
| RA | 0.481 | 0.207 | 0.021 | 1.615 | 1.075-2.429 |
| Vitamin C | 0.314 | 0.146 | 0.030 | 1.368 | 1.032-1.818 |
| Vitamin D | 0.491 | 0.238 | 0.039 | 1.637 | 1.028-2.603 |
| Frequency of coffee intake | -0.565 | 0.135 | < 0.001 | 0.569 | 0.436-0.741 |
Note: HTN - hypertension, CAD - coronary artery disease, DM - diabetes mellitus, RA - Rheumatoid arthritis.
The comparison of prevalence of OP among groups according to model 1 reported that the prevalence of OP was 30.66%, 12.04% and 16.84% in the three groups, respectively (Figure 2A). There were significant differences among the three groups (P value < 0.001). Significant differences among groups according to model 2 were also reported (Figure 2B, P value < 0.001 for model 2). Univariate analysis demonstrates a negative correlation between frequency of coffee intake and OP.
Figure 2.

Comparison of prevalence of osteoporosis among groups according to frequency of coffee intake. A. The results of comparison of prevalence of osteoporosis among groups according to Model 1 (Model 1: frequency of coffee intake were categorized by group 1: seldom, group 2: sometimes, group 3: always). The prevalence of osteoporosis was 30.66%, 12.04% and 16.84% in the three groups, respectively. There were significantly differences among the three groups (P value < 0.001). B. The results of comparison of prevalence of osteoporosis between groups according to Model 2 (Model 2: frequency of coffee intake were categorized by low frequency and high frequency groups). The prevalence of osteoporosis was 30.66% and 14.29% between the two groups, respectively. There were significantly differences between the two groups (P value < 0.001).
Multivariate logistic regression analyses were employed to evaluate the association between frequency of coffee intake and the OP outcome. After adjustment for relevant potential confounding factors, the multivariate logistic regression analyses detected significant associations (p-value = 0.017 for model 1; and p-value = 0.007 for model 2, Table 5). In participants with high frequency of coffee intake, the OR for OP was 0.541 (95% CI: 0.347-0.843).
Table 5.
Multiple variables logistic regression analysis for associations between frequency of coffee intake and osteoporosis
| Model | Variable | β | S.E. | P value | OR | 95% CI |
|---|---|---|---|---|---|---|
| Model 1 | Frequency of coffee intake | -0.346 | 0.145 | 0.017 | 0.708 | 0.533-0.940 |
| Model 2 | Frequency of coffee intake | 0.614 | 0.226 | 0.007 | 0.541 | 0.347-0.843 |
Note: Model 1: frequency of coffee intake were categorized by group 1: seldom, group 2: sometimes, group 3: always; Model 2: frequency of coffee intake were categorized by low frequency and high frequency groups; and all models adjusted for age, smoking, alcohol intake, education, exercise, medical and therapy history.
Discussion
We conducted a large-scale, community-based, cross-sectional study to investigate the associations between coffee consumption and OP in postmenopausal women. One of the most important strengths of our study is that we collected data from a large population-based cohort of postmenopausal women. BMD was evaluated using the QUS, which has many advantages in assessing osteoporosis. Specifically, the modality is small, no ionizing radiation is involved, measurements can be made quickly and easily, and the cost of the device is low compared with DXA and quantitative computed tomography devices. Several confounding factors such as age, education, and medical history were detected under univariate regression analysis or clinical analysis in practice. Multiple regression analysis adjustment for relevant potential confounding factors offered evidence that there were independent and significant associations between coffee consumption and outcome. In addition, coffee consumption was negatively correlated with the prevalence of OP.
The data on the association between coffee consumption and the prevalence of OP are inconclusive. Early results supported that coffee consumption was positively correlated with risk of OP. However, different results have shown no associations between coffee consumption and OP, or moderate coffee consumption may be negatively correlated with the risk of OP. Choi et al. conducted a population-based cross-sectional study to assess the relationship between coffee consumption and BMD in Korean premenopausal women [9]. Their findings did not support the hypothesis that coffee is a risk factor for impaired bone health in Korean premenopausal women. Similarly, Tavani and colleagues performed a case-control study in northern Italy to explore the possible relation between coffee and hip fracture and found no associations between hip fractures among women and the consumption of regular or decaffeinated coffee, tea, or cola [15]. In another study, Lloyd et al. aimed to evaluate in a cross-sectional study whether dietary caffeine intake was a risk factor for bone loss in postmenopausal women [16]. This study did not support the notion that caffeine is a risk factor for bone loss in healthy postmenopausal women. Additionally, in an animal study, Sakamoto et al. investigated the effects of coffee on bone metabolism and found that coffee does not stimulate bone loss in rats [17]. Similar results were reported in our study concerning the associations between moderate coffee consumption and OP.
In contrast, several studies have shown that heavy coffee consumption is associated with the risk of OP. Meyer et al. analyzed dietary data from a prospective study and found that women who drank 9 or more cups of coffee per day also had an increased risk of fracture, while there was no association between coffee consumption and hip fracture in men [12]. In addition, Hallström et al. explored whether coffee, tea, and caffeine consumption was associated with osteoporotic fracture risk in a cohort of 31,527 Swedish women aged 40-76 years and found that daily intake of 330 mg of caffeine, equivalent to 4 cups (600 ml) of coffee or more, may be associated with a modestly increased risk of osteoporotic fracture [11]. In an animal study, Lacerda et al. investigated the effects of coffee on bone metabolism in rats [18] and found that coffee/caffeine intake caused serious adverse effects on calcium metabolism in rats that led to a delay in the bone repair process. Meta-analyses suggested that daily consumption of coffee is associated with an increased risk of fracture in women and a contrasting decreased risk in men [10,19]. However, the current data are insufficient to reach a convincing conclusion, and further research must be conducted. The inconsistent results found in the literature may be attributed to incompleteness and/or inaccuracies in data collection regarding sample size, age of subjects, or the studies’ gender-specific nature; inclusion of cigarette smoking as a covariate; the method of coffee preparation (boiled or filtered); genetic differences in coffee metabolism and the caffeine content of the coffee beverage consumed; and lack of information regarding intake of other ingredients. According to these studies, heavy coffee consumption was associated with an increased risk of OP in women. Moderate coffee consumption may help prevent OP. Our data showed that moderate coffee consumption was independently and significantly associated with the prevalence of OP and negatively correlated with outcome. A slight increase in coffee consumption might be beneficial in the prevention of OP among Chinese postmenopausal women.
Coffee contains the stimulant caffeine, as well as antioxidants and other plant chemicals, all of which affect disease risk. The mechanism of caffeine-induced bone mineral density loss is still unclear; however, several possible causes have been elucidated by researchers. The cytotoxicity of caffeine may be due to its ability to trigger apoptosis [20]. A number of cysteine proteases, known as caspases, play important roles in apoptosis [21,22], similar to members of the Bcl-2 family [23] , which regulate mitochondrial membrane potential changes and the release of cytochrome C by modulating the permeability of the outer mitochondrial membrane. Heavy caffeine intake increases the urinary excretion of calcium, whereas moderate coffee consumption (1 or 2 cups per day) does not appear to significantly impact calcium imbalance in postmenopausal women [24] . Some studies have shown that coffee consumption seems to be a non-harmful habit for individuals who drink it regularly and in moderation, and in fact, coffee consumption may even be beneficial for most people. The most recently available evidence suggests that coffee consumption can help reduce the risk of several diseases, most notably type 2 diabetes, Parkinson’s disease, Alzheimer’s disease, cardiovascular disease, and cancer [4-7], although the underlying mechanisms of these effects are still being investigated. Regarding coffee’s other beneficial properties, some studies have shown that coffee may help reduce inflammation and pain more than the use of some painkillers alone [25]. Moreover, based on other findings, coffee may exert beneficial effects on bone health due to its high polyphenol composition; the impacts may be especially prominent in men, who are resistant to caffeine-induced bone loss [26,27].
This study has several potential limitations. First, this investigation was based on data obtained from a self-administered questionnaire. The questionnaire did not include references to the size of a cup of coffee, which could have led to misclassification. Additionally, genetically-determined differences in caffeine metabolism might be important for studying how BMD is affected by coffee [28]. However, our study did not involve the genotyping of participants. Finally, the study’s sample was composed entirely of Chinese postmenopausal women, and thus, the generalizability of our results is limited.
In conclusion, our findings suggested that frequency of coffee consumption was independently and significantly associated with OP. The prevalence of OP was more common in Chinese postmenopausal women who did not consume coffee. This study suggests that a change in preference for coffee consumption might be beneficial in the prevention of OP in Chinese postmenopausal women.
Acknowledgements
We thank the grant from Shanghai Tongji Hospital to support the study. Grants from the Clinical Medicine Foundation of Shanghai Tongji Hospital. ClinicalTrials.gov Identifier: NCT02451397.
Disclosure of conflict of interest
None.
Abbreviations
- BM-MNC
Bone mass density
- BM-MNC
Bone marrow-derived mononuclear cell
- BMI
Body mass index
- CAD
Coronary artery disease
- CI
Confidence intervals
- DM
Diabetes
- DXA
Dual-energy X-ray
- HTN
Hypertension
- GFR
Glomerular filtration rate
- OR
Odds ratios
- OP
Osteoporosis
- QUS
Quantitative ultrasound
- RA
Rheumatoid arthritis
References
- 1.Sattui SE, Saag KG. Fracture mortality: associations with epidemiology and osteoporosis treatment. Nat Rev Endocrinol. 2014;10:592–602. doi: 10.1038/nrendo.2014.125. [DOI] [PubMed] [Google Scholar]
- 2.Nguyen ND, Ahlborg HG, Center JR, Eisman JA, Nguyen TV. Residual lifetime risk of fractures in women and men. J Bone Miner Res. 2007;22:781–788. doi: 10.1359/jbmr.070315. [DOI] [PubMed] [Google Scholar]
- 3.Zhu K, Prince RL. Lifestyle and osteoporosis. Curr Osteoporos Rep. 2015;13:52–59. doi: 10.1007/s11914-014-0248-6. [DOI] [PubMed] [Google Scholar]
- 4.Larsson SC, Orsini N. Coffee consumption and risk of stroke: a dose-response meta-analysis of prospective studies. Am J Epidemiol. 2011;174:993–1001. doi: 10.1093/aje/kwr226. [DOI] [PubMed] [Google Scholar]
- 5.Larsson SC, Virtamo J, Wolk A. Coffee consumption and risk of stroke in women. Stroke. 2011;42:908–912. doi: 10.1161/STROKEAHA.110.603787. [DOI] [PubMed] [Google Scholar]
- 6.Huxley R, Lee CM, Barzi F, Timmermeister L, Czernichow S, Perkovic V, Grobbee DE, Batty D, Woodward M. Coffee, decaffeinated coffee, and tea consumption in relation to incident type 2 diabetes mellitus: a systematic review with meta-analysis. Arch Intern Med. 2009;169:2053–2063. doi: 10.1001/archinternmed.2009.439. [DOI] [PubMed] [Google Scholar]
- 7.Je Y, Liu W, Giovannucci E. Coffee consumption and risk of colorectal cancer: a systematic review and meta-analysis of prospective cohort studies. Int J Cancer. 2009;124:1662–1668. doi: 10.1002/ijc.24124. [DOI] [PubMed] [Google Scholar]
- 8.Hallstrom H, Byberg L, Glynn A, Lemming EW, Wolk A, Michaëlsson K. Long-term coffee consumption in relation to fracture risk and bone mineral density in women. Am J Epidemiol. 2013;178:898–909. doi: 10.1093/aje/kwt062. [DOI] [PubMed] [Google Scholar]
- 9.Choi EJ, Kim KH, Koh YJ, Lee JS, Lee DR, Park SM. Coffee consumption and bone mineral density in korean premenopausal women. Korean J Fam Med. 2014;35:11–18. doi: 10.4082/kjfm.2014.35.1.11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lee DR, Lee J, Rota M, Ahn HS, Park SM, Shin D. Coffee consumption and risk of fractures: a systematic review and dose-response meta-analysis. Bone. 2014;63:20–28. doi: 10.1016/j.bone.2014.02.007. [DOI] [PubMed] [Google Scholar]
- 11.Hallstrom H, Wolk A, Glynn A, Michaelsson K. Coffee, tea and caffeine consumption in relation to osteoporotic fracture risk in a cohort of Swedish women. Osteoporos Int. 2006;17:1055–1064. doi: 10.1007/s00198-006-0109-y. [DOI] [PubMed] [Google Scholar]
- 12.Meyer HE, Pedersen JI, Loken EB, Tverdal A. Dietary factors and the incidence of hip fracture in middle-aged Norwegians. A prospective study. Am J Epidemiol. 1997;145:117–123. doi: 10.1093/oxfordjournals.aje.a009082. [DOI] [PubMed] [Google Scholar]
- 13.Cummings SR, Nevitt MC, Browner WS, Stone K, Fox KM, Ensrud KE, Cauley J, Black D, Vogt TM. Risk factors for hip fracture in white women. Study of Osteoporotic Fractures Research Group. N Engl J Med. 1995;332:767–773. doi: 10.1056/NEJM199503233321202. [DOI] [PubMed] [Google Scholar]
- 14.Kanis J, Johnell O, Gullberg B, Allander E, Elffors L, Ranstam J, Dequeker J, Dilsen G, Gennari C, Vaz AL, Lyritis G, Mazzuoli G, Miravet L, Passeri M, Perez Cano R, Rapado A, Ribot C. Risk factors for hip fracture in men from southern Europe: the MEDOS study. Osteoporos Int. 1999;9:45–54. doi: 10.1007/s001980050115. [DOI] [PubMed] [Google Scholar]
- 15.Tavani A, Negri E, La Vecchia C. Coffee intake and risk of hip fracture in women in northern Italy. Prev Med. 1995;24:396–400. doi: 10.1006/pmed.1995.1064. [DOI] [PubMed] [Google Scholar]
- 16.Lloyd T, Rollings N, Eggli DF, Kieselhorst K, Chinchilli VM. Dietary caffeine intake and bone status of postmenopausal women. Am J Clin Nutr. 1997;65:1826–1830. doi: 10.1093/ajcn/65.6.1826. [DOI] [PubMed] [Google Scholar]
- 17.Sakamoto W, Nishihira J, Fujie K, Iizuka T, Handa H, Ozaki M, Yukawa S. Effect of coffee consumption on bone metabolism. Bone. 2001;28:332–336. doi: 10.1016/s8756-3282(00)00444-0. [DOI] [PubMed] [Google Scholar]
- 18.Lacerda SA, Matuoka RI, Macedo RM, Petenusci SO, Campos AA, Brentegani LG. Bone quality associated with daily intake of coffee: a biochemical, radiographic and histometric study. Brazilian Dental Journal. 2010;21:199–204. doi: 10.1590/s0103-64402010000300004. [DOI] [PubMed] [Google Scholar]
- 19.Liu H, Yao K, Zhang W, Zhou J, Wu T, He C. Coffee consumption and risk of fractures: a meta-analysis. Arch Med Sci. 2012;8:776–783. doi: 10.5114/aoms.2012.31612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Fernandez MJ, Lopez A, Santa-Maria A. Apoptosis induced by different doses of caffeine on Chinese hamster ovary cells. J Appl Toxicol. 2003;23:221–224. doi: 10.1002/jat.910. [DOI] [PubMed] [Google Scholar]
- 21.Martins LM, Kottke T, Mesner PW, Basi GS, Sinha S, Frigon N Jr, Tatar E, Tung JS, Bryant K, Takahashi A, Svingen PA, Madden BJ, McCormick DJ, Earnshaw WC, Kaufmann SH. Activation of multiple interleukin-1beta converting enzyme homologues in cytosol and nuclei of HL-60 cells during etoposide-induced apoptosis. J Biol Chem. 1997;272:7421–7430. doi: 10.1074/jbc.272.11.7421. [DOI] [PubMed] [Google Scholar]
- 22.Nicholson DW, Thornberry NA. Caspases: killer proteases. Trends Biochem Sci. 1997;22:299–306. doi: 10.1016/s0968-0004(97)01085-2. [DOI] [PubMed] [Google Scholar]
- 23.Tsujimoto Y, Shimizu S. Bcl-2 family: life-or-death switch. FEBS Lett. 2000;466:6–10. doi: 10.1016/s0014-5793(99)01761-5. [DOI] [PubMed] [Google Scholar]
- 24.Hasling C, Sondergaard K, Charles P, Mosekilde L. Calcium metabolism in postmenopausal osteoporotic women is determined by dietary calcium and coffee intake. J Nutr. 1992;122:1119–1126. doi: 10.1093/jn/122.5.1119. [DOI] [PubMed] [Google Scholar]
- 25.Lopez JR, Dominguez-Ramirez AM, Cook HJ, Bravo G, Diaz-Reval MI, Déciga-Campos M, López-Muñoz FJ. Enhancement of antinociception by co-administration of ibuprofen and caffeine in arthritic rats. Eur J Pharmacol. 2006;544:31–38. doi: 10.1016/j.ejphar.2006.06.041. [DOI] [PubMed] [Google Scholar]
- 26.Yano K, Heilbrun LK, Wasnich RD, Hankin JH, Vogel JM. The relationship between diet and bone mineral content of multiple skeletal sites in elderly Japanese-American men and women living in Hawaii. Am J Clin Nutr. 1985;42:877–888. doi: 10.1093/ajcn/42.5.877. [DOI] [PubMed] [Google Scholar]
- 27.Holbrook TL, Barrett-Connor E, Wingard DL. Dietary calcium and risk of hip fracture: 14-year prospective population study. Lancet. 1988;2:1046–1049. doi: 10.1016/s0140-6736(88)90065-7. [DOI] [PubMed] [Google Scholar]
- 28.Hallstrom H, Melhus H, Glynn A, Lind L, Syvanen AC, Michaëlsson K. Coffee consumption and CYP1A2 genotype in relation to bone mineral density of the proximal femur in elderly men and women: a cohort study. Nutr Metab (Lond) 2010;7:12. doi: 10.1186/1743-7075-7-12. [DOI] [PMC free article] [PubMed] [Google Scholar]


