Abstract
Objective: To evaluate whether the efficacy of high-dose chemotherapy in the treatment of primary well-differentiated osteosarcoma is superior to moderate-dose chemotherapy. Methods: Cochrane systematic review method was used to retrieve literatures from MEDLINE, Embase, OVID, Cochrane Library database of clinical trials, Chinese Biomedical Literature Database CD-ROM, as well as manual searching from “China Oncology”, “Chinese Journal of Clinical Oncology”, “Cancer” etc. Meta-analysis was performed using the RevMan 5.0 software. Results: A total of four studies, 937 cases of primary, non-metastatic, well-differentiated limb osteosarcoma patients were enrolled in the study. Meta-analysis results suggested that compared with moderate-dose group, 5-year disease-free survival, 5-year overall survival rate, the local recurrence rate, proportion of histologic response in good status, limb salvage rate showed no significant difference in high-dose chemotherapy group (All P > 0.05); good and poor response of preoperative chemotherapy tumor histologic of 5-year disease-free survival showed statistical difference (RR = 1.55; 95% CI: 1.19-2.00; P = 0.0009). Conclusion: High-dose chemotherapy for the treatment of primary osteosarcoma is not better than moderate-dose chemotherapy. It is expected that high quality of randomized controlled trials were performed to provide more reliable evidence in the future.
Keywords: Chemotherapy, osteosarcoma, systematic review, meta-analysis
Introduction
Osteosarcoma is a malignant tumor derived from mesenchymal tissue, characterized by the generation of spindle stromal cells of bone tissues, and with osteoid tissues directly produced by stromal sarcoma cells as a diagnostic basis [1]. Osteosarcoma is the most common malignance in bone tumors, accounting for about 20% [2]. Before the 1970s, the standard treatment for osteosarcoma was surgical amputation [3]; the prognosis was poor, and 5-year survival rate was less than 20% [4,5]. Lung metastasis is the main cause of death; in the initial surgery, about more than 80% of patients had lung micrometastases [6]. In 6 to 12 months after amputation, they often died due to lung metastasis progression [6]. After the 1970s, the use of adjuvant chemotherapy improves tumor control and survival of patients [7,8]. With the development of diagnostic imaging and surgical reconstruction techniques, and the improvement of tumor stage and grade, limb salvage surgery has been widely used in clinical practice as a safe surgical mode [9,10]; especially neoadjuvant chemotherapy combined with surgery for limb osteosarcoma increases the 5-year survival rate of patients (event-free-survival, EFS) increased from 10%~20% to 70%~75% [11-15]. Some scholars believe that high-dose chemotherapy (the so-called high-dose chemotherapy refers to that the dose of some or all chemotherapy drugs in each course or the accumulated dose of all the courses is greater than the recommended standard dosage) can improve the histological response of tumor cells, thus improving the patient’s survival [16]; but some researchers believe that there is no evidence to show the superiority of high-dose chemotherapy; the inducing chemotherapy-induced necrosis rate levels may reflect the inherent sensitivity of tumor cells to chemotherapeutic drugs, which cannot be changed by increasing chemotherapy dose [17-19]. Given the sample size of a single sample and even multi-center study is limited, bias is inevitable; therefore, it is necessary to conduct a systematic review for these studies, expecting to find the evidence for that high-dose chemotherapy is superior to conventional-dose chemotherapy, and make possible clinical explanation.
Materials and methods
Inclusion and exclusion criteria
We included the studies 1) Which were randomized, quasi-randomized clinical controlled trials. 2) The subjects of these studies with initial diagnosis, untreated primary and well-differentiated osteosarcoma, less than 50 years and there was no evidence of pulmonary metastasis. Osteosarcoma diagnosis was based on clinical manifestations, imaging (X-ray, CT, MRI) and tumor tissue biopsy. Seriously impaired function of heart, liver and kidney and those who cannot perform a given chemotherapy were excluded. Osteosarcoma associated with other tumors were excluded; 3) In the treatment group and the control group, chemotherapy may be the same and there may be some differences. In the treatment group dosage were higher than the recommended scheme, while in the control group recommended solutions of standard dose were used. Both groups were all underwent preoperative chemotherapy and prescribed surgery (limb salvage or amputation). According to assessment of the rate of tumor cell necrosis under pathological findings, we determined the programs and courses of postoperative chemotherapy.
Metrics and definitions of the key metrics
1). 5-year disease-free survival without tumor (5 years event-free-survival, EFS): it refers to the included subjects from the start of chemotherapy before surgery, surgery, chemotherapy after surgery to at least 5 year follow-up of survival without tumor recurrence and metastasis evidence; 2). 5-year overall survival (5 years overall-survival, OS): it refers to overall survival rate of the subjects from chemotherapy, surgery, postoperative chemotherapy to 5-year follow-up including the number of tumor-bearing survival. 3). histologic response (histological response): Detection of postoperative tumor tissue necrosis after preoperative chemotherapy. Tumor necrosis rate equal to or more than 90% was for good response; tumor necrosis rate less than 90% was for adverse reaction; 4). local recurrence: recurrence caused by local jump stoves and other tumor cell residual; 5). limb salvage rate: it refers to the ratio of after the assessment of preoperative chemotherapy which was suitable for localized completely resection and limb reconstruction restoration.
Search strategy
We retrieved literatures from MEDLINE, Embase, OVID, Cochrane Library database of clinical trials, Chinese Biomedical Literature Database CD-ROM database, and traced all the references of incorporated documents. We manual searched “China Oncology”, “Chinese Journal of Clinical Oncology”, “Cancer”. We also searched the papers at bone tumor professional conferences and unpublished gray literature. The language of the literature was not limited.
We used the key words as follow: “osteosarcoma” or “primary osteosarcoma” or “well-differentiated osteosarcoma” and “chemotherapy” or “high-dose chemotherapy” or “adjuvant chemotherapy” or “neoadjuvant chemotherapy“ and “methotrexate” or “cyclophosphamide” or “A doxorubicin” or “doxorubicin” or “vincristine” or “actinomycin D” or “ifosfamide” and “cisplatin” or “carboplatin”.
Statistical analysis
Cochrane systematic review software RevMan 5.0 was used for meta-analysis. Firstly, χ2 test was used to analysis heterogeneity between studies, with P equal to 0.1 for cut-off point of statistical difference. While I2 test were used for heterogeneity quantitative analysis between studies. When I2 was more than 50%, there may be heterogeneity. Fixed effect model was used for merging the data from the literatures without heterogeneity; if heterogeneity existed, we analyzed the reasons for heterogeneity and processed with sensitivity analysis; if the heterogeneity of literature still cannot be eliminated, random effect model was used to merge the data. RR and 95% CI were used for dichotomous variables; continuous variables using WMD and 95% CI to express the effect size, and the results were listed with the forest plot.
Results
Overview of included studies
736 documents were initially retrieved; by reading the title and summary, 631 clinical researches were screened out; and through reading the full text and rescreening, 76 documents inconsistent with the inclusion criteria were excluded due to non-control studies, reviews and the varied main measure indicators. Finally four literature were included [17-20], three of which were RCT [18-20] and 1 [17] of which was quasi-randomized controlled clinical trial as shown in Figure 1. All were English literature. General information of included studies was shown in Table 1. The 4 studies included 937 cases of patients younger than 50 years old, and all studies had provided the inclusion criteria. 3 RCTs [18-20] did not explicitly describe the randomly assigned method, and only one study [18] described the method of allocation concealment; all of them did not explain the use of blinding; three studies had reported the failure in follow-up, and the quality class was C. All studies provided the baseline of treatment group and the control group; the difference between the two groups was not statistically significant.
Figure 1.
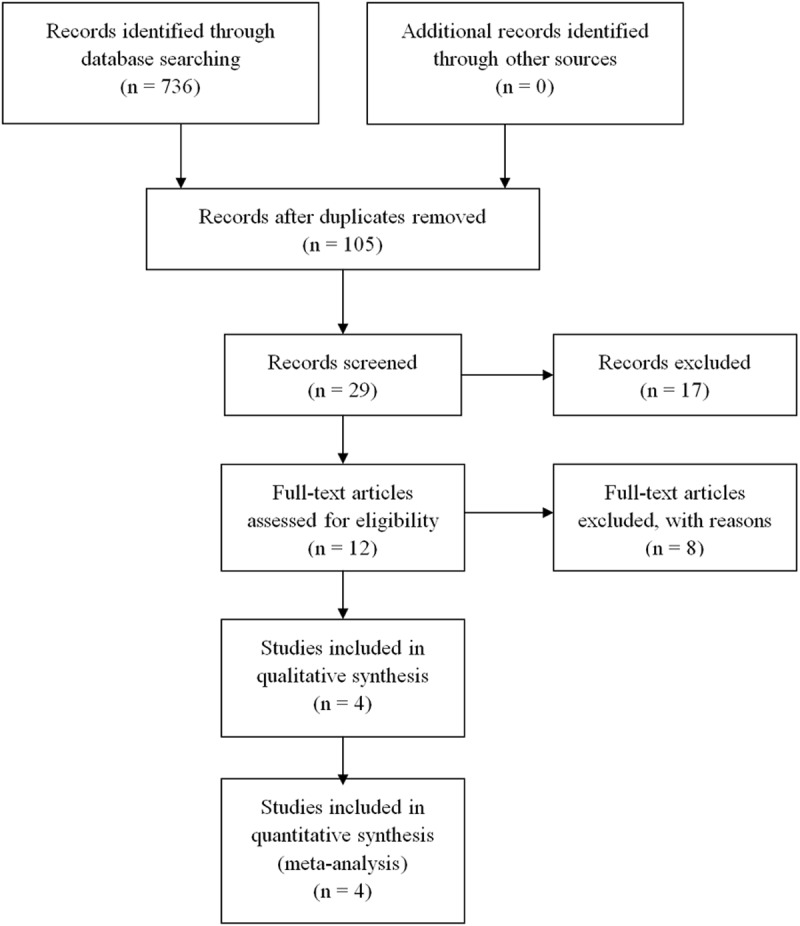
The follow chart of literatures identification.
Table 1.
Characteristics of the included studies
| Studies | N | Interventions | Outcome measure | Quality assessment of methodology | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
|
|
|
||||||||||
| Trial group | Control group | Design | Level | Randomization | Allocation concealment | Blinding | Loss to follow-up | Baseline | |||
| Bacci 2003 | 367 | IOR/OS-N5 | IOR/OS-N4 | 5-year disease free survival, local recurrence | CCT | C | Inadequate | Unclear | Not used | Unclear | Comparable |
| Meyers 1998 | 73 | Regimen II | Regimen I | 5-year disease free survival, local recurrence, Histological response | RCT | C | Inadequate | Clear | Unclear | Yes | Comparable |
| Bacci 1986 | 106 | Regimen II | Regimen I | 5-year disease free survival, Histological response, 5-year overall survival, percentage of limb salvage | RCT | C | Inadequate | Unclear | Unclear | Yes | Comparable |
| Rober 1997 | 391 | Regimen II | Regimen I | 5-year disease free survival, local recurrence, Histological response, 5-year overall survival, percentage of limb salvage | RCT | C | Inadequate | Unclear | Unclear | Yes | Comparable |
Meta-analysis results
5-year tumor-free survival
Four studies [17-20] have reported the 5-year disease-free survival of patients with primary limb osteosarcoma in moderate-dose chemotherapy group and high-dose chemotherapy group; there were a total of 923 cases, including 459 cases of moderate-dose chemotherapy and 464 cases of high-dose chemotherapy. No heterogeneity had been found among studies, so the fixed-effects model was used; the results showed no statistically significant difference in 5-year tumor-free survival between the two chemotherapy regimens, indicating that increasing cumulative doses of chemotherapy drugs cannot correspondingly increase 5-year tumor-free survival of patients. The results of Meta-analysis were shown in Table 2.
Table 2.
Meta-analysis of the efficacy of high-dose versus moderate-dose chemotherapy in treating osteosarcoma
| Sample size (n) | Test of association | Test for heterogeneity | |||||
|---|---|---|---|---|---|---|---|
|
|
|||||||
| Genetic model | High-dose | Moderate-dose | RR | 95% CI | P | P | I2 |
| 5-year tumor-free survival | 464 | 459 | 1.11 | 0.95-1.27 | 0.27 | 0.65 | 0% |
| The local recurrence rate | 282 | 264 | 0.94 | 0.54-1.58 | 0.72 | 0.45 | 0% |
| The rate of good local histologic response | 361 | 345 | 0.96 | 0.81-1.24 | 0.32 | 0.64 | 0% |
| 5-year survival | 423 | 405 | 1.09 | 0.97-1.21 | 0.21 | 0.35 | 5.2% |
| Limb salvage rate | 388 | 370 | 0.98 | 0.92-1.05 | 0.18 | 0.93 | 0% |
RR: Relative Risk, CI: confidence interval, vs.: versus.
The local recurrence rate
Three studies [17-19] reported the local recurrence rate after 5 years of follow-up, and no heterogeneity had been found among studies, so a fixed effects model was used; the results showed no significant difference in 5-year local recurrence rate between the two chemotherapy regimens, indicating that increasing cumulative dose of chemotherapy drugs did not improve the safety of the local operation. The results of Meta-analysis were shown in Table 2.
The rate of good local histologic response
Three studies [17,18,20] described the proportion of patients with good histologic response after moderate-dose and high-dose chemotherapy; no heterogeneity had been found among studies, so a fixed effects model was used; the results showed no significant difference in the proportion of patients with good histologic response between the two chemotherapy regimens, indicating that increasing cumulative dose of chemotherapy drugs did not improve the tumor histologic response to chemotherapy drugs. The results of Meta-analysis were shown in Table 2.
5-year survival
Three studies [17,18,20] reported the 5-year overall survival rates of the two methods; no heterogeneity had been found among studies, so a fixed effects model was used; the results showed no significant difference in 5-year overall survival rate between the two chemotherapy regimens, indicating that increasing cumulative dose of chemotherapy drugs did not increase the 5-year overall survival rate of patients with osteosarcoma. The results of Meta-analysis were shown in Table 2.
Meta-analysis of limb salvage rate
Two studies [17,20] reported the limb salvage rates of the two methods; no heterogeneity had been found between studies, so a fixed effects model was used; the results showed no significant difference in limb salvage rate between the two chemotherapy regimens. The results of Meta-analysis were shown in Table 2.
Preoperative chemotherapy histology reaction degree for tumor
Three studies [17,18,20] described 5-year disease-free survival of preoperative chemotherapy on tumor histology good and adverse reactions. The heterogeneity existed between studies (P = 0.06, I2 = 63.6%). By analyzing the sources of heterogeneity, we found Gaetano Bacci [17] study was designed as CCT. Therefore we divided it into another subgroup analysis, eliminating the heterogeneity, and fixed-effects model were used for analysis with statistical difference (P < 0.00001 and P < 0.007), indicating that the tumor correlated with response of preoperative chemotherapy histology and 5-year survival rate. Patients with good histologic response showed a higher 5-year survival rate, indicating that the tumor to histological response of preoperative chemotherapy was an independent prognostic factor for osteosarcoma. The result of meta-analysis was shown in Figure 2.
Figure 2.
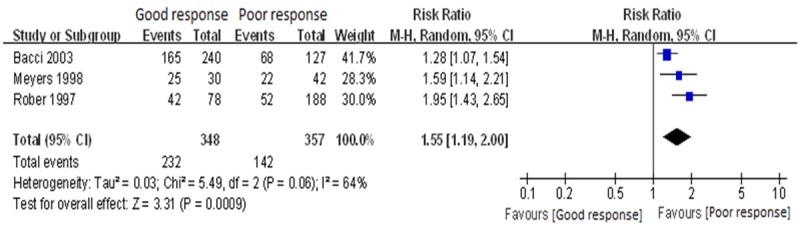
Meta-analysis of 5-year EFS for good vs. poor histological response for preoperative chemotherapy, the horizontal lines correspond to the study-specific RR and 95% CI, respectively. The area of the squares reflects the study-specific weight. The diamond represents the pooled results of RR and 95% CI.
Discussion
In the present study, we did not found significant difference in 5-year disease-free survival, 5-year overall survival rate, the local recurrence rate, proportion of histologic response in good status, and limb salvage rate between high-dose and moderate-dose chemotherapy in treating osteosarcoma.
Improving the survival rate of patients with osteosarcoma by systemic chemotherapy has been widely recognized [21,22], but the maximum dose of chemotherapy drugs has not yet been determined. Increasing the dose of chemotherapy is designed to improve the ratio of good histologic response to chemotherapy in patients and reduce the proportion of patients with resistance to chemotherapy, which are thus further converted to increase the survival rate of patients with osteosarcoma. In Sloan-Kettering Cancer Center, Rosen et al [23] firstly used T-10 program for treatment; they reported that the poor histologic response of patients can be improved by changing the postoperative chemotherapy regimen. But long-term follow-up results were not sure. Other researchers also conducted similar studies: individuals with general response were given various strengthening scheme to improve the therapeutic effect. But most such studies have failed to repeat the results of Rosen et al, and adjustments of chemotherapy regimen also failed to improve survival. Strengthened treatment during the preoperative therapy to increase the number of patients with good response also failed to change the long-term outcome of these patients, and after extending the current treatment, histologic response also lost its prognostic value. Meta-analysis showed that between the low-dose chemotherapy and high-dose chemotherapy groups, there were no statistically significant differences in 5-year tumor-free survival, 5-year overall survival, local recurrence, limb salvage rate and the proportion of patients with good response to chemotherapy.
Meta-analysis of five-year survival rate and extent of histologic response to chemotherapy in patients showed that there was statistically significant difference in 5-year tumor-free survival between patients with good and adverse histologic response to preoperative chemotherapy, and the 5-year tumor-free survival of patients with good histologic response was higher, indicating that tumor histological response to preoperative chemotherapy is an independent prognostic factor for osteosarcoma.
In conclusion, the existing research results showed that tumor for preoperative chemotherapy histological response is an independent prognostic factor for osteosarcoma. High-dose chemotherapy for the treatment of primary osteosarcoma is not better than low moderate -dose chemotherapy. But due to the bias of the included studies, performance bias, as well as the moderate possibility of publication bias, they were likely to affect the reliability of the results. It is expected that high quality of randomized controlled trials were performed to provide more reliable evidence in the future.
Disclosure of conflict of interest
None.
References
- 1.Nedelcu D, Andreescu N, Boeriu E, Stefanescu R, Arghirescu S, Puiu M. Retrospective study on osteosarcoma and ewing sarcoma - our experience. Maedica (Buchar) 2014;9:151–6. [PMC free article] [PubMed] [Google Scholar]
- 2.Obalum DC, Giwa SO, Banjo AF, Akinsulire AT. Primary bone tumours in a tertiary hospital in Nigeria: 25 year review. Niger J Clin Pract. 2009;12:169–72. [PubMed] [Google Scholar]
- 3.Mei J, Zhu XZ, Wang ZY, Cai XS. Functional outcomes and quality of life in patients with osteosarcoma treated with amputation versus limb-salvage surgery: a systematic review and meta-analysis. Arch Orthop Trauma Surg. 2014;134:1507–16. doi: 10.1007/s00402-014-2086-5. [DOI] [PubMed] [Google Scholar]
- 4.Ferguson PC, McLaughlin CE, Griffin AM, Bell RS, Deheshi BM, Wunder JS. Clinical and functional outcomes of patients with a pathologic fracture in high-grade osteosarcoma. J Surg Oncol. 2010;102:120–4. doi: 10.1002/jso.21542. [DOI] [PubMed] [Google Scholar]
- 5.Bhagat S, Sharma H, Pillai DS, Jane MJ. Pelvic Ewing’s sarcoma: a review from Scottish Bone Tumour Registry. J Orthop Surg (Hong Kong) 2008;16:333–8. doi: 10.1177/230949900801600313. [DOI] [PubMed] [Google Scholar]
- 6.Szewczyk M, Lechowski R, Zabielska K. What do we know about canine osteosarcoma treatment? - review. Vet Res Commun. 2015;39:61–7. doi: 10.1007/s11259-014-9623-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Briccoli A, Rocca M, Salone M, Guzzardella GA, Balladelli A, Bacci G. High grade osteosarcoma of the extremities metastatic to the lung: long-term results in 323 patients treated combining surgery and chemotherapy, 1985-2005. Surg Oncol. 2010;19:193–9. doi: 10.1016/j.suronc.2009.05.002. [DOI] [PubMed] [Google Scholar]
- 8.Nardin A, Lefebvre ML, Labroquère K, Faure O, Abastado JP. Liposomal muramyl tripeptide phosphatidylethanolamine: Targeting and activating macrophages for adjuvant treatment of osteosarcoma. Curr Cancer Drug Targets. 2006;6:123–33. doi: 10.2174/156800906776056473. [DOI] [PubMed] [Google Scholar]
- 9.Bielack SS, Carrle D, Hardes J, Schuck A, Paulussen M. Bone tumors in adolescents and young adults. Curr Treat Options Oncol. 2008;9:67–80. doi: 10.1007/s11864-008-0057-1. [DOI] [PubMed] [Google Scholar]
- 10.Shenoy R, Pillai A, Sokhi K, Porter D, Ried R. Survival trends in osteosarcoma of humerus. Eur J Cancer Care (Engl) 2008;17:261–9. doi: 10.1111/j.1365-2354.2007.00854.x. [DOI] [PubMed] [Google Scholar]
- 11.Campanacci M, Bacci G, Bertoni F, Picci P, Minutillo A, Franceschi C. The treatment of osteosarcoma of the extremities: twenty years’ experience at the Istituto Ortopedico Rizzoli. Cancer. 1981;48:1569–1581. doi: 10.1002/1097-0142(19811001)48:7<1569::aid-cncr2820480717>3.0.co;2-x. [DOI] [PubMed] [Google Scholar]
- 12.Hudson M, Jaffe MR, Jaffe N, Ayala A, Raymond AK, Carrasco H, Wallace S, Murray J, Robertson R. Pediatric osteosarcoma: therapeutic strategies, results and alprognostic factors derived from a 10-year experience. J. Clin. Oncol. 1990;8:1988–1997. doi: 10.1200/JCO.1990.8.12.1988. [DOI] [PubMed] [Google Scholar]
- 13.Jaffe N. Adjuvant chemotherapy in osteogenic sarcoma. J. Clin. Oncol. 1984;2:1179–1181. doi: 10.1200/JCO.1984.2.10.1179. [DOI] [PubMed] [Google Scholar]
- 14.Meyers PA, Heller G, Healey J. Chemotherapy for non-metastatic osteosarcoma: the Memorial Sloan-Kettering experience. J. Clin. Oncol. 1992;10:5–15. doi: 10.1200/JCO.1992.10.1.5. [DOI] [PubMed] [Google Scholar]
- 15.Eilber F, Giuliano A, Eckardt J, Patterson K, Moseley S, Goodnight J. Adjuvant chemotherapy for osteosarcoma: a randomised prospective trial. J. Clin. Oncol. 1987;5:21–26. doi: 10.1200/JCO.1987.5.1.21. [DOI] [PubMed] [Google Scholar]
- 16.Whelan JS, Jinks RC, McTiernan A, Sydes MR, Hook JM, Trani L, Uscinska B, Bramwell V, Lewis IJ, Nooij MA, van Glabbeke M, Grimer RJ, Hogendoorn PC, Taminiau AH, Gelderblom H. Survival from high-grade localised extremity osteosarcoma: combined results and prognostic factors from three European Osteosarcoma Intergroup randomised controlled trials. Ann Oncol. 2012;23:1607–16. doi: 10.1093/annonc/mdr491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bacci G, Forni C, Ferrari S, Longhi A, Bertoni F, Mercuri M, Donati D, Capanna R, Bernini G, Briccoli A, Setola E, Versari M. Neoadjuvant chemotherapy forosteosarcoma of the extremity: intensification of preoperative treatment does not increase the rate of good histologic response to the primary tumor or improve the final outcome. J Pediatr Hematol Oncol. 2003;25:845–853. doi: 10.1097/00043426-200311000-00006. [DOI] [PubMed] [Google Scholar]
- 18.Meyers PA, Gorlick R, Hellr G, Casper E, Lane J, Huvos AG, Healey JH. Intensification of preoperative chemotherapy for osteogenic sarcoma:rsults of the memorial sloan-kettering (t12) protocol. J. Clin. Oncol. 1998;16:2452–2458. doi: 10.1200/JCO.1998.16.7.2452. [DOI] [PubMed] [Google Scholar]
- 19.Bacci G, Gherlinzoni F, Picci P, Van Horn JR, Jaffe N, Guerra A, Ruggieri P, Biagini R, Capanna R, Toni A, et al. Adriamycin-methotrexae high dose as verss adriamycin-methotrexae moderate dose as adjuvant chemotherapy for osteosarcoma of the extremities: a randomized study. Eur J Cancer Clin Oncol. 1986;22:1337–1345. doi: 10.1016/0277-5379(86)90142-2. [DOI] [PubMed] [Google Scholar]
- 20.Souhami L, Craft AW, Van der Eijken JW, Nooij M, Spooner D, Bramwell VH, Wierzbicki R, Malcolm AJ, Kirkpatrick A, Uscinska BM, Van Glabbeke M, Machin D. Randomized trial of two regmens of chemotherapy in operative osteosarcoma: a study of the European osteosarcoma intergroup. Lancet. 1997;350:911–917. doi: 10.1016/S0140-6736(97)02307-6. [DOI] [PubMed] [Google Scholar]
- 21.Granowetter L, Womer R, Devidas M, Krailo M, Wang C, Bernstein M, Marina N, Leavey P, Gebhardt M, Healey J, Shamberger RC, Goorin A, Miser J, Meyer J, Arndt CA, Sailer S, Marcus K, Perlman E, Dickman P, Grier HE. Dose-intensified compared with standard chemotherapy for nonmetastatic Ewing sarcoma family of tumors: a Children’s Oncology Group Study. J. Clin. Oncol. 2009;27:2536–41. doi: 10.1200/JCO.2008.19.1478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Meyers PA, Gorlick R. Osteosarcoma. Pediatr Clin North Am. 1997;44:973–989. doi: 10.1016/s0031-3955(05)70540-x. [DOI] [PubMed] [Google Scholar]
- 23.Rosen G, Marcove RC, Caparros B, Nirenberg A, Kosloff C, Huvos AG. Primary osteogenic sarcoma:the rational for preoperative chemotherapy and delayed surgery. Cancer. 1979;43:2163–2177. doi: 10.1002/1097-0142(197906)43:6<2163::aid-cncr2820430602>3.0.co;2-s. [DOI] [PubMed] [Google Scholar]


