Abstract
High-dose chemotherapy (HDC) applied together with autologous stem cell transplantation (ASCT) is a commonly used treatment modality in patients with malignant lymphoma. At present, there is a limited number of studies which compare toxicity and efficacy of various high-dose regimens applied in the treatment of malignant lymphoma. For this reason, the aim of this study was to investigate the efficacy and toxicity of BuCyE (busulfan, cyclophosphamide and etoposide) and BEAM (carmustine, etoposide, cytarabine and melphalan) preparative regimens in the patients with malignant lymphoma scheduled for autologous stem cell transplantation. Between November, 2010 and April, 2015, 42 patients with relapsed or refractory malignant lymphoma who underwent autologous stem cell transplantation following BEAM (n=11) and BuCyE (n=31) preparative regimens were analyzed at Bone Marrow Transplantation Unit of TurgutOzal Medicine Center in Turkey. The groups were compared in terms of patient characteristics, hematopoietic engraftment time, toxicity profiles and survival. No significant differences were detected between the groups with regard to age, gender distribution, international prognostic index, ASCT indications, disease status at the time of ASCT and type of lymphoma (P>0.05). Median number of infused CD34+ cells/kg, neutrophil and platelet engraftment statuses of BuCyE and BEAM groups were found to be similar (P>0.05). More patients in BuCyE group developed mucositis and nausea, but this difference was not statistically significant (P>0.05). A similar statistically insignificant difference was seen in that infectious complications occurred more commonly in BEAM group (P>0.05). Overall survival and event-free survival rates were not significantly different between the groups (P>0.05). BuCyE is a conditioning regimen which can be effectively used as an alternative to BEAM in the patients with malignant lymphoma undergoing ASCT. Moreover, toxicity rates of both regimens are similar. In order to comprehend the effect of each HDC regimen, further evidence-based data obtained from the studies involving larger sample sizes are required.
Keywords: BuCyE, BEAM, autologous transplantation, lymphoma
Introduction
High-dose chemotherapy followed by autologous stem cell transplantation is still the standard treatment modality for malignant lymphoma patients which is resistant to initial treatment or has relapsed after initial treatment applied [1-3]. At present, no data is available on how HDC affects the success of ASCT [4,5]. The most commonly used high-dose conditioning regimens in malignant lymphoma patients are BEAM (carmustine [BCNU], etoposide, cytarabine, and melphalan), BEAC (BCNU, etoposide, cytarabine, and cyclophosphamide), CBV (cyclophosphamide, BCNU, etoposide), BuCyE (busulfan, cyclophosphamide, etoposide) and combination regimen with total body irradiation. BEAM is being the most commonly preferred HDC regimen among these [6,7]. Since there is no available clinical study comparing the toxicity and efficacy of different conditioning regimens, we have little information on this subject [7,8]. Favorable long-term survival rates have been achieved in malignant lymphoma by BuCyE regimen followed by ASCT [9]. Although there are many studies on the side effects and toxicity profiles of BEAM [10,11] and BuCyE regimens, there is still lack of information about comparative toxicity and efficacy profiles of different HDC regimens in the patients with malignant lymphoma. In the literature, only few studies are present comparing BuCyE and BEAM regimens [12,13].
In this study, we aimed to compare toxicity and efficacy profiles of BuCyE and BEAM preparative regimens in the patients with malignant lymphoma scheduled for autologous stem cell transplantation.
Materials and methods
This study was conducted between December 2010 and April 2015 in TurgutÖzal Medical Center, Bone Marrow Transplantation Unit of Inonu University, School of Medicine and included the patients with relapsed or refractory Non Hodgkin lymphoma (NHL) or Hodgkin lymphoma (HL) who scheduled for autologous stem cell transplantation following salvage chemotherapy. The patients with relapsed or refractory NHL and HL who had been diagnosed histopathologically were accepted as suitable candidates for ASCT. All cases enrolled in the study were assessed in terms of chemosensitivity. The other inclusion criteria of the study were age <70 years, adequate heart, lung, liver, and kidney reserves, sufficient hematopoietic function and Eastern Cooperative Oncology Group performance status of one or zero prior to ASCT. The study involved a total number of 42 patients with malignant lymphoma scheduled for ASCT. Among these patients, 31 cases received BuCyE regimen, while BEAM was applied to 11 patients as preparative regimen prior to ASCT (Table 1).
Table 1.
BuCyE and BEAM chemotherapy regimens
| BuCyE protocol | BEAM protocol | ||
|---|---|---|---|
| Busulfan (mg/kg) | 16 (-7, -6, -5, -4. days) | BCNU (mg/m2) | 200 (-6. day) |
| Siklofosfamid (mg/kg) | 120 (-3, -2. days) | Etoposid (mg/m2) | 800 (-5, -4, -3, -2. days) |
| Etoposid mg/m2/day | 400 (-3, -2. days) | Ara-C (mg/m2) | 800 (-5, -4, -3, -2. days) |
| Melfalan (mg/m2) | 140 (-1. day) | ||
Response rates prior to transplantation, transplantation-related antifungal treatment, time of engraftment and treatment response rates in post-transplantation period were analyzed. Post-transplant response rates were evaluated by neck, thoracic, abdominal computed tomography or positron emission tomography scans performed on the 30th day.
Treatment responses obtained from initial induction chemotherapy, salvage chemotherapy and HDC were evaluated according to International Workshop Criteria [14]. Chemosensitivity was defined as a reduction in the measurable disease which at least meets the criteria for partial response (PR) after salvage chemotherapy prior to transplantation.
A successful neutrophil engraftment was accepted as an absolute neutrophil count of ≥1×109/L attained for one day, while platelet count ≥20×109/L without a need for platelet transfusion on the first consecutive three days after platelet engraftment was considered to be a successful platelet engraftment procedure. Treatment response was firstly evaluated one month after ASCT performed, then by 3-months intervals within the first 2 years, by 6-months intervals during the next 3 years and annually or whenever clinically required thereafter.
Statistical analysis
Categorical variables are expressed as count and percentage. The comparison of gender distribution between groups was made by continuity corrected chi-square test. The groups were compared by Fisher’s Exact test due to the Lymphoma type, performance status at ASCT, indications of ASCT, status of disease at ASCT, mucositis, nausea, vomiting, diarrhea, infectious complications, treatment-related mortality, refractory, complete remission, relapse and disease related mortality distributions.
Normal distribution of the continuous variables was assessed by Shapiro-Wilk test. Normally distributed data were summarized with mean and standard deviation, for non-normal data median, minimum and maximum values were used. The mean age of the groups was compared by independent samples t test. The comparison of the number of infused CD34+ cells, engraftment time of neutrophils, engraftment time of platelets, transfused RBC during ASCT and transfused PLT during ASCT distributions between groups were made by Mann Whitney-U test.
For survival analysis Kaplan-Meier method was used and event free survival, survival after ASCT and overall survival times of groups were compared by Log-Rank test. Event-free survival (EFS) was defined as the time from ASCT to disease progression, relapse or death, or the last time point in which a patient was known to be in remission. Treatment-related mortality was defined as death occurred within 30 days after transplantation procedure without the role of an underlying disease. In all analysis significance level was considered to be 0.05.
Results
Among 42 patients who underwent autologous stem cell transplantation during the study period due to malignant lymphoma, 31 patients received BuCyE and BEAM regimen was given to 11 cases as conditioning regimen. The mean age of the study group was 38.40±12.82 years. In BuCyE group, there were 14 male patients and 17 female patients with a mean age of 40.87±14.05 years. On the other hand, in the group which received BEAM as conditioning regimen, 5 patients were female and 6 were male, whose mean age was 35.00±11.22 years. There was no statistically significant difference between the groups in terms of mean age, gender, type of lymphoma, performance status at the time of ASCT and ASCT indication. The detailed data on the characteristics of the groups were presented in Table 2.
Table 2.
Patient characteristics of all patients (N= 42)
| BuCyE | BEAM | P value | ||
|---|---|---|---|---|
| Number of patients | 31 | 11 | ||
| Mean of age | 40.87±14.05 | 35.00±11.22 | 0.219 | |
| Gender | Male | 14 (45.2%) | 6 (54.5%) | 0.854 |
| Female | 17 (54.8%) | 5 (45.5%) | ||
| Lymphoma Type | HL | 8 (25.8%) | 3 (27.3%) | 1.000 |
| NHL | 23 (74.2%) | 8 (72.7%) | ||
| Performance status at ASCT | 0 | 11 (35.5%) | 3 (27.3%) | 0.723 |
| 1 | 20 (64.5%) | 8 (72.7%) | ||
| Indications of ASCT | Refractory | 2 (6.5%) | 1 (9.1%) | 1.000 |
| Relapsed | 29 (93.5%) | 10 (90.9%) | ||
| Status of disease at ASCT | Complete Remission | 29 (93.5%) | 10 (90.9%) | 1.000 |
| Partial Remission | 2 (6.5%) | 1 (9.1%) | ||
Between-group comparison was performed with regard to median neutrophil and platelet engraftment time, number of infused CD34+ cells, transfused red blood cells and transfused platelets, which showed no significant differences between the groups (Table 3).
Table 3.
Transfused red blood cells (RBC) and platelet (PLT) during ASCT, hematopoietic engraftment time after ASCT
| BuCyE (N= 31) median (min-max) | BEAM (N= 11) median (min-max) | P value | |
|---|---|---|---|
| Number of infused CD34+ cells (x106/kg) | 9 (2-42) | 11 (2-14) | 0.653 |
| Engraftment time of neutrophils >1000/mm3 (days) | 12 (8-14) | 13 (9-18) | 0.873 |
| Engraftment time of platelets >20,000/mm3 (days) | 14.5 (10-32) | 15 (11-29) | 0.552 |
| Transfused RBC during ASCT (units) | 2 (0-10) | 3 (1-8) | 0.822 |
| Transfused PLT during ASCT (units) | 3 (1-14) | 4 (1-9) | 1.000 |
Toxicities related to the regimens given were rated according to National Cancer Institute Common Toxicity Criteria version 3.0 grading system. In two groups, grade 2 or higher mucositis, nausea, vomiting, diarrhea and infectious complications were not statistically significant (P>0.05). None of the patients experienced veno-occlusive disease in both groups. The numbers of the patients who died within 30 days following transplantation secondary to treatment related complications in BEAM and BuCyE groups were one and two, respectively (Table 4).
Table 4.
Toxicity associated with BuCyE and BEAM conditioning regimen
| BuCyE (N= 31) count (%) | BEAM (N= 11) count (%) | P value | |
|---|---|---|---|
| Mucositisa | 12 (38.7) | 3 (27.3) | 0.717 |
| Nauseaa | 13 (41.9) | 4 (36.4) | 1.000 |
| Vomitinga | 13 (41.9) | 5 (45.5) | 1.000 |
| Diarrheaa | 4 (12.9) | 2 (18.2) | 0.644 |
| Infectious complications | 24 (77.4) | 11 (100) | 0.161 |
| Treatment-related mortality | 2 (6.5) | 1 (9.1) | 1.000 |
Response rates and survival outcomes of two groups were compared (Figure 1A-C), which showed that survival rate was better in BEAM group when compared to BuCyE group, but this was not statistically significant (Table 5).
Figure 1.
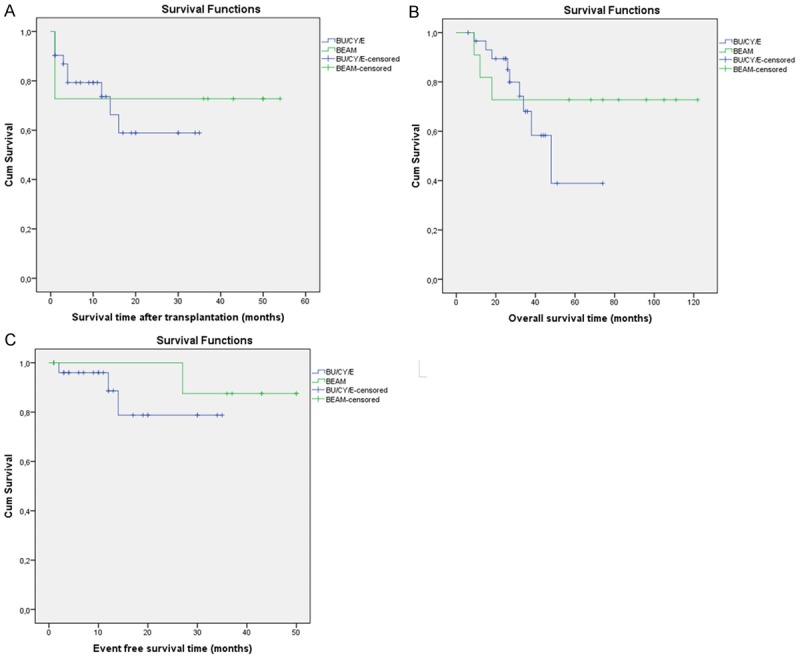
Survival time (A) and event-free survival (B) after high-dose chemotherapy with BuCyE or BEAM, followed by autologous stem cell transplantation and overall survival time (C) in both of groups from initial treatment.
Table 5.
Response and survival outcomes in both groups
| BuCyE | BEAM | P value | |
|---|---|---|---|
| Refractory, number of patients (%) | 2 (6.5%) | 0 (0%) | 1.000 |
| Complete Remission, number of patients (%) | 21 (67.7%) | 7 (63.6%) | 1.000 |
| Relapse, number of patients (%) | 3 (9.7%) | 1 (9.1%) | 1.000 |
| Event Free Survival, mean ± sem, months | 29.91±2.69 | 47.13±2.69 | 0.420 |
| Disease related mortality, number of patients (%) | 3 (33.3%) | 0 (0%) | 0.509 |
| Non disease related mortality, number of patients (%) | 6 (66.7%) | 3 (100%) | |
| Survival After ASCT, mean ± sem, months | 24.01±2.97 | 39.55±7.12 | 0.691 |
| Overall Survival, mean ± sem, months | 49.73±6.09 | 92.27±14.65 | 0.441 |
Discussion
High-dose chemotherapy supported by autologous stem cell transplantation is a commonly used procedure in the treatment of malignant lymphoma. We have limited information obtained from the studies comparing toxicity and efficacy profiles of different high-dose regimens applied in the treatment of NHL and HL. For this reason, in this study, we performed a retrospective analysis of toxicities and efficacies of these regimens in two groups of patients with similar age, gender distribution, International Prognostic Index, ASCT indications, type of lymphoma and the median number of infused CD34+ cells/kg.
In the study by Kim et al., efficacy and toxicity profiles of BEAM and BuCyE high-dose chemotherapy regimens applied prior to autologous stem cell transplantation were compared in patients with non-Hodgkin’s lymphoma. In this study, 65 patients with non-Hodgkin’s lymphoma received either BEAM (n=43) or BuCyE (n=22) regimens. The study concluded that there were no statistically significant differences between the groups in terms of age, gender distribution, International Prognostic Index, status of disease at the time of ASCT and median number of infused CD34+ cells/kg. Furthermore, a similar ratio of the patients experienced mucositis, nausea/vomiting, diarrhea, bleeding and infection in both groups. The study also revealed that median overall and event-free survival rates were not significantly different between two groups, while hematologic recovery was found to be significantly faster in the group who received BuCyE as conditioning regimen. The authors concluded that BuCyE was an effective conditioning regimen which had survival outcomes and toxicity profile similar to that of BEAM regimen [12]. In our study, engraftment time of neutrophils and platelets were shorter in BuCyE group, but this was not statistically significant.
In the study by Kim JG et al., which was a multi-center study published in 2005, BuCyE was evaluated as a conditioning regimen for autologous stem cell transplantation in patients with non-Hodgkin’s lymphoma. The study sample was composed of 64 patients most of whom had diffuse large B-cell lymphoma. It was reported that median time values to reach neutrophil and platelet engraftment were 12 and 13 days, respectively. The same study also revealed that 4 patients had hepatic veno-occlusive disease, while 15 patients (23.4%) developed relapse or progressed within a median follow-up of 16.4 months. In addition, 13 patients (20.3%) died of disease and estimated 3-years overall and progression-free survival rates were 72.1% and 70.1%, respectively. The authors suggested that BuCyE conditioning regimen was well tolerated and provided effective results in patients with aggressive NHL [8]. Among the patients received BuCyE, 9 (29.0%) patients died, 2 (6.5%) patients had refractory disease, while 3 (9.7%) developed relapse. The findings of our study were consistent with the results of that study.
A study by Jantunen E. et al. involved 71 consecutive patients with NHL and investigated drug related toxicity and outcomes. In that study, the patients were given either BEAC (high-dose BCNU, etoposide, cytarabine and cyclophosphamide, n=36) or BEAM (n=35). Among these patients, the occurrence of mucositis, diarrhea and septicemia was found to be more frequent in the patients receiving BEAM and the peak CRP value was also higher in this group (140 vs. 113 mg/l, P=0.034). The researchers concluded that the levels of anti-tumor activities of BEAC and BEAM appears to be equal in patients with NHL, while BEAM seems to have more toxic effects on gastrointestinal tract [15]. When we compared the results of our study groups in terms of treatment related complications, we found that mucositis and nausea were more common in BuCyE group, while more patients in BEAM group had infectious complications, vomiting and diarrhea, but these differences were found to be statistically insignificant. Moreover, between-group comparison for CRP level was not performed.
Hanel M et al. conducted a study on 53 patients with HL or NHL who received high dose BuCyE conditioning regimen and investigated the efficacy and toxicity of BuCyE used as a preparative regimen prior to ASCT. 10 of these 53 patients had HL, while 43 patients had the diagnosis of NHL (median age: 46 years, range: 18-64) and they all received BuCyE prior to ASCT. Among these patients, complete response or partial remission (n=25) was achieved in 50 patients (94%), while 3 patients had chemoresistant disease prior to BuCyE. In the evaluation of toxicities, mucositis (79%) and hepatic toxicity (15%) were found to be the most common non-hematological toxicities which were seen in 52 subjects, while 3 patients (5.8%) experienced severe veno-occlusive disease. In that study, the rate of treatment-related mortality was found as 3.8%. The authors concluded that BuCyE was an effective and well-tolerated conditioning regimen in patients with HL and NHL [7]. In our study, none of the patients had veno-occlusive disease and in BuCyE group, the rate of mucositis was 38.7% (n=12) and treatment-related mortality rate was 6.5%.
In their study, Copelan EA et al. aimed to investigate toxicity profile and efficacy of BuCyE as a novel preparative regimen and analyzed the data of 382 patients with non-Hodgkin’s lymphoma who received BuCyE prior to autologous transplantation. They concluded that BuCyE, as a novel preparative regimen, provided good results and low treatment related mortality in the treatment of patients with NHL [4].
In the study by Zhang CY et al., 70 patients with multiple myeloma who were eligible for ASCT were enrolled in the study, 32 of the patients were given high dose melphelan, while BuCyE was applied as conditioning regimen in 38 patients. It was concluded that in the patients with multiple myeloma who are candidates for ASCT, BuCyE conditioning regimen was an effective and safe option [16].
Our study has some limitations in that it was a retrospective study and included a small sample size. In addition, the groups were not well-matched in terms of the rates of lymphoma types. However, patient and disease characteristics and the median numbers of peripheral blood stem cells infused were similar in both groups. The ASCT protocol applied and supportive care given following ASCT were also same in both groups.
Our findings reveal that OS, EFS and toxicity profiles of both BuCyE and BEAM preparatory regimens were similar. The groups were also similar in that mucositis, infectious complications, VOD and treatment related mortality occurred at a similar rate.
In conclusion, BuCyE conditioning regimen may be used as an alternative to BEAM conditioning regimen in autologous stem cell transplantation in patients with relapsed and refractory lymphoma. It is an effective and feasible strategy with acceptable side effect profile.
Acknowledgements
This study was conducted at the Inonu University Faculty of Medicine Turgut Ozal Medical Center with the approval of the ethics committee. This study was not supported by any financial sources.
Disclosure of conflict of interest
None.
Abbreviations
- BEAM
Carmustine (BCNU), etoposide, cytarabine and melphalan
- BCNU
Carmustine
- BuCyE
Busulfan, cyclophosphamide and etoposide
- NHL
Non-Hodgkin’s lymphoma
- HL
Hodgkin lymphoma
- ASCT
Autologous stem cell transplantation
- HDC
High dose chemotherapy
- BEAC
BCNU, etoposide, cytarabine, and cyclophosphamide
- CBV
Cyclophosphamide, carmustine, etoposide
- DHAP
Cisplatin, cytarabine, dexamethasone
- RBC
Red blood cells
- PLT
Platelet
References
- 1.Milpied N, Deconinck E, Gaillard F, Delwail V, Foussard C, Berthou C, Gressin R, Lucas V, Colombat P, Harousseau JL GroupeOuest-Est des Leucémies et des Autres Maladies du Sang. Initial treatment of aggressive lymphoma with high-dose chemotherapy and autologous stem-cell support. N Engl J Med. 2004;350:1287–1295. doi: 10.1056/NEJMoa031770. [DOI] [PubMed] [Google Scholar]
- 2.Salar A, Sierra J, Gandarillas M, Caballero MD, Marín J, Lahuerta JJ, García-Conde J, Arranz R, León A, Zuazu J, García-Laraña J, López-Guillermo A, Sanz MA, Grañena A, García JC, Conde E GEL/TAMO Spanish Cooperative Group. Autologous stem cell transplantation for clinically aggressive non-Hodgkin’s lymphoma: the role of preparative regimens. Bone Marrow Transplant. 2001;27:405–412. doi: 10.1038/sj.bmt.1702795. [DOI] [PubMed] [Google Scholar]
- 3.Wadehra N, Farag S, Bolwell B, Elder P, Penza S, Kalaycio M, Avalos B, Pohlman B, Marcucci G, Sobecks R, Lin T, Andrèsen S, Copelan E. Long-term outcome of Hodgkin disease patients following high-dose busulfan, etoposide. cyclophosphamide, and autologous stem cell transplantation. Biol Blood Marrow Transplant. 2006;12:1343–1349. doi: 10.1016/j.bbmt.2006.08.039. [DOI] [PubMed] [Google Scholar]
- 4.Copelan EA, Penza SL, Pohlman B, Avalos BR, Goormastic M, Andresen SW, Kalaycio M, Bechtel TP, Scholl MD, Elder PJ, Ezzone SA, O’Donnell LC, Tighe MB, Risley GL, Young DC, Bolwell BJ. Autotransplantation following busulfan, etoposide and cyclophosphamide in patients with non-Hodgkin’s lymphoma. Bone Marrow Transplant. 2000;25:1243–1248. doi: 10.1038/sj.bmt.1702433. [DOI] [PubMed] [Google Scholar]
- 5.A predictive model for aggressive non-Hodgkin’s lymphoma. The International Non-Hodgkin’s Lymphoma Prognostic Factors Project. N Engl J Med. 1993;329:987–994. doi: 10.1056/NEJM199309303291402. [DOI] [PubMed] [Google Scholar]
- 6.Weaver CH, Schwartzberg L, Rhinehart S, West J, Zhen B, West WH, Buckner CD. Highdose chemotherapy with BUCY or BEAC and unpurged peripheral blood stem cell infusion in patients with low-grade non-Hodgkin’s lymphoma. Bone Marrow Transplant. 1998;21:383–389. doi: 10.1038/sj.bmt.1701101. [DOI] [PubMed] [Google Scholar]
- 7.Hänel M, Kröger N, Sonnenberg S, Bornhäuser M, Krüger W, Kroschinsky F, Hänel A, Metzner B, Birkmann J, Schmid B, Hoffknecht MM, Fiedler F, Ehninger G, Zander AR. Busulfan, cyclophosphamide, andetoposide as high-dose conditioningregimen in patients with malignant lymphoma. Ann Hematol. 2002;81:96–102. doi: 10.1007/s00277-001-0413-8. [DOI] [PubMed] [Google Scholar]
- 8.Kim JG, Sohn SK, Chae YS, Yang DH, Lee JJ, Kim HJ, Shin HJ, Jung JS, Kim WS, Kim DH, Suh C, Kim SJ, Eom HS, Bae SH. Multicenter study of intravenous busulfan, cyclophosphamide, and etoposide (i. v. Bu/Cy/E) as conditioning regimen for autologous stem cell transplantation in patients with non-Hodgkin’s lymphoma. Bone Marrow Transplant. 2007;40:919–924. doi: 10.1038/sj.bmt.1705841. [DOI] [PubMed] [Google Scholar]
- 9.Kröger N, Hoffknecht M, Hänel M, Krüger W, Zeller W, Stockschläder M, de Wit M, Weh HJ, Kabisch H, Erttmann R, Zander AR. Busulfan, cyclophosphamide and etoposide as high-dose conditioning therapy in patients with malignant lymphoma and prior dose-limiting radiation therapy. Bone Marrow Transplant. 1998;21:1171–1175. doi: 10.1038/sj.bmt.1701245. [DOI] [PubMed] [Google Scholar]
- 10.Buser AS, Stern M, Bucher C, Arber C, Heim D, Halter J, Meyer-Monard S, Stussi G, Lohri A, Ghielmini M, Tichelli A, Passweg JR, Gratwohl A. High-dose chemotherapy using BEAM without autologous rescue followed by reduced intensity conditioning allogeneic stem-cell transplantation for refractory or relapsing lymphomas: a comparison of delayed versus immediate transplantation. Bone Marrow Transplant. 2007;39:335–340. doi: 10.1038/sj.bmt.1705597. [DOI] [PubMed] [Google Scholar]
- 11.Caballero MD, Rubio V, Rifon J, Heras I, García-Sanz R, Vázquez L, Vidriales B, del Cañizo MC, Corral M, Gonzalez M, León A, Jean-Paul E, Rocha E, Moraleda JM, San Miguel JF. BEAM chemotherapy followed by autologous stem cell support in lymphoma patients: analysis of efficacy, toxicity and prognostic factors. Bone Marrow Transplant. 1997;20:451–458. doi: 10.1038/sj.bmt.1700913. [DOI] [PubMed] [Google Scholar]
- 12.Kim JE, Lee DH, Yoo C, Kim S, Kim SW, Lee JS, Park CJ, Huh J, Suh C. BEAM or BuCyE high-dose chemotherapy followed by autologous stem cell transplantation in non-Hodgkin’s lymphoma patients: a single center comparative analysis of efficacy and toxicity. Leuk Res. 2011;35:183–187. doi: 10.1016/j.leukres.2010.07.016. [DOI] [PubMed] [Google Scholar]
- 13.Crilley P, Lazarus H, Topolsky D, Ciobanu N, Creger RJ, Fox RM, Bulova SI, Shina DC, Gucalp R, Cooper BW, et al. Comparison of preparative transplantation regimens using carmustine/etoposidecisplatin or busulfan/etoposide/cyclophosphamide in lymphoid malignancies. Semin Oncol. 1993;20:50–54. [PubMed] [Google Scholar]
- 14.Cheson BD, Horning SJ, Coiffier B, Shipp MA, Fisher RI, Connors JM, Lister TA, Vose J, Grillo-López A, Hagenbeek A, Cabanillas F, Klippensten D, Hiddemann W, Castellino R, Harris NL, Armitage JO, Carter W, Hoppe R, Canellos GP. Report of an international work shop to standardize response criteria for nonHodgkin’s lymphomas. NCI Sponsored International Working Group. J. Clin. Oncol. 1999;17:1244. doi: 10.1200/JCO.1999.17.4.1244. [DOI] [PubMed] [Google Scholar]
- 15.Jantunen E, Kuittinen T, Nousiainen T. BEAC or BEAM for high-dose therapy in patients with non-Hodgkin’s lymphoma? A single centre analysis on toxicity and efficacy. Leuk Lymphoma. 2003;44:1151–1158. doi: 10.1080/1042819031000083028. [DOI] [PubMed] [Google Scholar]
- 16.Zhang CY, Fu WJ, Xi H, Zhou LL, Jiang H, Du J, Fan JL, Li R, Jin LN, Zeng TM, Hou J. Busulfan, cyclophosphamide and etoposide as conditioning for autologous stem cell transplantation in multiple myeloma. Zhonghua Xue Ye Xue Za Zhi. 2013;34:313–316. doi: 10.3760/cma.j.issn.0253-2727.2013.04.014. [DOI] [PubMed] [Google Scholar]


