Abstract
To determine if cetuximab combined with chemotherapy is beneficial for patients with advanced NSCLC after failure of first-line chemotherapy and EGFR-tyrosine kinase inhibitors (TKI). Twenty patients were treated with cetuximab in combination with pemetrexed, and 14 patients were treated with cetuximab in combination with docetaxel. Short-term response rates and long-term survival after salvage therapy were evaluated. Partial response (PR) occurred in 4 patients, stable disease (SD) occurred in 13 patients, and progressive disease (PD) occurred in 17 patients. No patient achieved a complete response (CR). The objective response rate (ORR) was 11.8% (4/34) and the disease control rate (DCR) was 50.0% (17/34). The disease progression rate (DPR) was 50% (17/34). Further analyses showed that the DCR was significantly higher in patients treated with EGFR-TKI for ≥6 months compared to patients treated with EGFR-TKI for <6 months (P=0.031). The median follow-up time was 5.5 months. The median progression-free survival (PFS) was 4.1 months. PFS was significantly longer in patients treated with EGFR-TKI for ≥ 6 months compared to those treated <6 months with EGFR-TKI (5.9 vs. 3.0 months; P=0.004). In general, however, patients tolerated this therapy well and there were no therapy-related deaths. As a salvage therapy, cetuximab combined with chemotherapy is indeed beneficial for patients with advanced NSCLC after first-line chemotherapy and subsequent EGFR-TKI treatment failure. In particular, this salvage regimen is beneficial for patients who were treated with EGFR-TKI for ≥6 months, and is well tolerated in these patients.
Keywords: Lung cancer, cetuximab, chemotherapy, salvage therapy, EGFR
Introduction
Non-small cell lung cancer (NSCLC) accounts for 80%-85% of newly diagnosed lung cancers. Approximately 65% of NSCLC patients are in advanced stages at the time of diagnosis, and therefore, the opportunity for surgical resection is lost [1-3]. Epidermal growth factor receptor (EGFR)-targeted therapy has been widely used to treated lung cancer and has achieved measurable effects in these patients [4-6]. EGFR is a 170 kDa transmembrane protein that is widely expressed in many malignancies, including lung, colon, and breast cancers [7-11]. EGFR signaling is one of the most studied pathways in cancer progression. Phosphorylation of EGFR leads to activation of its downstream target genes. These genes are involved in a variety of cellular processes, including promotion of cell proliferation, angiogenesis induction, and apoptosis inhibition [12-14].
EGFR tyrosine kinase inhibitors (EGFR-TKI) are a group of inhibitors that target EGFR tyrosine kinase activity. Gefitinib and erlotinib are two widely used EGFR-TKIs. These inhibitors are selective EGFR tyrosine kinase inhibitors and have specific effects on cancer cell growth, metastasis, and angiogenesis by blocking EGFR tyrosine kinase phosphorylation [15-18]. A variety of cancer treatment guidelines have recommended EGFR-TKI therapy as first-line, second-line, or third-line agents to treat NSCLC and to maintain chemotherapy. Most advanced NSCLC patients receive EGFR-TKI therapy at certain stages during the disease process. Acquired resistance to EGFR-TKI therapy is common during the treatment course [19-21]. Subsequent treatment in patients with advanced NSCLC after acquiring EGFR-TIK-resistance is a significant concern.
Currently, antibody therapy combined with chemotherapy is an important alternative treatment strategy for cancer patients. It is becoming more widely accepted, particularly as a salvage therapy for those patients resisting first-line chemotherapy. Cetuximab (Erbitux) is the first approved monoclonal antibody (IgG1) against EGFR. It binds to the EGFR ligand and blocks downstream pathways of EGFR signal transduction, thereby inhibiting cancer cell proliferation and growth [22-24]. The combination of cetuximab and chemotherapeutic agents to treat stage I-II NSCLC after failed EGFR-TKI treatment has been reported, and cetuximab can sensitize early stage NSCLC to subsequent chemotherapy [7,25]. However, whether a similar outcome occurs in EGFR-TKI-resistant advanced NSCLC is not yet known.
The primary objective of this study was to determine if salvage therapy based on combining cetuximab with chemotherapeutic agents can improve the therapeutic efficacy in patients with advanced NSCLC after resistance to initial first-line chemotherapy and subsequent EGFR-TKI treatments. For this purpose, we conducted a retrospective study in 34 patients with advanced NSCLC whose first-line platinum-based chemotherapy and EGFR-TKI failed.
Materials and methods
Eligibility criteria
For inclusion in this study, all patients satisfied the following criteria: 1) Pathologically confirmed advanced NSCLC according to the 2009 International Association for the Study of Lung Cancer staging system [26]; 2) Refractory to first-line standard chemotherapy containing platinum and resistant to subsequent erlotinib or gefitinib treatment; 3) An Eastern Cooperative Oncology Group (ECOG) performance status score of ≤2 [27]; 4) Predicted survival time was longer than three months; and (5) Normal bone marrow, liver, and kidney function. A total of 34 patients was collected from March 2007 to May 2013. This study and all treatments were conducted in accordance with the guidelines of Clinical Research Ethics Board at our hospital. Written informed consent was obtained from all patients before the inclusion in the study. Our hospital approved this study.
Study design
All patients received salvage therapy. Salvage treatment consisted of cetuximab combined with either pemetrexed (regimen A) or docetaxel (regimen B). An initial loading dose of cetuximab at 400 mg/m2 was administered over 2 hours. Cetuximab was intravenously injected weekly at a dose of 250 mg/m2. The delivery speed did not exceed 5 ml/min. In addition to cetuximab, 20 patients (58.8%) in regimen A were concurrently given 500 mg/m2 pemetrexed on day 1, continuing once every 3 weeks. Fourteen patients (41.2%) in regimen B were concurrently given 75 mg/m2 docetaxel on day 1, continuing once every 3 weeks. Standard premedications consisted of 0.4 g cimetidine, 5 mg diphenhydramine, and 5 mg dexamethasone. Patients participated in the study until the disease progressed, unacceptable toxicity, death, or withdrawal of consent. Two patients experienced disease progression after one treatment cycle. The remaining 32 patients were treated for at least two cycles. The median number of treatment cycles was three cycles (1-6 cycles).
Patient baseline information
Baseline clinical and pathological characteristics of patients were collected, including sex, age, pathological classification, tumor stage, smoking status, PS score, first-line chemotherapy, and duration of EGFR-TKI treatment. Additionally, physical examinations, weight measurements, hematology and chemistry tests, urinalyses, electrocardiograms, and Eastern Cooperative Oncology Group (ECOG) performance status were assessed at baseline. Imaging studies (computed tomography or magnetic resonance imaging) were performed at baseline and every 6 weeks or longer.
Treatment assessments
The lung cancer remission rate (RR) was assessed according to a modified version of the Response Evaluation Criteria in Solid Tumors 1.0 guidelines [28], and were divided into complete remission (complete response, CR), partial remission (partial response, PR), stable (stable disease, SD), and progression (progression disease, PD). Duration of response was defined as meeting time measurement criteria for CR/PR until the date of PD or death. Patients who were alive and without progression were censored at the day of their last tumor assessments. Objective response rate (objective response rate, ORR) refers to the percentage of CR+PR in patients. Disease control rate (disease control rate, DCR) refers to the percentage of CR+PR+SD in patients. Progression free survival (PFS) is defined as the time from randomization until the date of PD or death from any cause. Overall survival (OS) is defined as the time from randomization to death. During the study, weekly evaluations included vital sign measurements and toxicity evaluations [classified by the Medical Dictionary for Regulatory Activities and graded by the National Cancer Institute (NCI) Common Terminology Criteria for Adverse Events version 3.0].
Statistical analyses
SPSS 15.0 statistical software was used to analyze all data in this study. P<0.05 is considered statistically significant. The participating investigators determined PFS. Adverse events were assessed according to the NCI Common Terminology Criteria for Adverse Events (CTCAE) version 3.0. Only events that were reported as possibly, probably, or definitely related to treatment protocols were included in these analyses. Progression-free survival was estimated using the Kaplan-Meier product-limit method [29].
Results
Patient characteristics
A total of 34 patients participated in this retrospective study. The majority of patients were male (67.6%), were treated with EGFR-TKI for less than 6 months (64.7%), had PS scores of 0-1 (64.7%), and had histological diagnoses of adenocarcinoma (70.6%). The percentage of patients who smoked (either currently or in the past) was similar to patients who did not smoke or never smoked (41.2% vs. 58.8%). EGFR-TKI was given as second-line treatment in 52% of patients, and as first-line treatment in 48% of patients. The majority of patients (55.9%) received gefitinib treatment, whereas 44.1% of patients received erlotinib treatment. Baseline clinical and pathological characteristics are presented in Table 1.
Table 1.
Baseline characteristics of the study population
| Parameters | No. of patients | % |
|---|---|---|
| Sex | ||
| Male | 23 | 67.6 |
| Female | 11 | 32.4 |
| PS score | ||
| 0-1 | 22 | 64.7 |
| 2 | 12 | 35.3 |
| Pathological diagnosis | ||
| Adenocarcinoma | 24 | 70.6 |
| Squamous cell carcinoma | 6 | 17.6 |
| Unknown | 4 | 11.8 |
| Smoking status | ||
| Smoker | 14 | 41.2 |
| Non-smoker | 20 | 58.8 |
| EGFR-TKI agent | ||
| Gefitinib | 19 | 55.9 |
| Erlotinib | 15 | 44.1 |
| EGFR-TKI preference | ||
| Second-line | 18 | 52 |
| Above second-line | 16 | 48 |
| EGFR-TKI duration | ||
| ≥6 Months | 12 | 35.3 |
| <6 Months | 22 | 64.7 |
Note: EGFR-TKI, epidermal growth factor receptor tyrosine kinase inhibitor.
Responses to salvage therapy
Short-term tumor responses to salvage therapy were evaluated in all patients. We observed PR in 4 cases, SD in 13 cases, PD in 17 cases, and CR was not recorded in any patient. The ORR was 11.8% (4/34 patients) and DCR was 50.0% (17/34 patients). The disease progression rate was 50% (17/34 patients). Further analyses showed that the DCR was significantly higher in patients that were treated with EGFR-TKI for ≥6 months compared to patients who were treated with EGFR-TKI for <6 months (P=0.031). ORR and DCR were not significantly different in patients with different sex, PS scores, or chemotherapeutic regimens (P=0.05) (Table 2).
Table 2.
Efficacy of salvage therapy in 34 patients
| ORR | DCR | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
|
|
|||||||||||
| Cases | PR | SD | PD | % | χ2 | P | % | χ2 | P | ||
| Gender | Men | 23 | 3 | 8 | 12 | 13.0 | 0.112 | 0.738 | 47.8 | 0.134 | 0.714 |
| Women | 11 | 1 | 5 | 5 | 9.1 | 54.5 | |||||
| PS score | 0-1 | 22 | 3 | 10 | 9 | 13.6 | 0.210 | 0.647 | 59.1 | 2.061 | 0.151 |
| ≥2 | 12 | 1 | 3 | 8 | 8.3 | 33.3 | |||||
| TKI time, months | ≥6 | 12 | 3 | 6 | 3 | 25.0 | 1.469 | 0.226 | 75.0 | 4.636 | 0.031 |
| <6 | 22 | 1 | 7 | 14 | 4.5 | 36.4 | |||||
| Regimen | A | 20 | 2 | 9 | 9 | 10.0 | 0.146 | 0.703 | 55.0 | 1.630 | 0.728 |
| B | 14 | 2 | 4 | 8 | 14.3 | 42.9 | |||||
Note: No., number of patients. EGFR-TKI, epidermal growth factor receptor tyrosine kinase inhibitor. PR: Partial response, SD: stable disease, PD: progressive disease, CR: complete response, ORR: objective response rate, DCR: disease control rate.
Survival after salvage therapy
The median follow-up period was 5.5 months (2.0-20 months) in all patients. The median PFS was 4.1±0.5 months (range, 3.1 to 5.1 months). The overall PFS curve for all patients is shown in Figure 1A. Further analyses showed that the median PFS was 5.9 months in patients treated with EGFR-TKI for ≥ 6 months compared to 3 months in patients treated with EGFR-TKI for <6 months, which was a statistically significant difference (P=0.004; Figure 1B). There were no significant differences among patients with different sex (4.0 months in males vs. 4.1 months in female), PS scores (4.0 months in patients with 0-1 scores vs. 4.1 months in patients with 2 scores), or regimens (4.2 months for regimen A patients vs. 3.8 months for regimen B patients) (P>0.05; Figure 1C-E).
Figure 1.
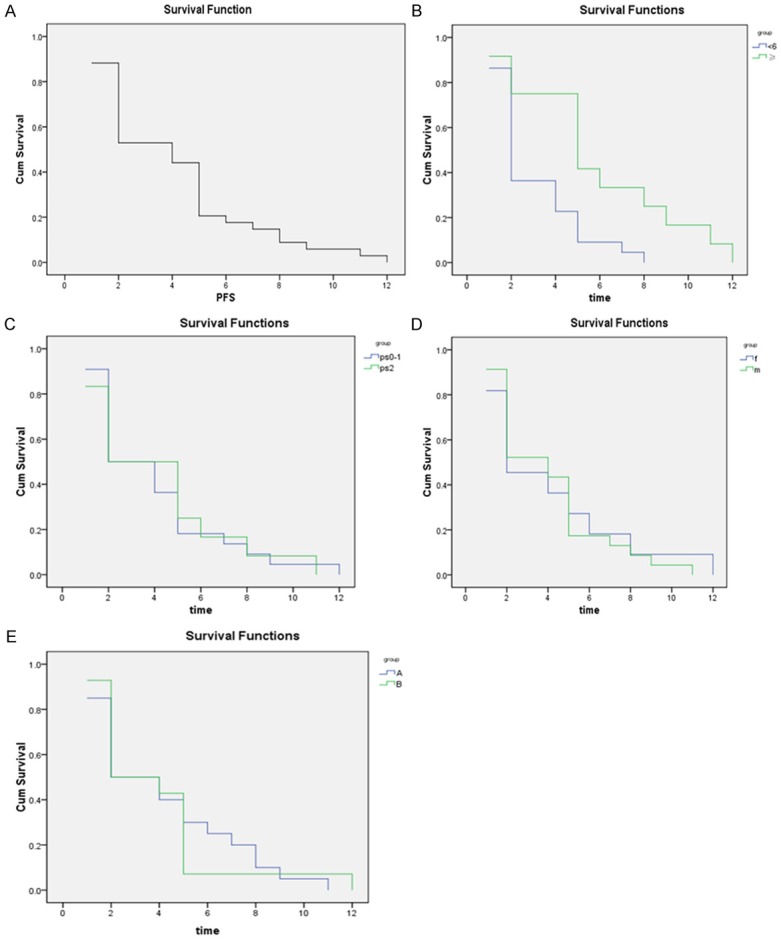
Progression-free survival (PFS) in 34 patients with advanced non-small cell lung cancer (NSCLC) after salvage therapy following failed first-line chemotherapy and EGFR-TKI therapy. Salvage therapy included a combination of cetuximab with either pemetrexed (regimen A) or docetaxel (regimen B). A. Overall PFS in 34 patients. The median follow-up time was 5.5 months. The median PFS was 4.147±0.494 months. B. PFS comparisons in patients with initial EGFR-TKI treatment times longer than six months versus shorter than six months. EGFR-TKI treatment times shorter than six months correlated with inferior PFS (P=0.004). ≥, EGFR-TKI treatment time ≥6 months; <6, GFR-TKI treatment time <6 months. C. PFS comparisons in patients with PS scores of 0-1 and 2 after salvage therapy. PFS did not differ between patients with different PS scores (P=0.931). PS0-1, PS score of 0-1; PS2, PS score of 2. D. PFS comparisons between male and female patients with advanced NSCLC after salvage therapy. PFS in males was similar to that in females (P=0.833). f, female; m, male. E. PFS comparisons in patients treated with different salvage regimens. PFS of patients treated with cetuximab combined with pemetrexed (regimen A) is similar to that of patients treated with cetuximab combined with docetaxel (regimen B) (P=0.980). (A. regimen A, B. regimen B).
Adverse reactions
Adverse reactions to salvage therapy were evaluated in all patients. The predominant symptom was bone marrow suppression. Twenty patients who received regimen A received concurrent pemetrexed treatment. Pemetrexed was reduced from an original dose of 500 mg/m2 to 375 mg/m2 in one patient due to grade 3 neutropenia and grade 4 thrombocytopenia. Fourteen patients who received the group B regimen received the concurrent docetaxel treatment. One patient had grade 3 peripheral neuropathy, and two patients were given blood transfusions to increase their red blood cell counts due to anemia. Docetaxel was reduced from 75 mg/m2 to 60 mg/m2 in three patients due to grade 3 and 4 neutropenia. No chemotherapy-related deaths occurred in this study (Table 3).
Table 3.
Adverse reactions in patients treated with different salvage regimens
| No. of patients | Neutropenia (n, %) | Anemia (n, %) | Thrombocytopenia (n, %) | Fever (n, %) | Peripheral neuropathy (n, %) | |
|---|---|---|---|---|---|---|
| Regimen | ||||||
| A | 20 | 2 (10.0) | 1 (5.0) | 1 (5.0) | 1 (5.0) | 0 (0) |
| B | 14 | 4 (28.6) | 2 (14.3) | 1 (7.14) | 2 (14.3) | 1 (7.14) |
| Statistical analyses | ||||||
| X 2 | 0.885 | 0.106 | 0.068 | 0.106 | 0.033 | |
| P | 0.347 | 0.745 | 0.794 | 0.745 | 0.856 |
Note: All data in the regimen groups are expressed as the number of patients (%) unless otherwise indicated.
Discussion
The present study shows that salvage therapy using cetuximab in combination with the chemotherapeutic agents pemetrexed or docetaxel improved overall response rates and prolonged survival in patients with advanced NSCLC after resistance to chemotherapy and EGFR-TKI treatment. Our data suggest that salvage therapy is beneficial for these patients, particularly for patients who were treated with EGFR-TKI for longer than 6 months. This is likely related to changes in the molecular behavior of the tumor induced by prior EGFR-TKI treatment.
To study the efficacy of cetuximab in combination with different chemotherapeutic agents, we compared the responses of patients to two regimens: cetuximab combined with pemetrexed or cetuximab combined with docetaxel. We found that ORR and PFS are similar among these patients. This result suggests that cetuximab combined with either pemetrexed or docetaxel can achieve similar improved response rates in patients with advanced NSCLC. Clinically, the PS score is considered a good marker for predicting chemotherapy efficacy [30]. However, the present study showed that ORR and PFS were similar among patients with different PS scores. Therefore, the PS score was not a meaningful predictor in our study. This finding may require further validation due to the small number of patients enrolled in this study.
Salvage therapy in advanced lung cancer patients with prior treatment failure is becoming a promising alternative. In 2007, Cho et al. [31] used erlotinib as a rescue therapy in patients after gefitinib failure. Since then, a number of similar studies have been reported. These studies suggest that after the initial gefitinib failure, erlotinib applications were able to increase the sensitivity of cancer cells to subsequent chemotherapy and improve the overall remission rate in patients [32-36]. However, some studies had opposing findings, and reported that erlotinib treatment failed to improve the remission rate in these patients [32,37].
Currently, the focus of salvage therapy after failed EGFR-TKI treatment has shifted to broader areas; for example, the application of irreversible EGFR-TKI, multi-targeted tyrosine kinase inhibitors, c-Met amplification inhibitors, and T790M mutation inhibitors. Kim et al. [38] reported that 31% of patients received docetaxel chemotherapy and 15% of patients received other chemotherapy after gefitinib failure. Despite the fact that increasing numbers of patients are receiving salvage therapy after first-line chemoimmunotherapy fails, few comprehensive studies have evaluated the efficacy of salvage therapy after EGFR-TKI failure. In addition, there are no standard guidelines to advise second-line or third-line chemotherapies in these drug-resistant patients. Wu et al. [39] conducted a retrospective study in 195 patients with advanced NSCLC after gefitinib first-line treatment failure. The follow-up data showed that there was better efficacy using platinum-based agents or paclitaxel as second-line chemotherapy. Chang et al. [40] reported that pemetrexed was given as a third- or fourth-line agent to 110 patients with advanced NSCLC. These authors found the ORR was 16.3%, SD was 37.3%, PFS was 3.2 months, and overall survival (OS) was 11.6 months.
In vitro studies have shown that EGFR-TKl combined with the anti-EGFR monoclonal antibody cetuximab synergistically affects cancer cells after the failure of EGFR-TKI [41]. This is likely due to enhanced downregulation of key enzymes controlling EGFR signaling. Some studies reported different conclusions. Jianjigian et al. [41] reported that cetuximab combined with EGFR-TKI was not beneficial to patients with advanced lung adenocarcinoma after prior EGFR-TKI treatment failure. Our present study showed that cetuximab combined with chemotherapy is beneficial to patients with advanced NSCLC after the first-line EGFR-TKI treatment failure. The achieved ORR and PFS in our study are consistent with previous salvage therapy reports [40,42].
The current data found that the predominant adverse effect of salvage therapy was bone marrow suppression. Other minor side effects included anemia, thrombocytopenia, fever, and peripheral neuropathy. Chemotherapy-related death did not occur in this study. Therefore, our data suggest that cetuximab combined with either pemetrexed or docetaxel is well tolerated by these patients.
In conclusion, cetuximab combined with chemotherapy is beneficial as a salvage therapy for patients with advanced NSCLC after first-line chemotherapy and EGFR-TKI treatment failure. Salvage therapy is particularly beneficial for patients who were treated with EGFR-TKI ≥6 months.
Disclosure of conflict of interest
None.
References
- 1.Kosmidis P. Chemotherapy in NSCLC: historical review. Lung Cancer. 2002;38(Suppl 3):S19–22. doi: 10.1016/s0169-5002(02)00261-1. [DOI] [PubMed] [Google Scholar]
- 2.De Geer A, Eriksson J, Finnern HW. A cross-country review of data collected on non-small cell lung cancer (NSCLC) patients in cancer registries, databases, retrospective and non-randomized prospective studies. J Med Econ. 2013;16:134–149. doi: 10.3111/13696998.2012.703631. [DOI] [PubMed] [Google Scholar]
- 3.Tsim S, O’Dowd CA, Milroy R, Davidson S. Staging of non-small cell lung cancer (NSCLC): a review. Respir Med. 2010;104:1767–1774. doi: 10.1016/j.rmed.2010.08.005. [DOI] [PubMed] [Google Scholar]
- 4.Gelsomino F, Agustoni F, Niger M, Valota M, Haspinger ER. Epidermal growth factor receptor tyrosine kinase inhibitor treatment in patients with EGFR wild-type non-small-cell lung cancer: the never-ending story. J. Clin. Oncol. 2013;31:3291–3293. doi: 10.1200/JCO.2013.50.2617. [DOI] [PubMed] [Google Scholar]
- 5.Galvani E, Alfieri R, Giovannetti E, Cavazzoni A, La Monica S, Galetti M, Fumarola C, Bonelli M, Mor M, Tiseo M, Peters GJ, Petronini PG, Ardizzoni A. Epidermal growth factor receptor tyrosine kinase inhibitors: current status and future perspectives in the development of novel irreversible inhibitors for the treatment of mutant non-small cell lung cancer. Curr Pharm Des. 2013;19:818–832. [PubMed] [Google Scholar]
- 6.Gao G, Ren S, Li A, Xu J, Xu Q, Su C, Guo J, Deng Q, Zhou C. Epidermal growth factor receptor-tyrosine kinase inhibitor therapy is effective as first-line treatment of advanced non-small-cell lung cancer with mutated EGFR: A meta-analysis from six phase III randomized controlled trials. Int J Cancer. 2012;131:E822–829. doi: 10.1002/ijc.27396. [DOI] [PubMed] [Google Scholar]
- 7.Schmid-Bindert G, Gebbia V, Mayer F, Arriola E, Marquez-Medina D, Syrigos K, Biesma B, Leschinger MI, Frimodt-Moller B, Ripoche V, Myrand SP, Nguyen TS, Hozak RR, Zimmermann A, Visseren-Grul C, Schuette W. Phase II study of pemetrexed and cisplatin plus cetuximab followed by pemetrexed and cetuximab maintenance therapy in patients with advanced nonsquamous non-small cell lung cancer. Lung Cancer. 2013;81:428–434. doi: 10.1016/j.lungcan.2013.05.010. [DOI] [PubMed] [Google Scholar]
- 8.Charpidou A, Blatza D, Anagnostou V, Syrigos KN. Review. EGFR mutations in non-small cell lung cancer--clinical implications. In Vivo. 2008;22:529–536. [PubMed] [Google Scholar]
- 9.Saif MW. Colorectal cancer in review: the role of the EGFR pathway. Expert Opin Investig Drugs. 2010;19:357–369. doi: 10.1517/13543781003593962. [DOI] [PubMed] [Google Scholar]
- 10.Rabinowits G, Haddad RI. Overcoming resistance to EGFR inhibitor in head and neck cancer: a review of the literature. Oral Oncol. 2012;48:1085–1089. doi: 10.1016/j.oraloncology.2012.06.016. [DOI] [PubMed] [Google Scholar]
- 11.Zahorowska B, Crowe PJ, Yang JL. Com-bined therapies for cancer: a review of EGFR-targeted monotherapy and combination treatment with other drugs. J Cancer Res Clin Oncol. 2009;135:1137–1148. doi: 10.1007/s00432-009-0622-4. [DOI] [PubMed] [Google Scholar]
- 12.Yewale C, Baradia D, Vhora I, Patil S, Misra A. Epidermal growth factor receptor targeting in cancer: a review of trends and strategies. Biomaterials. 2013;34:8690–8707. doi: 10.1016/j.biomaterials.2013.07.100. [DOI] [PubMed] [Google Scholar]
- 13.Govindan R. A review of epidermal growth factor receptor/HER2 inhibitors in the treatment of patients with non-small-cell lung cancer. Clin Lung Cancer. 2010;11:8–12. doi: 10.3816/CLC.2010.n.001. [DOI] [PubMed] [Google Scholar]
- 14.Herbst RS. Review of epidermal growth factor receptor biology. Int J Radiat Oncol Biol Phys. 2004;59:21–26. doi: 10.1016/j.ijrobp.2003.11.041. [DOI] [PubMed] [Google Scholar]
- 15.Arnold D, Peinert S, Voigt W, Schmoll HJ. Epidermal growth factor receptor tyrosine kinase inhibitors: present and future role in gastrointestinal cancer treatment: a review. Oncologist. 2006;11:602–611. doi: 10.1634/theoncologist.11-6-602. [DOI] [PubMed] [Google Scholar]
- 16.Ou SH. Second-generation irreversible epidermal growth factor receptor (EGFR) tyrosine kinase inhibitors (TKIs): a better mousetrap? A review of the clinical evidence. Crit Rev Oncol Hematol. 2012;83:407–421. doi: 10.1016/j.critrevonc.2011.11.010. [DOI] [PubMed] [Google Scholar]
- 17.Garassino MC, Borgonovo K, Rossi A, Mancuso A, Martelli O, Tinazzi A, Di Cosimo S, La Verde N, Sburlati P, Bianchi C, Farina G, Torri V. Biological and clinical features in predicting efficacy of epidermal growth factor receptor tyrosine kinase inhibitors: a systematic review and meta-analysis. Anticancer Res. 2009;29:2691–2701. [PubMed] [Google Scholar]
- 18.Glover KY, Perez-Soler R, Papadimitradopoulou VA. A review of small-molecule epidermal growth factor receptor-specific tyrosine kinase inhibitors in development for non-small cell lung cancer. Semin Oncol. 2004;31:83–92. doi: 10.1053/j.seminoncol.2003.12.019. [DOI] [PubMed] [Google Scholar]
- 19.Remon J, Moran T, Majem M, Reguart N, Dalmau E, Marquez-Medina D, Lianes P. Acquired resistance to epidermal growth factor receptor tyrosine kinase inhibitors in EGFR-mutant non-small cell lung cancer: A new era begins. Cancer Treat Rev. 2014;40:93–101. doi: 10.1016/j.ctrv.2013.06.002. [DOI] [PubMed] [Google Scholar]
- 20.Hammerman PS, Janne PA, Johnson BE. Resistance to Epidermal Growth Factor Receptor Tyrosine Kinase Inhibitors in Non-Small Cell Lung Cancer. Clin Cancer Res. 2009;15:7502–7509. doi: 10.1158/1078-0432.CCR-09-0189. [DOI] [PubMed] [Google Scholar]
- 21.Wozniak AJ. Resistance to epidermal growth factor receptor tyrosine kinase inhibitors. J Thorac Oncol. 2009;4:S1084–1085. doi: 10.1097/01.JTO.0000361760.11575.54. [DOI] [PubMed] [Google Scholar]
- 22.Hoyle M, Crathorne L, Peters J, Jones-Hughes T, Cooper C, Napier M, Tappenden P, Hyde C. The clinical effectiveness and cost-effectiveness of cetuximab (mono- or combination chemotherapy), bevacizumab (combination with non-oxaliplatin chemotherapy) and panitumumab (monotherapy) for the treatment of metastatic colorectal cancer after first-line chemotherapy (review of technology appraisal No. 150 and part review of technology appraisal No. 118): a systematic review and economic model. Health Technol Assess. 2013;17:1–237. doi: 10.3310/hta17140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lin H, Jiang J, Liang X, Zhou X, Huang R. Chemotherapy with cetuximab or chemotherapy alone for untreated advanced non-small-cell lung cancer: a systematic review and meta-analysis. Lung Cancer. 2010;70:57–62. doi: 10.1016/j.lungcan.2010.01.009. [DOI] [PubMed] [Google Scholar]
- 24.Blick SK, Scott LJ. Cetuximab: a review of its use in squamous cell carcinoma of the head and neck and metastatic colorectal cancer. Drugs. 2007;67:2585–2607. doi: 10.2165/00003495-200767170-00008. [DOI] [PubMed] [Google Scholar]
- 25.Gatzemeier U, von Pawel J, Vynnychenko I, Zatloukal P, de Marinis F, Eberhardt WE, Paz-Ares L, Schumacher KM, Goddemeier T, O’Byrne KJ, Pirker R. First-cycle rash and survival in patients with advanced non-small-cell lung cancer receiving cetuximab in combination with first-line chemotherapy: a subgroup analysis of data from the FLEX phase 3 study. Lancet Oncol. 2011;12:30–37. doi: 10.1016/S1470-2045(10)70278-3. [DOI] [PubMed] [Google Scholar]
- 26.Detterbeck FC, Boffa DJ, Tanoue LT. The new lung cancer staging system. Chest. 2009;136:260–271. doi: 10.1378/chest.08-0978. [DOI] [PubMed] [Google Scholar]
- 27.Oken MM, Creech RH, Tormey DC, Horton J, Davis TE, McFadden ET, Carbone PP. Toxicity and response criteria of the Eastern Cooperative Oncology Group. Am J Clin Oncol. 1982;5:649–655. [PubMed] [Google Scholar]
- 28.Therasse P, Arbuck SG, Eisenhauer EA, Wanders J, Kaplan RS, Rubinstein L, Verweij J, Van Glabbeke M, van Oosterom AT, Christian MC, Gwyther SG. New guidelines to evaluate the response to treatment in solid tumors. European Organization for Research and Treatment of Cancer, National Cancer Institute of the United States, National Cancer Institute of Canada. J Natl Cancer Inst. 2000;92:205–216. doi: 10.1093/jnci/92.3.205. [DOI] [PubMed] [Google Scholar]
- 29.Kaplan EL, Meier P. Nonparametric estimation from incomplete observations. J Am Statis Assoc. 1958;53:457–481. [Google Scholar]
- 30.Paesmans M, Sculier JP, Libert P, Bureau G, Dabouis G, Thiriaux J, Michel J, Van Cutsem O, Sergysels R, Mommen P, et al. Prognostic factors for survival in advanced non-small-cell lung cancer: univariate and multivariate analyses including recursive partitioning and amalgamation algorithms in 1,052 patients. The European Lung Cancer Working Party. J. Clin. Oncol. 1995;13:1221–1230. doi: 10.1200/JCO.1995.13.5.1221. [DOI] [PubMed] [Google Scholar]
- 31.Cho BC, Im CK, Park MS, Kim SK, Chang J, Park JP, Choi HJ, Kim YJ, Shin SJ, Sohn JH, Kim H, Kim JH. Phase II study of erlotinib in advanced non-small-cell lung cancer after failure of gefitinib. J. Clin. Oncol. 2007;25:2528–2533. doi: 10.1200/JCO.2006.10.4166. [DOI] [PubMed] [Google Scholar]
- 32.Watanabe S, Tanaka J, Ota T, Kondo R, Tanaka H, Kagamu H, Ichikawa K, Koshio J, Baba J, Miyabayashi T, Narita I, Yoshizawa H. Clinical responses to EGFR-tyrosine kinase inhibitor retreatment in non-small cell lung cancer patients who benefited from prior effective gefitinib therapy: a retrospective analysis. BMC Cancer. 2011;11:1. doi: 10.1186/1471-2407-11-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wong MK, Lo AI, Lam B, Lam WK, Ip MS, Ho JC. Erlotinib as salvage treatment after failure to first-line gefitinib in non-small cell lung cancer. Cancer Chemother Pharmacol. 2010;65:1023–1028. doi: 10.1007/s00280-009-1107-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Saito H, Murakami S, Kondo T, Oshita F, Noda K, Yamada K. Effectiveness of erlotinib in advanced non-small cell lung cancer in cases of gefitinib resistance after treatment of more than 6 months. Onkologie. 2012;35:18–22. doi: 10.1159/000335736. [DOI] [PubMed] [Google Scholar]
- 35.Kaira K, Yamamoto N. Erlotinib after failure of gefitinib treatment of more than 6 months in advanced non-small cell lung cancer. Onkologie. 2012;35:8–9. doi: 10.1159/000336306. [DOI] [PubMed] [Google Scholar]
- 36.Maruyama R, Wataya H, Seto T, Ichinose Y. Treatment after the failure of gefitinib in patients with advanced or recurrent non-small cell lung cancer. Anticancer Res. 2009;29:4217–4221. [PubMed] [Google Scholar]
- 37.Kaira K, Naito T, Takahashi T, Ayabe E, Shimoyama R, Kaira R, Ono A, Igawa S, Shukuya T, Murakami H, Tsuya A, Nakamura Y, Endo M, Yamamoto N. Pooled analysis of the reports of erlotinib after failure of gefitinib for non-small cell lung cancer. Lung Cancer. 2010;68:99–104. doi: 10.1016/j.lungcan.2009.05.006. [DOI] [PubMed] [Google Scholar]
- 38.Kim ES, Hirsh V, Mok T, Socinski MA, Gervais R, Wu YL, Li LY, Watkins CL, Sellers MV, Lowe ES, Sun Y, Liao ML, Osterlind K, Reck M, Armour AA, Shepherd FA, Lippman SM, Douillard JY. Gefitinib versus docetaxel in previously treated non-small-cell lung cancer (INTEREST): a randomised phase III trial. Lancet. 2008;372:1809–1818. doi: 10.1016/S0140-6736(08)61758-4. [DOI] [PubMed] [Google Scholar]
- 39.Wu JY, Shih JY, Chen KY, Yang CH, Yu CJ, Yang PC. Gefitinib therapy in patients with advanced non-small cell lung cancer with or without testing for epidermal growth factor receptor (EGFR) mutations. Medicine (Baltimore) 2011;90:159–167. doi: 10.1097/MD.0b013e31821a16f4. [DOI] [PubMed] [Google Scholar]
- 40.Chang MH, Ahn JS, Lee J, Kim KH, Park YH, Han J, Ahn MJ, Park K. The efficacy of pemetrexed as a third- or fourth-line therapy and the significance of thymidylate synthase expression in patients with advanced non-small cell lung cancer. Lung Cancer. 2010;69:323–329. doi: 10.1016/j.lungcan.2009.12.002. [DOI] [PubMed] [Google Scholar]
- 41.Janjigian YY, Azzoli CG, Krug LM, Pereira LK, Rizvi NA, Pietanza MC, Kris MG, Ginsberg MS, Pao W, Miller VA, Riely GJ. Phase I/II trial of cetuximab and erlotinib in patients with lung adenocarcinoma and acquired resistance to erlotinib. Clin Cancer Res. 2011;17:2521–2527. doi: 10.1158/1078-0432.CCR-10-2662. [DOI] [PubMed] [Google Scholar]
- 42.Hanna N, Shepherd FA, Fossella FV, Pereira JR, De Marinis F, von Pawel J, Gatzemeier U, Tsao TC, Pless M, Muller T, Lim HL, Desch C, Szondy K, Gervais R, Shaharyar , Manegold C, Paul S, Paoletti P, Einhorn L, Bunn PA Jr. Randomized phase III trial of pemetrexed versus docetaxel in patients with non-small-cell lung cancer previously treated with chemotherapy. J. Clin. Oncol. 2004;22:1589–1597. doi: 10.1200/JCO.2004.08.163. [DOI] [PubMed] [Google Scholar]


