Abstract
Aims and objectives: The purpose of this study was to examine the sleep quality and health-related quality of life (HRQOL) in patients after renal transplantation and to explore the relationship between the quality of sleep and the HRQOL. Background: Sleep disorders are still an important clinical problem after renal transplantation. Previous studies mainly focused on patients’ sleep quality before kidney transplant. More studies are needed to document sleep quality after renal transplantation. Design: A cross-sectional design was used in this study. Methods: A convenience sample of renal transplant recipients was recruited at an outpatient transplant clinic of a general hospital in Beijing, China. The Pittsburgh Sleep Quality Index (PSQI) was used to measure quality of sleep. The Medical Outcomes Study 36-item Short Form (MOS SF-36) was used to measure health-related quality of life. Results: The average PSQI score of the 204 renal transplant recipients was 5.81±3.52, significantly lower than the norm. Fifty (24.5%) recipients were classified as having poor sleep quality (global PSQI > 7). The mean scores of renal transplant recipients for SF-36 Mental Component Summary (MCS) and Physical Component Summary (PCS) were 47.57±6.71 and 48.26±9.66 respectively. Compared with residents in Sichuan province, recipients’ scores for SF-36 dimensions were statistically lower except the dimension of mental health. SF-36 scores of poor sleepers (PSQI > 7) were significantly lower than the good sleepers (PSQI ≤ 7) in both the MCS and PCS. Significant differences exist between the groups in physical function, bodily pain, vitality, and mental health dimensions. Conclusions: Sleep quality and HRQOL of patients after renal transplantation were lower than the norm. Poor sleep is associated with lower HRQOL. Relevance to clinical practice: Health professionals need to pay attention to sleep quality and HRQOL in renal transplant recipients and take appropriate measures to improve patients’ sleep quality and HRQOL.
Keywords: Health-related quality of life, quality of sleep, recipients, renal transplantation
Introduction
For patients with chronic kidney disease and end-stage renal disease, sleep disorder is a very common problem [1,2]. Sleeping disorders include insomnia, sleep apnea, restless legs syndrome, periodic limb movement disorder, excessive daily sleeping, sleepwalking, nightmares, and narcolepsy. Previous studies found that renal transplantation can prolong patients’ survival time [3], while helping solve some pre-transplant sleep problems, such as restless legs syndrome [4,5] sleep apnea [6], sleep-related breathing disorder [7] and chronic insomnia [8]. However, transplantation cannot completely eliminate sleep problems. Evidence is emerging that sleep disorders are still an important clinical problem after renal transplantation [9]. High prevalences and incidences of insomnias, such as difficult to stay asleep (49.4%), difficult to fall asleep (32.1%) negatively impacted the daytime functionality of renal transplant recipients [9].
Health-related quality of life (HRQOL) has become recognized as an important outcome measure in patients with organ transplantation. Successful renal transplantation provides a better patient outcome in terms of HRQOL. Evidence shows that HRQOL of renal transplant recipients improved significantly when compared with their HRQOL in the preoperative dialysis period [10]. The improvement in HRQOL after renal transplantation may be attributable to many factors, such as, effective functioning of renal graft, fewer medical complications, lifestyle changes afforded by medical treatment and so on [10]. There are several factors in renal transplant recipients which have a negative impact on HRQOL, sleep disorders belong to this group of factors. Sleep is an important index of health, and its main function is to promote mental and physical recovery. Poor sleep may reduce a person’s immunity, lead to endocrine disorders, and lead to more negative emotions, finally impacting negatively on the patient’s activity and quality of life during the daytime [11]. Some evidence indicated that there were an association between sleep-disordered breathing and cardiovascular diseases [12]. The presence of sleep problems has repeatedly been shown to be associated with impaired HRQOL in dialysis patients [13,14], and increased morbidity and mortality in other populations [15,16]. Therefore, the sleep quality of patients after renal transplantation may influence their postoperative recovery, and ultimately affect their quality of life.
Because of the heavy financial burden, postoperative pain, side effects of immunosuppressant therapy, decreased postoperative physical activity and, increased frequency of night urination [17], patients often experience certain short term sleep disorders after renal transplantation. Over time these issues generally subside and sleep may improve. However, some influencing factors such as demographic factors, ESRD duration prior to transplant [18] would may not change after renal transplantation, which may still have an effect on their sleep after renal transplantation. Some sleep risk factors such as medical comorbidity and poor emotional state [19] may begin to appear or change gradually as times go on, and their effect on renal transplant recipients’ sleep may change accordingly. Previous studies mainly focused on patients’ sleep quality before kidney transplant [11] or short-term after renal transplantation [10]. More studies are needed to document sleep quality three months or more after renal transplantation.
This cross-sectional study examines the sleep quality and HRQOL of renal transplant recipients three months or more after renal transplantation and explores the relationship between the quality of sleep and the HRQOL, thereby providing a basis for health professionals to facilitate the development and implementation of specific interventions to improve sleep quality and HRQO of renal transplant recipients.
Subjects and methods
Subjects
Using a convenience sampling strategy, 210 renal transplant recipients were recruited when they visited transplant follow-up clinics in one general hospital in Beijing, China from March 2013 to February 2014 as study participants. Inclusion criteria were (1) 3 months or more post renal transplantation, (2) functional renal graft (meaning the patient does not need dialysis), (3) patient at least 18 years old, (4) ability to speak and read Chinese, and (5) willingness to participate in this study. Patients who had multiple organ transplants or who had more than one renal transplant were excluded from this study.
Measurement
Patients completed two standardized questionnaires in the assessment of sleep quality and HRQOL. Questionnaires were completed in the transplant follow-up clinics. Demographic information was collected at the same time, including current age, gender, employment status, education, marital status, whether the transplant was self-paid or national insurance paid and family financial income. Transplant specific information, such as the date of transplant and whether the kidney was from a living or cadaveric donor, was also collected.
Quality of sleep
The Pittsburgh Sleep Quality Index (PSQI) was adopted to assess the transplant recipient’s sleep quality during the past month. The scale was developed by Buysse (1989) and demonstrated good internal consistency (Cronbach’s α = 0.83) and test-retest reliability (r = 0.85) [20]. Liu and colleagues [21] examined the reliability and validity of PSQI in the Chinese population and found that the sensitivity and the specificity of PSQI were respectively 98.3% and 90.2% when the cutoff score was 7. This 19-item scale is composed 7 components: subjective sleep quality, sleep latency, sleep duration, sleep efficiency, sleep disturbance, use of sleep medications, and daytime dysfunction. Scores of each component range from 0 to 3. The sum of seven components scores is global sleep quality score (range = 0~21), with higher score indicating a lower quality of sleep. A global sleep quality score is calculated with a cutoff score > 7 used to identify poor sleep quality. In this study, the Cronbach α coefficient of PSQI was 0.751. The correlation coefficient between each component score and global PSQI score ranged from 0.535 (sleep disturbance and daytime dysfunction) to 0.753 (sleep latency), namely, a statistically positive correlation (P < 0.01).
Health-related quality of life
Health-related quality of life was measured by the Medical Outcomes Study 36-item Short Form (MOS SF-36) (Cronbach’s α = 0.91), a 36-item self-administered brief questionnaire [22]. The questionnaire was already translated into Chinese. It covers 8 domains of physical functioning, role-physical, bodily pain, general health, vitality, social functioning, role-emotional, and mental health. SF-36 consisted Physical Component Summary (PCS) including physical functioning, role-physical, bodily pain, and general health subscales and Mental Component Summary (MCS) including vitality, social functioning, role-emotional, and mental health subscales [22]. Scores of each domain ranged from 0 to 100, with higher scores reflecting higher HRQOL. The MCS and PCS scores are standardized to a mean of 50, with scores above and below 50 indicating above and below average functioning, respectively. The Cronbach α coefficient of SF-36 PCS and MCS in this study were 0.712 and 0.811 respectively, and the correlation coefficient between PCS and its four domains, between MCS and its four domains ranged from 0.624 to 0.737, 0.680 to 0.879, both showing a statistically positive correlation (P < 0.01).
Ethical considerations
The study was approved by the university ethics committee, which requires processes to ensure the confidentiality of all data. The purpose, risks and benefits of this study were explained to the patients before they were asked to participate. The patients were assured that participation was voluntary, and that choosing not to participate would not influence their clinical care.
Data collection procedures
Investigators were trained before the survey to make sure that they were familiar with the requirements and methods of data collection. The principal investigator prepared survey questionnaires. Only packet identification codes appeared on the surveys. Survey packets and a cover letter with a description of the project, response confidentiality, consent procedure, and investigator contact information were packaged in unsealed envelopes. Packets were distributed at the time of check in and patients were invited to participate. Consent was demonstrated by their completing and returning the surveys. The investigators were present at the clinic to answer patients’ questions. Patients returned the survey packet after they completed at the clinic. Patients did not put their name or any other identifying information on the surveys.
Statistical analysis
Original data were input into Excel software and checked by two research assistants. Data was analyzed using SPSS 21.0 software. Continuous variables were expressed as mean ± standard deviation (SD). Discrete variables were reported as frequency and percentage. Student’s t test was conducted to compare the mean scores of PSQI and SF-36 between renal transplant recipients and health people, and to compare the mean scores of SF-36 between good and poor sleepers of renal transplant recipients. Statistical significance was set at P < 0.05.
Results
Participant characteristics
A total of 210 questionnaires were distributed and all were returned (the return rate is 100%); of which 6 were incomplete and therefore invalid. Information from the remaining 204 patients was included in the analysis.
The characteristics of the 204 recipients are shown in Table 1. There were 127 (62.3%) men and 77 (37.7%) women. They were between 19 and 71 years of age, with average age 43.33±11.29. One-hundred and nine (53.4%) recipients were still employed. One hundred and sixty-four recipients were married. Twenty paid “out of pocket” for their healthcare. Forty-eight of the recipients had a middle school education or below, 55 attended high school or technical secondary school and 101 had a college degree or above. Thirty (14.7%) recipients received their graft from living donors. The range of time since transplantation was 3.23~204.67 months.
Table 1.
Socio-demographic characteristics of renal transplant recipients
| Variables | N (%) | Mean/SD | Range | |
|---|---|---|---|---|
| Age (years) | 43.33/11.29 | 19~71 | ||
| Gender | Male | 127 (62.3) | ||
| Female | 77 (37.7) | |||
| Employed | Yes | 109 (53.4) | ||
| Not | 95 (46.6) | |||
| Education | Middle school or below | 48 (23.5) | ||
| High school or technical secondary school | 55 (27.0) | |||
| College degree or above | 101 (49.5) | |||
| Marital status | Married | 164 (80.4) | ||
| Single/widowed/divorced | 40 (19.6) | |||
| Medical payment | By self | 20 (9.8) | ||
| Public service or medical insurance | 184 (90.2) | |||
| Family income | ≤ 3000 | 79 (38.7) | ||
| (CNY/month) | 3000~6000 | 75 (36.8) | ||
| > 6000 | 50 (24.5) | |||
| Donor | Deceased | 174 (85.3) | ||
| Living | 30 (14.7) | |||
| Duration after RT (month) | 39.22/30.76 | 3.23~204.67 | ||
Sleep quality
The mean (SD) global and component PSQI scores of recipients are shown in Table 2. The average global score was 5.81±3.52 (range = 0~17). Comparing the PSQI scores of renal transplant recipients with the scores of the Chinese general public (3.88±2.52) [21], the T value of Student’s t test was 7.823 (P < 0.05), indicating the sleep quality of renal transplant patients was lower than the Chinese general public. Fifty (24.5%) recipients were poor sleepers (global PSQI > 7), while the remaining 154 (75.5%) recipients were good sleepers. Ranking component PSQI scores in descending order, the seven dimensions were sleep latency, sleep disturbance, sleep quality, sleep efficiency, daytime dysfunction, sleep duration and use of sleep medications.
Table 2.
Renal transplantation recipients’ scores on the PSQI
| Range | Mean | SD | |
|---|---|---|---|
| Sleep quality | 0~3 | 1.11 | 0.78 |
| Sleep latency | 0~3 | 1.45 | 1.00 |
| Sleep duration | 0~3 | 0.41 | 0.77 |
| Sleep efficiency | 0~3 | 0.67 | 1.01 |
| Sleep disturbance | 0~3 | 1.36 | 0.56 |
| Use of sleep medications | 0~3 | 0.25 | 0.71 |
| Daytime dysfunction | 0~3 | 0.57 | 0.64 |
| Global score | 0~17 | 5.81 | 3.52 |
Health-related quality of life
The SF-36 scores of the 204 renal transplant recipients are shown in Table 3. The mean score of PCS was 47.57±6.71. Four dimensions scores of PCS ordered from high to low are physiological functioning, bodily pain, role-physical and general health. The mean score of MCS is 48.26±9.66. Four dimensions scores of MCS ordered from high to low are mental health, role-emotional, social functioning and vitality. Comparing the SF-36 scores of patients with the scores of residents in Sichuan province [23] with Student’s t test, there were significant differences in all SF-36 dimensions except mental health (t = 0.453, P > 0.05). Scores of patients in the remaining seven dimensions were statistically higher than Sichuan province residents’ scores (P < 0.05).
Table 3.
Comparisons of the mean scores of SF-36 between renal transplant recipients and Sichuan province norm
| Mean ± SD | t | P | ||
|---|---|---|---|---|
| PCS | recipients | 47.57±6.71 | ||
| Physical functioning | recipients | 80.15±15.41 | -9.709 | 0.000* |
| Sichuan Norm | 90.62±15.40 | |||
| Role-physical | recipients | 63.33±24.20 | -9.551 | 0.000* |
| Sichuan Norm | 79.51±34.70 | |||
| Bodily pain | recipients | 71.11±19.32 | -10.720 | 0.000* |
| Sichuan Norm | 85.61±18.37 | |||
| General health | recipients | 51.54±23.83 | -10.791 | 0.000* |
| Sichuan Norm | 69.55±21.32 | |||
| MCS | recipients | 48.26±9.66 | ||
| Vitality | recipients | 65.93±20.27 | -3.072 | 0.002* |
| Sichuan Norm | 70.29±17.07 | |||
| Social functioning | recipients | 71.26±25.14 | -8.858 | 0.000* |
| Sichuan Norm | 86.85±17.28 | |||
| Role-emotional | recipients | 71.98±23.54 | -2.714 | 0.007* |
| Sichuan Norm | 76.45±38.47 | |||
| Mental health | recipients | 73.21±17.70 | 0.453 | 0.651 |
| Sichuan Norm | 72.65±16.81 |
P < 0.05;
Sichuan Norm: SF-36 scores of residents in Sichuan province (Liu et al. 1996).
Relationship between sleep quality and HRQOL
The 204 recipients were divided into two groups by whether their global PSQI scores were more or less than 7. Sleep quality was categorized as ‘poor’ (global PSQI score > 7) or ‘good’ (global PSQI score ≤ 7). Figure 1 and Table 4 provide a comparison of the quality of life scores between two groups with Student’s t test. The differences of both PCS (P = 0.038 < 0.05) and MCS (P = 0.004 < 0.05) composite scores were statistically significant between two groups. The PCS and MCS scores of poor sleepers were significantly less than good sleepers’ scores. There were significant differences between two groups in dimensions scores of physical functioning, bodily pain, vitality, and mental health (P < 0.05).
Figure 1.
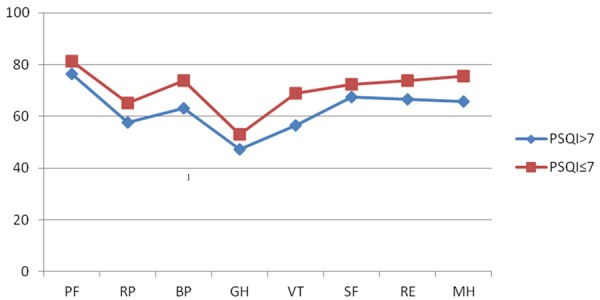
Comparisons of the mean scores of SF-36 between good and poor sleepers of PSQI. (PF, physical functioning; RP, role-physical; BP, bodily pain; GH, general health; VT, vitality; SF, social functioning; RE, role-emotional; MH, mental health).
Table 4.
Comparisons of the mean scores of SF-36 between good and poor sleepers of PSQI
| PSQI > 7 | PSQI ≤ 7 | t | P | |
|---|---|---|---|---|
| Physical functioning | 76.30±15.93 | 81.40±15.08 | -2.048 | 0.042* |
| Role-physical | 57.62±24.07 | 65.18±24.03 | -1. 930 | 0.055 |
| Bodily pain | 63.12±18.02 | 73.70±19.07 | -3.454 | 0.001* |
| General health | 47.30±27.02 | 52.92±22.63 | -1.328 | 0.188 |
| Vitality | 56.50±20.59 | 68.99±19.25 | -3.919 | 0.000* |
| Social functioning | 67.50±27.43 | 72.48±24.31 | -1.220 | 0.224 |
| Role-emotional | 66.67±21.89 | 73.70±23.86 | -1.847 | 0.066 |
| Mental health | 65.80±18.28 | 75.62±16.87 | -3.501 | 0.001* |
| PCS | 45.87±6.58 | 48.13±6.68 | -2.087 | 0.038* |
| MCS | 44.89±9.55 | 49.35±9.46 | -2.893 | 0.004* |
P < 0.05.
Discussion
Sleep quality of renal transplant recipients is lower than that of the Chinese general public
Findings suggested that sleep quality of renal transplant recipients was lower than the Chinese general public and sleep disorders were still highly prevalent after transplantation. About one quarter of the participants had poor sleep quality. The most common problems were the incubation period of fallen asleep extended, sleep disturbance and poor subjective sleep quality. Fewer recipients used sleep medications during the past month. This result is similar to that of Eryilmaz and colleagues’ study [24]. A poor quality of sleep was common among kidney transplantation recipients. Kachuee and colleagues found that 62% patients were poor sleepers (PSQI > 5) in their study [19]. The implication is that it is not easy for renal transplant recipients to fall asleep at night and their sleep is interrupted frequently for going to the bathroom, uncomfortable breathing, coughing or snoring loudly. Poor sleep quality may be related to the long-term use of immunosuppressive drugs after transplantation. Previous studies show that commonly used immunosuppressant tacrolimus (FK506) and cyclosporine (CsA) have effects that are toxic to the human central nervous system and that patients who use them in long term may suffer from some side effects such as headache, tremor and insomnia [25]. In addition, patients after renal transplantation may have some emotional-psychological problems such as fear of graft rejection, worries about graft function, worries about family economic burden and these factors may be reduced their sleep quality as well [26].
HRQOL of renal transplant recipients is lower than the Chinese general public
The results showed that PCS and MCS scores of renal transplant recipients were 47.57±6.71 and 48.26±9.66, respectively, both below 50. Besides, the SF-36 scores of renal transplant recipients were significantly lower than the scores of Sichuan residents in seven dimensions of the SF-36, meaning that the HRQOL of recipients after renal transplantation is apparently poor. This was similar to other research results. Many studies showed that quality of life of patients who underwent successful renal transplantation was apparently higher than that of patients utilizing dialysis [27], however, quality of life was still lower than the general public [13,28]. Fernández-Jimeniz and colleagues studied patients after renal transplantation and found that even a year later after transplantation patients’ SF-36 scores in physical functioning, social functioning and general health dimensions were still lower than the general public [29]. Various factors may affect the health-related quality of life of renal transplant recipients. Gentile and colleagues found that variables such as being female, being unemployed, having lower education, living alone, having a high BMI, diabetes, recent critical illness, or hospitalization, being non-compliance, or having a long history of dialysis, and treatment side effects decreased patients’ HRQOL score [30]. Medical staff should focus on the postoperative quality of life of renal transplant recipients, especially those who possess any of these characteristics.
Sleep quality of renal transplant recipients is related to their health-related quality of life
Many factors may affect renal transplant recipients’ quality of life. In this study, good sleepers’ HRQOL were significantly higher than those of poor sleepers, indicating that the good sleep quality in renal transplant recipients positively affects their quality of life. The better their sleep quality is, the better their quality of life is. This result is similar to that of Eryilmaz and colleagues’ study [24]. Kachuee also found poor sleepers group had a higher total medical comorbidity score, more bodily pain, poorer general mental health and less physical function on SF-36 than good sleepers group [19]. Another study found strong associations between quality of sleep and quality of life among dialysis patients, which both the mental field and physical field of QOL were associated with dimensions of sleep quality such as sleep disturbance, use of sleep medications, and daytime dysfunction [13]. Because of poor sleep quality at night, patients often felt tired and found it hard to concentrate in the daytime; their exercise decreased, finally affecting their physiological function. In addition to this, patients’ emotional and psychological well-being was affected by poor sleep. They were inclined to be irritable and depressed. The appropriate diagnosis and management of sleep disorders such as exercise training [31] and bright light therapy [32] may improve QOL in renal transplantation patients.
Limitations and recommendations
This study had certain limitations such as being a single-center cross-sectional survey. Additional longitudinal studies of quality of sleep and health-related quality of life in renal transplant recipients are needed to explore the influences of demographic characteristics such as age and gender, and transplant characteristics such as the source of the graft, and the time since transplantation.
Conclusion
The current study showed that renal transplant recipients’ sleep quality was lower than that of the Chinese general public. Poor sleep quality negatively influences the recipient’s quality of life.
Relevance to clinical practice
Sleep, as an important indicator of health, is conducive to renewing strength and restoring vigor. Excellent sleep quality is essential for the rehabilitation of patients after renal transplantation. Health professionals and patients’ family members should have a comprehensive understanding of patients’ sleep condition and take appropriate measures, like encouraging the rational use of sleep medicine and reducing the effects of factors that may interfere with sleep quality. These measures should improve renal transplant recipients’ sleep quality and quality of life.
Acknowledgements
This study was supported by the National Natural Science Foundation of China, grant number: 81171860. The authors declare that there is no conflict of interest. Special thanks to the participants in this study and the doctors who help the data collection.
Disclosure of conflict of interest
None.
References
- 1.Roumelioti ME, Argyropoulos C, Buysse DJ, Nayar H, Weisbord SD, Unruh ML. Sleep quality, mood, alertness and their variability in CKD and ESRD. Nephron Clin Pract. 2010;114:c277–c287. doi: 10.1159/000276580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ahmad S, Gupta M, Gupta R, Dhyani M. Prevalence and correlates of insomnia and obstructive sleep apnea in chronic kidney disease. N Am J Med Sci. 2013;5:641–646. doi: 10.4103/1947-2714.122306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Rabbat CG, Thorpe KE, Russell JD, Churchill DN. Comparison of mortality risk for dialysis patients and cadaveric first renal transplant recipients in Ontario, Canada. J Am Soc Nephrol. 2000;11:917–922. doi: 10.1681/ASN.V115917. [DOI] [PubMed] [Google Scholar]
- 4.Azar SA, Hatefi R, Talebi M. Evaluation of effect of renal transplantation in treatment of restless legs syndrome. Transplant Proc. 2007;39:1132–1133. doi: 10.1016/j.transproceed.2007.03.097. [DOI] [PubMed] [Google Scholar]
- 5.Kahvecioglu S, Yildiz D, Buyukkoyuncu N, Celik H, Tufan F, Kılıç AK, Gul B, Yildiz A. Effect of renal transplantation in restless legs syndrome. Exp Clin Transplant. 2014 doi: 10.6002/ect.2014.0163. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 6.Beecroft JM, Zaltzman J, Prasad R, Meliton G, Hanly PJ. Impact of kidney transplantation on sleep apnoea in patients with end-stage renal disease. Nephrol Dial Transplant. 2007;22:3028–3033. doi: 10.1093/ndt/gfm309. [DOI] [PubMed] [Google Scholar]
- 7.Lee JJ, Kim GS, Kim JA, Kim SJ, Kang JG, Kim GH, Jung HH. Improvement of sleep-related breathing disorder in patients with end-stage renal disease after kidney transplantation. Clin Transplant. 2011;25:126–130. doi: 10.1111/j.1399-0012.2009.01174.x. [DOI] [PubMed] [Google Scholar]
- 8.Novak M, Molnar MZ, Ambrus C, Kovacs AZ, Koczy A, Remport A, Szeifert L, Szentkiralyi A, Shapiro CM, Kopp MS, Mucsi I. Chronic insomnia in kidney transplant recipients. Am J Kidney Dis. 2006;47:655–665. doi: 10.1053/j.ajkd.2005.12.035. [DOI] [PubMed] [Google Scholar]
- 9.Burkhalter H, Brunner DP, Wirz-Justice A, Cajochen C, Weaver TE, Steiger J, Fehr T, Venzin RM, De Geest S. Self-reported sleep disturbances in renal transplant recipients. BMC Nephrol. 2013;14:220. doi: 10.1186/1471-2369-14-220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cameron JI, Whiteside C, Katz J, Devins GM. Differences in quality of life across renal replacement therapies: a meta-analytic comparison. Am J Kidney Dis. 2000;35:629–637. doi: 10.1016/s0272-6386(00)70009-6. [DOI] [PubMed] [Google Scholar]
- 11.Zhang Y, Liu J, Li L, Tao H, Chen J. Insomnia and its related factors in maintenance hemodialysis patients. Chinese Journal of Blood Purification. 2007;6:645–648. [Google Scholar]
- 12.Roebuck T, Solin P, Kaye DM, Bergin P, Bailey M, Naughton MT. Increased long-term mortality in heart failure due to sleep apnoea is not yet proven. Eur Respir J. 2004;23:735–740. doi: 10.1183/09031936.04.00060404. [DOI] [PubMed] [Google Scholar]
- 13.Iliescu EA, Coo H, McMurray MH, Meers CL, Quinn MM, Singer MA, Hopman WM. Quality of sleep and health-related quality of life in haemodialysis patients. Nephrol Dial Transplant. 2003;18:126–132. doi: 10.1093/ndt/18.1.126. [DOI] [PubMed] [Google Scholar]
- 14.Mucsi I, Molnar MZ, Rethelyi J, Vamos E, Csepanyi G, Tompa G, Barotfi S, Marton A, Novak M. Sleep disorders and illness intrusiveness in patients on chronic dialysis. Nephrol Dial Transplant. 2004;19:1815–1822. doi: 10.1093/ndt/gfh130. [DOI] [PubMed] [Google Scholar]
- 15.Mallon L, Broman JE, Hetta J. Relationship between insomnia, depression, and mortality: a 12-year follow-up of older adults in the community. Int Psychogeriatr. 2000;12:295–306. doi: 10.1017/s1041610200006414. [DOI] [PubMed] [Google Scholar]
- 16.Mallon L, Broman JE, Hetta J. Sleep complaints predict coronary artery disease mortality in males: a 12-year follow-up study of a middle-aged Swedish population. J Intern Med. 2002;251:207–216. doi: 10.1046/j.1365-2796.2002.00941.x. [DOI] [PubMed] [Google Scholar]
- 17.Lu J, Ma D, Tan Q, Diao Y. Reason and nursing intervention of patients after renal transplantation with sleep disorders. West China Medical Journal. 2009;24:1007–1009. [Google Scholar]
- 18.Pourfarziani V, Taheri S, Sharifi-Bonab MM, Mohammadzadeh M. Assessment of sleep disturbance in renal transplant recipients and associated risk factors. Saudi J Kidney Dis Transpl. 2010;21:433–437. [PubMed] [Google Scholar]
- 19.Kachuee H, Ameli J, Taheri S, Assari S, Riahipour F, Khedmat H, Saadat AR. Sleep quality and its correlates in renal transplant patients. Transplant Proc. 2007;39:1095–1097. doi: 10.1016/j.transproceed.2007.04.001. [DOI] [PubMed] [Google Scholar]
- 20.Buysse DJ, Reynolds CF 3rd, Monk TH, Berman SR, Kupfer DJ. The Pittsburgh Sleep Quality Index: a new instrument for psychiatric practice and research. Psychiatry Research. 1989;28:193–213. doi: 10.1016/0165-1781(89)90047-4. [DOI] [PubMed] [Google Scholar]
- 21.Liu X, Tang M, Hu L, Wang A, Wu H, Zhao G, Gao C, Li W. Reliability and validity of the Pittsburgh sleep quality index. Chin J Psychiatry. 1996;29:103–107. [Google Scholar]
- 22.Ware JE Jr, Sherbourne CD. The MOS 36-item short-form health survey (SF-36). I. Conceptual framework and item selection. Med Care. 1992;30:473–483. [PubMed] [Google Scholar]
- 23.Li N, Liu C, Li J, Ren X. The Norms of SF-36 scale scores in urban and rural residents of Sichuan province. Journal of West China University of Medical Sciences. 2001;32:43–47. [PubMed] [Google Scholar]
- 24.Eryilmaz MM, Ozdemir C, Yurtman F, Cilli A, Karaman T. Quality of sleep and quality of life in renal transplantation patients. Transplant Proc. 2005;37:2072–2076. doi: 10.1016/j.transproceed.2005.03.084. [DOI] [PubMed] [Google Scholar]
- 25.Taylor AL, Watson CJ, Bradley JA. Immunosuppressive agents in solid organ transplantation: Mechanisms of action and therapeutic efficacy. Crit Rev Oncol Hematol. 2005;56:23–46. doi: 10.1016/j.critrevonc.2005.03.012. [DOI] [PubMed] [Google Scholar]
- 26.Molnar MZ, Novak M, Mucsi I. Sleep disorders and quality of life in renal transplant recipients. Int Urol Nephrol. 2009;41:373–382. doi: 10.1007/s11255-009-9527-z. [DOI] [PubMed] [Google Scholar]
- 27.Emmanuel J, Unni VN, Deepa AR, Aboobacker S. Evaluation of quality of life in hemodialysis and renal transplant patients. Int J Pharm and Health Sci. 2010;1:77–83. [Google Scholar]
- 28.Dong J, Gu P, Chen L. Research progress on quality of life of patients undergoing renal transplant. Chinese General Nursing. 2009;7:169–171. [Google Scholar]
- 29.Fernández-Jiménez E, Pérez-San-Gregorio MA, Martín-Rodríguez A, Pérez-Bernal J, Izquierdo G. Evolution of quality of life in renal transplant recipients and patients with multiple sclerosis: a follow-up study. Transplant Proc. 2013;45:3616–3619. doi: 10.1016/j.transproceed.2013.10.027. [DOI] [PubMed] [Google Scholar]
- 30.Gentile S, Beauger D, Speyer E, Jouve E, Dussol B, Jacquelinet C, Briançon S. Factors associated with health-related quality of life in renal transplant recipients: results of a national survey in France. Health Qual Life Outcomes. 2013;11:88. doi: 10.1186/1477-7525-11-88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Pooranfar S, Shakoor E, Shafahi M, Salesi M, Karimi M, Roozbeh J, Hasheminasab M. The Effect of Exercise Training on Quality and Quantity of Sleep and Lipid Profile in Renal Transplant Patients: A Randomized Clinical Trial. Int J Organ Transplant Med. 2014;5:157–165. [PMC free article] [PubMed] [Google Scholar]
- 32.Burkhalter H, Wirz-Justice A, Denhaerynck K, Fehr T, Steiger J, Venzin RM, Cajochen C, Weaver TE, De Geest S. The effect of bright light therapy on sleep and circadian rhythms in renal transplant recipients: a pilot randomized, multicentre wait-list controlled trial. Transpl Int. 2015;28:59–70. doi: 10.1111/tri.12443. [DOI] [PubMed] [Google Scholar]


