Abstract
Background: Concurrent chemoradiotherapy (CCRT) plays an important role in multimodality therapy for non-small cell lung cancer. However, esophagitis often develops as a complication of CCRT, causing treatment delays and reducing the patient’s quality of life. We examined the efficacy of polaprezinc (PZ), zinc L-carnosine used for the therapy of gastric ulcer, against the onset of esophagitis caused by CCRT for lung cancer. Patients and Methods: Patients who concurrently underwent chemotherapy with carboplatin and paclitaxel and thoracic radiotherapy at Gifu University Hospital during a period of January 2011 and May 2015 were the subjects of the present study. Patients received a mixture of sodium alginate solution and aluminum-magnesium hydroxide gel with (PZ group) or without (control group) PZ for prevention of radiation esophagitis. Results: PZ significantly inhibited the development of grade ≥2 radiation esophagitis (HR 0.397, 95% confidence interval, 0.160-0.990; P=0.047). In addition, PZ lowered the incidence of grade ≥2 esophagitis at the time point of 40 Gy irradiation (26.3% versus 63.2%, P=0.05). However, there were no significant differences in the incident rates of other adverse events associated with chemoradiotherapy between the PZ group and control group. Moreover, PZ had no significant influence on the tumor response rate. Conclusion: PZ significantly retarded the development as well as the incidence of grade ≥2 esophagitis without affecting the tumor response.
Keywords: Polaprezinc, radiation esophagitis, concurrent chemoradiotherapy, non-small cell lung cancer
Introduction
In the radiation therapy for the treatment of lung cancer, the mediastinum is irradiated in most cases, which often leads to the development of radiation esophagitis. As compared with radiation therapy alone or sequential chemotherapy and irradiation, concurrent chemoradiotherapy (CCRT) has been reported to result in a greater incidence of side effects, especially of radiation esophagitis during the acute phase [1,2]. The radiation esophagitis not only leads to interruption or cessation of treatment, but also causes intense pain and dysphagia. As a result, it may aggravate the nutritional status due to the decrease in food intake, thereby causing considerable deterioration in the patient’s QOL [3].
Mucosal edema, infiltration of inflammatory cells and ulcerations have been reported as histological findings of radiation esophagitis during the acute phase [4]. Animal experiments conducted on mice have shown that, in radiation esophagitis, there is an increased cytokine expression of m-RNAs such as IL-1, TNFα, and IFN-γ. The increased expression of cytokines is associated with the apoptosis of basal cells under the esophageal mucosa, DNA damage, micro-ulcers and esophagitis. In addition, the administration of superoxide dismutase, an enzyme responsible for the degradation of reactive oxygen species produced in the cells, has been reported to inhibit esophagitis and apoptosis [5]. Therefore, it is suggested that the inhibition of reactive oxygen species in the esophagus during radiation therapy is a useful means of preventing radiation esophagitis.
To date, various interventions, including improvement of dietary patterns or oral administration of mucoprotective agents, analgesics, antispasmodics, mucosal anesthetics or antioxidants, have been investigated for prevention of radiation esophagitis [6,7]. However, none of them has been proven to be effective. Polaprezinc (PZ) is zinc containing compound (zincL-carnosine) and used for the treatment of gastric ulcers. Several mechanisms underlying the anti-ulcer effect of PZ have been postulated: a direct protective action on the gastrointestinal mucosa, a wound healing-promoting effect, and a free radical scavenging effect. Moreover, it has been shown in animal experiments that PZ is effective in prevention of oral mucositis [8,9]. We previously found that PZ suspension with sodium alginate is highly effective for prevention of oral mucositis caused by the chemoradiotherapy in patients with head and neck cancers [10].
In our hospital, a combination of sodium alginate solution and aluminum-magnesium hydroxide gel was used conventionally for prevention of radiation esophagitis. In the present study, the effect of PZ in combination with sodium alginate solution and aluminum-magnesium hydroxide gel on the development of radiation esophagitis was evaluated by a retrospective chart review.
Patients and methods
Patients
Patients with non-small cell lung cancer (NSCLC) who received a weekly administration of carboplatin (CBDCA) and paclitaxel (PTX) combination chemotherapy with a concurrent thoracic radiotherapy at the Department of Respiratory Medicine, Gifu University Hospital between January 2011 and May 2015, were analyzed retrospectively. A group of patients who did not receive PZ between January 2011 and December 2012 were included as the control group (19 patients), and a group of patients who were concurrently treated with PZ between January 2013 and May 2015 were included as the PZ group (19 patients). This study was carried out after receiving approval from the ethical review board of Gifu University Graduate School of Medicine (approval number 26-57).
Chemoradiotherapy
Thoracic radiotherapy was delivered once daily fractions of 2 Gy, five fractions a week. Irradiation was conducted using a 10-MV X-ray linear accelerator as the medical therapeutic device, consisting of anterior-posterior opposing portal irradiation. In principle, the irradiation field was centered mainly on the primary tumor. When ipsilateral hilar, ipsilateral mediastinal, or contralateral mediastinal lymph node metastases were present, these were included in the irradiation field. In patients with supraclavicular lymph node metastasis, the metastasis was also included in the irradiation field. The medulla spinalis was excluded from the therapy field with a 40 Gy radiation dose.
Chemotherapy consisted of CBDCA (area under the plasma concentration-time curve [AUC] 2) and PTX (40 mg/m2), which were administered through intravenous drip infusion on a weekly basis.
Prophylactic medication regimen for esophagitis
In the control group, patients received sodium alginate solution (Alloid-G® 5%, Kaigen Pharmaceutical Co., Osaka, Japan), (20 ml per dose), which was administered orally before each meal, as well as aluminum-magnesium hydroxide gel (Malfa®, Ono Pharmaceutical Co., Osaka, Japan) (10 ml per dose, three times per day), which was administered orally between meals. In the PZ group, patients received a mixture of 60 ml sodium alginate solution and 150 mg PZ (Promac granules®15%, ZERIA Pharmaceutical Co., Tokyo, Japan), which was administered orally with doses divided into 3 times per day before every meal, as well as an aluminum-magnesium hydroxide gel (10 ml per dose, administered orally 3 times a day between meals). The oral administration of the prophylactic medication was started on the irradiation day, and was continued until the completion of radiation therapy.
Evaluation of toxicity and response
The grade of esophagitis was assessed according to the Common Terminology Criteria for Adverse Events (CTCAE) version 4.0 as follows. Grade 1: symptoms are absent; only clinical findings or laboratory findings are present; and medical treatment is not required, Grade 2: symptoms are present; there is an apparent deterioration of feeding and swallowing functions; and medical treatment is required, Grade 3: feeding and swallowing functions are severely reduced, and there is need for enteral tube feeding/total parenteral nutrition (TPN)/hospitalization, Grade 4: the patient’s condition is life-threatening. Our evaluations of the symptoms of esophagitis and the amount of dietary intake were based on the written records of physicians, nurses, and pharmacists (as stored in electronic medical records).
Hematological toxicities such as neutropenia, leukopenia, anemia, thrombocytopenia, and febrile neutropenia and non-hematological adverse events, including nausea and vomiting, constipation, diarrhea, peripheral neuropathy, and malaise, were also evaluated on the basis of CTCAE version 4.0 from the laboratory test values and the records left by physicians, nurses, and pharmacists in the patients’ electronic medical records.
Therapeutic efficacy was evaluated on the basis of the Response Evaluation Criteria in Solid Tumors (RECIST) version 1.1. The response rate was evaluated at one month after the completion of CCRT.
Statistical analysis
Data were analyzed using the Statistics Program for Social Science for Windows (SPSS II, version 11, SPSS Inc.). Patients’ data were compared between PZ group and non PZ group by χ2-test for non-parametric data such as gender, incidence of esophagitis and other side effects, or by Mann-Whitney U-test for non-parametric data, including age, clinical stage, histological type, the number of chemotherapy courses, and response rate. Parametric data such as dosimetric parameters and relative dose intensity of chemotherapy were compared between the two groups by Student’s t-test. The time course of development of radiation esophagitis was compared by using the Mantel-Cox log rank test. P-value less than 0.05 was considered as the level of statistical significance.
Results
Patients’ characteristics
Characteristics of patients are shown in Table 1. Both the control and the PZ groups consisted of 19 patients. All of them had an Eastern Cooperative Oncology Group performance status of 0 or 1. No significant difference was found between the two groups in terms of gender, age, clinical stage and histological type. Furthermore, there was no significant difference in any of the following parameters: the total dose of radiation, percentage of esophagus volume treated to ≥40 Gy (V40), the maximum (Dmax) and mean doses (Dmean) delivered to esophagus, the number of chemotherapy courses, or the relative dose intensity between the two study groups.
Table 1.
Patients’ characteristics
| Control n = 19 | Polaprezinc n = 19 | P-value | |||
|---|---|---|---|---|---|
|
|
|||||
| No. | % | No. | % | ||
| Gender | 0.421 | ||||
| Male | 13 | 68.4 | 16 | 84.2 | |
| Female | 6 | 31.6 | 3 | 15.8 | |
| Age (years) | 0.642 | ||||
| Median (Range) | 63.7 | (45-83) | 64.4 | (43-79) | |
| Clinical stage | 0.172 | ||||
| IIA | 1 | 5.3 | 1 | 5.3 | |
| IIB | 2 | 10.5 | 0 | 0.0 | |
| IIIA | 6 | 31.6 | 13 | 68.4 | |
| IIIB | 4 | 21.1 | 5 | 26.3 | |
| IV | 6 | 31.6 | 0 | 0.0 | |
| Histology | 0.302 | ||||
| Adenocarcinoma | 7 | 36.8 | 10 | 52.6 | |
| Squamous cell carcinoma | 9 | 47.4 | 8 | 42.1 | |
| Others | 3 | 15.8 | 1 | 5.3 | |
| Radiation doses (Gy) | 0.383 | ||||
| Mean (Range) | 55.7 (40.0-63.0) | 52.8 (40.0-70.0) | |||
| Number of chemotherapy courses administered | 0.542 | ||||
| Mean (Range) | 5.2 (3-6) | 5.5 (4-8) | |||
| Relative dose intensity (Mean, %) | |||||
| Carboplatin | 94.2 | 95.8 | 0.653 | ||
| Paclitaxel | 92.1 | 90.9 | 0.723 | ||
| Dosimetric parameters | |||||
| Dmax (Gy) | 49.8 ± 11.5 | 51.5 ± 13.3 | 0.773 | ||
| Dmean (Gy) | 17.7 ± 9.0 | 19.3 ± 8.7 | 0.603 | ||
| V40 (%) | 21.8 ± 20.0 | 20.8 ± 19.6 | 0.883 | ||
Data on the dosimetric parameters represent the mean ± SD.
χ2-test;
Mann-Whitney U-test;
t-test.
Effect of PZ on radiation esophagitis
As shown in Figure 1, the development of grade ≥2 radiation esophagitis was significantly retarded by PZ (HR, 0.397; 95% confidence interval [CI], 0.160-0.990; P=0.047). The median onset of grade ≥2 esophagitis was 21.0 days (95% CI: 16.7-25.3) in the control group, while not reached in the PZ group. In addition, as shown in Table 2, PZ inhibited the incidence of grade ≥2 radiation esophagitis at the time point of 40 Gy irradiation (26.3% versus 63.2%, P=0.05), although the difference in the incidence of radiation esophagitis was not significant at the time point of 60 Gy irradiation (42.1% versus 63.2%, P=0.33).
Figure 1.
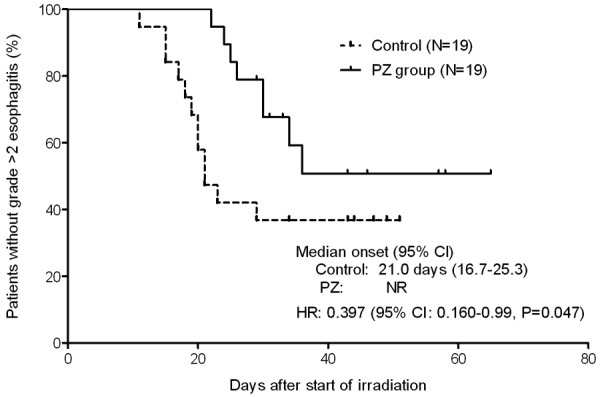
Kaplan-Meier plots showing the effect of PZ on the development of grade ≥2 radiation esophagitis in patients with NSCLC who received chemoradiotherapy. The onset of radiation esophagitis was compared between the two groups by Mantel-Cox log rank test.
Table 2.
Effect of PZ on the incidence of radiation esophagitis at the point of 40 Gy irradiation or 60 Gy irradiation in NSCLC patients who received chemoradiotherapy
| 40 Gy | 60 Gy | |||||
|---|---|---|---|---|---|---|
|
|
|
|||||
| Control | Polaprezinc | P-value | Control | Polaprezinc | P-value | |
| Grade 0 | 5 (26.3) | 3 (15.8) | 3 (15.8) | 2 (10.5) | ||
| Grade 1 | 2 (10.5) | 11 (57.9) | 4 (21.1) | 9 (47.4) | ||
| Grade 2 | 11 (57.9) | 5 (26.3) | 11 (57.9) | 8 (42.1) | ||
| Grade 3 | 1 (5.3) | 0 (0) | 1 (5.3) | 0 (0) | ||
| Grade ≥2 | 12 (63.2) | 5 (26.3) | 0.05 | 12 (63.2) | 8 (42.1) | 0.33 |
Data were statistically compared by χ2-test.
Effect of PZ on other adverse events
The incidence rates of grade ≥2 other adverse events are shown in Table 3. No significant differences in the incidence of hematological or non-hematological adverse events were observed between the two groups.
Table 3.
Comparison of incidence of grade >2 other side effects between PZ group and control group
| Toxicity (Grade ≥2) | Control | Polaprezinc | P-value | ||
|---|---|---|---|---|---|
|
|
|
||||
| No. | % | No. | % | ||
| Hematological toxicities | |||||
| Neutropenia | 5 | 26 | 5 | 26 | 0.72 |
| Leukopenia | 15 | 79 | 14 | 74 | 0.72 |
| Anemia | 4 | 21 | 2 | 11 | 0.66 |
| Thrombocytopenia | 1 | 5 | 1 | 5 | 1.00 |
| Febrile neutropenia | 0 | 0 | 0 | 0 | 1.00 |
| Non-hematological toxicities | |||||
| Nausea | 3 | 16 | 4 | 21 | 1.00 |
| Vomiting | 0 | 0 | 0 | 0 | 1.00 |
| Constipation | 9 | 47 | 8 | 42 | 1.00 |
| Diarrhea | 1 | 5 | 1 | 5 | 1.00 |
| Peripheral sensory neuropathy | 1 | 5 | 0 | 0 | 1.00 |
| Malaise | 0 | 0 | 1 | 5 | 1.00 |
Data were statistically compared by X2-test.
Effect of PZ on the tumor response
As shown in Table 4, tumor response rate (partial response) was not significantly different between the two groups, although the response rate tended to be higher in PZ group as compared with the control group (68.4% for control group versus 94.7% for PZ group, P=0.094). Therefore, it is unlikely that the concurrent use of PZ for the prevention of radiation esophagitis interferes with the therapeutic efficacy.
Table 4.
Comparison of therapeutic efficacy between PZ group and control group
| Response | Control | Polaprezinc | P-value | ||
|---|---|---|---|---|---|
|
|
|
||||
| No. | % | No. | % | ||
| PR | 13 | 68.4 | 18 | 94.7 | 0.094 |
| SD | 6 | 31.6 | 1 | 5.3 | |
| PD | 0 | 0.0 | 0 | 0.0 | |
PR: partial response; SD: stable disease; PD: progressive disease. Data were statistically compared by X2-test.
Discussion
Chemoradiotherapy including platinum compound is recommended for patients who have locally advanced NSCLC [11]. CCRT has a stronger combined effect than sequential chemoradiotherapy [12], although the therapy has some drawbacks from a safety point of view, particularly high incident rate of esophagitis [2]. In Japan, weekly CBDCA +PTX chemotherapy regimen combined with concurrent radiation therapy is one of the standard treatments for unresectable stage III NSCLC [13]. In our hospital, this therapeutic approach is primarily used when conducting CCRT. In addition, preoperative chemoradiotherapy is among the treatment options for patients with clinical stage IIIA (N2) disease and are eligible for lobectomy [14,15]. Mascarenhas et al. [16] reported in lung cancer patients who undertook radiation therapy that the incidence of radiation esophagitis is 45% after irradiation of 30-40 Gy. In addition, Suzuki et al. [3] showed that the incidence of all grades esophagitis is 77.4% in patients receiving radiation therapy for lung cancer, in which the average dose of radioactivity is 29.7 Gy at the time of the onset of esophagitis and that the grade ≥2 symptom occurs in 63% of patients. In generally consistent with the data reported by Suzuki et al. [3], the incidence of grade ≥2 esophagitis was 63.2% and the median onset of grade ≥2 symptom was 21 days in the control group of the present study.
Radiation esophagitis was often neglected in the past, perhaps because there have been virtually no fatal cases in the radioactive esophagitis as a complication of radiation therapy. Indeed, no treatment has been established for radiation esophagitis. However, pain during swallowing and dysphagia due to esophagitis may sometimes lead to the difficulty in feeding, which reduces the patient’s QOL. In severe cases, esophagitis leads to the cessation or discontinuation of the therapy. Machtay et al. [17] demonstrated in stage III and selected medically inoperable Stage II NSCLC patients who received CCRT that the prolonged treatment due to the occurrence of severe radiation esophagitis is associated with a poor survival outcome (HR, 1.02; 95% CI, 1.003-1.03; P=0.01 for overall survival; HR, 1.02; 95% CI, 1.002-1.02; P=0.02 for progression-free survival). Therefore, the prevention of esophagitis is particularly important from the viewpoint of the avoidance of treatment delay or cessation in addition to the improvement of patient’s QOL.
It was notable that the development of grade ≥2 radiation esophagitis was significantly inhibited by PZ (HR, 0.397; 95% CI, 0.160-0.990; P=0.047). The median onset was 21.0 days for the control group, while the value did not reach in the PZ group. Moreover, PZ reduced the incidence of grade >2 esophagitis at the time point of 40 Gy but not 60 Gy, thereby suggesting that PZ causes a delay in the onset of grade ≥2 esophagitis.
Zhang et al. [2] reported that dosimetric parameters such as Vmax, Vmean and V40 could predict the incidence of grade ≥2 radiation esophagitis in NSCLC patients receiving CCRT. However, in the present study, no significant differences in Vmax, Vmean or V40 were found between the PZ group and the control group.
PZ is a zinc-containing compound (zinc L-carnosine) and has been used for the treatment of gastric ulcer. The agent acts directly on the damaged area of the gastric mucosa. PZ exerts a direct cytoprotective action through its anti-oxidative effect and membrane-stabilizing action without affecting the secretion of gastric acid. In addition, PZ has a wound healing-promoting action [18]. Preclinical studies have demonstrated that PZ is effective in preventing the incidence of oral mucositis in hamster cheek pouch model induced by fluorouracil or acetic acid [8,9]. We previously reported in patients with head and neck cancer that oral rinse followed by ingestion of PZ-containing sodium alginate solution is dramatically effective for prevention of severe oral mucositis associated with chemoradiotherapy [10]. On the other hand, it is likely that radiation esophagitis is based on the development of mucosal inflammation as in the case of radiation-induced oral mucositis. Sonis et al. [19] reported that there are following 5 stages in the development of mucosal damage in response to the chemoradiotherapy: 1) the emergence of reactive oxygen species, 2) the activation of nuclear factor-kappa B, which is followed by the production of inflammatory cytokines such as TNF-α, IL-1β and IL-6, 3) the synthesis of ceramide and subsequent activation of caspases, 4) development of inflammation and ulceration, and 5) healing. It has also been reported in animal model of gastric mucosal damage that PZ protects gastric mucosa by inhibiting the expression of TNF-α [20,21]. Taken together, it is suggested that PZ inhibits the development of radiation esophagitis possibly via inhibition of reactive oxygen species and cytokines such as TNF-α.
On the other hand, it has been demonstrated that zinc itself possesses the membrane-stabilizing action [22], anti-oxidative effect [23,24], and wound healing-promoting effect [25]. It has also been shown that zinc alone reveals an inhibitory effect on the radiation stomatitis [26]. Moreover, L-carnosine has been reported to possess a healing-promoting effect and immunoregulatory activity [27,28]. PZ exerts its pharmacological actions after attachment to the gastric mucosa in the stomach, followed by the dissociation into zinc and L-carnosine, and subsequent penetration into the mucosal cells [29]. Yoneta et al. [30] compared the pharmacological effect of zinc with PZ and showed that PZ is more potent in revealing pharmacological actions and longer in duration of the attachment to the mucosa, while the tissue irritating and corrosive actions are far less potent, than zinc [30]. Therefore, both zinc and L-carnosine appear to be requisite to fulfill pharmacological actions of PZ.
In our study, the sodium alginate was used in combination with PZ, resulting in high viscosity. Sodium alginate is known to possess tissue-protective action. Although sodium alginate has long been used for the treatment of esophagitis and stomatitis associated with radiotherapy, its efficacy in the prevention of esophagitis still remains unclear [31]. In addition, inclusion of sodium alginate into PZ may allow PZ to attach to the esophageal mucosa for a longer period because of the high viscosity of sodium alginate. This could potentially increase the prophylactic efficacy of PZ against esophagitis. Furthermore, aluminum-magnesium hydroxide gel is effective against gastroduodenal ulcers, gastritis, and upper gastrointestinal tract dysfunction. However, few evidence has been accumulated suggesting that aluminum-magnesium hydroxide gel has a preventive effect against radiation esophagitis, although this solution is reportedly effective in reducing the reflux esophagitis due to gastric acid [32].
In the present study, PZ had no influence on the incidence of other adverse events. Moreover, the tumor response rate was not changed or even higher in PZ group as compared with the control group, thereby indicating that PZ has little, if any, influence on the clinical outcome of the chemoradiotherapy.
The present study has several limitations: this was a single center retrospective study with a small number of patients. Therefore, a prospective randomized control study on a large scale is warranted to demonstrate the prophylactic effect of PZ against esophagitis induced by chemoradiotherapy in patients with NSCLC.
In conclusion, PZ in combination with sodium alginate and aluminum-magnesium hydroxide gel was found to be highly effective in suppressing the development of radiation esophagitis without affecting the tumor response rate in NSCLC patients receiving CCRT. Therefore, PZ may become a useful supportive option that helps to prevent radiation esophagitis from the viewpoints of improvement of patient’s QOL and maintenance of the treatment course without dose reduction.
Acknowledgements
This study was supported in part by a Grant-in-Aid for Scientific Research (26929001) from the Ministry of Education, Science, Sport and Culture, Japan.
Disclosure of conflict of interest
None.
References
- 1.Palma DA, Senan S, Oberije C, Belderbos J, de Dios NR, Bradley JD, Barriger RB, Moreno-Jiménez M, Kim TH, Ramella S, Everitt S, Rengan R, Marks LB, De Ruyck K, Warner A, Rodrigues G. Predicting esophagitis after chemoradiation therapy for non-small cell lung cancer: an individualpatient data meta-analysis. Int J Radiat Oncol Biol Phys. 2013;87:690–696. doi: 10.1016/j.ijrobp.2013.07.029. [DOI] [PubMed] [Google Scholar]
- 2.Zhang Z, Xu J, Zhou T, Yi Y, Li H, Sun H, Huang W, Wang D, Li B, Ying G. Risk factors of radiation-induced acute esophagitis in non-small celllung cancer patients treated with concomitant chemoradiotherapy. Radiat Oncol. 2014;9:54. doi: 10.1186/1748-717X-9-54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Suzuki M, Kitamura S. Radiation esophagitis in the treatment for lung cancer. The Japan Lung Cancer Society. 1998;38:807–813. [Google Scholar]
- 4.Hishikawa Y, Mitsunobu M, Uematsu K, Miura T. Histological findings of esophageal injury induced by intracavitary irradiation. Radiat Med. 1985;3:112–117. [PubMed] [Google Scholar]
- 5.Epperly MW, Gretton JA, DeFilippi SJ, Greenberger JS, Sikora CA, Liggitt D, Koe G. Modulation of radiation-induced cytokine elevation associated with esophagitis and esophageal stricture by manganese superoxide dismutase-plasmid/liposome (SOD2-PL) gene therapy. Radiat Res. 2001;155:2–14. doi: 10.1667/0033-7587(2001)155[0002:morice]2.0.co;2. [DOI] [PubMed] [Google Scholar]
- 6.Vidal-Casariego A, Calleja-Fernández A, Ballesteros-Pomar MD, Cano-Rodríguez I. Efficacy of glutamine in the prevention of oral mucositis and acute radiation-induced esophagitis: a retrospective study. Nutr Cancer. 2013;65:424–429. doi: 10.1080/01635581.2013.765017. [DOI] [PubMed] [Google Scholar]
- 7.Northway MG, Libshitz HI, Osborne BM, Feldman MS, Mamel JJ, West JH, Szwarc IA. Radiation esophagitis in the opossum: radioprotection with indomethacin. Gastroenterology. 1980;78:883–892. [PubMed] [Google Scholar]
- 8.Mitsuhashi H, Suemaru K, Li B, Cui R, Araki H. Evaluation of topical external medicine for 5-fluorouracil-induced oral mucositis in hamsters. Eur J Pharmacol. 2006;551:152–155. doi: 10.1016/j.ejphar.2006.09.005. [DOI] [PubMed] [Google Scholar]
- 9.Katayama S, Nishizawa K, Hirano M. Effect of polaprezinc on healing of acetic acid-induced stomatitis in hamsters. J Pharm Pharm Sci. 2000;3:114–117. [PubMed] [Google Scholar]
- 10.Watanabe T, Ishihara M, Matsuura K, Mizuta K, Itoh Y. Polaprezinc prevents oral mucositis associated with radiochemotherapy in patients with head and neck cancer. Int J Cancer. 2010;127:1984–1990. doi: 10.1002/ijc.25200. [DOI] [PubMed] [Google Scholar]
- 11.Marino P, Preatoni A, Cantoni A. Randomized trials of radiotherapy alone versus combined chemotherapy and radiotherapy in stages IIIa and IIIbnonsmall cell lung cancer. A meta-analysis. Cancer. 1995;76:593–601. doi: 10.1002/1097-0142(19950815)76:4<593::aid-cncr2820760409>3.0.co;2-n. [DOI] [PubMed] [Google Scholar]
- 12.Aupérin A, Le Péchoux C, Rolland E, Curran WJ, Furuse K, Fournel P, Belderbos J, Clamon G, Ulutin HC, Paulus R, Yamanaka T, Bozonnat MC, Uitterhoeve A, Wang X, Stewart L, Arriagada R, Burdett S, Pignon JP. Meta-analysis of concomitant versus sequential radiochemotherapy in locally advanced non-small-cell lung cancer. J. Clin. Oncol. 2010;28:2181–2190. doi: 10.1200/JCO.2009.26.2543. [DOI] [PubMed] [Google Scholar]
- 13.Yamamoto N, Nakagawa K, Nishimura Y, Tsujino K, Satouchi M, Kudo S, Hida T, Kawahara M, Takeda K, Katakami N, Sawa T, Yokota S, Seto T, Imamura F, Saka H, Iwamoto Y, Semba H, Chiba Y, Uejima H, Fukuoka M. Phase III Study Comparing second and third generation regimens with concurrent thoracic radiotherapy in patients with unresectable stage III non-small-cell lung cancer. J. Clin. Oncol. 2010;28:3739–3745. doi: 10.1200/JCO.2009.24.5050. [DOI] [PubMed] [Google Scholar]
- 14.Albain KS, Swann RS, Rusch VW, Turrisi AT 3rd, Shepherd FA, Smith C, Chen Y, Livingston RB, Feins RH, Gandara DR, Fry WA, Darling G, Johnson DH, Green MR, Miller RC, Ley J, Sause WT, Cox JD. Radiotherapy plus chemotherapy with or without surgical resection for stage III non-small-cell lung cancer: a phase III randomised controlled trial. Lancet. 2009;374:379–386. doi: 10.1016/S0140-6736(09)60737-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Katakami N, Tada H, Mitsudomi T, Kudoh S, Senba H, Matsui K, Saka H, Kurata T, Nishimura Y, Fukuoka M. A phase 3 study of induction treatment with concurrent chemoradiotherapy versus chemotherapy before surgery in patients with pathologically confirmed N2 stage IIIA non-small cell lung cancer (WJTOG9903) Cancer. 2012;118:6126–6135. doi: 10.1002/cncr.26689. [DOI] [PubMed] [Google Scholar]
- 16.Mascarenhas F, Silvestre ME, Sá da Costa M, Grima N, Campos C, Chaves P. Acute secondary effects in the esophagus in patients undergoing radiotherapy for carcinoma of the lung. Am J Clin Oncol. 1989;12:34–40. doi: 10.1097/00000421-198902000-00008. [DOI] [PubMed] [Google Scholar]
- 17.Machtay M, Hsu C, Komaki R, Sause WT, Swann RS, Langer CJ, Byhardt RW, Curran WJ. Effect of overall treatment time on outcomes after concurrent chemoradiation for locally advanced non-small-cell lung carcinoma: analysis of the Radiation Therapy Oncology Group (RTOG) experience. Int J Radiat Oncol Biol Phys. 2005;63:667–671. doi: 10.1016/j.ijrobp.2005.03.037. [DOI] [PubMed] [Google Scholar]
- 18.Yoshikawa T, Naito Y, Tanigawa T, Yoneta T, Kondo M. The antioxidant properties of a novel zinc-carnosine chelate compound, N-(3-aminopropionyl)-L-histidinato zinc. Biochim Biophys Acta. 1991;1115:15–22. doi: 10.1016/0304-4165(91)90005-2. [DOI] [PubMed] [Google Scholar]
- 19.Sonis ST, Elting LS, Keefe D, Peterson DE, Schubert M, Hauer-Jensen M, Bekele BN, Raber-Durlacher J, Donnelly JP, Rubenstein EB. Mucositis Study Section of the Multinational Association for Supportive Care in Cancer; International Society for Oral Oncology. Perspectives on cancer therapy-induced mucosal injury: pathogenesis, measurement, epidemiology, and consequences for patients. Cancer. 2004;100:1995–2025. doi: 10.1002/cncr.20162. [DOI] [PubMed] [Google Scholar]
- 20.Naito Y, Yoshikawa T, Yagi N, Matsuyama K, Yoshida N, Seto K, Yoneta T. Effects of polaprezinc on lipid peroxidation, neutrophil accumulation, and TNF-alphaexpression in rats with aspirin-inducedgastric mucosal injury. Dig Dis Sci. 2001;46:845–851. doi: 10.1023/a:1010716804594. [DOI] [PubMed] [Google Scholar]
- 21.Shimada T, Watanabe N, Ohtsuka Y, Endoh M, Kojima K, Hiraishi H, Terano A. Polaprezinc down-regulates proinflammatory cytokine-induced nuclearfactor-kappaBactivation and interleukin-8 expression in gastric epithelial cells. J Pharmacol Exp Ther. 1999;291:345–352. [PubMed] [Google Scholar]
- 22.Ludwing J, Chvapil M. Reversible stabilization of liver lysosomes by zinc ions. J Nutr. 1980;110:945–953. doi: 10.1093/jn/110.5.945. [DOI] [PubMed] [Google Scholar]
- 23.Girotti AW, Thomas JP, Jordan JE. Inhibitory effect of zinc (II) on free radical lipid peroxidation in erythrocyte membranes. J Free Radic Biol Med. 1985;1:395–401. doi: 10.1016/0748-5514(85)90152-7. [DOI] [PubMed] [Google Scholar]
- 24.Bray TM, Bettger WJ. The physiological role of zinc as an antioxidant. Free Radic Biol Med. 1990;8:281–291. doi: 10.1016/0891-5849(90)90076-u. [DOI] [PubMed] [Google Scholar]
- 25.Cho CH, Ogle CW, Dai S. Effects of zinc chloride on the gastric secretion and ulcer formation in pylorus-occluded rats. Eur J Pharmacol. 1976;38:337–341. doi: 10.1016/0014-2999(76)90337-x. [DOI] [PubMed] [Google Scholar]
- 26.Ertekin MV, Koç M, Karslioglu I, Sezen O. Zinc sulfate in the prevention of radiation-induced oropharyngeal mucositis: a prospective, placebo-controlled, randomized study. Int J Radiat Oncol Biol Phys. 2004;58:167–174. doi: 10.1016/s0360-3016(03)01562-1. [DOI] [PubMed] [Google Scholar]
- 27.Nagai K, Suda T, Kawasaki K, Matsuura S. Action of carnosine and beta-alanine on wound healing. Surgery. 1986;100:815–821. [PubMed] [Google Scholar]
- 28.Nagai K, Suda T. Immunoregulative effects of carnosine and beta-alanine. J Physiol Soc Jpn. 1986;48:564–571. [PubMed] [Google Scholar]
- 29.Matsukura T, Tanaka H. Applicability of zinc complex of L-carnosine for medical use. Biochemistry (Moscow) 2000;65:817–823. [PubMed] [Google Scholar]
- 30.Yoneta T, Hori Y, Morita H, Seto K, Ikeda Y. Structural advantage of polaprezinc (Z-103) as a chelate compound: comparison with a combination of its relative compound on various experimental models of gastric lesions in rats. JPn Pharmacol Ther. 1994;22:4647–4656. [Google Scholar]
- 31.Adachi H, Ono K, Abe M, Inoue T, Imura T, Onoyama Y, Tanaka Y, Narabayashi I, Yoshikawa Y. Clinical studies on the effect of sodium arginate on radiation esophagitis and proctitis. Nihon Gan Chiryo Gakkai Shi. 1985;20:618–624. [PubMed] [Google Scholar]
- 32.Grebenev AL, Sheptulin AA, Okhlobystin AV. A comparative evaluation of the antacid properties of the preparations Maalox and Almagel. Ter Arkh. 1994;66:44–47. [PubMed] [Google Scholar]


