Abstract
Objectives: To compare the cytokine profile in RA patients and healthy control by using two methods-FlowCytomix assay and traditional ELISA. Methods: Cytokine levels were evaluated by FlowCytomix assay and ELISA in serum and supernatants of peripheral blood mononuclear cells (PBMC) cultures with and without stimulation by phytohaemagglutinin (PHA). Results: The levels of IL-6, IL-1β, and TNF-α were significantly higher in sera of RA patients than those of healthy controls. The levels of IL-22, IL-6, IL-1β, TNF-α, and IL-10 were higher in unstimulated PBMC culture supernatant of RA patients than those of healthy controls. PHA stimulation significantly increased the production of proinflammatory cytokines from PBMC with RA patients. Compared with detectable cytokine levels in sera, cytokine concentration in the supernatant of PBMCs was remarkably higher. FlowCytomix and ELISA showed significant correlation in detecting cytokines. However, the FlowCytomix assay detected more cytokines than ELISA. Conclusion: The supernatant of PBMCs provide a fine condition for the study of cytokine production because of the lack of interference factors in sera. The FlowCytomix assay is more sensitive than ELISA in detecting cytokines from RA patients. Multiple cytokine signatures using FlowCytomix assay may represent a more realistic approach in the future of personalized medicine in RA.
Keywords: Cytokine profile, FlowCytomix assay, ELISA, rheumatoid arthritis
Introduction
Rheumatoid arthritis (RA) is one of the most common systemic autoimmune diseases. RA characterized by chronic inflammation in the synovium, resulting in progressive destruction of joints and cartilage. Although the pathophysiology of RA has not been completely understood yet, increasing numbers of cytokines have been found to be involved in RA pathology. Hence, cytokine-blocking therapies are being considered for prevention of inflammation and cartilage and bone destruction [1]. Several detection methods (ELISA, immunohistochemistry) have identified TNF-α and IL-1 as major players in the network of cytokines, notably directly expressed at the disease site in joint fluid tissue. Recently, more cytokines, including IL-6, IFN-γ, GM-CSF, IL-17, IL-21, IL-18 and IL-15, have also been found to be involved in the RA pathology [2,3]. Thus, increased levels of cytokines such as IL-1, IL-6 and TNF have been interpreted as an indicator of inflammatory state [1]. Furthermore, as an expensive cytokine therapy, there are up to 40% non-response patients. Das and colleagues [4] reported that serum IL-6 was significantly higher at baseline in rituximab non responders and that a significant reduction followed treatment in responders. Response to anti-TNF (etanercept) was associated with reduced levels of IL-6 and increased IL-23 and IL-32 post-treatment while there was no change in non-responders [5]. Thus, cytokines may as predictive biomarkers for therapy outcome would allow tailoring therapy to the individual. It is unlikely that single immune mediators correlate with inflammatory state or disease biological processes on their own. Instead, multiple cytokines are involved in pathological and immunological processes of RA, reflecting the different capacities and functions of immune cells, such as immunoregulation, proliferation or activation [6]. Understanding the complex cytokine networks that contribute to the autoimmune processes associated with RA has become more relevant since the advent of biologic therapy [1,7].
Methods of detection for cytokines vary considerably. Enzyme-linked immunosorbent assays (ELISAs) have long been considered the “gold standard”, but could only measure one analyte at a time. Furthermore, traditional ELISA for a single cytokine could take up to 4 h to complete; therefore, performing multiple ELISA assays for a number of different cytokines could be very expensive and time-consuming.
Currently, development of multiplexing technology has allowed simultaneous investigation of multiple cytokines from a small amount of sample material, reducing any inter-assay variation, analysis, cost, sample material, and human error. A number of commercial kits are available. Flow cytometric multiplexed bead assays using fluorescently-labeled microbeads coated antibodies that specifically react with different analytes in a multiplex system could be differentiated by size and distinct fluorescence intensity using flow cytometry, and allow for automated high-throughput screening, potentially improving reproducibility and reducing human error. Although FlowCytomix assay is widely used for detection of cytokines [8-11]; however, few comparative studies of this assay with traditional ELISA methods have been performed. Furthermore, most of the studies comparing different multiplex kits have measured cytokines in serum or the supernatants of peripheral blood mononuclear cells (PBMC) [12-14]; few comparative studies of serum with PBMC supernatant have been reported.
In this study, we employed the Bender MedSystems FlowCytomix platform to determine the cytokines from serum and supernatants of PBMC with RA, and then compared with traditional ELISA. This study provides useful information that could be used as a guide in the selection of appropriate multiple analyte technology, which allows discovery of patterns and relevant correlations and may be more powerful in capturing the emergence of complex responses associated to inflammation or pathology in RA.
Materials and methods
Patients
Thirty-five patients with active RA based on the criteria of the American College of Rheumatology [15] and fifteen age- and sex-matched healthy controls were enrolled in study. Disease activity was assessed using the 28-joint disease activity score (DAS28) [16]; all patients were free of infectious diseases, malignant diseases, cardiovascular complaints, or other inflammatory diseases. Table 1 presents the characteristics of the RA patients and the healthy controls. Informed consent was obtained from all patients and healthy controls. This study was approved by the Ethics Committee of the Affiliated Hospital, Jiangsu University (Permit Number: JSU 12-098) and abides by the Helsinki Declaration on ethical principles for medical research involving human subjects.
Table 1.
Characteristics of RA patients and healthy controls
| Demographics | Active RA (n = 35) | Healthy (n = 15) |
|---|---|---|
| Age (years), mean (range) | 54.1 (35-71) | 52.6 (32-68) |
| Sex, female/male | 29/6 | 12/3 |
| Disease duration (years), Mean (range) | 7.5 (2-16) | - |
| RF, positive/negative | 21/14 | - |
| ESR (mm/h), mean (range) | 38.9 (28-69) | 7.6 (4-16) |
| CRP (mg/L), mean (range) | 62.8 (27-135) | 3.6 (1-8) |
| Mean DAS28 | 6.2 (4.5-7.3) | - |
RF Rheumatoid factor, ESR Erythrocyte sedimentation rate, CRP C-reactive protein; DAS28 Disease Activity Score in 28 joints.
Blood sample preparation
Approximately 8 ml of venous blood was drawn by venipuncture from the patients and healthy controls. Up to 6 ml of the 8 ml sample was heparinized for isolation of PBMC, while the remaining 2 ml was centrifuged at 800× g for 10 min, aliquot, and stored at -80°C for preparation of serum.
PBMC were isolated from healthy controls and RA patients by Ficoll-Hypaque (Amersham Pharmacia Biotech, Uppsala, Sweden) density gradient centrifugation. The cells were washed three times with sterile phosphate buffered saline (PBS) and suspended in a concentration of 2×106 cells/ml in RPMI 1640 (Life Technologies) supplemented with 10% heat-inactivated fetal calf serum, 2 mM L-glutamine, and 1% penicillin-streptomycin (HyClone, Logan, Utah, USA). PBMCs were stimulated in a concentration of 2×105 cells per well in 200 μl complete culture medium with 5 μg/ml phytohaemagglutinin (PHA) in U bottom 96-well plates at 37°C. The supernatant was collected after incubation for 24 h, and then frozen at -80°C until assay.
The concentrations of the cytokines in the sera and the supernatants of PBMCs were determined using the FlowCytomix Human Cytokine kit (Bender MedSystems, Vienna, Austria) and ELISA kit (Bender Med Systems, Vienna, Austria).
Cytokine measurement by FlowCytomix human cytokine Kit
The sera and PBMC supernatant were assayed to determine the concentrations of cytokines with Human Th1/Th2/Th9/Th17/Th22 13-plex FlowCytomixTM Kit (Bender MedSystems, Vienna, Austria) according to the instructions of the manufacturer. The kit allowed simultaneous quantification of 13 cytokines, including Th1 cytokines (IFN-γ, IL-2, and TNF-α), Th2 cytokines (IL-10, IL-4, IL-5, IL-6, and IL-13), Th17 cytokines (IL-17 and IL-23), Th9 cytokines (IL-9), and Th22 cytokines (IL-12p70 and IL-22). Data were collected on a FACSCalibur flow cytometer using CellQuest (BD Biosciences, Franklin Lakes, NJ) and analyzed with FlowCytomix Pro 2.2 Software (Bender MedSystems). Standard curves were determined for each cytokine from a range of 27-20000 pg/ml. The concentration of each cytokine was expressed as pg/ml. The lower limit of detection according to the manufacturer is 1.2-43.3 pg/ml depending on the analyte.
Cytokine measurement by ELISA
The measurement of cytokines in sera and PBMC supernatant by ELISA kit (Bender Med Systems, Vienna, Austria) was performed according to manufacturer’s instructions of the manufacturer. Dilutions of recombinant human cytokines following the instructions from the manufacturer were used to create a standard curve. All samples were assayed in duplicate. Concentrations below the detection limit were considered undetectable.
Statistical analysis
Statistical analyses were performed with GraphPad Prism 5.01 (GraphPad Software, 2007, CA, USA). Data are expressed as the mean ± standard error of the mean (SEM). Nonparametric Mann-Whitney U-test was used to calculate the significance difference between the groups and P < 0.05 (two-tailed) was considered statistically significant. Pearson correlation analysis was used to calculate the correlation coefficient between FlowCytomix and ELISA. The significance was set at P < 0.05.
Results
Increased pro-inflammatory cytokines in the serum from RA patients by FlowCytomix assay
The serum cytokine environment of the RA patients and the healthy controls were measured using FlowCytomix assay. Table 2 shows that the active RA group exhibited higher levels of serum IL-6, IL-1β, and TNF-α than those of the healthy controls (all P < 0.05). Serum levels of the other cytokines tested were almost exclusively less than the detection limit of the assay system. These results suggested that an increased pro-inflammatory cytokines in the serum from active RA patients.
Table 2.
Concentrations of serum cytokines determined by FlowCytomix assay
| Cytokines (pg/ml) | Control | Active RA |
|---|---|---|
| IL-6 | 46.24±15.71 | 91.65±24.82* |
| IL-1β | 182.75±55.27 | 412.09±67.92* |
| TNF-α | 59.62±27.18 | 162.35±46.78* |
P < 0.05, significance between RA patients and healthy control.
Increased pro-inflammatory cytokines in the supernatant of PBMCs from RA patients by FlowCytomix assay
To determine the cytokine supernatant profiles, PBMCs from RA patients and healthy controls were cultured for 48 h in the presence or absence of PHA stimulation; the cytokine production in the supernatants was evaluated. IL-22, IL-6, IL-1β, TNF-α and IL-10 levels were higher in the unstimulated PBMC culture supernatant of RA patients compared with those of the healthy controls (Table 3). The Th1-associated cytokine IFN-γ, Th2-associated cytokine IL-4, IL-5, and IL-13, Th9-associated cytokine IL-9, and Th17-associated cytokine IL-17 were almost not detected in the unstimulated PBMC culture supernatants of the patients and controls. Consistent with results described previously by our group [9], PHA stimulation induced significant increase in the production of proinflammatory cytokines from PBMC with RA patients in this experiment. In response to PHA stimulation, PBMC from RA patients released more IL-22, IL-6, IL-1β, TNF-α, and IL-10 (Table 3). Furthermore, PHA stimulation resulted in the PBMC of RA patients secreting higher levels of IL-17 than those of healthy controls (all P < 0.05). However, IFN-γ levels decreased and IL-2, IL-13, IL-4, IL-5 and IL-10 levels were not significantly changed. IL-9 and IL-12p70 were undetectable (Table 3). Compared with detectable cytokine levels in sera, the concentration of cytokines in the supernatant of PBMCs were remarkably high. Collectively, these results suggest that increased levels of pro-inflammatory cytokines in the supernatant of PBMCs from RA patients regardless of PHA stimulation.
Table 3.
Concentrations of cytokines in culture supernatants of PBMCs with or without PHA stimulation by FlowCytomix assay
| Cytokines (pg/ml) | Control | Active RA | ||
|---|---|---|---|---|
|
| ||||
| Unstimulated | stimulated | Unstimulated | Stimulated | |
| IFN-γ | / | 665.35±213.24 | / | 339.51±135.16¥ |
| IL-17 | / | 73.68±28.61 | / | 172.05±82.64¥ |
| IL-2 | / | 277.19±84.86 | / | 353.54±75.24 |
| IL-22 | 51.25±12.26 | 89.10±21.15 | 112.38±25.56* | 177.83±51.55¥,§ |
| IL-6 | 1252.09±93.10 | 1386.35±625.75 | 1894.75±832.55* | 2606.19±721.61¥,§ |
| IL-13 | / | 216.79±41.63 | / | 263.45±33.65 |
| IL-4 | / | 29.58±17.41 | / | 23.82±8.53 |
| IL-5 | / | 76.47±45.50 | / | 84.12±67.93 |
| IL-1β | 1318.44±181.12 | 2465.32±525.86 | 1967.32±247.71* | 3693.09±866.99¥,§ |
| TNF-α | 441.31±79.17 | 1189.76±462.38 | 903.50±413.12* | 1795.84±718.18¥,§ |
| IL-10 | 89.98±19.30 | 735.26±224.35 | 352.42±124.18* | 713.16±237.91§ |
P < 0.05, significance between RA patients and healthy control from PBMCs without stimulation.
P < 0.05, significance between RA patients and healthy control from PBMCs with stimulation.
P < 0.05, significance between with and without stimulation from RA patients PBMCs.
Comparison of FlowCytomix and ELISA for cytokine measurement in RA patients
Given of limited detection of cytokines in serum and unstimulated PBMC culture supernatant of RA patients and healthy control, FlowCytomix and ELISA were compared based on their ability to detect cytokine production in PBMC after PHA stimulation. Among the 13 cytokines measured, IL-2, IL-4, IL-5, IL-9, IL-12p70 and IL-13 were undetectable by the ELISA method, whereas IFN-γ, IL-17, IL-22, IL-6, IL-1β, TNF-α, and IL-10 could be detected by the FlowCytomix and ELISA. Table 4 shows the concentrations of cytokines in culture supernatants of PHA-stimulated PBMCs by ELISA. A similar trend for cytokine levels was observed using the FlowCytomix (Table 3) and ELISA (Table 4) among the detectable cytokines, such as IFN-γ, IL-17, IL-22, IL-6, IL-1β, TNF-α and IL-10. However, the FlowCytomix detected higher quantities of IFN-γ, IL-17, IL-22, IL-6, IL-1β, TNF-α and IL-10, and the correlation between the FlowCytomix and ELISA is graphically represented in Figure 1. Table 5 shows the corresponding linear relationships between the FlowCytomix and ELISA readings on the detectable cytokines (IFN-γ, IL-17, IL-22, IL-6, IL-1β, TNF-α, and IL-10) in supernatant PHA-stimulated PBMCs from RA patients. The FlowCytomix assay detected more cytokines with higher quantities than the ELISA. Therefore, the FlowCytomix assay may be more sensitive than ELISA in detecting cytokines from patients with inflammatory diseases, such as RA.
Table 4.
Concentrations of cytokines in culture supernatants of PHA-stimulated PBMCs by ELISA
| Cytokines (pg/ml) | Control | Active RA |
|---|---|---|
| IFN-γ | 67.38±12.33 | 15.09±7.07* |
| IL-17 | 19.91±7.14 | 43.69±7.94* |
| IL-22 | 18.18±2.97 | 35.07±3.95** |
| IL-6 | 196.94±27.15 | 352.12±32.23** |
| IL-1β | 380.61±54.83 | 570.40±53.31* |
| TNF-α | 262.52±44.12 | 417.41±40.60* |
| IL-10 | 205.8±25.69 | 254.2±29.24 |
P < 0.05, significance between RA patients and healthy control;
P < 0.01, significance between RA patients and healthy control.
Figure 1.
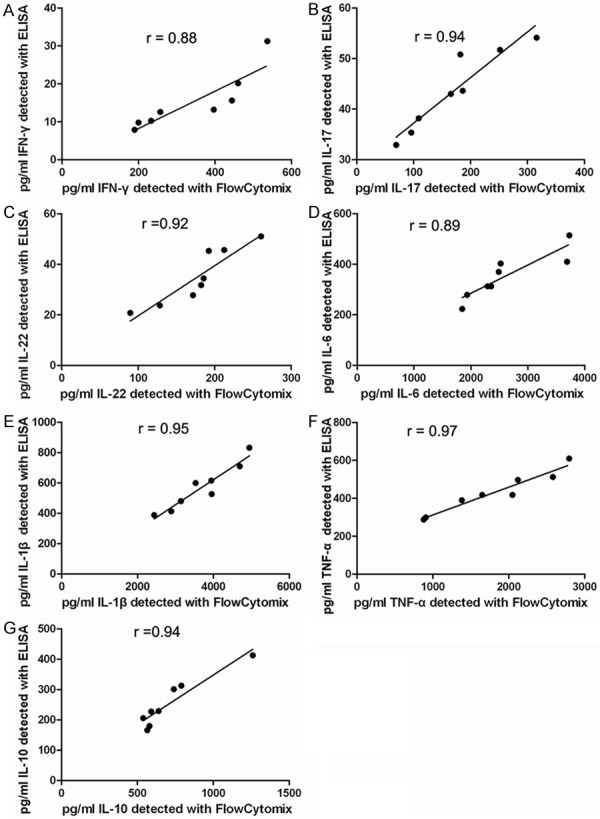
Correlation of cytokines in supernatants of PHA-stimulated PBMCs from RA patients detected between FlowCytomix and ELISA. Correlation of pg/ml of IFN-γ (A), IL-17 (B), IL-22 (C), IL-6 (D), IL-1β (E), TNF-α (F), and IL-10 (G) detected between the FlowCytomix and ELISA. The two methods significantly correlated with one another for the detection of IFN-γ (Pearson r = 0.88, P = 0.004), IL-17 (Pearson r = 0.94, P = 0.0005), IL-22 (Pearson r = 0.92, P = 0.0014), IL-6 (Pearson r = 0.89, P = 0.0031), IL-1β (Pearson r = 0.95, P = 0.0003), TNF-α (Pearson r = 0.97, P < 0.001), and IL-10 (Pearson r = 0.94, P =0.0006).
Table 5.
Correlation of cytokines in culture supernatants of PHA-stimulated PBMCs from RA patients detectedbetween FlowCytomix and ELISA
| Cytokines | ELISA values relative to FlowCytomix |
|---|---|
| IFN-γ | Y = 0.049x-1.613 |
| IL-17 | Y = 0.09x+28.17 |
| IL-22 | Y = 0.20x-0.19 |
| IL-6 | Y = 0.11x+61.82 |
| IL-1β | Y = 0.14x+165.8 |
| TNF-α | Y = 0.17x-39.56 |
| IL-10 | Y = 0.32x+21.73 |
Discussion
Currently, the use of multiplexed methods to simultaneously measure multiple analytes for better understanding of the underlying biological mechanisms of various diseases and the mechanism of action of therapeutic agents has received a great deal of interest [17,18]. These methods facilitate the evaluation of multiple analytes and collection of large amount of scientific information despite limited sample volumes [19,20]. However, to determine the best method, a thorough knowledge of assay performance in the sample matrix intended for analysis and comparison with traditional methods is essential. The performance of the FlowCytomix assay in sera or culture supernatant of stimulated PBMCs isolated from normal donors or patients have been fairly well characterized [9,21,22]. However, few studies that provide a thorough evaluation of the performance of these assays to compare the serum cytokine level with ex vivo spontaneous and induced PBMC cytokine production that would be analyzed during a clinical study and how assay performance is affected in samples collected from patients with different disease states have been reported. Assay performance with clinically relevant sample types is critical in determining the proper application of these methods to understand disease process and drug action.
The method described in this study was evaluated for quantification of thirteen cytokines in culture supernatant from unstimulated and stimulated PBMCs and in serum samples from RA patients and healthy controls. We found that FlowCytomix assay for the cytokine measurement in sera or supernatant from RA patients and healthy control is quite reliable. However, the detection range and concentration of cytokines in supernatant was far superior to that in sera. Normal human serum has reduces the sensitivity of some commercial sources of antibody pairs [23], and the presence of animal proteins such as bovine serum albumin could also interfere with the assays. These results are consistent with another study demonstrating lower quantities of cytokines in sera than that in supernatant by LINCOplex [19] and MILLIplex kits [24], which may be related to the presence of large dynamic concentration ranges of proteins and other interfering factors in sera [19].
PBMC culture may allow the study of cytokine production in vitro spontaneously or after activation of specific cells, reflecting their secretory potential in an attempt to identify a specific cytokine “signature” to RA patients. Consistent with a previous study reporting that IL-6, IL-1β, and TNF-α are abundant in the inflamed joint in patients with active RA and are directly involved in the destruction of cartilage and bone [25], our study demonstrated that PBMCs from RA patients without stimulation could secrete higher levels of IL-22, IL-6, IL-1β and TNF-α compared with healthy donors. IL-22, a Th17-related cytokines, is also produced by Th22 cells, innate lymphocytes (lymphoid tissue-inducer cells, γ/δ T cells and natural killer cells) [26], has been found to be elevated in the periphery and synovial of RA patients [27-29] and has a pathogenic role in RA [26]. Furthermore, consistent with our previous study [9], we also found that PBMCs from RA patients stimulated by PHA, a polyclonal stimulator of T cells, could secrete higher levels of IL-17, IL-6, TNF-α and IL-1β compared with healthy donors. However, IFN-γ, a Th1-related cytokine was decreased, while IL-2, IL-13, IL-4, IL-5 and IL-10, other Th1 or Th2-related cytokines, were not changed in RA patients compared with those in healthy controls (Table 4). This result was consistent with the concept that the function of Th2 cells in RA was impaired [25], however, it is in contrast to a study that demonstrated that Th1 and Th17 cells play an important role in the progression of RA [30,31]. Similarly, decreased IFN-γ level in RA patients was found in another study [32], suggesting that Th17, rather than Th1 cells, is the pathogenic culprit in RA [31].
Although multiplexed assays provide the ability to collect large amount of information from limited samples, the results highlight the point that “real world” assay performance must be evaluated to help in understanding how the results of the assays should be used. This understanding will be especially important when trying to compare results obtained with different methods. FlowCytomix assay was able to detect more and higher quantities cytokines than ELISA. Good concordance was observed between the two methods when detecting IFN-γ, IL-17, IL-22, IL-6, IL-1β, TNF-α and IL-10; a linear correlation was also observed between the two methods in the above cytokine detection. These correlations could be attributed to the high level of cytokines detected by all assays, assuming that the levels were well above the limits of detection, thus, the quantities are more accurately determined. These results were consistent with another study demonstrating that values from cytokine flow cytometry assays were consistently higher than those from an Enzyme-linked immunospot (ELISPOT) [33] or ELISA [34].
Because cytokines form part of a network, the profile obtained is better understood if all or most variables contributing to it are analyzed simultaneously. Multiplex techniques can simultaneously measure a number of different parameters using a small amount of sample thereby reducing any inter-assay variation, sample material, analysis time, cost and human error. Multiplex techniques better reflect the “real world” in vivo from patients than single-measurement ELISAs. Although we did not compare FlowCytomix assay with other multiplex cytokine detection, Berthoud et al. [35] compared two Luminex methods and two flow cytometry methods, including the Bender FlowCytomix kit, for detection of cytokines in supernatants from human leukocytes stimulated with viable Plasmodium falciparum infected red blood cells or P. falciparum schizont lysates. They found that the two flow cytometry-based kits showed significant correlation in detection of IFN-γ, IL-2, TNF, IL-10 and IL-6. The Bender FlowCytomix kit detected significantly higher amounts of IFN-γ and TNF-α, but significantly lower amounts of IL-6 and IL-10 than the BD kit. Young et al. [34] compared the BD kit, ELISA and Bender MedSystems kit in measurement of cytokines in rodent bronchoalveolar lavage, and reported that although correlations could be good between multiplex cytokine kits and ELISA, a large fold difference (up to 11-fold) in the quantity of cytokine detected could be observed. Differences in the absolute quantity of cytokine present in the samples may be explained by contaminants in the matrix that differentially affect the standard curves and efficiency of different monoclonal antibody pairs. Thus, when much information is needed from a complex inflammatory disease such as RA, a different method such as FlowCytomix assay should be considered.
Conclusion
In summary, this work demonstrates that the supernatant of PBMCs provide a fine condition for the study of cytokine production because of the lack of interference factors in sera. FlowCytomix assay provides reliable, high throughput and rapid screening for cytokine signature profiles in inflammatory disease such as RA even when using small samples. Further investigations based on multiple samplings are necessary. Furthermore, the complicated mechanism and the relationships of the cytokines and clinical characteristics of RA need to be further investigated in the future.
Acknowledgements
This work was supported by a grant from the Natural Science Foundation of Jiangsu (BK20141295), the “333” Projects of Jiangsu Province (BRA2014172), and a grant from Key Medical Personnel of Zhenjiang to Xuefeng Wang. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Disclosure of conflict of interest
None.
References
- 1.Burska A, Boissinot M, Ponchel F. Cytokines as biomarkers in rheumatoid arthritis. Mediators Inflamm. 2014;2014:545493. doi: 10.1155/2014/545493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Niu X, Chen G. Clinical biomarkers and pathogenic-related cytokines in rheumatoid arthritis. J Immunol Res. 2014;2014:698192. doi: 10.1155/2014/698192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Mcinnes IB, Schett G. Cytokines in the pathogenesis of rheumatoid arthritis. Nat Rev Immunol. 2007;7:429–442. doi: 10.1038/nri2094. [DOI] [PubMed] [Google Scholar]
- 4.Das S, Vital EM, Horton S, Bryer D, EI-Sherbiny Y, Rawstron AC, Ponchel F, Emery P, Buch MH. Abatacept or tocilizumab after rituximab in rheumatoid arthritis? An exploratory study suggests non-response to rituximab is associated with persistently high IL-6 and better clinical response to IL-6 blocking therapy. Ann Rheum Dis. 2014;73:909–912. doi: 10.1136/annrheumdis-2013-204417. [DOI] [PubMed] [Google Scholar]
- 5.Zivojinovic SM, Pejnovic NN, Sefik-Bukilica MN, Kovacevic LV, Soldatovic II, Damjanov NS. Tumor necrosis factor blockade differentially affects innate inflammatory and Th17 cytokines in rheumatoid arthritis. J Rheumatol. 2012;39:18–21. doi: 10.3899/jrheum.110697. [DOI] [PubMed] [Google Scholar]
- 6.Brennan FM, Mcinnes IB. Evidence that cytokines play a role in rheumatoid arthritis. J Clin Invest. 2008;118:3537–45. doi: 10.1172/JCI36389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Connor V. Anti-TNF therapies: a comprehensive analysis of adverse effects associated with immunosuppression. Rheumatol Int. 2011;31:327–37. doi: 10.1007/s00296-009-1292-x. [DOI] [PubMed] [Google Scholar]
- 8.Sarapik A, Velthut A, Haller-Kikkatalo K, Faure GC, Bene MC, de Carvalho Bittencourt M, Massin F, Uibo R, Salumets A. Follicular proinflammatory cytokines and chemokines as markers of IVF success. Clin Dev Immunol. 2012;2012:606459. doi: 10.1155/2012/606459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Dong L, Wang X, Tan J, Li H, Qian W, Chen J, Chen Q, Wang J, Xu W, Tao C, Wang S. Decreased expression of microRNA-21 correlates with the imbalance of Th17 and Treg cells in patients with rheumatoid arthritis. J Cell Mol Med. 2014;18:2213–24. doi: 10.1111/jcmm.12353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wang X, Dong L, Ni H, Zhou S, Xu Z, Hoellwarth JS, Chen X, Zhang R, Chen Q, Liu F, Wang J, Su C. Combined TLR7/8 and TLR9 ligands potentiate the activity of a Schistosoma japonicum DNA vaccine. PLoS Negl Trop Dis. 2013;7:e2164. doi: 10.1371/journal.pntd.0002164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wang X, Liu F, Zhou S, Xu Z, Hoellwarth J, Chen X, He L, Zhang R, Liu F, Wang J, Su C. Partial regulatory T cell depletion prior to schistosomiasis vaccination does not enhance the protection. PLoS One. 2012;7:e40359. doi: 10.1371/journal.pone.0040359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Richens JL, Urbanowicz RA, Metcalf R, Corne J, O’Shea P, Fairclough L. Quantitative validation and comparison of multiplex cytokine kits. J Biomol Screen. 2010;15:562–8. doi: 10.1177/1087057110362099. [DOI] [PubMed] [Google Scholar]
- 13.Khan IH, Krishnan VV, Ziman M, Janatpour K, Wun T, Luciw PA, Tuscano J. A comparison of multiplex suspension array large-panel kits for profiling cytokines and chemokines in rheumatoid arthritis patients. Cytometry B Clin Cytom. 2009;76:159–68. doi: 10.1002/cyto.b.20452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Moncunill G, Aponte JJ, Nhabomba AJ, Dobano C. Performance of multiplex commercial kits to quantify cytokine and chemokine responses in culture supernatants from Plasmodium falciparum stimulations. PLoS One. 2013;8:e52587. doi: 10.1371/journal.pone.0052587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Arnett FC, Edworthy SM, Bloch DA, McShane DJ, Fries JF, Cooper NS, Healey LA, Kaplan SR, Liang MH, Luthra HS. The American Rheumatism Association 1987 revised criteria for the classification of rheumatoid arthritis. Arthritis Rheum. 1988;31:315–24. doi: 10.1002/art.1780310302. [DOI] [PubMed] [Google Scholar]
- 16.Prevoo ML, van’tHof MA, Kuper HH, van Leewen MA, van de Putte LB, van Riel PL. Modified disease activity scores that include twenty-eight-joint counts. Development and validation in a prospective longitudinal study of patients with rheumatoid arthritis. Arthritis Rheum. 1995;38:44–8. doi: 10.1002/art.1780380107. [DOI] [PubMed] [Google Scholar]
- 17.Bastarache JA, Koyama T, Wickersham NE, Mitchell DB, Mernaugh RL, Ware LB. Accuracy and reproducibility of a multiplex immunoassay platform: a validation study. J Immunol Methods. 2011;367:33–9. doi: 10.1016/j.jim.2011.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Toedter G, Hayden K, Wagner C, Brodmerkel C. Simultaneous detection of eight analytes in human serum by two commercially available platforms for multiplex cytokine analysis. Clin Vaccine Immunol. 2008;15:42–8. doi: 10.1128/CVI.00211-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Prabhakar U, Eirikis E, Reddy M, Silvestro E, Spitz S, Pendley C 2nd, Davis HM, Miller BE. Validation and comparative analysis of a multiplexed assay for the simultaneous quantitative measurement of Th1/Th2 cytokines in human serum and human peripheral blood mononuclear cell culture supernatants. J Immunol Methods. 2004;291:27–38. doi: 10.1016/j.jim.2004.04.018. [DOI] [PubMed] [Google Scholar]
- 20.Ray CA, Bowsher RR, Smith WC, Devanarayan V, Willey MB, Brandt JT, Dean RA. Development, validation, and implementation of a multiplex immunoassay for the simultaneous determination of five cytokines in human serum. J Pharm Biomed Anal. 2005;36:1037–44. doi: 10.1016/j.jpba.2004.05.024. [DOI] [PubMed] [Google Scholar]
- 21.Zhang JS, Zhao QM, Zuo SQ, Jia N, Guo XF. Cytokine and chemokine responses to Japanese encephalitis live attenuated vaccine in a human population. Int J Infect Dis. 2012;16:e285–8. doi: 10.1016/j.ijid.2011.12.010. [DOI] [PubMed] [Google Scholar]
- 22.Chen H, Wen F, Zhang X, Su SB. Expression of T-helper-associated cytokines in patients with type 2 diabetes mellitus with retinopathy. Mol Vis. 2012;18:219–26. [PMC free article] [PubMed] [Google Scholar]
- 23.Kellar KL, Kalwar RR, Dubois KA, Crouse D, Chafin WD, Kane BE. Multiplexed fluorescent bead-based immunoassays for quantitation of human cytokines in serum and culture supernatants. Cytometry. 2001;45:27–36. doi: 10.1002/1097-0320(20010901)45:1<27::aid-cyto1141>3.0.co;2-i. [DOI] [PubMed] [Google Scholar]
- 24.Ibrahim JN, Jounblat R, Delwail A, Abou-Ghoch J, Salem N, Chouery E, Megarbane A, Medlej-Hashim M, Lecron JC. Ex vivo PBMC cytokine profile in familial Mediterranean fever patients: Involvement of IL-1beta, IL-1alpha and Th17-associated cytokines and decrease of Th1 and Th2 cytokines. Cytokine. 2014;69:248–54. doi: 10.1016/j.cyto.2014.06.012. [DOI] [PubMed] [Google Scholar]
- 25.Toh ML, Miossec P. The role of T cells in rheumatoid arthritis: new subsets and new targets. Curr Opin Rheumatol. 2007;19:284–8. doi: 10.1097/BOR.0b013e32805e87e0. [DOI] [PubMed] [Google Scholar]
- 26.Yang X, Zheng SG. Interleukin-22: a likely target for treatment of autoimmune diseases. Autoimmun Rev. 2014;13:615–20. doi: 10.1016/j.autrev.2013.11.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Colin EM, Asmawidjaja PS, van Hamburg JP, Mus AM, van Driel M, Hazes JM, van Leeuwen JP, Lubberts E. 1,25-dihydroxyvitamin D3 modulates Th17 polarization and interleukin-22 expression by memory T cells from patients with early rheumatoid arthritis. Arthritis Rheum. 2010;62:132–42. doi: 10.1002/art.25043. [DOI] [PubMed] [Google Scholar]
- 28.Zhang L, Li YG, Li YH, Qi L, Liu XG, Yuan CZ, Hu NW, Ma DX, Li ZF, Yang Q, Li W, Li JM. Increased frequencies of Th22 cells as well as Th17 cells in the peripheral blood of patients with ankylosing spondylitis and rheumatoid arthritis. PLoS One. 2012;7:e31000. doi: 10.1371/journal.pone.0031000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Zhao L, Jiang Z, Jiang Y, Ma N, Zhang Y, Feng L, Wang K. IL-22+ CD4+ T cells in patients with rheumatoid arthritis. Int J Rheum Dis. 2013;16:518–26. doi: 10.1111/1756-185X.12099. [DOI] [PubMed] [Google Scholar]
- 30.Park H, Li Z, Yang XO, Chang SH, Nurieva R, Wang YH, Wang Y, Hood L, Zhu Z, Tian Q, Dong C. A distinct lineage of CD4 T cells regulates tissue inflammation by producing interleukin 17. Nat Immunol. 2005;6:1133–41. doi: 10.1038/ni1261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Gaston JS. Cytokines in arthritis--the ‘big numbers’ move centre stage. Rheumatology (Oxford) 2008;47:8–12. doi: 10.1093/rheumatology/kem203. [DOI] [PubMed] [Google Scholar]
- 32.Nissinen R, Leirisalo-Repo M, Peltomaa R, Palosuo T, Vaarala O. Cytokine and chemokine receptor profile of peripheral blood mononuclear cells during treatment with infliximab in patients with active rheumatoid arthritis. Ann Rheum Dis. 2004;63:681–7. doi: 10.1136/ard.2003.008599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Karlsson AC, Martin JN, Younger SR, Bredt BM, Epling L, Ronquillo R, Varma A, Deeks SG, McCune JM, Nixon DF, Sinclair E. Comparison of the ELISPOT and cytokine flow cytometry assays for the enumeration of antigen-specific T cells. J Immunol Methods. 2003;283:141–53. doi: 10.1016/j.jim.2003.09.001. [DOI] [PubMed] [Google Scholar]
- 34.Young SH, Antonini JM, Roberts JR, Erdely AD, Zeidler-Erdely PC. Performance evaluation of cytometric bead assays for the measurement of lung cytokines in two rodent models. J Immunol Methods. 2008;331:59–68. doi: 10.1016/j.jim.2007.11.004. [DOI] [PubMed] [Google Scholar]
- 35.Berthoud TK, Manaca MN, Quelhas D, Aguilar R, Guinovart C, Puyol L, Barbosa A, Alonso PL, Dobano C. Comparison of commercial kits to measure cytokine responses to Plasmodium falciparum by multiplex microsphere suspension array technology. Malar J. 2011;10:115. doi: 10.1186/1475-2875-10-115. [DOI] [PMC free article] [PubMed] [Google Scholar]


