Abstract
To investigate the association of serum semaphorin 4D (Sema4D) levels with lumbar spine bone mineral density (BMD) and bone turnover markers in patients with postmenopausal osteoporosis (PO). Lumbar spine BMD was measured by dual-energy X-ray absorptiometry in 257 PO patients (aged from 50 to 75) and 90 healthy controls (aged from 51 to 83). Serum Sema4D, BAP, BGP and TRACP-5b levels were measured by enzyme linked immunosorbent assay. Serum cross linked N-telopeptides of type I (NTX), 25-hydroxyvitamin D (25(OH)D) and N-mid fragment of osteocalcin (N-MID-OT) levels were measured using automated electrochemiluminescence system. Sema4D level was significantly higher in PO women compared to healthy controls (1.40±0.33 vs. 0.58±0.18 μg/L, P=0.006). Sema4D level was positively correlated with serumTRACP-5b and NTX levels and negatively correlated with lumbar spine BMD and serum BAP and BGP levels. There were no correlations between Sema4D level and age, body mass index, and serum 25(OH)D and N-MID-OT levels. Lumbar spine BMD (β=-0.354, P<0.001) and serum BAP level (β=0.127, P=0.019) were independent predictors of serum Sema4D level in PO patients. Sema4D may be involved in the pathogenesis of PO and play a critical role in bone formation and resorption. Sema4D may represent a novel therapeutic target for treatment of PO and function as a predictive indicator of PO.
Keywords: Sema4D, postmenopausal osteoporosis, bone mineral density, bone turnover markers
Introduction
Osteoporosis is one of the most common skeletal disorders. It is characterized by low bone mass and deterioration of micro-architectural bone structure, which causes bone fragility and susceptibility to fracture [1]. Primary osteoporosis includes age-related osteoporosis and idiopathic osteoporosis. Age-related osteoporosis is often referred as postmenopausal osteoporosis (PO), since more women than men are affected by it.
PO negatively affects the quality of life of aging women and results in various complications, such as fractures, chest tightness, shortness of breath, coughing, bloating, and constipation. These complications are resulted from disorders of the heart, lungs, digestive system, and impaired blood flow due to thoracic and lumbar protrusion [2-4]. Consequently, various treatment options for PO have been proposed. Current PO treatments focus on the regulation of estrogen and calcium absorption, and include supplementation of calcium and vitamin D [5,6], hormone replacement therapy (HRT) [7,8], and treatment with bisphosphonates [9], calcitonin [10], fluoride preparations selective estrogen receptor modulators (SERM) [11], and parathyroid hormone (PTH) [12,13]. Different therapeutic outcomes have been reported from studies using these agents, but all were beneficial for PO patients.
Bone is subjected to continuous renewal by bone remodeling, during which bone resorption is followed by bone formation [14]. Bone loss occurs when the balance between resorption and formation is disrupted. Estrogen deficiency in postmenopausal women prevents absorption of calcium and phosphorus, resulting in bone loss and osteoporosis [15-17]. The balance between bone resorption and bone formation can be indirectly evaluated by bone turnover markers. Recently, Love et al. found strong similarity between the semaphorin 4D (Sema4D) homodimer and αVβ3 integrin heterodimer, which is the most abundant integrin receptor expressed in bone resorbing osteoclasts [18]. Negishi-Koga et al. showed that bone volume, trabecular thickness, and bone strength were all greater in mice lacking Sema4D compared to wild-type animals. In ovariectomized mice, treatment with an anti-Sema4D antibody promoted bone formation without affecting resorption [14]. Sema4D is mainly expressed in the nervous system, platelets, and ovaries and may have important functions in neurite proliferation, neuronal migration, and stimulation of angiogenesis [19-21]. Sema4D is also involved in follicle maturation in the mouse ovary.
To the best of our knowledge, there are no published clinical reports of a correlation between serum Sema4D levels and osteoporosis in postmenopausal women. Therefore, the objective of the present study was to investigate the association of serum Sema4D levels with lumbar spine bone mineral density (BMD) and bone turnover markers in PO patients.
Materials and methods
Study population
A total of 257 PO patients (age range, 50 to 75 years) and 90 healthy postmenopausal women as controls (age range, 51 to 83 years) were enrolled in this study. For PO patients, inclusion criteria were: (1) the evidence of >1 year of menopause; (2) the average BMD in PO patients with at least 2.5 standard deviation lower than in normal adults. Exclusion criteria were: (1) secondary osteoporosis; (2) congenital bone deformities, polio, severe liver or kidney diseases, hyperthyroidism or hypothyroidism, collagen diseases, diabetes, bone cancer, bone softening disease, and other related bone and joint diseases; (3) treatment with drugs affecting bone metabolism, including calcium, calcitonin, vitamin D, estrogen, bisphosphonates, and raloxifene within one year; or (4) previous radiation therapy. Healthy postmenopausal women were identified following a physical examination at our Hospital, which excluded them from osteoporosis and other related bone diseases that could affect bone metabolism. The study was approved by the institutional review board of our Hospital. Written informed consent was obtained from each participant.
Bone mineral density
BMD is considered as an indicator of bone mass and is useful for evaluating the degree of osteoporosis. In the present study, BMD of the lumbar spine (L1-L4) was measured by dual-energy X-ray absorptiometry (DEXA) (Norland XR-46 Excell plus, USA). Osteoporosis was defined as a T-score of <-2.5. Intra-and inter-assay coefficients of variation (CVs) were <1.4% and <2.6%, respectively.
Biochemical assays
Serum Sema4D, bone alkaline phosphatase (BAP), bone gla protein (BGP), and tartrate resistant acid phosphatase (TRACP-5b) levels were measured by enzyme-linked immunosorbent assay (ELISA, R & D, USA). Intra-assay CVs were 3.6-4.4%, 3.7-4.3%, 3.3-4.8%, and 4.0-5.1%, respectively, and inter assay CVs were <6%. Serum cross-linked N-telopeptides of type I (NTX), 25-hydroxyvitamin D (25(OH)D), and N-mid fragment of osteocalcin (N-MID-OT) levels were measured using the automated Roche electrochemiluminescence system. Intra-assay CVs were 3.4-4.2%, 3.7-4.5%, 4.2-5.5% and 3.8-5.3%, respectively, and the inter assay CVs were <6%.
Statistical analysis
All statistics were analyzed using SPSS 13.0 (SPSS Inc., Chicago, Illinois, USA). Data are described as mean ± standard deviation (SD). The differences between groups were evaluated by independent-samples t-test. Correlation analyses were performed using Spearman’s coefficient of correlation. Multiple regression analysis was conducted to determine the influence of each independent variable after correcting other factors. A p-value of <0.05 was considered statistically significant.
Results
Demographic and clinical characteristics of the study population are shown in Table 1. There were no statistically significant differences in age (P=0.383), age of menopause (P=0.227), weight (P=0.432), height (P=0.374), and body mass index (BMI, P=0.188) between PO patients and healthy controls. Serum Sema4D, TRACP-5b, and NTX levels were significantly higher in PO patients compared to controls (P=0.006, P=0.023, and P=0.035, respectively). Serum BAP and BGP levels were significantly lower in PO patients compared to controls (P=0.008 and P=0.017, respectively). There were no significant differences in serum 25(OH)D and N-MID-OT levels between PO patients and controls (P=0.582 and P=0.633, respectively).
Table 1.
Characteristic and biochemical data of study population
| Variables | Control (n=90) | PO (n=257) | P values |
|---|---|---|---|
| Age (years) | 61.36±3.75 | 62.73±3.94 | 0.383 |
| Age of menopause (years) | 53.13±2.94 | 53.75±2.76 | 0.227 |
| Weight (kg) | 57.59±7.21 | 57.47±7.73 | 0.432 |
| Height (m) | 1.54±0.09 | 1.55±0.07 | 0.374 |
| BMI (kg/m2) | 24.77±4.13 | 24.65±3.67 | 0.188 |
| spine BMD (g/cm2) | 0.99±0.15 | 0.79±0.19 | 0.013 |
| T-score | -1.58±0.45 | -3.42±0.83 | 0.008 |
| Sema4D (μg/L) | 0.58±0.18 | 1.40±0.33 | 0.006 |
| BAP (U/L) | 60.28±15.33 | 33.17±9.22 | 0.008 |
| BGP (μg/L) | 7.75±1.36 | 4.82±0.77 | 0.017 |
| TRACP-5b (U/L) | 3.40±0.82 | 5.61±1.07 | 0.023 |
| NTX (μmol BCE/L) | 14.49±3.88 | 23.02±7.41 | 0.035 |
| 25(OH)D (ng/mL) | 15.77±6.33 | 16.02±7.13 | 0.582 |
| N-MID-OT (ng/mL) | 17.07±6.44 | 16.96±5.96 | 0.633 |
Body mass index (BMI) was calculated as weight in kilograms divided by square of the height in meters.
Correlation analyses are shown in Table 2 and Figure 1. Serum Sema4D levels were positively correlated with serum TRACP-5b and NTX levels (r=0.365, P=0.014; r=0.287, P=0.019; respectively) and negatively correlated with lumbar spine BMD and serum BAP and BGP levels (r=-0.212, P<0.001; R=-0.396, P=0.007; r=-0.411, P=0.006; respectively). There were no correlations between serum Sema4D levels and age, BMI, serum 25(OH)D, and N-MID-OT levels (r=0.005, P=0.768; r=-0.073, P=0.161; r=0.058, P=0.227; r=0.045, P=0.169; respectively).
Table 2.
Correlations of serum Sema4D with age, BMI, BMD, and bone turnover markers in PO patients
| Variables | R | P-values |
|---|---|---|
| Age | 0.005 | 0.768 |
| BMI | -0.073 | 0.161 |
| BMD | -0.572 | <0.001 |
| BAP | -0.396 | 0.007 |
| BGP | -0.411 | 0.006 |
| TRACP-5b | 0.365 | 0.014 |
| NTX | 0.287 | 0.019 |
| 25(OH)D | 0.058 | 0.227 |
| N-MID-OT | 0.045 | 0.169 |
Figure 1.
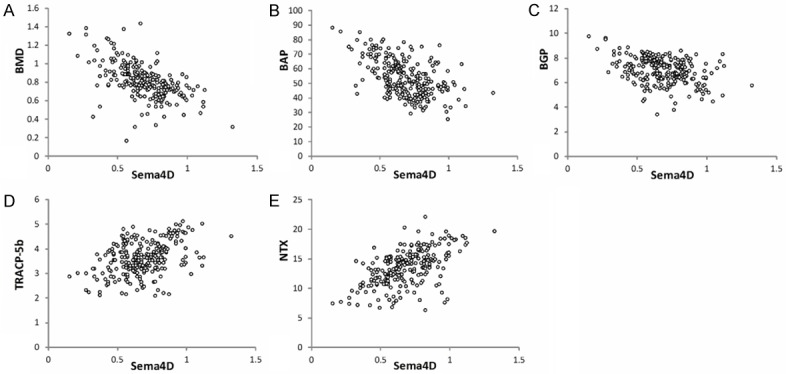
Correlations of serum Sema4D levels with A. BMD, B. BAP, C. BGP, D. TRACP-5b and E. NTX levels in PO patients. Serum Sema4D levels were negatively correlated with BMD (r=-0.212, P<0.001), BAP (r=-0.396, P=0.007) and BGP (r=-0.411, P=0.006) and positively correlated with TRACP-5b (r=0.365, P=0.014) and NTX (r=0.287, P=0.019).
Multiple regression analyses are shown in Table 3. The dependent variable was serum Sema4D levels, and the independent variables were age, BMI, lumbar spine BMD, serum BAP, BGP, TRACP-5b, NTX, 25(OH)D, and N-MID-OT levels. The results showed that lumbar spine BMD (β=-0.354, P<0.001) and serum BAP levels (β=0.127, P=0.019) were independent predictors of serum Sema4D levels in PO patients.
Table 3.
Results of multiple regression in PO patients
| Variables | B | SE | β | t | P-values |
|---|---|---|---|---|---|
| Age | -0.031 | 0.077 | -0.019 | -0.041 | 0.762 |
| BMI | -0.024 | 0.172 | -0.005 | -0.087 | 0.883 |
| BMD | -33.122 | 4.270 | -0.354 | -7.574 | <0.001 |
| BAP | 4.331 | 1.205 | 0.127 | 2.044 | 0.019 |
| BGP | 2.117 | 0.743 | 0.039 | 0.932 | 0.078 |
| TRACP-5b | -0.176 | 0.175 | -0.083 | -1.277 | 0.097 |
| NTX | -0.433 | 0.072 | -0.065 | -0.921 | 0.106 |
| 25(OH)D | 0.067 | 0.059 | 0.034 | 0.771 | 0.459 |
| N-MID-OT | -0.056 | 0.037 | -0.073 | -0.945 | 0.330 |
Discussion
The present study demonstrated that serum Sema4D levels were significantly higher in PO patients, serum BAP and BGP levels were significantly lower in PO patients, and serum TRACP-5b and NTX levels were much higher in PO patients compared to controls. A positive correlation between serum Sema4D levels and serum TRACP-5b and NTX levels and a negative correlation between serum Sema4D levels and BMD and serum BAP and BGP levels were observed, but no significant correlations between serum Sema4D levels and age, BMI, serum 25(OH)D, and N-MID-OT levels were identified. These data provide new insight into the mechanisms of regulating the balance between bone formation and bone resorption in postmenopausal women.
Our observations confirmed and extended the results reported in previous studies. Dacquin et al. published in vitro data showing Sema4D-deficient primary osteoclasts had impaired spreading, adhesion, migration, and resorption. In vivo, Sema4D deletion in sexually mature female mice led to a high bone mass phenotype due to defective bone resorption by osteoclasts. In ovariectomized Sema4D-/-mice, the bone resorption phenotype was abrogated, providing evidence that the high bone mass phenotype was dependent on ovarian function [1]. Negishi-Koga et al. showed that Sema4D-specific antibody treatment markedly prevented bone loss in a mouse model of PO [14]. Taken together, the results of these studies suggest that Sema4D is emerging as a new therapeutic target for the discovery and development of bone anabolic drugs.
As preclinical scientific investigations continue to reveal a role of Sema4D in bone remodeling, there is a need for preliminary clinical data. To the best of our knowledge, there are no published clinical studies that correlate serum Sema4D level with BMD. We substantiated observations of a negative correlation between Sema4D and BMD with measurements of bone turnover markers. BAP mediates osteoblast proliferation, and BGP is involved in the maintenance of bone mineralization rate. Both showed a negative correlation with serum Sema4D levels in our population of PO patients [22,23]. TRACP-5b and NTX determine bone resorption [24]. Accordingly, both were positively correlated with serum Sema4D levels in the PO patients in our study.
Despite being the first clinical study reporting on the correlation between serum Sema4D level and BMD in PO patients, this study was associated with several limitations. First, the results should be further verified by large-scale investigations, since only one published study showed that Sema4D acts as a potent inhibitor of bone formation in a PO mouse model [14]. Second, the association between serum Sema4D levels and local factors in osteoporotic bone tissue remains to be elucidated. Third, mechanistic studies beyond the use of an anti-Sema4D antibody to promote bone formation in ovariectomized mice are required. Clinical research similar to that conducted with anti-romosozumab monoclonal antibody, which increased BMD and bone formation and reduced bone resorption in postmenopausal women with low bone mineral density, is needed [25,26].
In conclusion, our results suggest that Sema4D might be involved in the pathogenesis of PO and play a critical role in bone formation and resorption. Sema4D might be a novel therapeutic target for the treatment of PO. Serum Sema4D level may function as a predictive indicator of PO and be used in monitoring anti-osteoporosis treatment.
Disclosure of conflict of interest
None.
References
- 1.Dacquin R, Domenget C, Kumanogoh A, Kikutani H, Jurdic P, Machuca-Gayet I. Control of bone resorption by semaphorin 4D is dependent on ovarian function. PLoS One. 2011;6:e26627. doi: 10.1371/journal.pone.0026627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Fathilah SN, Abdullah S, Mohamed N, Shuid AN. Labisia pumila Prevents Complications of Osteoporosis by Increasing Bone Strength in a Rat Model of Postmenopausal Osteoporosis. Evid Based Complement Alternat Med. 2012;2012:948080. doi: 10.1155/2012/948080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Viegas M, Costa C, Lopes A, Griz L, Medeiro MA, Bandeira F. Prevalence of osteoporosis and vertebral fractures in postmenopausal women with type 2 diabetes mellitus and their relationship with duration of the disease and chronic complications. J Diabetes Complications. 2011;25:216–221. doi: 10.1016/j.jdiacomp.2011.02.004. [DOI] [PubMed] [Google Scholar]
- 4.Taggart H, Cheng J, Archbold P. Respiratory complications associated with IV zoledronic acid infusion in the treatment of postmenopausal osteoporosis. Osteoporos Int. 2010;21:1621–1622. doi: 10.1007/s00198-009-1088-6. [DOI] [PubMed] [Google Scholar]
- 5.Fan T, Nocea G, Modi A, Stokes L, Sen SS. Calcium and vitamin D intake by postmenopausal women with osteoporosis in Spain: an observational calcium and vitamin D intake (CaVIT) study. Clin Interv Aging. 2013;8:689–696. doi: 10.2147/CIA.S41335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Reymondier A, Caillet P, Abbas-Chorfa F, Ambrosi V, Jaglal SB, Chapurlat R, Schott AM. MENOPOST--calcium and vitamin D supplementation in postmenopausal osteoporosis treatment: a descriptive cohort study. Osteoporos Int. 2013;24:559–566. doi: 10.1007/s00198-012-1999-5. [DOI] [PubMed] [Google Scholar]
- 7.Hayashi T, Ina K, Maeda M, Nomura H. The effects of selective estrogen receptor modulator treatment following hormone replacement therapy on elderly postmenopausal women with osteoporosis. Nitric Oxide. 2011;24:199–203. doi: 10.1016/j.niox.2011.04.002. [DOI] [PubMed] [Google Scholar]
- 8.Campbell IA, Douglas JG, Francis RM, Prescott RJ, Reid DM. Hormone replacement therapy (HRT) or etidronate for osteoporosis in postmenopausal asthmatics on glucocorticoids: a randomised factorial trial. Scott Med J. 2009;54:21–25. doi: 10.1258/rsmsmj.54.1.21. [DOI] [PubMed] [Google Scholar]
- 9.Ellis AG, Reginster JY, Luo X, Cappelleri JC, Chines A, Sutradhar S, Jansen JP. Bazedoxifene versus oral bisphosphonates for the prevention of nonvertebral fractures in postmenopausal women with osteoporosis at higher risk of fracture: a network meta-analysis. Value Health. 2014;17:424–432. doi: 10.1016/j.jval.2014.01.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Traynor K. Experts recommend against calcitoninsalmon for postmenopausal osteoporosis. Am J Health Syst Pharm. 2013;70:648–650. doi: 10.2146/news130028. [DOI] [PubMed] [Google Scholar]
- 11.Gennari L, Merlotti D, Nuti R. Selective estrogen receptor modulator (SERM) for the treatment of osteoporosis in postmenopausal women: focus on lasofoxifene. Clin Interv Aging. 2010;5:19–29. doi: 10.2147/cia.s6083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Makras P, Polyzos SA, Papatheodorou A, Kokkoris P, Chatzifotiadis D, Anastasilakis AD. Parathyroid hormone changes following denosumab treatment in postmenopausal osteoporosis. Clin Endocrinol (Oxf) 2013;79:499–503. doi: 10.1111/cen.12188. [DOI] [PubMed] [Google Scholar]
- 13.Li Y, Xuan M, Wang B, Yang J, Zhang H, Zhang XZ, Guo XH, Lu XF, Xue QY, Yang GY, Ji QH, Liu ZM, Li CJ, Wu TF, Sheng ZY, Li PQ, Tong JC. Comparison of parathyroid hormone (1-34) and elcatonin in postmenopausal women with osteoporosis: an 18-month randomized, multicenter controlled trial in China. Chin Med J (Engl) 2013;126:457–463. [PubMed] [Google Scholar]
- 14.Negishi-Koga T, Shinohara M, Komatsu N, Bito H, Kodama T, Friedel RH, Takayanagi H. Suppression of bone formation by osteoclastic expression of semaphorin 4D. Nat Med. 2011;17:1473–1480. doi: 10.1038/nm.2489. [DOI] [PubMed] [Google Scholar]
- 15.Xu XJ, Shen L, Yang YP, Lu FR, Zhu R, Shuai B, Li CG, Wu MX. Serum sclerostin levels associated with lumbar spine bone mineral density and bone turnover markers in patients with postmenopausal osteoporosis. Chin Med J (Engl) 2013;126:2480–2484. [PubMed] [Google Scholar]
- 16.Sachdeva A, Seth S, Khosla AH, Sachdeva S. Study of some common biochemical bone turnover markers in postmenopausal women. Indian J Clin Biochem. 2005;20:131–134. doi: 10.1007/BF02893058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Dogan E, Posaci C. Monitoring hormone replacement therapy by biochemical markers of bone metabolism in menopausal women. Postgrad Med J. 2002;78:727–731. doi: 10.1136/pmj.78.926.727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Love CA, Harlos K, Mavaddat N, Davis SJ, Stuart DI, Jones EY, Esnouf RM. The ligand-binding face of the semaphorins revealed by the high-resolution crystal structure of SEMA4D. Nat Struct Biol. 2003;10:843–848. doi: 10.1038/nsb977. [DOI] [PubMed] [Google Scholar]
- 19.Zhu L, Bergmeier W, Wu J, Jiang H, Stalker TJ, Cieslak M, Fan R, Boumsell L, Kumanogoh A, Kikutani H, Tamagnone L, Wagner DD, Milla ME, Brass LF. Regulated surface expression and shedding support a dual role for semaphorin 4D in platelet responses to vascular injury. Proc Natl Acad Sci U S A. 2007;104:1621–1626. doi: 10.1073/pnas.0606344104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Conrotto P, Valdembri D, Corso S, Serini G, Tamagnone L, Comoglio PM, Bussolino F, Giordano S. Sema4D induces angiogenesis through Met recruitment by Plexin B1. Blood. 2005;105:4321–4329. doi: 10.1182/blood-2004-07-2885. [DOI] [PubMed] [Google Scholar]
- 21.Sierra JR, Corso S, Caione L, Cepero V, Conrotto P, Cignetti A, Piacibello W, Kumanogoh A, Kikutani H, Comoglio PM, Tamagnone L, Giordano S. Tumor angiogenesis and progression are enhanced by Sema4D produced by tumor-associated macrophages. J Exp Med. 2008;205:1673–1685. doi: 10.1084/jem.20072602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Cundy T, Horne A, Bolland M, Gamble G, Davidson J. Bone formation markers in adults with mild osteogenesis imperfecta. Clin Chem. 2007;53:1109–1114. doi: 10.1373/clinchem.2006.083055. [DOI] [PubMed] [Google Scholar]
- 23.Aonuma H, Miyakoshi N, Hongo M, Kasukawa Y, Shimada Y. Low serum levels of undercarboxylated osteocalcin in postmenopausal osteoporotic women receiving an inhibitor of bone resorption. Tohoku J Exp Med. 2009;218:201–205. doi: 10.1620/tjem.218.201. [DOI] [PubMed] [Google Scholar]
- 24.Takada J, Iba K, Imoto K, Yamashita T. Changes in bone resorption markers among Japanese patients with postmenopausal osteoporosis treated with alendronate and risedronate. J Bone Miner Metab. 2007;25:142–146. doi: 10.1007/s00774-006-0739-3. [DOI] [PubMed] [Google Scholar]
- 25.McClung MR, Grauer A, Boonen S, Bolognese MA, Brown JP, Diez-Perez A, Langdahl BL, Reginster JY, Zanchetta JR, Wasserman SM, Katz L, Maddox J, Yang YC, Libanati C, Bone HG. Romosozumab in postmenopausal women with low bone mineral density. N Engl J Med. 2014;370:412–420. doi: 10.1056/NEJMoa1305224. [DOI] [PubMed] [Google Scholar]
- 26.McClung MR, Grauer A. Romosozumab in postmenopausal women with osteopenia. N Engl J Med. 2014;370:1664–1665. doi: 10.1056/NEJMc1402396. [DOI] [PubMed] [Google Scholar]


