Abstract
Objective: CD36 is involved in oxidant stress, hyperlipidemia, and thrombosis in the pathology of stroke. CD 36 single nucleotide polymorphisms (SNPs) were reported to be associated with abnormalities of serum FA, triglyceride level and to increase risk of metabolic syndrome, coronary artery disease and type 2 diabetes. Based on these finding we hypothesized that CD36 is an important candidate gene of stroke; therefore, we set out a case-control study to explore the association of CD36 SNPs with ischemic stroke. Methods: We enrolled 374 patients with atherothrombotic stroke as cases and 1,013 people without stroke as controls. CD36 rs3211842, rs3211870, rs1761667, rs9784998, and rs10499859 loci were detected by PCR-ligase detection reaction. Results: Only rs1761667 (P=0.042) and rs10499859 (P=0.038) polymorphisms were associated with cases of ischemic stroke. Under a dominant genetic model, logistic regression analysis revealed a 1.34-fold increased risk (95% CI 1.05-1.72) of ischemic stroke with rs1761667 A than non-A carriers (P=0.020); the adjusted odds ratio (AOR) was 1.38 (95% CI 1.06-1.78) after adjusting for the covariates age, gender, body mass index (BMI), cigarette smoking, hypertension, and diabetes. For rs10499859, the risk was increased 1.36-fold for G than non-G carriers (P=0.016), and the AOR was 1.39 (95% CI 1.08-1.81) (P=0.012). The 5 SNPs were in strong linkage disequilibrium. CD36 SNPs may have no association with plasma lipid levels and thromboxane B2 (TXB2) expression. Conclusion: CD36 rs1761667 and rs10499859 may indicate genetic susceptibility to ischemic stroke among Chinese Han.
Keywords: Ischemic stroke, CD36, single nucleotide polymorphism, association
Introduction
CD36 is a multi-ligand scavenger receptor expressed in various cell types including monocytes, platelets, adipocytes, myocytes, hepatocytes and vascular epithelial cells [1,2]. Signal transduction triggered by binding CD36 contributes to multiple cellular effects in pathways related to lipid utilization, insulin resistance, inflammation, atherosclerosis, platelet activation and thrombosis [3-5]. CD36 is involved in oxidant stress, hyperlipidemia, and thrombosis in the pathology of stroke [6]. A genome-wide association study (GWAS) of the association of 2,194,468 SNPs and ischemic stroke in 4 large cohorts of 19,602 white people (1,544 cases of stroke) found CD36 SNPs significantly associated with stroke [7].
The human CD36 gene locates on band 11.2 of long arm of chromosome 7 and contains ≥15 exons, 12 of which are coding [8,9]. CD36 is highly polymorphic. The impact of the CD36 null mutation and polymorphisms in human biology are not well characterized. CD 36 SNPs were reported to be associated with abnormalities of serum FA, triglyceride level and increase risk of metabolic syndrome, coronary artery disease and type 2 diabetes [10-14]. Based on these finding we hypothesized that CD36 is an important candidate gene in stroke; therefore, we set out a hospital-based case-control study to explore the association of CD36 SNPs with ischemic stroke. Five SNPs were chosen based on former functional association study. SNP rs3211842, rs3211870, rs1761667, rs9784998, and rs10499859 were reported to affect CD36 expression and to be associated with serum lipid level [10]. In the current study, we investigate the association of these five CD36 SNPs and ischemic stroke in Chinese Han people.
Materials and methods
Patients with ischemic stroke
374 Patients of atherothrombotic stroke were prospectively recruited in the emergency and neurology departments from January 2010 to December 2013. Stroke is defined according to Trial of Org 10172 in Acute Stroke Treatment [TOAST] criteria as rapidly developing signs of a focal or global disturbance of cerebral function with symptoms lasting ≥24 h or leading to death, with no apparent cause other than vascular origin [15]. All patients received a diagnosis of ischemic stroke providing the patient had a clinical diagnosis of stroke and a CT/MR scan of the brain after onset of symptoms either was normal or showed the relevant infarct. Patients with cardioembolic strokes, hemorrhagic stroke, transient ischemic attack, cerebral venous thrombosis, hemorrhagic transformation of an infarct were excluded.
We randomly selected 1,013 age and sex-matched apparently healthy controls who were undergoing physical health check-up at the hospital during the same period. We included subjects with vascular risk factors, such as hypertension, diabetes mellitus, but not history of stroke, ischemic cardiac diseases or peripheral arterial disease. We excluded patients with a history of platelet disorders, with platelet count <150000/uL, or with hemoglobin level <10 g/100 mL or hematocrit <30% or who had a major surgical procedure in the week before enrollment.
The study was approved by the hospital human ethics committee. All procedures performed in studies involving human participants were in accordance with the latest version of Declaration of Helsinki. Informed consent was obtained from all participants in this study.
Collection of clinical data
Hypertension was diagnosed with blood pressure ≥140 mmHg systolic and/or 90 mmHg diastolic on repeated measure when blood-pressure-lowering agents were not used. Diabetes mellitus was defined by the World Health Organization criteria [16]. Smoking history was defined as ever-smoked more than 5 cigarettes/day for at least a year.
Biochemical analysis
Serum concentrations of glucose, triglycerides (TG), total cholesterol (TC), low-density lipoprotein cholesterol (LDL-C), and HDL cholesterol (HDL-C) were measured by methods of the Clinical Laboratory of Jinan Central Hospital.
DNA genotyping
Genomic DNA was extracted from peripheral blood leukocytes by standard techniques (Qiagen GmbH, Germany) and stored at -20°C. The 5 CD36 SNPs were genotyped by PCR/ligase detection reaction assay [17]. Primers were synthesized by Shanghai Sangon Biological Engineering Technology & Services. Each set of ligase detection reaction probes comprised one common probe and 2 discriminating probes for the 2 types (Table 1). The target DNA sequences were amplified by a multiplex PCR method. PCR for each subject sample was performed in a final volume of 20 μL containing 1× PCR buffer, 3.0 m mol/l MgCl2, 2.0 m mol/L deoxynucleotide triphosphates, 0.4 μL primers, 0.2 μL Qiagen HotStarTaq Polymerase (QIAGEN, China), 4 μL 1× Q-solution, and 20 ng genomic DNA. Thermal cycling involved the Gene Amp PCR 9600 system (PerkinElmer) at 95°C for 15 min, then 35 cycles at 94°C for 30 s, 56°C for 1 min, and 65°C for 1 min, then 65°C for 10 min. The ligase detection reaction for each subject sample involved a final volume of 10 μL containing 1× NEB Taq DNA ligase buffer, 12.5 p mol each probe mix, 0.05 μL Taq DNA ligase (NEB Biotechnology, Beijing), and 1 μL multi-PCR product for 35 cycles at 95°C for 2 min, 94°C for 15 s, and 50°C for 25 s. Fluorescent products were differentiated by use of ABI sequencer 377 (ABI).
Table 1.
Primer sequences and ligase detection reaction (LDR) probe sequences for single nucleotide polymorphisms (SNPs) in CD36 gene
| SNP | PCR primer sequence | LDR probe sequence |
|---|---|---|
| rs3211842 | F: TGCTTTAGGGAAGAAGCCACT | P-CCTTCATACTTTGCTACGCATTTTTTTTTTTTTTTTTT-FAM |
| R: CAGATGCTTGTTGGGACCTT | A: TTTTTTTTTTTTTTTTGACAACTTTCATTATTAAAATGT | |
| G: TTTTTTTTTTTTTTTTTTGACAACTTTCATTATTAAAATGC | ||
| rs9784998 | F: CTGCATGTGAAGTCCTTCCTC | P-TCACTTATAGAAGTATTAAATTTTTTTTTTTTTTTTTTTTT-FAM |
| R: CCAATGTAAAACCAGGCAACA | C: TTTTTTTTTTTTTTTTTTAACATTTTTCAGTTGCCAGCTCG | |
| T: TTTTTTTTTTTTTTTTTTTTAACATTTTTCAGTTGCCAGCTCA | ||
| rs3211870 | F: GGAGATTGAGGTGCCAAAAA | P-CTAAAACTACAAATTATAAATTTTTTTTTTTTTTTTTTTTTTTT-FAM |
| R: GGGTGTGAATTTTCAATCTGG | C: TTTTTTTTTTTTTTTTTTTTCGATAACCAAAACAGATAAGATG | |
| T: TTTTTTTTTTTTTTTTTTTTTTCGATAACCAAAACAGATAAGATA | ||
| rs1761667 | F: TCCATTGAAGCCCTTCTGTT | P-GCTGGCATGCAAAGATGAATTTTTTTTTTTTTTTTTTTTTTTTTTTT-FAM |
| R: CCCGCCTTAGAATATTTTGG | A: TTTTTTTTTTTTTTTTTTTTTTATACTCCAGGCTTTGAGCATGGT | |
| G: TTTTTTTTTTTTTTTTTTTTTTTTATACTCCAGGCTTTGAGCATGGC | ||
| rs10499859 | F: TCATTCCCACTGCTGTTCAA | P-GTGGAGCTATTAATGTGGTATTTTTTTTTTTTTTTTTTTTTTTTTTTTTT-FAM |
| R: TGCTTGGCTGGTTAGTTTCC | A: TTTTTTTTTTTTTTTTTTTTTTTTCGAAAGTTTCTCCATATGTTGAT | |
| G: TTTTTTTTTTTTTTTTTTTTTTTTTTCGAAAGTTTCTCCATATGTTGAC |
Determination of TXB2 in plasma
A volume of 2 ml whole blood was spun at 1000 rpm for 15 min. The supernatants were stored at -80°C before ELISA. The thromboxane A2 (TXA2) hydrolysis product TXB2 was measured in plasma by ELISA (Cayman Chemicals, Michigan USA). We excluded samples for subjects who had taken aspirin, ticlopidine, dipyridamole, steroids, cyclooxygenase-2 (COX-2) inhibitors or glycoprotein IIb/IIIa inhibitors within 7 days before enrollment.
Statistical analysis
Data are expressed as mean ± SD (normal distribution), median ± IQR (skewed distribution) or number (%). Deviation from Hardy-Weinberg equilibrium was analyzed by chi-square test. Data for continuous variables were compared by one-way ANOVA and categorical variables by chi-square test. Logistic regression analysis was used to estimate the odds ratio (OR) and 95% confidence intervals (CIs) for stroke, conducted under the assumption of different genetic models (dominant, additive, and recessive). Data were also adjusted for relevant variables. Statistical analyses involved use of SPSS v17.0 (SPSS Inc., Chicago, IL). To construct the related haplotype, genotype data were used to estimate inter-marker linkage disequilibrium (LD), measure pair-wise D’ and r2 and define LD blocks. Haplotype association tests were estimated by use of Haploview 4.2 and SHE sis [18].
Results
General and biochemical characteristics
The study cohort consisted of 374 patients with atherothrombotic stroke and 1013 controls. The characteristics of subjects are in Table 2. Patients and controls did not differ in age or gender. As expected, patients more often than controls had hypertension (P<0.001) and diabetes mellitus (P<0.001). The demographic data support a role for hypertension and diabetes mellitus in the predisposition to stroke. TC and LDL-C levels (P<0.05) and platelet count (P>0.05) were higher for patients than controls, whereas HDL-C level (P=0.036) was lower.
Table 2.
Clinical characteristics of Chinese Han patients with ischemic stroke and controls
| Ischemic stroke patients (n=374) | Controls (n=1013) | P value | |
|---|---|---|---|
| Age, mean ± SD | 75.73±8.10 | 75.01±7.57 | 0.121 |
| Male/female | 304/70 | 825/188 | 0.501 |
| Body mass index, mean ± SD | 25.52±3.42 | 25.29±3.48 | 0.277 |
| Hypertension, no. (%) | 112 (29.94) | 195 (19.25) | <0.001 |
| Diabetes mellitus, no (%) | 107 (28.61) | 176 (17.37) | <0.001 |
| Smoking cigarettes, no. (%) | 79 (21.12) | 210 (20.73) | 0.444 |
| TC, m mol/L, mean ± SD | 5.08±0.99 | 4.95±1.02 | 0.031 |
| TG, m mol/L, median ± IQR | 1.15±0.73 | 1.11±0.70 | 0.156 |
| HDL-C, m mol/L, mean ± SD | 1.343±0.346 | 1.388±0.352 | 0.036 |
| LDL-C, m mol/L, mean ± SD | 3.088±0.798 | 2.477±0.827 | 0.025 |
| Platelet count, ×109/L, mean ± SD | 196.22±49.14 | 194.82±50.42 | 0.644 |
Genotype distribution, haplotype distribution and allele frequency of CD36 SNPs
The genotype distribution for all 5 polymorphisms was in Hardy-Weinberg equilibrium and is in Table 3. The minor allele frequencies ranged from 26.8% to 49.6%. Patients and controls differed in genotype distribution for rs1761667 (P=0.042) and rs10499859 (P=0.038), with no association of the other 3 selected SNPs and ischemic stroke in Chinese Han.
Table 3.
Association of CD36 SNPs and ischemic stroke
| SNPs | Genotype frequency (%) | Allele frequency (%) | |||||
|---|---|---|---|---|---|---|---|
|
| |||||||
| Major homozygote | Heterozygote | Minor homozygote | * P value | Major allele | Minor allele | ** P value | |
| rs1761667 | GG | GA | AA | 0.042 | G | A | 0.113 |
| Cases | 128 (34.9) | 190 (51.8) | 49 (13.4) | 446 (60.8) | 288 (39.2) | ||
| Controls | 415 (41.8) | 441 (44.5) | 136 (13.7) | 1271 (64.1) | 713 (35.9) | ||
| rs10499859 | AA | AG | GG | 0.038 | A | G | 0.088 |
| Cases | 125 (34.0) | 191 (51.9) | 52 (14.1) | 441 (59.9) | 295 (40.1) | ||
| Controls | 409 (41.1) | 444 (44.7) | 141 (14.2) | 1262 (63.5) | 726 (36.5) | ||
| rs3211870 | TT | TC | CC | 0.701 | T | C | 0.113 |
| Cases | 90 (24.5) | 179 (48.8) | 98 (26.7) | 359 (48.9) | 375 (51.9) | ||
| Controls | 251 (25.3) | 499 (50.3) | 243 (24.5) | 1001 (50.4) | 985 (49.6) | ||
| rs9784998 | CC | CT | TT | 0.219 | C | T | 0.192 |
| Cases | 194 (52.7) | 151 (41.0) | 23 (6.3) | 539 (73.2) | 19726.8 | ||
| Controls | 501 (50.6) | 399 (40.3) | 91 (9.2) | 1401 (70.7) | 581 (29.3) | ||
| rs3211842 | AA | AG | GG | 0.321 | A | G | 0.166 |
| Cases | 99 (27.1) | 186 (50.8) | 81 (22.1) | 384 (52.5) | 348 (47.5) | ||
| Controls | 307 (31.2) | 476 (48.4) | 200 (20.4) | 1090 (55.4) | 876 (44.6) | ||
For additive genetic models;
comparing alleles.
The Odds ratios (ORs) and adjusted ORs (AORs) for association of CD36 SNPs and ischemic stroke (in recessive model and dominant model) is in Table 4. Under a dominant genetic model, logistic regression analysis revealed a 1.34-fold increased risk (95% CI 1.05-1.72) of ischemic stroke with rs1761667 A than non-A carriers (P=0.020); the adjusted odds ratio (A OR) was 1.38 (95% CI 1.06-1.78) after adjusting for the covariates age, gender, body mass index (BMI), cigarette smoking, hypertension, and diabetes (Table 4). For rs10499859, the risk was increased 1.36-fold for G than non-G carriers (P=0.016), and the AOR was 1.39 (95% CI 1.08-1.81) (P=0.012).
Table 4.
Odds ratios (ORs) and adjusted ORs (AORs) for association of CD36 SNPs and ischemic stroke in Chinese Han (in recessive model and dominant model)
| OR (95% CI) | P value | A OR (95% CI) | P value | |
|---|---|---|---|---|
| rs1761667 (dominant) | 1.34 (1.05-1.72) | 0.020 | 1.38 (1.06-1.78) | 0.016 |
| rs1761667 (recessive) | 0.97 (0.68-1.38) | 0.864 | 0.97 (0.68-1.40) | 0.882 |
| rs10499859 (dominant) | 1.36 (1.06-1.75) | 0.016 | 1.39 (1.08-1.81) | 0.012 |
| rs10499859 (recessive) | 0.99 (0.71-1.40) | 0.980 | 1.03 (0.72-1.67) | 0.885 |
| rs3211870 (dominant) | 1.04 (0.79-1.37) | 0.776 | 0.82 (0.58-1.14) | 0.231 |
| rs3211870 (recessive) | 1.12 (0.86-1.48) | 0.399 | 1.13 (0.85-1.51) | 0.394 |
| rs9784998 (dominant) | 0.92 (0.72-1.17) | 0.479 | 0.86 (0.67-1.11) | 0.240 |
| rs9784998 (recessive) | 0.66 (0.41-1.06) | 0.085 | 0.62 (0.37-1.03) | 0.066 |
| rs3211842 (dominant) | 1.23 (0.94-1.60) | 0.137 | 1.26 (0.95-1.26) | 0.108 |
| rs3211842 (recessive) | 1.11 (0.83-1.49) | 0.473 | 1.17 (0.86-1.58) | 0.320 |
95% CI, 95% confidence interval; aOR, adjusted for age, gender, body mass index, cigarette smoking, hypertension, and diabetes.
The LD pattern of the 5 SNPs is in Figure 1. The 5 SNPs showed strong LD and formed two blocks: rs1761667, rs10499859 and rs9784998 in block one, and rs3211842 and rs3211870 in block 2. The sample size is too small to support haplotype analysis and no association was found (data not shown).
Figure 1.
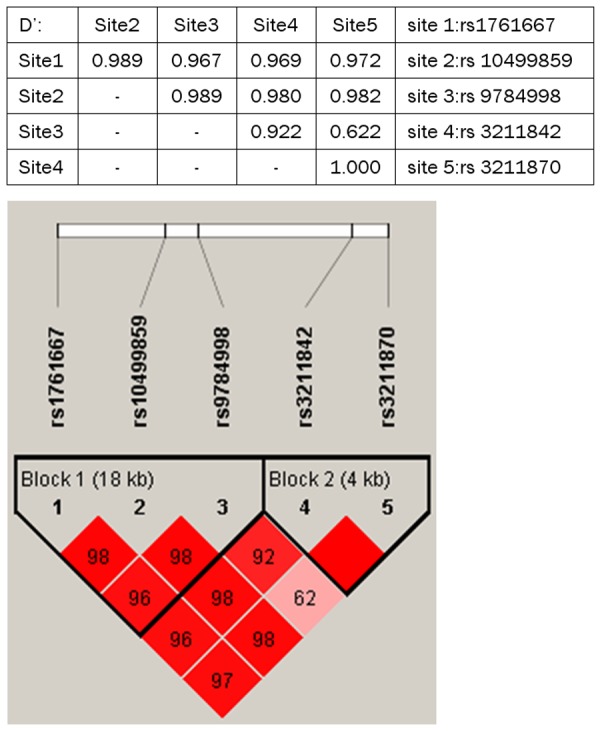
LD analysis. Each diamond for each SNP combination indicates pairwise LD between all SNPs. The correlation of LD (r 2) among all pairs of SNPs is shown by the shade of red.
Association among genotypes, plasma lipid and TXB2 levels
CD36 SNP has been documented to be associated with lipid levels and platelet aggregation [10,19], we exam in our local recruits the relationship between TG, TC, HDL, LDL, platelet count and TXB2. No significant association was found among genotypes, plasma and TXB2 level (Table 5).
Table 5.
Association of CD36 SNPs with plasma lipid, plate count and TXB2 levels
| SNP | TG | TC | HDL | LDL | Plate count | TXB2 | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
|
|||||||||||||
| Median ± IQR | P | mean ± SD | P | mean ± SD | P | mean ± SD | P | Mean ± SD | P | mean ± SD | P | ||
| rs1761667 | GG | 1.11±0.71 | 0.753 | 5.02±0.04 | 0.613 | 1.38±0.04 | 0.794 | 3.03±0.04 | 0.447 | 195.69±2.27 | 0.945 | 3135.93±326.65 | 0.297 |
| GA | 1.13±0.71 | 5.08±0.04 | 1.37±0.02 | 3.09±0.03 | 195.02±1.95 | 3145.13±278.62 | |||||||
| AA | 1.11±0.71 | 5.05±0.07 | 1.38±0.03 | 3.08±0.06 | 194.35±3.35 | 3257.12±379.21 | |||||||
| rs10499859 | AA | 1.11±0.71 | 0.870 | 5.01±0.04 | 0.620 | 1.38±0.02 | 0.859 | 3.02±0.04 | 0.333 | 195.52±2.29 | 0.960 | 3168.28±335.51 | 0.574 |
| AG | 1.12±0.71 | 5.06±0.04 | 1.37±0.01 | 3.07±0.03 | 194.83±1.94 | 3073.42±261.46 | |||||||
| GG | 1.13±0.67 | 5.08±0.07 | 1.37±0.02 | 3.12±0.06 | 194.49±3.38 | 2539.23±486.39 | |||||||
| rs3211870 | TT | 1.12±0.77 | 0.149 | 5.04±0.06 | 0.990 | 1.38±0.02 | 0.957 | 3.02±0.04 | 0.560 | 198.17±3.01 | 0.403 | 3243.67±421.33 | 0.440 |
| TC | 1.11±0.70 | 5.05±0.04 | 1.37±0.01 | 3.07±0.03 | 193.76±1.84 | 3135.40±251.41 | |||||||
| CC | 1.15±0.70 | 5.05±0.05 | 1.38±0.02 | 3.08±0.04 | 194.43±2.65 | 2595.02±398.59 | |||||||
| rs9784998 | CC | 1.13±0.70 | 0.277 | 5.05±0.04 | 0.647 | 1.38±0.01 | 0.976 | 3.07±0.03 | 0.791 | 195.50±1.85 | 0.956 | 2759.66±246.97 | 0.128 |
| CT | 1.11±0.72 | 5.05±0.05 | 1.38±0.02 | 3.05±0.03 | 194.65±2.23 | 2980.88±284.67 | |||||||
| TT | 1.06±0.70 | 4.96±0.11 | 1.38±0.03 | 3.02±0.08 | 194.90±4.68 | 2615.64±283.47 | |||||||
| rs3211842 | AA | 1.10±0.71 | 0.763 | 5.01±1.01 | 0.647 | 1.39±0.02 | 0.760 | 3.00±0.04 | 0.316 | 196.10±2.67 | 0.707 | 3160.16±396.91 | 0.140 |
| AG | 1.11±0.72 | 5.06±1.02 | 1.37±0.01 | 3.08±0.03 | 194.83±1.89 | 3275.28±264.79 | |||||||
| GG | 1.14±0.66 | 5.04±0.97 | 1.37±0.02 | 3.07±0.05 | 192.86±2.87 | 2860.90±362.54 | |||||||
Discussion
Here we demonstrated that under a dominant inheritance model, CD36 rs1761667 and rs10499859 but not rs3211842, rs3211870 and rs9784998 genotypes were associated with atherothrombotic stroke in Chinese Han. The rs1761667 A and rs10499859 G polymorphisms were more prevalent in patients with atherothrombotic stroke than healthy controls. The significance remained after adjusting for age, gender, BMI, cigarette smoking, alcohol drinking, hypertension, and diabetes. The 5 SNPs were in strong LD and were divided into 2 blocks, but analysis showed no haplotype association with atherothrombotic stroke. All 5 SNPs were not associated with serum lipid and TXB2 levels.
SNP rs1761667 locates in the 5’ flanking exon 1A. It is common in African Americans (minor allele frequency [MAF] =0.39) and Caucasians (MAF =0.48), although the minor alleles are reversed [10]. The A allele is the minor allele in our sample of Chinese Han and African Americans (versus G in Caucasians). The mutation did not change the amino acid sequence whereas the SNP was reported to be associated with protein expression, serum lipid level and some diseases [11-14,19,20]. The A allele was found associated with a 2.7 mg/dl increase in HDL in non-diabetic African Americans, and reduced monocyte CD36 mRNA level and total and surface protein levels of CD36 [10]. The SNP was associated with free fatty acid (FFA) levels in Caucasian men; men carrying the major allele (an allele) had higher FFA levels than men homozygous for the minor allele [11]. Carriage of the rs1761667 G allele was found more sensitive in detecting oleic acid and triolein than homozygosity for the A allele, associated with low CD36 expression, whereas heterozygosity for this allele was intermediate [20]. In two studies, CD36 rs1761667 SNP was reported significantly associated with type 2 diabetes mellitus, with the GA heterozygous genotype showing highest frequency among patients [12,13]. However, all the research are association study and none of them explored the molecular mechanism of the polymorphism.
SNP rs10499859 was in strong LD with rs1761667. The G allele was the minor allele in our sample of Chinese Han versus A in Caucasians [10]. The mutation did not change the amino acid sequence and carriers of the rs10499859 genotypes were reported differ in mean HDL-C levels [10]. The magnitude of the increase in HDL-C was positively associated with number of minor alleles. Mean HDL-C concentrations were increased between 1.5 and 2.5 mg/dl per allele [14]. A GWAS study for left ventricular mass index showed a borderline association of CD36 rs10499859 SNP and left ventricular hypertrophy [21]. The LD of rs10499859 has not been reported before.
CD36 is expressed in various types of cells and tissues, including microglia and astrocytes in the brain, monocytes/macrophages, platelets, microvascular endothelium, cardiac and skeletal muscle, adipocytes, and dendritic cells. It exhibits high affinity towards a wide variety of structurally distinct ligands, including oxidized or modified LDL (oxLDL, mLDL), long-chain fatty acids, thrombospondin (TSP) -1 and -2, fibrillar β-amyloid, Plasmodium falciparum-parasitized erythrocytes, and membranes of cells undergoing apoptosis [22-26]. CD36 has been implicated in a wide variety of normal and abnormal biological functions, including angiogenesis, atherosclerosis, inflammation, lipid metabolism, and removal of apoptotic cells. It was found to be a major platelet glycoprotein and named glycoprotein IV. OxLDL induces platelet activation via platelet CD36, and the level of CD36 expression in platelets modulates platelet activity [19,27]. Platelet CD36 also functions as a receptor for cell-derived microparticles and thereby contributes to thrombus formation in vascular injury and inflammation, when microparticles are generated [28].
CD36 expression is up-regulated with cerebral ischemia: CD36 expression occurs mainly on CD11b+ microglia or infiltrated macrophages within the infarct territory during infarct development [29]. With increased expression of CD36 and TSPs with ischemia [29-31], angiostatic signaling with excessive TSP-CD36 interaction may contribute to the pathology of stroke. Furthermore, mice lacking the CD36 receptor showed greatly reduced reactive oxygen species production after ischemia and are relatively protected against ischemic injury [29]. CD36 is involved in innate immune responses after cerebral ischemia [32]. Targeting CD36 to help attenuate innate immune responses under pathological conditions may be a therapeutic strategy for stroke. Using the cell-permeable anti-oxidant peptide SS31, which down-regulates CD36 pathways, injury size and GSH depletion were reduced in wild-type but not CD36-null mice after ischemia [33]. A subsequent study reported a shift toward a less pro-inflammatory state in the post-ischemic brain in the absence of CD36 and suggested that CD36 is a critical mediator eliciting pro-inflammatory responses following ischemia [34].
We found CD36 rs1761667 and 10499859 polymorphisms associated with ischemic stroke in Chinese Han. However, our study did not find association of these two mutations with serum lipid and TXB2 levels. Although the two identified SNPs did not change the amino acid sequence, it might be located in genomic areas important to CD36 gene transcription or mRNA translation. The SNPs were in strong LD with each other. Thus, these SNPs may be in LD with other rarer polymorphisms in the coding region of the gene that effect transcription, translation, mRNA stability, or protein stability [19]. Nonetheless, the results of association studies must always be interpreted with caution, especially when multiple comparisons are performed, and replication in other populations is needed before establishing a true link.
Conclusion
This is the first study to examine the association of CD36 polymorphisms and ischemic stroke in Chinese. Rs1761667 and rs10499859 SNPs are associated with ischemic stroke in this population.
Acknowledgements
This study was supported by grants from the National Natural Science Foundation of China (No. 81070919), and Jinan Youth Science and Technology Grant.
Disclosure of conflict of interest
None.
References
- 1.Greenwalt DE, Lipsky RH, Ockenhouse CF, Ikeda H, Tandon NN, Jamieson GA. Membrane glycoprotein CD36: a review of its roles in adherence, signal transduction, and transfusion medicine. Blood. 1992;80:1105–1111. [PubMed] [Google Scholar]
- 2.Nicholson AC, Han J, Febbraio M, Silversterin RL, Hajjar DP. Role of CD36, the macrophage, class B scavenger receptor, in atherosclerosis. Ann N Y Acad Sci. 2001;947:224–228. doi: 10.1111/j.1749-6632.2001.tb03944.x. [DOI] [PubMed] [Google Scholar]
- 3.Park YM, Febbraio M, Silverstein RL. CD36 modulates migration of mouse and human macrophages in response to oxidized LDL and may contribute to macrophage trapping in the arterial intima. J Clin Invest. 2009;119:136–145. doi: 10.1172/JCI35535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Rahaman SO, Lennon DJ, Febbraio M, Podrez EA, Hazen SL, Silverstein RL. A CD36-dependent signaling cascade is necessary for macrophage foam cell formation. Cell Metab. 2006;4:211–221. doi: 10.1016/j.cmet.2006.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kashyap SR, Ioachimescu AG, Gornik HL, Gopan T, Davidson MB, Makdissi A, Major J, Febbraio M, Silverstein RL. Lipid-induced insulin resistance is associated with increased monocyte expression of scavenger receptor CD36 and internalization of oxidized LDL. Obesity (Silver Spring) 2009;17:2142–2148. doi: 10.1038/oby.2009.179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kim E, Febbraio M, Bao Y, Tolhurst AT, Epstein JM, Cho S. CD36 in the periphery and brain synergizes in stroke injury in hyperlipidemia. Ann Neurol. 2012;71:753–764. doi: 10.1002/ana.23569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ikram MA, Seshadri S, Bis JC, Fornage M, DeStefano AL, Aulchenko YS, Debette S, Lumley T, Folsom AR, van den Herik EG, Bos MJ, Beiser A, Cushman M, Launer LJ, Shahar E, Struchalin M, Du Y, Glazer NL, Rosamond WD, Rivadeneira F, Kelly-Hayes M, Lopez OL, Coresh J, Hofman A, DeCarli C, Heckbert SR, Koudstaal PJ, Yang Q, Smith NL, Kase CS, Rice K, Haritunians T, Roks G, de Kort PL, Taylor KD, de Lau LM, Oostra BA, Uitterlinden AG, Rotter JI, Boerwinkle E, Psaty BM, Mosley TH, van Duijn CM, Breteler MM, Longstreth WT Jr, Wolf PA. Genome wide association studies of stroke. N Engl J Med. 2009;360:1718–28. doi: 10.1056/NEJMoa0900094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Armesilla AL, Vega MA. Structural organization of the gene for human CD36 glycoprotein. J Biol Chem. 1994;269:18985–18991. [PubMed] [Google Scholar]
- 9.Rac ME, Safranow K, Poncylijusz W. Molecular basis of CD36 gene mutations. Mol Med. 2007;13:288–296. doi: 10.2119/2006-00088.Rac. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Love-Gregory L, Sherva R, Schappe T, Qi JS, McCrea J, Klein S, Connelly MA, Abumrad NA. Common CD36 SNPs reduce protein expression and may contribute to a protective atherogenic profile. Hum Mol Genet. 2011;20:193–201. doi: 10.1093/hmg/ddq449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ma X, Bacci S, Mlynarski W, Gottardo L, Soccio T, Menzaghi C, Iori E, Lager RA, Shroff AR, Gervino EV, Nesto RW, Johnstone MT, Abumrad NA, Avogaro A, Trischitta V, Doria A. A common haplotype at the CD36 locus is associated with high free fatty acid levels and increased cardiovascular risk in Caucasians. Hum Mol Genet. 2004;13:2197–2205. doi: 10.1093/hmg/ddh233. [DOI] [PubMed] [Google Scholar]
- 12.Banerjee M, Gautam S, Saxena M, Bid HK, Agrawal CG. Association of CD36 gene variants rs1761667 (G > A) and rs1527483 (C > T) with Type 2 diabetes in North Indian population. Int J Diabetes Mellit. 2010:179–183. [Google Scholar]
- 13.Gautam S, Pirabu L, Agrawal CG, Banerjee M. CD36 genevariants and their association with type 2 diabetes in an Indian population. Diabetes Technol Ther. 2013;15:680–7. doi: 10.1089/dia.2012.0326. [DOI] [PubMed] [Google Scholar]
- 14.Love-Gregory L, Sherva R, Sun L, Wasson J, Schappe T, Doria A, Rao DC, Hunt SC, Klein S, Neuman RJ, Permutt MA, Abumrad NA. Variants in the CD36 gene associate with the metabolic syndrome and high-density lipoprotein cholesterol. Hum Mol Genet. 2008;17:1695–1704. doi: 10.1093/hmg/ddn060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Adams HP Jr, Bendixen BH, Kappelle LJ, Biller J, Love BB, Gordon DL, Marsh EE 3rd. Classification of subtype of acute ischemic stroke: definitions for use in a multicenter clinical trial: TOAST: Trial of Org 10172 in Acute Stroke Treatment. Stroke. 1993;24:35–41. doi: 10.1161/01.str.24.1.35. [DOI] [PubMed] [Google Scholar]
- 16.Alberti KG, Zimmet PZ. Definition, diagnosis, and classification of diabetes mellitus and its complications. Part I: diagnosis and classification of diabetes mellitus provisional report of a WHO consultation. Diabet Med. 1998;15:539–553. doi: 10.1002/(SICI)1096-9136(199807)15:7<539::AID-DIA668>3.0.CO;2-S. [DOI] [PubMed] [Google Scholar]
- 17.Deng L, Huang R, Chen Z, Wu L, Xu DL. A Study on Polymorphisms of Elastin Gene in Chinese Han Patients With Isolated Systolic Hypertension. Am J Hypertens. 2009;22:656–62. doi: 10.1038/ajh.2009.53. [DOI] [PubMed] [Google Scholar]
- 18.Shi YY, He L. SHEsis, a powerful software platform for analyses of linkage disequilibrium, haplotype construction, and genetic association at polymorphism loci. Cell Res. 2005;15:97–8. doi: 10.1038/sj.cr.7290272. [DOI] [PubMed] [Google Scholar]
- 19.Ghosh A, Murugesan G, Chen K, Zhang L, Wang Q, Febbraio M. Platelet CD36 surface expression levels affect functional responses to oxidized LDL and are associated with inheritance of specific genetic polymorphisms. Blood. 2011;117:6355–6366. doi: 10.1182/blood-2011-02-338582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Pepino MY, Love-Gregory L, Klein S, Abumrad NA. The fatty acid translocase gene CD36 and lingual lipase influence oral sensitivity to fat in obese subjects. J Lipid Res. 2012;53:561–566. doi: 10.1194/jlr.M021873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Arnett DK, Li N, Tang W, Rao DC, Devereux RB, Claas SA, Kraemer R, Broeckel U. Genome-wide association study identifies single-nucleotide 254 polymorphism in KCNB1 associated with left ventricular mass in humans: the Hyper GEN Study. BMC Med Genet. 2009;10:43–51. doi: 10.1186/1471-2350-10-43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Febbraio M, Hajjar DP, Silverstein RL. CD36: a class B scavenger receptor involved in angiogenesis, atherosclerosis, inflammation, and lipid metabolism. J Clin Invest. 2001;108:785–91. doi: 10.1172/JCI14006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Yamashita S, Hirano K, Kuwasako T, Janabi M, Toyama Y, Ishigami M, Sakai N. Physiological and pathological roles of a multi-ligand receptor CD36 in atherogenesis; insights from CD36-deficient patients. Mol Cell Biochem. 2007;299:19–22. doi: 10.1007/s11010-005-9031-4. [DOI] [PubMed] [Google Scholar]
- 24.Hirano K, Kuwasako T, Nakagawa-Toyama Y, Janabi M, Yamashita S, Matsuzawa Y. Pathophysiology of human genetic CD36 deficiency. Trends Cardiovasc Med. 2003;13:136–141. doi: 10.1016/s1050-1738(03)00026-4. [DOI] [PubMed] [Google Scholar]
- 25.Medeiros LA, Khan T, El Khoury JB, Pham CL, Hatters DM, Howlett GJ, Lopez R, O’Brien KD, Moore KJ. Fibrillar amyloid protein present in atheroma activates CD36 signal transduction. J Biol Chem. 2004;279:10643–10648. doi: 10.1074/jbc.M311735200. [DOI] [PubMed] [Google Scholar]
- 26.Febbraio M, Silverstein RL. CD36: implications in cardiovascular disease. Int J Biochem Cell Biol. 2007;39:2012–2030. doi: 10.1016/j.biocel.2007.03.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Podrez EA, Byzova TV, Febbraio M, Salomon RG, Ma Y, Valiyaveettil M, Poliakov E, Sun M, Finton PJ, Curtis BR, Chen J, Zhang R, Silverstein RL, Hazen SL. Platelet CD36 links hyperlipidemia, oxidant stress and a pro-thrombotic phenotype. Nat Me. 2007;13:1086–1095. doi: 10.1038/nm1626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ghosh A, Febbraio M, Espinola RG, McCrae KR, Silverstein RL. Platelet CD36 mediates interactions with endothelial cell-derived microparticles and contributes to thrombosis in vivo. J Clin Invest. 2008;118:1934–1943. doi: 10.1172/JCI34904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Cho S, Park EM, Febbraio M, Anrather J, Park L, Racchumi G, Silverstein RL, Iadecola C. The class B scavenger receptor CD36 mediates free radical production and tissue injury in cerebral ischemia. J Neurosci. 2005;25:2504–2512. doi: 10.1523/JNEUROSCI.0035-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hayashi T, Noshita N, Sugawara T, Chan PH. Temporal profile of angiogenesis and expression of related genes in the brain after ischemia. J Cereb Blood Flow Metab. 2003;23:166–180. doi: 10.1097/01.WCB.0000041283.53351.CB. [DOI] [PubMed] [Google Scholar]
- 31.Lin TN, Kim GM, Chen JJ, Cheung WM, He YY, Hsu CY. Differential regulation of thrombospondin-1 and thrombospondin-2 after focal cerebral ischemia/reperfusion. Stroke. 2003;34:177–186. doi: 10.1161/01.str.0000047100.84604.ba. [DOI] [PubMed] [Google Scholar]
- 32.Cho S, Kim E. CD36: A multi-modal target for acute stroke therapy. J Neurochem. 2009;109(Suppl 1):126–132. doi: 10.1111/j.1471-4159.2009.05801.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Cho S, Szeto HH, Kim E, Kim H, Tolhurst AT, Pinto JT. A novel cell-permeable antioxidant peptide, SS31, attenuates ischemic brain injury by down-regulating CD36. J Biol Chem. 2007;282:4634–4642. doi: 10.1074/jbc.M609388200. [DOI] [PubMed] [Google Scholar]
- 34.Kim E, Tolhurst AT, Qin LY, Chen XY, Febbraio M, Cho S. CD36/fatty acid translocase, an inflammatory mediator, is involved in hyperlipidemia-induced exacerbation in ischemic brain injury. J Neurosci. 2008;28:4661–4670. doi: 10.1523/JNEUROSCI.0982-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]


