Abstract
Objectives: This study aimed to evaluate the diagnostic accuracy and complication rates of contrast-enhanced ultrasound (CEUS)-guided biopsy of small subpleural nodules with SonoVue. Methods: CEUS-guided biopsies with SonoVue and conventional ultrasound were performed to determine nodule size, texture and biopsy route. After baseline ultrasonography, all patients received an intravenous injection of 4 mL of SonoVue, followed by 5 mL of saline flush. CEUS was obtained using a convex probe and contrast-specific imaging software. The lesion was observed using a contrast agent. Biopsies were performed during real-time visualisation of the target lesion. Results: A total of 51 patients (34 males and 17 females; average age, 54.8 ± 5.8 years) with subpleural nodules were enrolled. The median nodule size was 1.92 ± 0.75 cm (0.9-2.5 cm). Forty-eight of 51 procedures (94.1%) provided adequate material for histological analysis. Thirty patients (62.5%) were malignant and 18 patients (37.5%) were benign at the definitive diagnosis. The true positive and true negative result were 28 (58.3%) and 18 (37.5%), no false positive result was seen and two (4.2%) provided a false negative result. The sensitivity, specificity, positive and negative predictive values for the malignant diagnosis were 93.3, 100, 100 and 90%, respectively. The diagnostic accuracy was 95.8% (46/48), the standard error and the 95% CI were 2.8% and 86%-99%. An asymptomatic pneumothorax was present in one patient with no chest tube placement required. A small amount of hemoptysis was observed in another patient, which stopped spontaneously without treatment. Conclusions: CEUS-guided biopsy with SonoVue exhibits high diagnostic accuracy and low complication rates. It is especially advantageous for biopsies of small subpleural nodules.
Keywords: Contrast-enhanced ultrasound, SonoVue, biopsy, subpleural nodules
Introduction
Small subpleural nodules (less than 2.5 cm) are common in physicians’ practices. However, the aetiology and diagnostic approach for these nodules are highly difficult. Definitive diagnosis relies on histological proof obtained via nodule biopsy. For subpleural nodules, CT-guided percutaneous transthoracic biopsy is a well-established technique of acquiring sufficient tissue for histopathological diagnosis [1,2]. However, biopsies of small subpleural nodules have been reported to be technically difficult and associated with a higher risk of pneumothorax [3] if the nodules are located in the lower lobes, where nodules are subjected to major respiratory movements and/or the needle tract is obstructed by overlying bony structures [1].
Thoracic ultrasound (US) is a non-invasive and portable diagnostic tool with important application for pulmonary specialists. US is suited to visualise nodules arising from the chest wall and peripheral lung nodules abutting the chest wall or adjacent to the pleural surface [4,5]. US-guided biopsy is effective and offers many advantages, such as real-time monitoring throughout the procedure; it can even be performed in general wards [5-8]. Despite being a validated and well-established imaging modality, US-guided biopsy is still not utilised to its full potential by pulmonary specialists. Contrast materials, such as SonoVue (Bracco SpA, Milan, Italy), a sulphur hexafluoride-filled microbubble contrast agent, have been recently used to expand the diagnostic yield of peripheral lung and mediastinal lesions and avoid puncture in necrotic areas [9-13]. However, no reports have been made to determine the utility of contrast-enhanced ultrasound (CEUS) with SonoVue in small subpleural nodules. This paper presents the experiences in the utility of CEUS-guided biopsy with SonoVue as an effective procedure for the diagnosis of small subpleural nodules.
Materials and methods
Patients
The study design and protocol were approved by the Ethics Committee of the First Affiliated Hospital of Guangzhou Medical University. Written informed consent for publication and use of accompanying images were obtained from each patient before the contrast-enhanced sonography and biopsy procedures. From January 2011 to July 2013, a total of 51 subpleural nodule biopsies were performed in 51 consecutive patients. All patients had subpleural nodules (less than 2.5 cm in diameter) adjacent to and/or abutting the pleura. Information on material adequacy and specific diagnosis were collected. Pre-interventional chest CT scans showed small subpleural nodules, and the clinical data are shown in Table 1.
Table 1.
Demographic characteristics of the patients, nodules characteristics
| Parameter | value |
|---|---|
| Number of patients | 51 |
| Sex (m/f) | 34/17 |
| Age [median, range (years)] | 54.8 ± 5.8, (22-91) |
| Number of procedures | 51 |
| Nodules size [median, range (cm)] | 1.92 ± 0.75, (0.9-2.5) |
| Nodules number [median, range] | 1.46, (1-4) |
| Location distance from pleura [median, range (mm)] | 1.11, (0-3) |
Datas were number of patients unless otherwise stated, enumeration data were given as means ± standard deviation.
Sonographic procedures and analysis
US units offer 2D, motion-mode and colour flow Doppler scanning (Esaote Mylab 90, Italy). A low-frequency (2-5 MHz) or high-frequency probe (5-10 MHz) can be used alternately. Patients were in a prone, supine or lateral decubitus position, depending on the location of the nodules. With the reference CT, conventional US was performed using the splenic echotexture as an in vivo reference. The following parameters were evaluated: the number of the nodules (solitary or multiple); maximal size of the nodules (in case of multiple nodules, the largest nodule was measured); and echotexture (hyperechoic, isoechoic, hypoechoic, echofree, or complex). Then Doppler US was also used to detect the blood flow of great vessels.
After baseline ultrasonography, all patients received an intravenous injection of 4 mL of SonoVue, followed by 5 mL of saline flush. CEUS was obtained using a low-frequency or high-frequency probe and contrast-specific imaging software. The lesion was observed for contrast agent uptake over 4 min after contrast agent administration. The following CEUS patterns of nodules were considered: extent of enhancement during the arterial phase between 1 and 30 s after injection; and extent of enhancement during the parenchymal phase between 1 and 4 min after injection (hyperechoic, isoechoic, hypoechoic, echofree, or complex). The dynamic image was recorded on the hard disk of the sonography machine. All US/CEUS were operated by a same experienced sonographer (Dazhi Zhou), patterns of enhancement were evaluated by two observers (Jinlin Wang and Dazhi Zhou). When disputes regarding the nodules areas occurred, a consensus was reached by discussion.
Biopsy technique
The target lesion was identified with US/CEUS. Information from the CEUS was used to select the entry site, route, sampling site, direction for the biopsy and depth for the biopsy. Immediately after the completion of CEUS, biopsies were performed with an 18-gauge spring-loaded automated cutting needle (MC1816, Bard Max. Core, Bard. Inc. USA). Local anaesthetic with 2% lidocaine was applied after planning and sterilising the skin. Under real-time visualisation, the cutting needle was inserted through the guiding channel and introduced into the margin of the target area. If the nodule was less than 2 cm in diameter, the tip of the needle was placed at least 20 mm away from the posterior margin of the lung lesion interface. All biopsies were performed by a single experienced physician (Jinlin Wang).
At least four specimens were obtained from each patient, placed in 10% formaldehyde solution and brought to the pathology department for histological examination and immunohistochemical studies. One specimen was immediately rolled on glass slides, and the other specimen was placed in a sterile tube and sent to be cultured for mycobacteria. All patients were examined with US immediately after the procedure to determine if pneumothorax had developed. If suspected, a chest ray was obtained to verify that no pneumothorax was present. Otherwise, a routine follow-up chest X-ray was obtained within 24 h after the biopsies to assess any possible complications. Figures 1 and 2 show the CT and US/CEUS images of biopsy procedures of small subpleural nodules.
Figure 1.
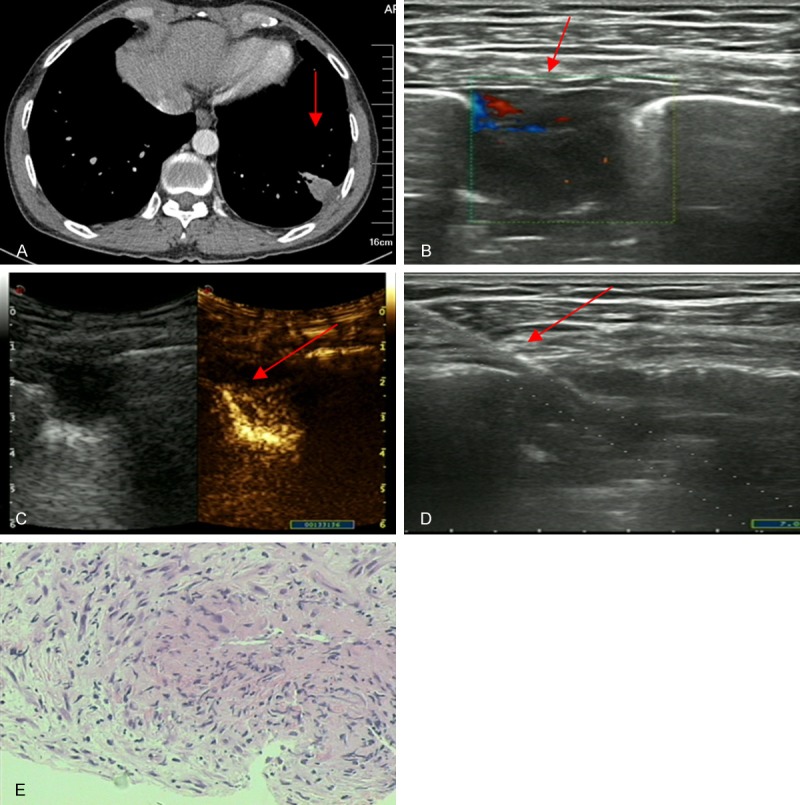
Images of a 42-year-old man with dry cough for one month. A. Chest CT revealed a subpleural nodule in the lower portion of the left lung (1.5 × 2.4 cm, arrowhead). B. US scan showed a subpleural nodule (arrowhead) with a low echo texture, the vessel of which is shown by colour flow Doppler scanning. C. CEUS with SonoVue showed enhancement of the nodule area (arrowhead). D. Real-time US-guided nodule biopsy (arrowhead) focused on the most vascularised area, avoiding avascular zones. E. Biopsy sample obtained from the subpleural nodule showed a tuberculoid nodule and caseous necrosis (H&E staining; magnification, × 100).
Figure 2.
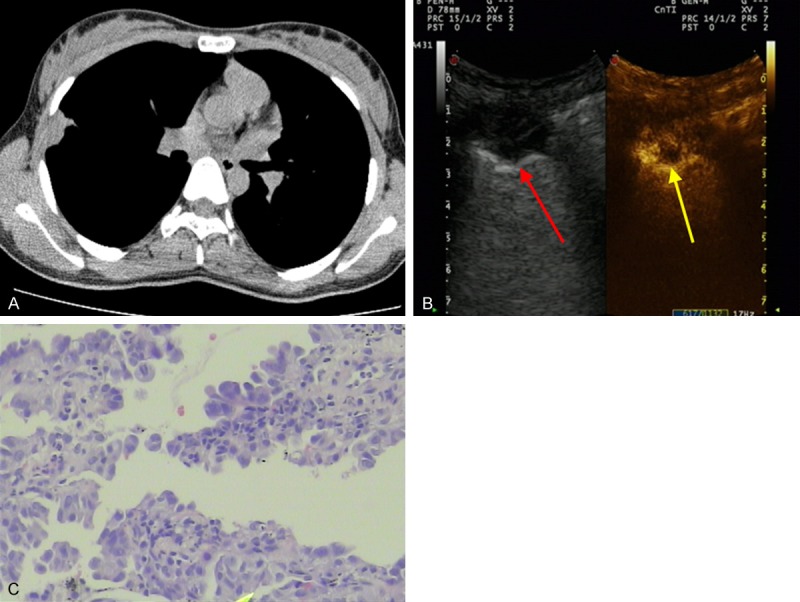
Images of a 56-year-old man with chest pain for two weeks. A. Chest CT revealed a subpleural nodule in the middle lobe of the right lung (1.1 × 2.0 cm, arrowhead). B. Conventional US scan showed a subpleural nodule (red arrowhead) with a low echo texture; CEUS with SonoVue showed the enhanced area and necrotic area (yellow arrowhead). C. Biopsy sample obtained from the subpleural nodule showed adenocarcinoma cells (H&E staining; magnification, × 100).
Data analysis
The definitive diagnosis of malignancy of the pulmonary nodules (true positive) was made by histopathological analysis of biopsy specimens, clinical follow up, and surgery, while benign diagnosis (true negative) was based on the following: (a) a benign histological diagnosis has been established with a precise etiology, (b) subsequent disappearance of the nodules or decrease in its size, or (c) follow-up chest radiographs or CT scans showing that the lesion remained stable for at least 18 months. Patients with benign histology remained under surveillance for 18 months to minimise the risk of potential false-negative results.
The clinical features of the patients, characteristics of the nodules, pathology reports of the biopsy specimens, results of the specimens’ cultured, definitive diagnosis and clinical outcomes of the patients were recorded.
Statistical analysis
Statistical analysis was carried out using SPSS 16.0 (IBM, Chicago, USA). Data were reported as number and percent for qualitative variables, enumeration data were given as means ± standard deviation. Categorical variables were analyzed, standard error and 95% confidence interval (CI) were calculated to demonstrate the utility of this procedure.
Results
Characteristics of patients
This study consisted of 51 patients (34 men, 17 women) with a mean age of 54.8 ± 5.8 years (age range, 22-91 years). Thirty-one patients had single subpleural nodule, whereas 17 patients had multiple pulmonary nodules, including at least one subpleural lesion. The median size of the biopsy nodules was 1.92 ± 0.75 cm (0.9-2.5 cm). The demographic data of the patients are summarised in Table 1.
Diagnostic accuracy
Forty-eight of 51 procedures (94.1%) provided adequate material for histological analysis. Thirty patients (62.5%) were malignant and 18 patients (37.5%) were benign at the definitive diagnosis. Three of 51 procedures had non-diagnostic or insufficient samples; two patients had benign clinical features on interval imaging with follow-up of 16 and 18 months, respectively; while the other patient was conducted to biopsy by video-assisted thoracic surgery (VATS) and revealed a malignant diagnosis.
Of 48 procedures, the true positive and true negative result were 28 (58.3%) and 18 (37.5%), no false positive result was seen and two (4.2%) provided a false negative result. Therefore, the sensitivity, specificity, positive and negative predictive values for the malignant diagnosis were 93.3, 100, 100 and 90%, respectively. The diagnostic accuracy was 95.8% (46/48), the standard error and the 95% CI were 2.8% and 86%-99% (Table 2). Of the two false negative patients, one patient in which biopsy yield a diagnosis of chronic inflammation turned out to be adenocarcinoma at examination of surgical sample, the other patient had initial benign result but had progressive disease clinically suggestive of mesothelioma and confirmed by biopsy at 9 months.
Table 2.
The diagnostic accuracy of the procedures performed with CEUS
| Procedures performed with CEUS (n = 48) | |
|---|---|
| False negative | 2 (4.2%) |
| True negative | 18 (37.5%) |
| True positive | 28 (58.3%) |
| False positive | 0 |
| Sensitivity | 93.3% |
| Specificity | 100% |
| PPV | 100% |
| NPV | 90% |
| Diagnostic accuracy | 95.8% |
| 95% CI | 86%-99% |
| Standard errors | 2.8% |
CEUS contrast-enhanced ultrasound, PPV positive predictive value, NPV negative predictive value. Categorical variables were analyzed, standard error and 95% confidence interval (CI) were calculated.
In 28 patients (58.3%), the histopathological results revealed malignant nodules (adenocarcinoma, n = 17; squamous cell carcinoma, n = 4; metastases from an extrathoracic malignancy, n = 3; small cell lung cancer, n = 2; mesothelioma of pleura, n = 1; pulmonary lymphoma, n = 1). For these patients, fourteen patients underwent standard surgical treatment by VATS after clinical evaluation and all were confirmed based on the surgical samples (twelve adenocarcinoma and two squamous carcinoma). Twelve patients selected chemotherapy for initial treatment after clinical evaluation, and all responded to systemic treatment during follow-up. Two patients suffered from a progressive disease after six months of support treatment (one was metastases from an extrathoracic malignancy and the other was pulmonary lymphoma at 91 years).
In 18 patients (37.5%), biopsy revealed specific benign diagnosis (tuberculosis, n = 7; organising pneumonia, n = 4; chronic inflammation, n = 3; round atelectases, n = 2; pulmonary aspergillosis, n = 1; neurogenic tumour, n = 1). One patient (2.1%) had identifiable microorganisms (Streptococcus pneumoniae) in the subsequent analysis of the biopsy specimen culture. All the patients were treated based on the benign results, and monitored at a mean follow-up period of 14 ± 3 months (range, 6-18 months). In 17 patients, the lesions were found to regress during the follow-up period. By contrast, the lesion in one patient was found to be neurogenic tumour.
Complications
Among 51 patients, only one (2.0%) suffered from a slight pneumothorax, which was suspected by US post biopsy and confirmed by chest radiography with no chest tube placement required. A small amount of hemoptysis was observed one patient (2.0%), which stopped spontaneously without treatment. All patients experienced no significant pain or other respiratory impairment.
Discussion
Microbubbles, such as SonoVue used for CEUS, promise to expand the range of functional imaging, enable small subpleural nodule detection and provide spatial information to avoid necrotic areas for US-guided biopsy. SonoVue is composed of gas-filled microbubbles, which are smaller than red blood cells, which can circulate freely in the capillaries without leaking out of the vessels; these microbubbles strongly increase the backscatter US signal and are used for the enhancement of blood echogenicity for the assessment of blood flow in the vasculature [14,15]. CEUS has gained a well-established role in the detection and characterisation of focal liver lesions [16]. Investigations also revealed that CEUS was beneficial in confirming the diagnosis of pleurisy and peripheral lesions [9-13]. CEUS could help operators in conducting interventional procedures to obtain satisfactory specimens and avoid necrotic area and damage to adjacent organs with high confidence and accuracy for challenging target lesions. In the study of Cao BS et al. [9], they showed that the diagnostic accuracy of CEUS-guided lung biopsy was statistically significant, contrast to the non-CEUS group (93.6% vs. 78%). In addition, Gorg C et al. [12] further classified CEUS patterns into the parenchymal and reduced parenchymal enhancement subgroups.
In this study, real-time US-guided subpleural small nodule biopsies with SonoVue were found to have a high diagnostic accuracy (95.8%, 46/48), which was greater than the yield described using CT-guided biopsies [1,3], and specimens adequate for histological examination were obtained from 48 of the 51 lesions (94.1%). Peripheral small thoracic lesions are usually not assessable with fiberoptic bronchoscopy, which has a low diagnostic yield even when guided by radial probe endobronchial ultrasonography [17] or electromagnetic navigation bronchoscopy [18]. Real-time US-guided biopsy allows for dynamic evaluation of localization and vessels which move during respiration, the tip of the needle can be monitored throughout the procedure, and fine adjustments can be made precisely and quickly. With SonoVue, small target lesions were more frequently detected, this ability is especially beneficial in biopsies of small nodules. In a study conducted by Wu et al. [19], CEUS before percutaneous focal liver lesion biopsy improves the diagnostic accuracy from 87.5% to 95.3% by providing important intralesional information and more accurate information about the site of biopsy even in lesions ≤ 2.0 cm. In our practice, conventional sonographic windows of small nodules was sometimes limited by the rib cage (Figure 1) and breathing motion in the lower lobes (Figure 2). In our study, the high diagnostic accuracy using CEUS may have resulted from the expanded detection of small subpleural nodules, obtained intralesional information and effectively guided biopsy procedures. On the other hand, most of scholars considered that CEUS-guided lung biopsy improved the diagnostic accuracy based on avoiding necrotic area, especially for large lesions [9-13], but for small nodules, we also can see more clearly the echo of the nodule, helping to obtain better tissue samples (Figure 2). As far as we know, this is the first report describing CEUS with SonoVue and small subpleural nodules biopsy.
The complication rates of US-guided biopsy of small subpleural nodules with SonoVue are mostly mild. Pneumothorax is a common complication, the rate of which was found to be considerably lower using CEUS than that using CT-guided biopsy [1,3,20]. In our study, only one patient (2.0%) had a mild pneumothorax, which did not need chest tube insertion. Some factors (e.g., pre-existing lung disease, patient age and needle gauge used) were found to be associated with the frequency of pneumothorax. In our study, the fact that many nodules were located peripherally may mainly contribute to the low incidence of pneumothorax, in addition, real-time monitoring helped to avoid puncturing the aerated lung.
Bleeding, such as pleural bleeding and hemoptysis, is another frequent complication. Pleural bleeding is always self-limiting. In our study, one patient developed mild hemoptysis, which stopped spontaneously without specific treatment. With SonoVue, CEUS-guided biopsy could be visualised and effectively avoid puncturing vessels. Furthermore, real-time monitoring of cutting biopsy specimens from nodules less than 20 mm in diameter may have enabled us to avoid cutting the lung parenchyma and reduce the rate of hemoptysis in our study.
Cutting-needle biopsy (CNB) using 20-or larger than 20-gauge aspirating needle and fine-needle aspiration biopsy (FNAB) using smaller than 20-gauge aspirating needle are the most commonly used for thoracic US guided biopsy. FNAB may be a safer and technically easier alternative to CNB, but specimens of FNAB sometimes is limited by a small sample or the lack of a histological pattern. Some studies have compared the value of FNAB and CNB for thoracic lesions. In a study performed by Diacon AH et al. [21], they concluded that CNB was superior in non-carcinomatous tumours and in benign lesions, similar to the result of Schubert P et al. [22]. In our practice, 18-gauge spring-loaded automated cutting needle was used to biopsy and good results have been obtained, in spite of this, which gauges (greater or smaller) are more suitable for thoracic US guided biopsy that requires more experiences and prospective studies.
Despite the relatively satisfactory results, our study had some limitations. The uncontrolled retrospective design only allowed limited comparability with historical data. No prospective study has compared the use of other imaging techniques with or without CEUS. Another limitation was our small number of patients, larger studies that require more experiences are recommended.
Acknowledgements
The authors would like to thank Yongfeng Luo for his assistance in proof reading of the present manuscript.
Disclosure of conflict of interest
None.
References
- 1.Prosch H, Oschatz E, Eisenhuber E, Wohlschlager H, Mostbeck GH. CT fluoroscopy guided transpleural cutting needle biopsy of small (≤ 2.5 cm) subpleural pulmonary nodules. Eur J Radiol. 2011;77:164–6. doi: 10.1016/j.ejrad.2009.07.021. [DOI] [PubMed] [Google Scholar]
- 2.Priola AM, Priola SM, Cataldi A, Errico L, Di Franco M, Campisi P, Molinaro L, Marci V, Novello S, Fava C. Accuracy of CT-guided transthoracic needle biopsy of lung lesions: factors affecting diagnostic yield. Radiol Med. 2007;112:1142–59. doi: 10.1007/s11547-007-0212-y. [DOI] [PubMed] [Google Scholar]
- 3.Gupta S, Krishnamurthy S, Broemeling LD, Morello FA Jr, Wallace MJ, Ahrar K, Madoff DC, Murthy R, Hicks ME. Small (≤ 2-cm) Subpleural Pulmonary lesions: short-versus long-needle-path CT-guided Biopsy--comparison of diagnostic yields and complications. Radiology. 2005;234:631–7. doi: 10.1148/radiol.2342031423. [DOI] [PubMed] [Google Scholar]
- 4.Koegelenberg CFN, Diacon AH, Bolliger CT. Transthoracic ultrasound of the chest wall, pleura, and the peripheral lung. In: Bolliger CT, Herth FJF, Mayo PH, Miyazama T, Beamis JF, editors. Progress in Respiratory Research. Clinical Chest Ultrasound. Basel: Karger; 2009. pp. 22–33. [Google Scholar]
- 5.Diacon AH, Theron J, Schubert P, Brundyn K, Louw M, Wright CA, Bolliger CT. Ultraound-assisted ransthoracic biopsy: fine-needle aspiration or cutting-needle biopsy? Eur Respir J. 2007;29:357–62. doi: 10.1183/09031936.00077706. [DOI] [PubMed] [Google Scholar]
- 6.Bandi V, Lunn W, Ernst A, Eberhardt R, Hoffmann H, Herth FJ. Ultrasound vs. CT in detecting chest wall invasion by tumor: a prospective study. Chest. 2008;133:881–6. doi: 10.1378/chest.07-1656. [DOI] [PubMed] [Google Scholar]
- 7.Koegelenberg CF, Diacon AH. Pleural controversy: close needle pleural biopsy or thoracoscopy-which first? Respirology. 2011;16:738–46. doi: 10.1111/j.1440-1843.2011.01973.x. [DOI] [PubMed] [Google Scholar]
- 8.Diacon AH, Schuurmans MM, Theron J, Schubert PT, Wright CA, Bolliger CT. Safety and yield of ultrasound-assisted transthoracic biopsy performed by pulmonologists. Respiration. 2004;71:519–22. doi: 10.1159/000080638. [DOI] [PubMed] [Google Scholar]
- 9.Cao BS, Wu JH, Li XL, Deng J, Liao GQ. Sonographically guided transthoracic biopsy of peripheral lung and mediastinal lesions: role of contrast-enhanced sonography. J Ultrasound Med. 2011;30:1479–90. doi: 10.7863/jum.2011.30.11.1479. [DOI] [PubMed] [Google Scholar]
- 10.Di Vece F, Tombesi P, Ermili F, Sartori S. Contrast-enhanced ultrasound (CEUS) and CEUS-guided biopsy in the diagnosis of lung abscess in a patient with achalasia: Case report. Interv Med Appl Sci. 2013;5:31–3. doi: 10.1556/IMAS.5.2013.1.6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sartori S, Nielsen I, Trevisani L, Tombesi P, Ceccotti P, Abbasciano V. Contrast-enhanced sonography as guidance for transthoracic biopsy of a peripheral lung lesion with large necrotic areas. J Ultrasound Med. 2004;23:133–6. doi: 10.7863/jum.2004.23.1.133. [DOI] [PubMed] [Google Scholar]
- 12.Gorg C, Bert T, Gorg K. Contrast-enhanced sonography for differential diagnosis of pleurisy and focal pleural lesions of unknown cause. Chest. 2005;128:3894–99. doi: 10.1378/chest.128.6.3894. [DOI] [PubMed] [Google Scholar]
- 13.Gorg C, Bert T, Kring R. Contrast-enhanced sonography of the lung for differential diagnosis of atelectasis. J Ultrasound Med. 2006;25:35–9. doi: 10.7863/jum.2006.25.1.35. [DOI] [PubMed] [Google Scholar]
- 14.Unnikrishnan S, Klibanov AL. Microbubbles as ultrasound contrast agents for molecular imaging: preparation and application. AJR Am J Roentgenol. 2012;199:292–9. doi: 10.2214/AJR.12.8826. [DOI] [PubMed] [Google Scholar]
- 15.Piscaglia F, Lencioni R, Sagrini E, Pina CD, Cioni D, Vidili G, Bolondi L. Characterization of focal liver lesions with contrast-enhanced ultrasound. Ultrasound Med Biol. 2010;36:531–50. doi: 10.1016/j.ultrasmedbio.2010.01.004. [DOI] [PubMed] [Google Scholar]
- 16.Claudon M, Dietrich CF, Choi BI, Cosgrove DO, Kudo M, Nolsøe CP, Piscaglia F, Wilson SR, Barr RG, Chammas MC, Chaubal NG, Chen MH, Clevert DA, Correas JM, Ding H, Forsberg F, Fowlkes JB, Gibson RN, Goldberg BB, Lassau N, Leen EL, Mattrey RF, Moriyasu F, Solbiati L, Weskott HP, Xu HX. Guidelines and good clinical practice recommendations for Contrast Enhanced Ultrasound (CEUS) in the liver-update 2012: A WFUMB-EFSUMB initiative in cooperation with representatives of AFSUMB, AIUM, ASUM, FLAUS and ICUS. Ultrasound Med Biol. 2013;39:187–210. doi: 10.1016/j.ultrasmedbio.2012.09.002. [DOI] [PubMed] [Google Scholar]
- 17.Oki M, Saka H, Kitagawa C, Kogure Y, Murata N, Adachi T, Ando M. Randomized study of endobronchial ultrasound-guided transbronchial biopsy: thin bronchoscopic methodversus guide sheath method. J Thorac Oncol. 2012;7:535–41. doi: 10.1097/JTO.0b013e3182417e60. [DOI] [PubMed] [Google Scholar]
- 18.Lamprecht B, Porsch P, Wegleitner B, Strasser G, Kaiser B, Studnicka M. Electromagnetic navigation bronchoscopy (ENB): Increasing diagnostic yield. Respir Med. 2012;106:710–15. doi: 10.1016/j.rmed.2012.02.002. [DOI] [PubMed] [Google Scholar]
- 19.Wu W, Chen MH, Yin SS, Yan K, Fan ZH, Yang W, Dai Y, Huo L, Li JY. The role of contrast-enhanced sonography of focal liver lesions before percutaneous biopsy. AJR Am J Roentgenol. 2006;187:752–61. doi: 10.2214/AJR.05.0535. [DOI] [PubMed] [Google Scholar]
- 20.Sconfienza LM, Mauri G, Grossi F, Truini M, Serafini G, Sardanelli F, Murolo C. Pleural and peripheral lung lesions: comparison of US- and CT-guided biopsy. Radiology. 2013;266:930–5. doi: 10.1148/radiol.12112077. [DOI] [PubMed] [Google Scholar]
- 21.Diacon AH, Theron J, Schubert P, Brundyn K, Louw M, Wright CA, Bolliger CT. Ultrasound-assisted transthoracic biopsy: fine-needle aspiration or cutting-needle biopsy? Eur Respir J. 2007;29:357–62. doi: 10.1183/09031936.00077706. [DOI] [PubMed] [Google Scholar]
- 22.Schubert P, Wright CA, Louw M, Brundyn K, Theron J, Bolliger CT, Diacon AH. Ultrasound-assisted transthoracic biopsy: cells or sections? Diagn Cytopathol. 2005;33:233–7. doi: 10.1002/dc.20342. [DOI] [PubMed] [Google Scholar]


