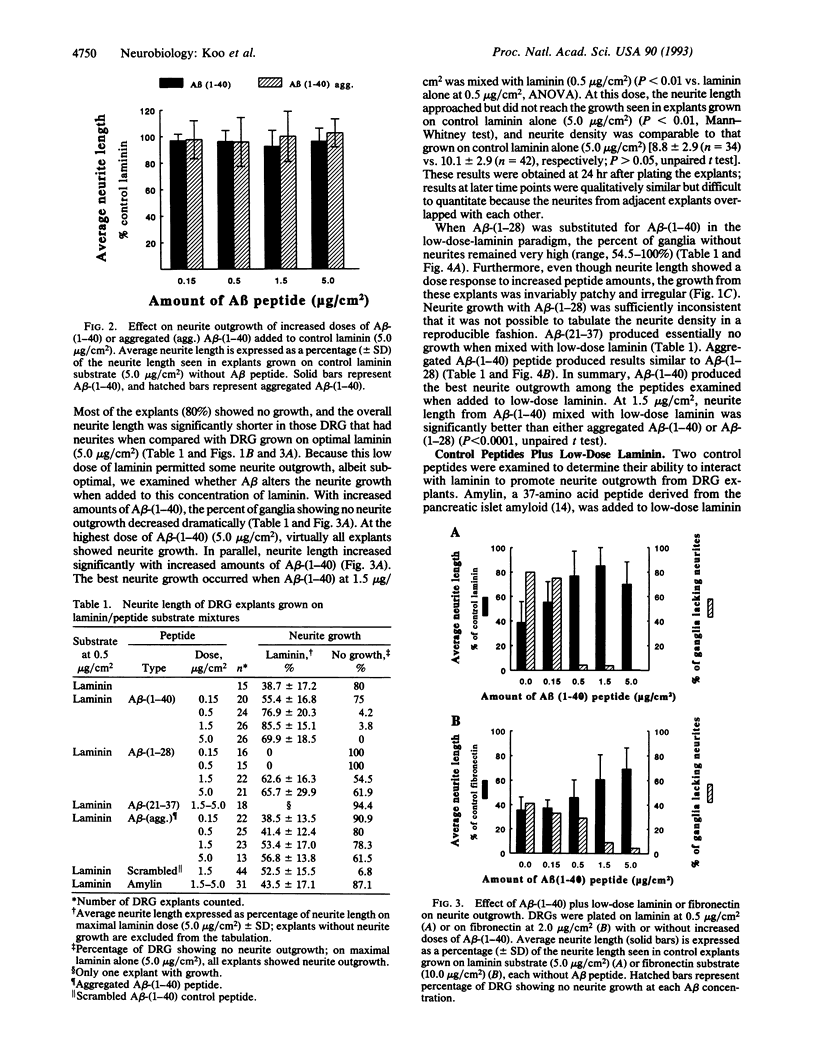
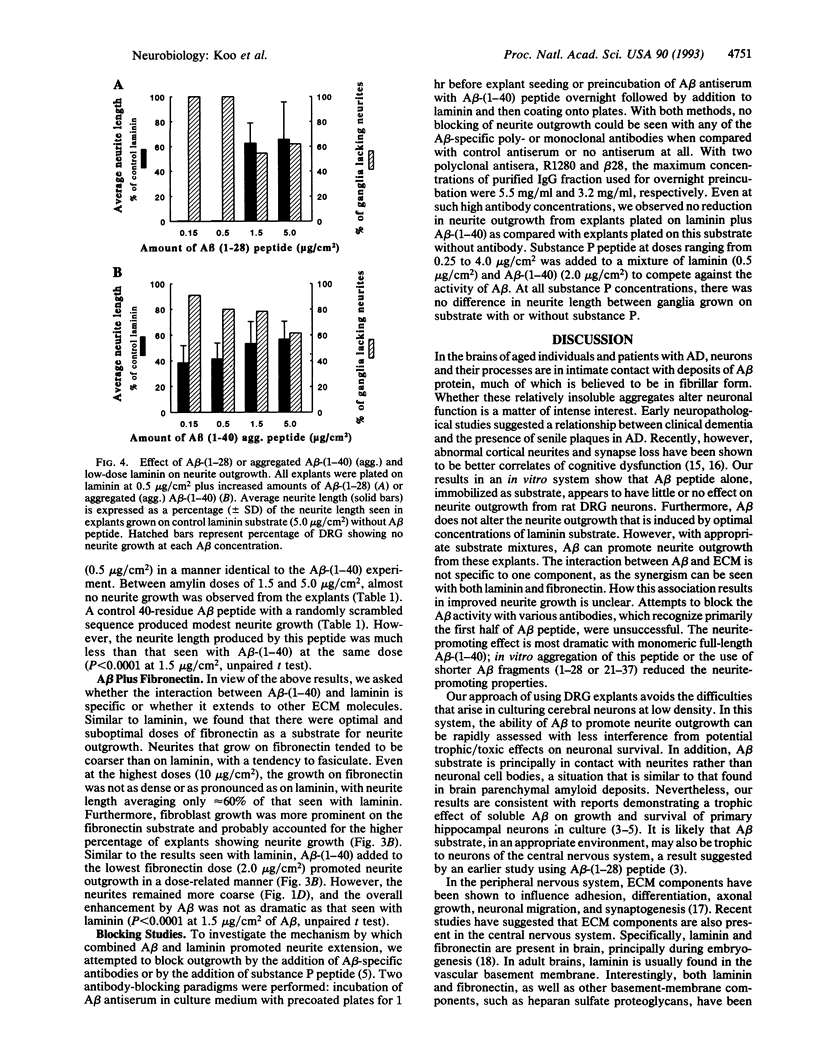
Abstract
Progressive deposition of amyloid beta-protein (A beta) in brain parenchyma and blood vessels is a characteristic feature of Alzheimer disease. Recent evidence suggests that addition of solubilized synthetic A beta to medium may produce toxic or trophic effects on cultured hippocampal neurons. Because soluble A beta may not accumulate in significant quantities in brain, we asked whether immobilized A beta peptide as a substrate alters neurite outgrowth from cultured rat peripheral sensory neurons. This paradigm may closely mimic the conditions in Alzheimer disease brain tissue, in which neurites contact insoluble, extracellular aggregates of beta-amyloid. We detected no detrimental effects of A beta substrate on neurite outgrowth. Rather, A beta in combination with low doses of laminin or fibronectin enhanced neurite out-growth from these neuronal explants. Our results suggest that insoluble A beta in the cerebral neuropil may serve as a neurite-promoting matrix, perhaps explaining the apparent regenerative response of neurites observed around amyloid plaques in Alzheimer disease. Moreover, in concert with the recent discovery of A beta production by cultured neurons, our data suggest that A beta plays a normal physiological role in brain by complexing with the extracellular matrix.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Buettner H. M., Pittman R. N. Quantitative effects of laminin concentration on neurite outgrowth in vitro. Dev Biol. 1991 Jun;145(2):266–276. doi: 10.1016/0012-1606(91)90125-m. [DOI] [PubMed] [Google Scholar]
- Bugiani O., Giaccone G., Verga L., Pollo B., Ghetti B., Frangione B., Tagliavini F. Alzheimer patients and Down patients: abnormal presynaptic terminals are related to cerebral preamyloid deposits. Neurosci Lett. 1990 Oct 30;119(1):56–59. doi: 10.1016/0304-3940(90)90754-w. [DOI] [PubMed] [Google Scholar]
- Geddes J. W., Anderson K. J., Cotman C. W. Senile plaques as aberrant sprout-stimulating structures. Exp Neurol. 1986 Dec;94(3):767–776. doi: 10.1016/0014-4886(86)90254-2. [DOI] [PubMed] [Google Scholar]
- Gilbert M. R., Harding B. L., Hoffman P. N., Griffin J. W., Price D. L., Troncoso J. C. Aluminum-induced neurofilamentous changes in cultured rat dorsal root ganglia explants. J Neurosci. 1992 May;12(5):1763–1771. doi: 10.1523/JNEUROSCI.12-05-01763.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haass C., Schlossmacher M. G., Hung A. Y., Vigo-Pelfrey C., Mellon A., Ostaszewski B. L., Lieberburg I., Koo E. H., Schenk D., Teplow D. B. Amyloid beta-peptide is produced by cultured cells during normal metabolism. Nature. 1992 Sep 24;359(6393):322–325. doi: 10.1038/359322a0. [DOI] [PubMed] [Google Scholar]
- Howard J., Pilkington G. J. Antibodies to fibronectin bind to plaques and other structures in Alzheimer's disease and control brain. Neurosci Lett. 1990 Oct 2;118(1):71–76. doi: 10.1016/0304-3940(90)90251-4. [DOI] [PubMed] [Google Scholar]
- Joachim C. L., Morris J. H., Selkoe D. J. Diffuse senile plaques occur commonly in the cerebellum in Alzheimer's disease. Am J Pathol. 1989 Aug;135(2):309–319. [PMC free article] [PubMed] [Google Scholar]
- Koh J. Y., Yang L. L., Cotman C. W. Beta-amyloid protein increases the vulnerability of cultured cortical neurons to excitotoxic damage. Brain Res. 1990 Nov 19;533(2):315–320. doi: 10.1016/0006-8993(90)91355-k. [DOI] [PubMed] [Google Scholar]
- Lander A. D. Understanding the molecules of neural cell contacts: emerging patterns of structure and function. Trends Neurosci. 1989 May;12(5):189–195. doi: 10.1016/0166-2236(89)90070-2. [DOI] [PubMed] [Google Scholar]
- Mann D. M., Jones D. Deposition of amyloid (A4) protein within the brains of persons with dementing disorders other than Alzheimer's disease and Down's syndrome. Neurosci Lett. 1990 Feb 5;109(1-2):68–75. doi: 10.1016/0304-3940(90)90539-l. [DOI] [PubMed] [Google Scholar]
- Masliah E., Mallory M., Hansen L., Alford M., Albright T., DeTeresa R., Terry R., Baudier J., Saitoh T. Patterns of aberrant sprouting in Alzheimer's disease. Neuron. 1991 May;6(5):729–739. doi: 10.1016/0896-6273(91)90170-5. [DOI] [PubMed] [Google Scholar]
- Mattson M. P., Cheng B., Davis D., Bryant K., Lieberburg I., Rydel R. E. beta-Amyloid peptides destabilize calcium homeostasis and render human cortical neurons vulnerable to excitotoxicity. J Neurosci. 1992 Feb;12(2):376–389. doi: 10.1523/JNEUROSCI.12-02-00376.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKee A. C., Kosik K. S., Kowall N. W. Neuritic pathology and dementia in Alzheimer's disease. Ann Neurol. 1991 Aug;30(2):156–165. doi: 10.1002/ana.410300206. [DOI] [PubMed] [Google Scholar]
- Murtomäki S., Risteli J., Risteli L., Koivisto U. M., Johansson S., Liesi P. Laminin and its neurite outgrowth-promoting domain in the brain in Alzheimer's disease and Down's syndrome patients. J Neurosci Res. 1992 Jun;32(2):261–273. doi: 10.1002/jnr.490320216. [DOI] [PubMed] [Google Scholar]
- Müller-Hill B., Beyreuther K. Molecular biology of Alzheimer's disease. Annu Rev Biochem. 1989;58:287–307. doi: 10.1146/annurev.bi.58.070189.001443. [DOI] [PubMed] [Google Scholar]
- Perlmutter L. S., Barrón E., Saperia D., Chui H. C. Association between vascular basement membrane components and the lesions of Alzheimer's disease. J Neurosci Res. 1991 Dec;30(4):673–681. doi: 10.1002/jnr.490300411. [DOI] [PubMed] [Google Scholar]
- Pike C. J., Walencewicz A. J., Glabe C. G., Cotman C. W. In vitro aging of beta-amyloid protein causes peptide aggregation and neurotoxicity. Brain Res. 1991 Nov 1;563(1-2):311–314. doi: 10.1016/0006-8993(91)91553-d. [DOI] [PubMed] [Google Scholar]
- Probst A., Brunnschweiler H., Lautenschlager C., Ulrich J. A special type of senile plaque, possibly an initial stage. Acta Neuropathol. 1987;74(2):133–141. doi: 10.1007/BF00692843. [DOI] [PubMed] [Google Scholar]
- Sanes J. R. Extracellular matrix molecules that influence neural development. Annu Rev Neurosci. 1989;12:491–516. doi: 10.1146/annurev.ne.12.030189.002423. [DOI] [PubMed] [Google Scholar]
- Selkoe D. J. The molecular pathology of Alzheimer's disease. Neuron. 1991 Apr;6(4):487–498. doi: 10.1016/0896-6273(91)90052-2. [DOI] [PubMed] [Google Scholar]
- Shoji M., Golde T. E., Ghiso J., Cheung T. T., Estus S., Shaffer L. M., Cai X. D., McKay D. M., Tintner R., Frangione B. Production of the Alzheimer amyloid beta protein by normal proteolytic processing. Science. 1992 Oct 2;258(5079):126–129. doi: 10.1126/science.1439760. [DOI] [PubMed] [Google Scholar]
- Snow A. D., Mar H., Nochlin D., Kimata K., Kato M., Suzuki S., Hassell J., Wight T. N. The presence of heparan sulfate proteoglycans in the neuritic plaques and congophilic angiopathy in Alzheimer's disease. Am J Pathol. 1988 Dec;133(3):456–463. [PMC free article] [PubMed] [Google Scholar]
- Terry R. D., Masliah E., Salmon D. P., Butters N., DeTeresa R., Hill R., Hansen L. A., Katzman R. Physical basis of cognitive alterations in Alzheimer's disease: synapse loss is the major correlate of cognitive impairment. Ann Neurol. 1991 Oct;30(4):572–580. doi: 10.1002/ana.410300410. [DOI] [PubMed] [Google Scholar]
- Westermark P., Wernstedt C., Wilander E., Hayden D. W., O'Brien T. D., Johnson K. H. Amyloid fibrils in human insulinoma and islets of Langerhans of the diabetic cat are derived from a neuropeptide-like protein also present in normal islet cells. Proc Natl Acad Sci U S A. 1987 Jun;84(11):3881–3885. doi: 10.1073/pnas.84.11.3881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whitson J. S., Glabe C. G., Shintani E., Abcar A., Cotman C. W. Beta-amyloid protein promotes neuritic branching in hippocampal cultures. Neurosci Lett. 1990 Mar 14;110(3):319–324. doi: 10.1016/0304-3940(90)90867-9. [DOI] [PubMed] [Google Scholar]
- Whitson J. S., Selkoe D. J., Cotman C. W. Amyloid beta protein enhances the survival of hippocampal neurons in vitro. Science. 1989 Mar 17;243(4897):1488–1490. doi: 10.1126/science.2928783. [DOI] [PubMed] [Google Scholar]
- Yamaguchi H., Nakazato Y., Hirai S., Shoji M., Harigaya Y. Electron micrograph of diffuse plaques. Initial stage of senile plaque formation in the Alzheimer brain. Am J Pathol. 1989 Oct;135(4):593–597. [PMC free article] [PubMed] [Google Scholar]
- Yankner B. A., Dawes L. R., Fisher S., Villa-Komaroff L., Oster-Granite M. L., Neve R. L. Neurotoxicity of a fragment of the amyloid precursor associated with Alzheimer's disease. Science. 1989 Jul 28;245(4916):417–420. doi: 10.1126/science.2474201. [DOI] [PubMed] [Google Scholar]
- Yankner B. A., Duffy L. K., Kirschner D. A. Neurotrophic and neurotoxic effects of amyloid beta protein: reversal by tachykinin neuropeptides. Science. 1990 Oct 12;250(4978):279–282. doi: 10.1126/science.2218531. [DOI] [PubMed] [Google Scholar]
- Yoshikawa K., Aizawa T., Hayashi Y. Degeneration in vitro of post-mitotic neurons overexpressing the Alzheimer amyloid protein precursor. Nature. 1992 Sep 3;359(6390):64–67. doi: 10.1038/359064a0. [DOI] [PubMed] [Google Scholar]




