Abstract
Objective: To evaluate the feasibility, safety and efficacy of artificial pleural effusion in percutaneous microwave ablation of hepatic tumors near the diaphragm under ultrasound guidance. Methods: For localization and navigation of tumors near the dome of the diaphragm by ultrasound during microwave ablation in 14 tumors of 11 cases, artificial pleural effusion was performed in the volume of 1000~1500 ml of Normal saline or 5% Glucose injection solution via the right thoracic cavity. The tumor marker, AFP was monitored before and after operation in 6 times in a period of 2 years. We analyzed the successful rate and effectiveness of artificial pleural effusion. Results: The successful rate of artificial pleural effusion was 100% without complications. Artificial hydrothorax on the right eliminated the interference of intrapulmonary gas to the visualization of hepatic tumors near the diaphragm on ultrasound. In the follow-up of 2 years, the ablation rate reached to 92.9% with no serious complications. The AFP value before operation was in significant statistical difference with the others after operation (P = 0.000). Conclusions: Artificial pleural effusion aids the visualization of hepatic tumors near the diaphragm on ultrasound. A good therapeutic effectiveness can be reached in percutaneous microwave ablation of tumors in the hepatic dome under the guidance of ultrasound.
Keywords: Artificial pleural effusion, hepatic tumors, microwave ablation, guidance, ultrasound
Introduction
Percutaneous microwave ablation of hepatic tumors has been acknowledged in minimally invasive, definite curative effect, repeated treatment and easy procedure, so it becomes one of the preferred treatment methods for non-operated hepatic malignant tumors. Because ultrasound can monitor the hepatic tumor in real-time easily and effectively, the ablation treatment guided by ultrasound has been used the most commonly [1]. However, if hepatic tumors locate in the liver dome, the lesion could not discerned clearly due to the interference of intrapulmonary gas in oblique scan below the inferior margin of the rib. And the gas in costophrenic angle can also affect the observation of ultrasound in oblique scan along the right intercostal space. Although deep breath may help the visualization of small hepatic tumors in the hepatic dome, holding breath can not last long enough for the ablation operation. In addition, puncture point below the costal margin increases the operating difficulties due to costal arch barrier, puncture distance and causing obvious pain. Artificial pleural effusion can solve these problems encountered in intercostal puncture. In this study, a retrospective analysis was made in percutaneous microwave ablation of hepatic tumors near the diaphragm guided by ultrasound in our department, in order to disclose its feasibility, complications and clinical application value of artificial hydrothorax.
Materials and methods
The object
From October, 2008 to February, 2012, there were 14 hepatic tumors of 11 patients who received percutaneous microwave ablation through artificial pleural effusion guided by ultrasound in our department. Among them, the tumors contained primary liver cancer in 4 cases, metastatic liver cancer in 7 cases. The lesions located on the liver segment IV in 2, segment VII in 9, segment VIII in 3 respectively. Because of the effect of intrapulmonary gas, these 14 tumors could not be showed clearly. After artificial hydrothorax, ultrasound could clearly display these 14 tumors, which ranged from 1.0 to 4.1 cm in diameter. All patients obtained informed consent.
Equipment and materials
For artificial hydrothorax, we used 16 G venous indwelling needle and Normal Saline or 5% Glucose Injection Solution. The model of ultrasound was Aloka SSD-alpha 10, Esaote Mylab Twice color Doppler apparatus with the probe frequency of 1.5-3.5 MHz. The instrument of microwave ablation used KY-2000 microwave ablation therapeutic equipment.
Methods
Artificial pleural effusion: The patients had routine preoperative pulmonary function tests had been ascertained nothing remarkable. After that, the bag of Normal saline or 5% Glucose injection solution was hanged on a stand in a height of 0.8-1.0 meter to the examination and operation table. The patient kept in supine position with elevation in head side in 30 degrees and a flexed right arm beside the head. Next, ultrasound scan confirmed the location of the right costophrenic angle in the 7th intercostal space between anterior axillary line and middle axillary line. Then, doctor disinfected the skin and laid the towels and made local anesthesia with 1% lidocaine. Needle puncture was performed under the guidance of ultrasound using 16 G venous indwelling needle. While the needle was closing to the costophrenic angle, the switch of transfusion was opened. To advance the needle slowly and observe the drip bucket carefully, if the liquid dropped and the feeling of resistance lost and even more the liquid flew smoothly and fluctuated with respiration, it indicated that the puncture needle entered into thoracic cavity. To close the switch of transfusion, doctor continued to transfer the indwelling tube and open the flow regulator to the maximum. Ultrasonic screen would display the compressed lung tissue and the water-filled costophrenic angle. As a result, ultrasound could observe the tumors near the diaphragm clearly. The whole process needed 15-20 min and 1000-1500 ml fluid. At last, the indwelling catheter was maintained and the hydrothorax was discharged by negative pressure.
Percutaneous microwave ablation under the guidance of ultrasound (Figure 1): The ablation was performed under local anesthesia in accompany with drugs of sedation and analgesia. Repeated the former procedure of artificial hydrothorax, doctor punctured the tumor in the condition that the needle did not pass through the thoracic cavity and the ablation electrode did not contact with the diaphragm directly. During the operation, microwave ablation adopted single puncture for the nodules in diameters below 2.5 cm and multiple punctures for the nodules in diameters larger than 2.5 cm. Three days later, ultrasonography was performed in the patients repeatedly.
Figure 1.
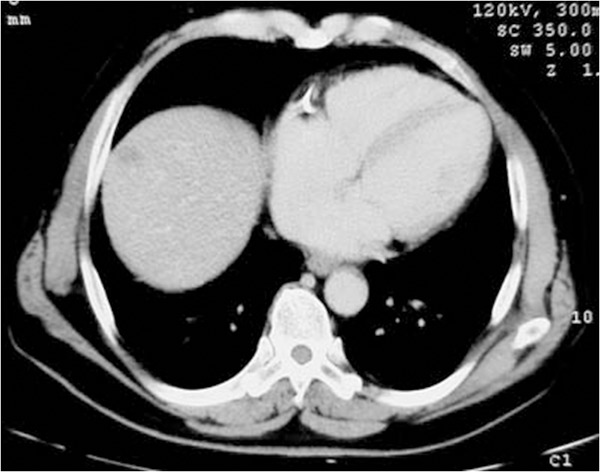
Male, 64-year-old. After surgical operation of cholangiocarcinoma, CT shows a new intrahepatic nodule half a year later. The nodule is in the right hepatic lobe near the dome of the diaphragm. It is difficult to be displayed by ultrasound because of effect of the surrounding intrapulmonary gas.
Observation index
Doctor recorded the time of artificial hydrothorax, the volume of injected fluid, the successful rate of operation and the blood oxygen saturation. To access the visualization of tumors and puncture route and the operation related complications, doctor observed the patients carefully whether the patient had chest pain, dyspnea and other symptoms. After operation, the patient had a chest X-ray film taken to rule out pneumothorax. Three days after the operation, ultrasound examination checked if there were pleural effusion. If the pleural effusion reached to medium degree, the case would be cataloged in operation related complication. Immediately after ablation, ultrasonic contrast examination was conducted to definite the range of the ablation and residual tumor. If there was a definitive residual tumor, a remedy ablation would be supplemented. After 1, 3, 6, 12 and 24 months respectively, contrast enhanced CT or MRI examination reexamined the ablated hepatic tumors and a comprehensive evaluation of ablation effect would be conducted. Meanwhile, the tumor marker, AFP was monitored in this period of time.
Results
Eleven patients were successfully completed the artificial hydrothorax. Through this fluid window, ultrasonography could display the hepatic tumors near the diaphragm clearly and find suitable puncture route for microwave ablation (Figures 2, 3, 4, 5 and 6). Ultrasound examination could monitor the whole process of puncture and microwave ablation of the tumors. After treatment, the echo of the tumors would be enhanced, while there were no remarkable changes in the echo of diaphragm and its surrounding tissues.
Figure 2.
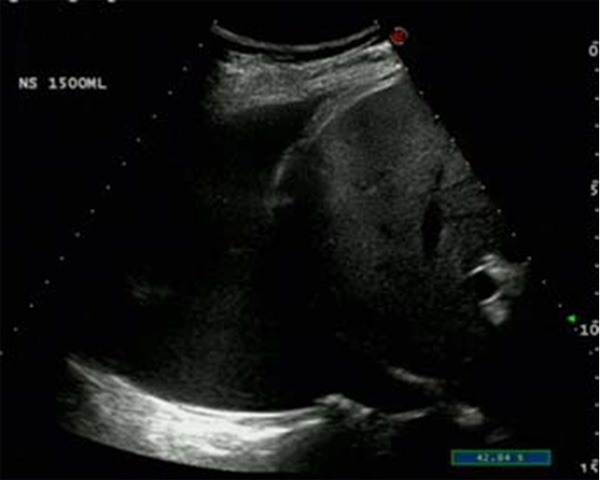
After injection of Normal saline in 1500 ml in the right thoracic cavity, ultrasonography displays the diaphragm and hyper echo nodule in the segment VIII of the liver via the scan in the intercostal space in the middle axillary line.
Figure 3.
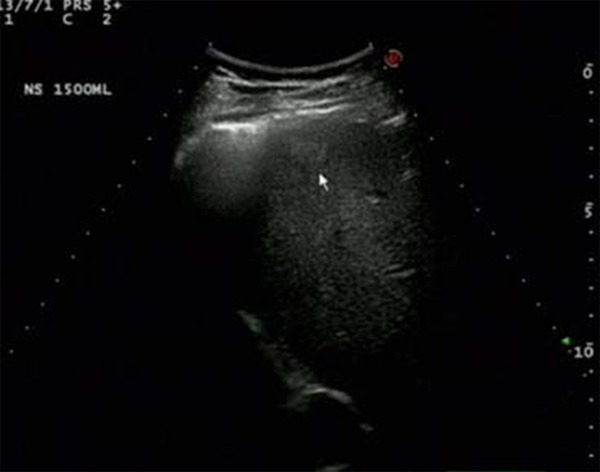
After artificial hydrothorax, ultrasonography displays a hyper echo nodule in the segment VIII of the liver via the scan in the intercostal space in the anterior axillary line.
Figure 4.
After intravenous injection of SonoVue, ultrasonography shows that the whole nodule has been enhanced evidently in arterial phase (21 s) via the scan in the intercostal space in the anterior axillary line and has a stronger signal than liver parenchyma.
Figure 5.
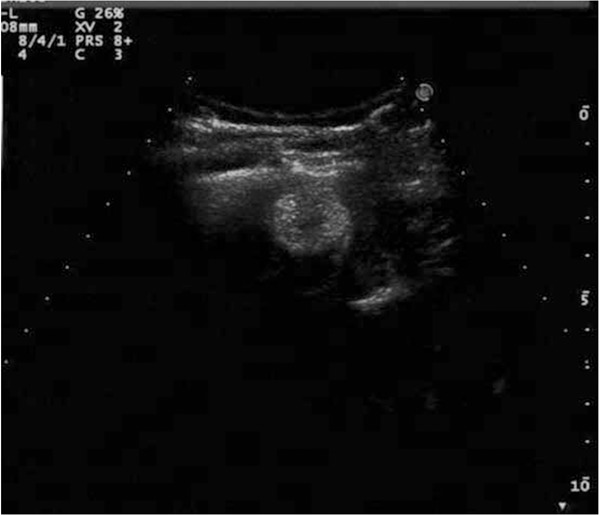
After intravenous injection of SonoVue, ultrasonography shows that the whole nodule has been enhanced slightly in portal phase (31 s) via the scan in the intercostal space in the anterior axillary line and has a lower signal than liver parenchyma remarkably.
Figure 6.
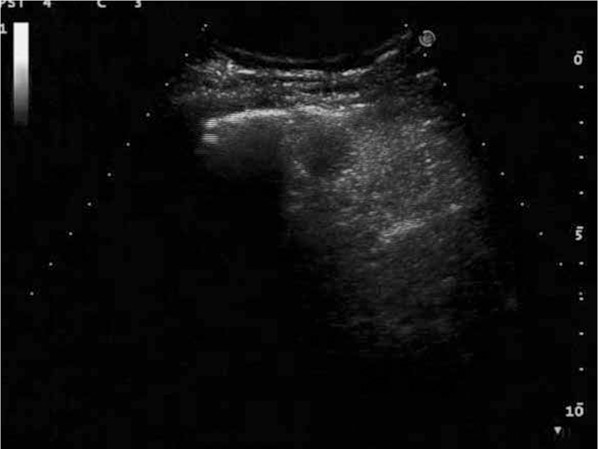
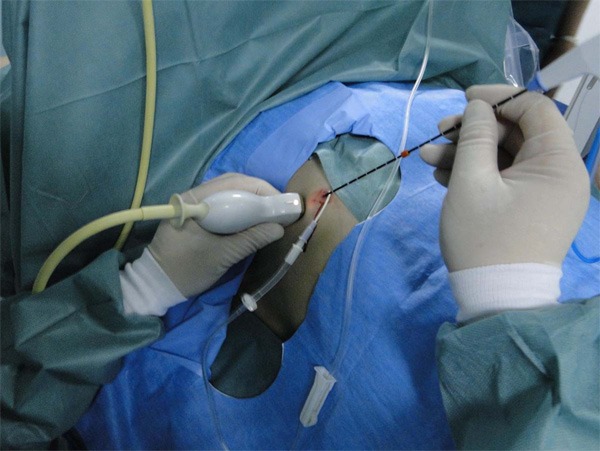
After artificial hydrothorax, the microwave ablation of liver tumor is being performed in the anterior axillary line guided by ultrasound.
Artificial pleural effusion had been driven out almost completely after operation without intrathoracic hemorrhage and pneumothorax. Three days later, 5 patients had a minor right pleural effusion and 6 patients had no pleural effusion.
The 14 hepatic tumors were successfully completed the microwave ablation treatment under the guidance of ultrasound. The result of postoperative contrast-enhanced examination and postoperative follow-up were satisfaction (Figure 7A-E). Two years after the operation, the ablation rate was 92.9% without severe complications.
Figure 7.
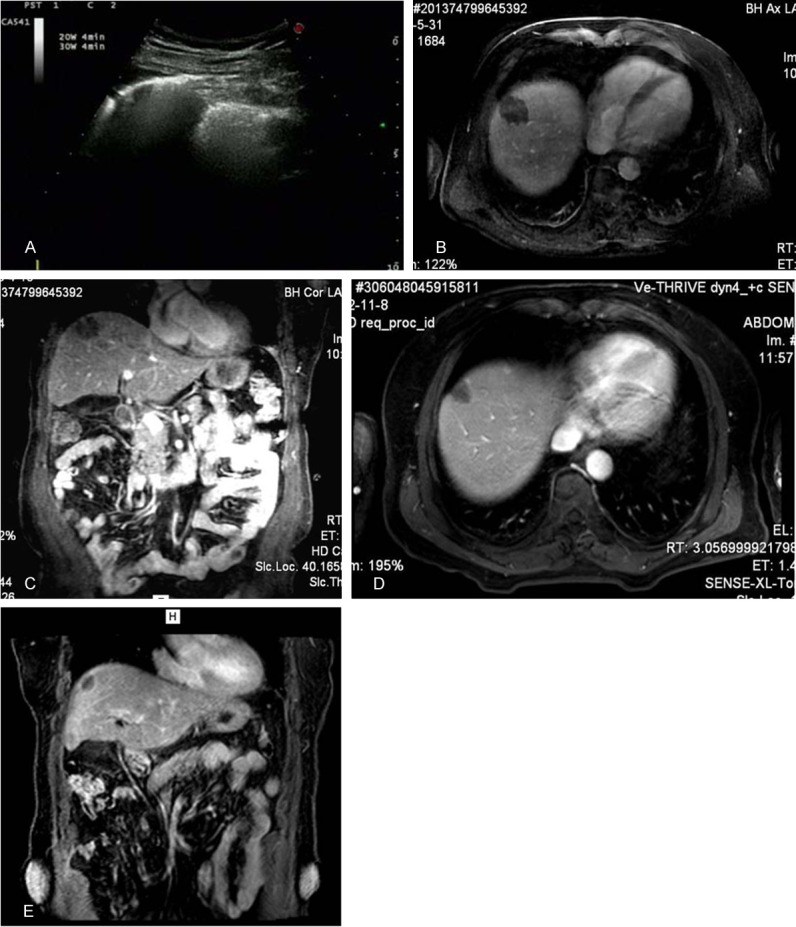
After microwave ablation of liver tumor guided by ultrasound, the whole nodule is covered by the gas (A). A day after microwave ablation, the nodule has no enhancement on MRI examination on axial image (B) and coronal image (C). Five months later, the nodule becomes smaller than before and has no enhancement either on axial image (D) and coronal image (E).
In total of 11 cases, 8 cases had available data of AFP (Normal value ≤20 μg/L) in following up. The average value of AFP was 182.6±128.4 μg/L, before the operation, and 15.9±10.8 μg/L, 9.4±2.6 μg/L, 8.9±2.3 μg/L, 8.8±2.5 μg/L and 8.2±2.2 μg/L after operation in the corresponding time of a month, 3 months, 6 months, 12 months and 24 months respectively. In ANOV test, only the value of AFP before operation had significant difference with the others (P = 0.000). However, the values of AFP has no statistic difference among the following up after operation. The data of AFP was shown in detail in Table 1.
Table 1.
The changes of the value of AFP before and after operation
| Normal value of AFP ≤20 μg/L | Within a week before operation | A month after operation | 3 months after operation | 6 months after operation | 12 months after operation L | 24 months after operation |
|---|---|---|---|---|---|---|
| 1 | 98.3 | 13.9 | 12.4 | 11.6 | 12.5 | 10.9 |
| 2 | 334.8 | 34.6 | 9.5 | 8.6 | 9.7 | 8.0 |
| 3 | 36.5 | 6.5 | 5.7 | 6.4 | 7.8 | 6.2 |
| 4 | 197.5 | 18.4 | 10.4 | 8.9 | 9.4 | 6.8 |
| 5 | 45.3 | 5.6 | 6.4 | 5.1 | 4.9 | 6.2 |
| 6 | 245.7 | 12.5 | 10.8 | 11.4 | 10.5 | 9.9 |
| 7 | 376.4 | 28.8 | 12.5 | 10.3 | 9.4 | 11.3 |
| 8 | 126.4 | 6.8 | 7.5 | 8.5 | 5.9 | 6.1 |
Discussion
Resection of hepatic tumor is still an effective and radical method at present, but some tumors are not appropriate for resection or the patient would not receive operative treatment. Percutaneous microwave ablation guided by ultrasound is a new technology in recent years for the treatment of hepatocellular carcinoma in a reliable effect and good compliance. As reported, microwave ablation of small hepatocellular carcinoma reached the necrosis rate of 80%~90%, while the local recurrence rate was 5%~15% [2-4]. The whole operational process is guided by ultrasound which has features of real-time monitoring, good accuracy, and light and flexible, especially for tumors in the “risk hepatic area” [5]. Because our patients presented the tumors near the diaphragm, conventional ultrasound could not show the tumors clearly or completely due to block of ultrasound wave by the surrounding intrapulmonary gas. Artificial pleural effusion created a good “window” to help ultrasound demonstrate the tumors clearly and indicate the suitable puncture route. According to strict inclusion criteria, all single tumor was less than 5 cm in diameter; all multiple tumor was less than or equal to 3 cm in diameter and no more than 3 pieces in number. Although the tumors were in the “risk hepatic area”, the ablation treatment could also be completed. In order to reduce local thermal injury to the diaphragm, doctor should review and assess the image data in detail in size of the tumor(s), the direction and angle and depth of the puncture, ablation time and power before operation. Ablation should be processed in a low power state and monitored in real-time by ultrasonography. Doctor kept close watch on the temperature control and scope of coagulative necrosis [6].
The formation of artificial pleural effusion was safe, simple under the guidance of ultrasound. When the puncture needle was located in the pleural cavity, doctor pushed the retaining deep vein catheter in a certain length and drew out the puncture needle, then to accelerate the velocity of liquid. The retaining deep vein catheter could also act as a drainage tube of the hydrothorax and indicate whether there was blood pneumothorax, pneumothorax and etc. For patients in normal pulmonary function, they could tolerate artificial pleural effusion under the observation of blood gas analysis and the blood oxygen saturation during the operation [7]. There were no intrathoracic hemorrhage and pneumothorax in 3 days after operation, but 5 patients occurred minor right pleural effusion. Application of artificial hydrothorax compressed the pulmonary gas help ultrasound show the tumors near the hepatic dome clearly and find a suitable puncture route. It broadened the microwave ablation range for tumor near the diaphragm, and taken away the heat produced during microwave ablation to reduce burning pain of patient [8]. In addition, for patient with abdominal operation, which may cause adhesion of diaphragmatic muscle and liver, artificial hydrothorax can be the first choice to help localize and puncture the tumors in the hepatic dome [9]. Artificial hydrothorax is an invasive operation. The following notices are very important to reduce or avoid complications: real-time observation of the needle tip under the guidance of ultrasound and putting the velocity switch to the maximum while the needle tip is closing to the pleura; slow and shallow respiration of patient to avoid the needle piercing the visceral pleura and causing pneumothorax or hemothorax.
Although CT/MRI could clearly show the lesions near the diaphragm, those can not conduct a real-time puncture. For large tumor in the hepatic dome, the chances of overlapping ablation will be increased as well as injury of the diaphragm. The injured diaphragm becomes weak and has a risk of hernia in the condition of high abdominal pressure in ascites of liver cirrhosis and refractory pleural effusion. However, in approach of artificial pleural effusion, it can protect the diaphragm from heat injury and decrease the risk rate [10]. A good therapeutic effect has been got in laparoscopic ablation for hepatic tumor near the diaphragm in the aid of artificial hydrothorax and ascites, but the operation is under general anesthesia and has possible complications, such as vascular injury, visceral damage, subcutaneous emphysema, hypercapnia, lower extremity deep venous congestion and thrombosis and etc [11]. In this group of patients, artificial hydrothorax was successful under the guidance of ultrasound in 14 tumors and helps the microwave ablation in a satisfactory effect based on the observation of ultrasonic enhancement in ablation rate of 92.9% without serious complications 2 years after the operation. Artificial pleural effusion provides a good “liquid window” to help display tumor in the hepatic dome as a ultrasound blind area, find a suitable puncture route, and broaden the therapeutic indication, so some hepatic tumors can got a chance to be treated. Artificial pleural effusion is a safe and feasible method to be worthy to recommand its clinical application. During 2 years following up, there were no signs of local recurrence or metastases according to the result of CT/MRI enhanced examination and tumor marker-AFP. In a long run, no complications can be found in secondary pleural effusion, pleural adhesion and seeding metastasis in the pleural cavity.
Disclosure of conflict of interest
None.
References
- 1.Rhim H, Lim HK. Radiofrequency ablation for hepatocellular carcinoma abutting the diaphragm: the value of artificial ascite. Abdom Imaging. 2009;34:371–380. doi: 10.1007/s00261-008-9408-4. [DOI] [PubMed] [Google Scholar]
- 2.Ng KK, Poon RT, Chan SC, Chok KS, Cheung TT, Tung H, Chu F, Tso WK, Yu WC, Lo CM, Fan ST. High-intensity focused ultrasound for hepatocellular carcinoma: a single-center experience. Ann Surg. 2011;253:981–987. doi: 10.1097/SLA.0b013e3182128a8b. [DOI] [PubMed] [Google Scholar]
- 3.Koda M, Ueki M, Maeda Y, Mimura K, Okamoto K, Matsunaga Y, Kawakami M, Hosho K, Murawaki Y. Percutaneous sonographically guided radiofrequeney ablation with artificial located under the diaphragm. AJR Am J Roentgenol. 2004;183:583–588. doi: 10.2214/ajr.183.3.1830583. [DOI] [PubMed] [Google Scholar]
- 4.Livraghi T, Solbiati L, Meloni MF, Gazelle GS, Halpern EF, Goldberg SN. Treatment of focal liver tumors with percutaneous radio frequency ablation: complications encountered in a multicenter study. Radiology. 2003;226:441–451. doi: 10.1148/radiol.2262012198. [DOI] [PubMed] [Google Scholar]
- 5.Maruyama H, Takahashi M, Ishibashi H, Okugawa H, Okabe S, Yoshikawa M, Yokosuka O. Ultrasound-guided treatments under low acoustic power contrast harmonic imaging for hepatocellular carcinomas undetected by B-mode ultrasonography. Liver Int. 2009;29:708–714. doi: 10.1111/j.1478-3231.2008.01875.x. [DOI] [PubMed] [Google Scholar]
- 6.Wijlemans JW, de Greef M, Schubert G, Moonen CT, van den Bosch MA, Ries M. Intrapleural fluid infusion for mr-guided high-intensity focused ultrasound ablation in the liver dome. Acad Radiol. 2014;21:1597–602. doi: 10.1016/j.acra.2014.06.015. [DOI] [PubMed] [Google Scholar]
- 7.Zhang D, Liang P, Yu X, Cheng Z, Han Z, Yu J, Liu F. The value of artificial pleural effusion for percutaneous microwave ablation of liver tumour in the hepatic dome: a retrospective case-control study. Int J Hyperthermia. 2013;29:663–670. doi: 10.3109/02656736.2013.833347. [DOI] [PubMed] [Google Scholar]
- 8.Kang TW, Rhim H, Lee MW, Kim YS, Choi D, Lee WJ, Lim HK. Radiofrequency ablation for hepatocellular carcinoma abutting the diaphragm: comparison of effects of thermal protection and therapeutic efficacy. AJR Am J Roentgenol. 2011;196:907–913. doi: 10.2214/AJR.10.4584. [DOI] [PubMed] [Google Scholar]
- 9.Lee MW, Lim HK, Kim YJ, Choi D, Kim YS, Lee WJ, Cha DI, Park MJ, Rhim H. Percutaneous sonographically guided radio frequency ablation of hepatocellular carcinoma: causes of mistargeting and factors affecting the feasibility of a second ablation session. J Ultrasound Med. 2011;30:607–615. doi: 10.7863/jum.2011.30.5.607. [DOI] [PubMed] [Google Scholar]
- 10.Groeschl RT, Pilgrim CH, Hanna EM, Simo KA, Swan RZ, Sindram D, Martinie JB, Iannitti DA, Bloomston M, Schmidt C, Khabiri H, Shirley LA, Martin RC, Tsai S, Turaga KK, Christians KK, Rilling WS, Gamblin TC. Microwave ablation for hepatic malignancies: a multiinstitutional analysis. Ann Surg. 2014;259:1195–1200. doi: 10.1097/SLA.0000000000000234. [DOI] [PubMed] [Google Scholar]
- 11.Song I, Rhim H, Lim HK, Kim YS, Choi D. Percutaneous radiofrequency ablation of hepatocellular carcinoma abutting the diaphragm and gastrointestinal tracts with the use of artificial ascites: safety and technical efficacy in 143 patients. Eur Radiol. 2009;19:2630–2640. doi: 10.1007/s00330-009-1463-x. [DOI] [PubMed] [Google Scholar]


