Abstract
Hashimoto’s encephalopathy (HE) is a very rate condition characterized by various clinical features consisting of psychiatric manifestations, seizures and focal neurologic deficits. In this study, three Hashimoto’s encephalopathy cases were reported, including two female patients and one male patient. The two female patients (22-year-old and 49-year-old) were presented with brainstem involvement and the symptoms progressed gradually. The 70-year-old male patient was diagnosed with space-occupying lesion that seemed to be malignant, but the symptom was substantially ameliorated right after glucocorticoid therapy. Pathological studies indicate abnormal blood vessels are important in the progression of the disease. Compared with current reports, the male case was the first patient diagnosed of Hashimoto’s encephalopathy but presented with malignant features and local occupying effect. Intracranial lesions were found in all of the three patients, but these lesions responded well to glucocorticoid therapy. And the lesions were remarkably reduced after treatment. Meanwhile, the prognosis of diffuse Hashimoto’s encephalopathy is better than the vascular type.
Keywords: Hashimoto’s encephalopathy, diffuse type, vascular type, glucocorticoid therapy, follow-up
Introduction
Hashimotob encephalopathy (HE), a rare autoimmune disease with unknown origin, is referred to as non-vasculitic autoimmune encephalopathy/meningoencephalitis. Hashimoto’s thyroiditis or steroid responsive encephalopathy associated with autoimmune. HE cases are often described with unexplained symptoms including psychiatric manifestations, seizures, cognitive impairment and coma. HE is a rare disease with an estimated prevalence of 2 per 100,000 people. The mean age of symptoms’ onset ranges from 45 to 55 years. The condition is more frequently found in females than in males, with a ratio of ~5:1. [1,2]. More than 100 scientific articles on Hashimoto’s Encephalopathy have been reported and published between 2000 and 2013 (http://hesaonline.org/links-to-scientific-articles-and-published-case-reports). But limited progress has been made in the exploration of effective therapies, and steroids remain the first line treatment for this condition. Here, three cases were covered here including 2 female and 1 male patients, and female patients were presented with brainstem involvement and the symptoms progressed gradually. The male one was diagnosed with space-occupying lesion in the brain that seemed to be malignant. However, the intracranial lesions were significantly reduced after glucocorticoid treatment.
Materials and methods
Case report
Case 1
A 49-year-old previously healthy woman was admitted to hospital with a history of hiccupping for 2 months, gait instability for 1 month, urinary retention for 4 days, and weakness of limbs accompanied with dyspnea for 1 day on Jan 15 2012. She has been hospitalized 2 months ago due to hiccupping, nausea and vomiting. After symptomatic treatment, symptoms were ameliorated. She also described vertigo, gait instability and diplopia which has lasted for one mouth. Urinary retention came out 4 days ago accompanied with dysphagia, cough and hoarseness. One day before hospitalization, the patient experienced limbs weakness, dyspnea, and discontinuous unconsciousness. The physical examination was otherwise normal except impaired consciousness, mild dysarthria, and bluntness of light reflex. Meanwhile, physical examination also revealed nystagmus, weakened extremities’ muscle strength (graded II), increased lower muscular tension, hyporeflexia in limb tendon, and positive Babinski sign. Her past medical history was unremarkable. Results of electromyography (EMG) and head CT were negative. Electroencephalogram (EEG) images indicated widespread mild abnormalities, and AQP4 test was negative. The results of cerebrospinal fluid tests showed that the concentration of protein was 0.4 g/L and the cell count was 5*106/L, there were no white blood cells. The concentrations of glucose, sodium and chlorine were normal. Magnetic resonance imaging (MRI) of the brain showed T2 (long) and FLAIR (high) signal in right dorsal side of medulla oblongata and connection between medulla oblongata and cervical spinal. And T2 (long) and FLAIR (high) signal with symmetric distribution were detected in both sides of pons (Figure 1A). Thyroid function tests were performed (FT3 4.1 pmol/L, FT4 12.9 pmol/L, TSH 1.0 μIU/mL, aTPOAb 686.9 U/mL). She was treated using r-globulin (0.4 g/kg/d) twice, from Jan 16 to Jan 20 and then from Jan 29 to Feb 1. Simultaneously, she was treated using methylprednisolone (500 mg/d) from Jan 20 to Jan 22, and the dose then was reduced to 250 mg/d, 125 mg/d, 60 mg/d, and 30 mg/d every three days. Finally, the dose was set to be methylprednisolone 16 mg/d for three days. After the treatment, the symptoms improved remarkably, and thyroid function tests indicated the same (FT3 3.9 pmol/L, FT4 12.8 pmol/L, TSH 0.9 μIU/mL, aTPOAb 269.6 U/mL). The patient continued methylprednisolone at a dose of 16 mg/d after discharged, and maintained treatment with gradually reduced dose. After 3 months, the patient was still on methylprednisolone, and the MRI revealed reductions of abnormal signals in the same places as described above (Figure 1B). Thyroid function came out as FT3 4.39 pmol/L, FT4 13.11 pmol/L, TSH 1.03 uIU/mL, aTPOAb 268 U/mL. The patient took hormone orally for two years, and there was no recurrence of symptoms. Repeats of MRI imaging showed that the abnormalities observed before in the brain was gone (Figure 1C).
Figure 1.
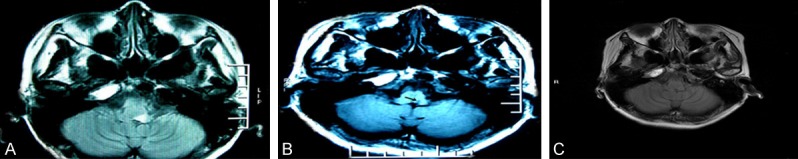
Head MRI in case 1. A. T2 (long) and FLAIR (high) signal with symmetric distribution were detected in both sides of pons at the time of diagnosis. B. After 3 months, the MRI revealed reductions of abnormal signals in the same places as described above. C. After two years, repeats of MRI imaging showed that the abnormalities observed before in the brain was gone.
Case 2
The 22-year-old female accountant complaining about dizziness and double vision for 3 months, hiccupping and vomiting for 1 month. She was hospitalized on Feb 28 2012. Physical examination: clear consciousness and eloquent speech, rough nystagmus in the right eye and slight nystagmus in the left eye, sensitive light reflex, blunt pharyngeal reflex, muscle strength of both upper limbs graded V, muscle strength of both lower limbs graded IV, active tendon reflexes in both upper limbs, tendon hyperreflexia in both lower limbs, positive ankle clonus, positive Babinski sign on both sides, positive Romberg sign, and hypaesthesia on the left side of body. Past medical history was unremarkable. The results of head MRI performed 3 months ago revealed high T2 signals and FLAIR signals in both dorsal sides of midbrain and right dorsal side of pons (Figure 2A). These abnormalities reduced or disappeared when examined again, but high FLAIR signals in right dorsal side of medulla oblongata and the junction between medulla oblongata and cervical spinal still exist (Figure 2B). Thyroid function tests after admission came out as FT3 4.93 pmol/L, FT4 17.15 pmol/L, TSH 18.39 μIU/mL, aTPOAb>1300 U/mL, ATG 2733 U/mL. The ultrasound imaging results of thyroid revealed enlargement of the echo, and low echo on the right lobe. Electroencephalogram and cerebrospinal fluid tests were negative, as well as the AQP4 tests The patient was diagnosed with Hashimoto’s thyroiditis, and subclinical hypothyroidism. She was treated with Thyroxine (25 μg/d), and methylprednisolone (1000 mg/d) from March 2 to March 4. Then, the dose of methylprednisolone was reduced to 500 mg/d and 60 mg/d every three days after. When the treatment was finished, the symptoms were ameliorated. The patient continued methylprednisolone after discharged, with its dose gradually reduced, and was treated with Tthyroxine as well at the same dose. 3 months later, the patient came back with methylprednisolone stopped for one month already. She reported blurred vision in her right eye. After examined with ophthalmofundoscope, mild edema and obscure was observed on the right side, and then the patient was diagnosed with optic nerve inflammation. Head MRI showed that abnormal signals in the brainstem disappeared, and thyroid function tests came out as FT3 4.04 pmol/L, FT4 14.26 pmol/L, TSH 37.96 μIU/mL, aTPOAb>1300 U/mL. Follow-up investigations showed no recurrence of the symptoms as she was kept on the treatment of Hashimoto encephalopathy.
Figure 2.
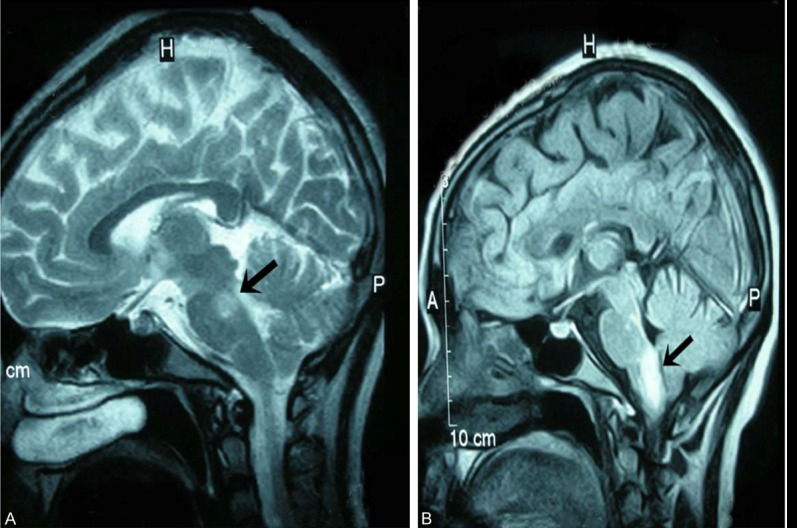
Head MRI in case 2. A. The head MRI performed at the time of diagnosis revealed high T2 signals and FLAIR signals in both dorsal sides of midbrain and right dorsal side of pons. B. After 3 months, high FLAIR signals in right dorsal side of medulla oblongata and the junction between medulla oblongata and cervical spinal still exist.
Case 3
A 70-year-old male retired worker was complaining with alalia for 40 days and admitted on Nov 14 2011. Physical examinations: clear consciousness, impaired speech, limb muscle strength graded V, normal limb muscular tension and limb tendon reflexes, negative Babinski sign. He was diagnosed with hypothyroidism for 8 years. Head MRI results indicated that the left frontal lobe veins was infarcted, and other lesions were not excluded (Figure 3A). After treated with therapies of antiplatelet, anticoagulation, and circulation improvement, symptoms was ameliorated and the patient was discharged. Then, the patient came back again on Apr 5 in 2012 with a complaint of progressive right-sided hemiparesis and logagnosia for 1 month, aconuresis and dysphagia for 6 days. Physical examinations: clear consciousness, deteriorated memory, slow response, motor aphasia, shallow nasolabial sulcus on the right side, drooping angulus oris on the right side, muscle strength of the right limbs graded III, muscle strength of the left limbs graded V, normal limb muscle tension, active limb tendon reflexes, and negative Babinski sign. Thyroid function results: FT3 2.37 pmol/L, FT4 8.04 pmol/L, TSH 78.95 uIU/mL, aTPOAb>1300 U/mL. Electroencephalogram showed extensive abnormalities, while both the AQP4 and cerebrospinal fluid testes were negative. Head MRI results showed occupying lesions in both frontal lobes and the left parietal lobe with uneven enhancement. Both metastatic tumors and glioblastoma were considered according to the results described as above. Both MRA and MRV revealed nothing (Figure 3B), as well as the CT of the lung. The patient was treated with Thyroxine (75 μg/d) and hexadecadrol (10 mg/d) from April 6 to April 14, and the dose of hexadecadrol was then reduced to 5 mg/d. The patient was discharged because the symptoms were obviously improved. He was advised to continue prednisone (30 mg/d) and Thyroxine, with the dose of prednisone gradually reduced. The patient came back 1 month later, still on methylprednisolone. Thyroid function tests: FT3 3.94 pmol/L, FT4 12.82 pmol/L, TSH 25.56 μIU/mL, aTPOAb 524.2 U/mL. Head MRI restuls indicated that areas of lesions and edema were obviously smaller in the same sites, and the midline shift was relieved (Figure 3C). The patient was on follow-up clinic for 2 years, and he was back to normal life with continuous medication. Rechecked brain MRI was shown in Figure 3D.
Figure 3.
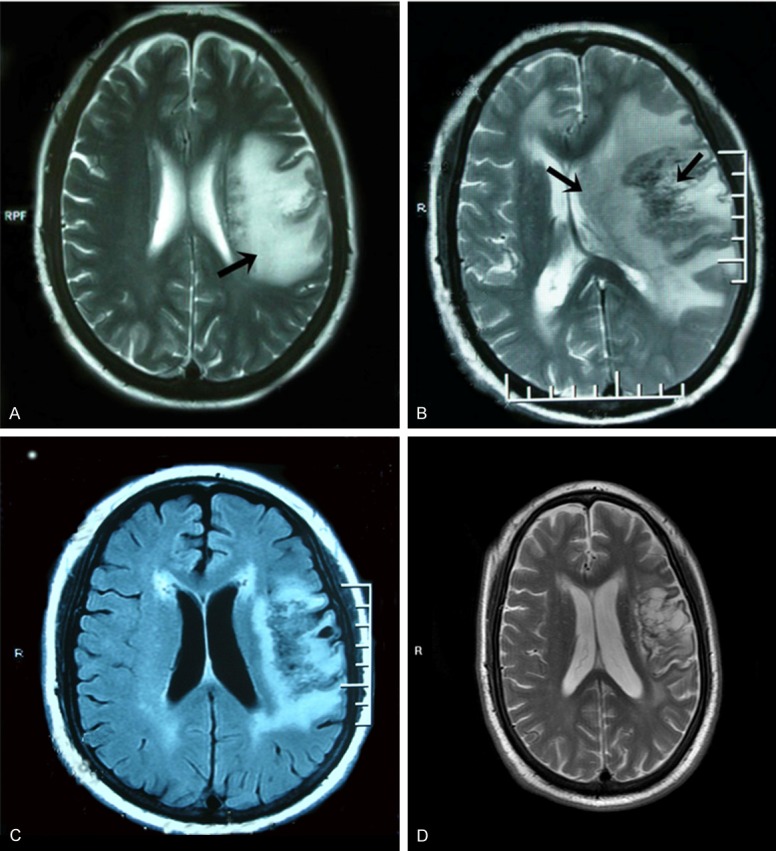
Head MRI in case 3. A. Head MRI indicated that the left frontal lobe veins was infarcted, and other lesions were not excluded. B. Head MRI showed occupying lesions in both frontal lobes and the left parietal lobe with uneven enhancement. C. Head MRI one month later indicated that areas of lesions and edema were obviously smaller in the same sites, and the midline shift was relieved. D. Rechecked brain MRI of the patient with continuous medication after two years.
Discussion
Case 1 came with intractable hiccupping which gradually progressed to stumbling and uroschesis, and her medulla and respiratory muscles were involved later on. Finally, unconsciousness and dyspnea appeared, and MRI results indicated abnormal signals in medulla and pons. The symptoms were obviously improved after the steroid therapy, his aTPOAb titter was reduced and encephalic lesions was obviously ameliorated as well. These results implied that damaged medulla could be the cause of initial symptoms in these patients with Hashimoto’s encephalopathy, and then progressed to a stage on which the corticospinal tract was involved. The patients can benefit from the steroid therapy, which may be an important feature of Hashimoto’s encephalopathy. Case 2 was admitted due to dizziness, which then processed to hiccupping and vomiting. Nervous system examination indicated damage in medulla, pons and cerebellar peduncle. MRI characteristics of the patient were: lesions in midbrain and pons were worse as the disease progressed (the possibility that the patient was ever treated using steroid was not excluded) and gradually disappeared, but the new lesion was detected in medulla and junction of medulla and cervical spinal (the new lesion was also derived from improper decrement or termination of the steroid). Similar to most patients with Hashimoto’s encephalopathy, the patient responded quickly to the steroid treatment, but she stopped the steroid by herself and this leaded to optic neuritis (The symptom was not accompanied with damaged spinal cord. According to previous medical history, diagnose of the optic nerve myelitis was not supported). Head MRI results was negative. The patient developed strong dependency on steroid, as shown by the increased aTPOAb titer when the optic neuritis appeared Case 3 was about a male patient. Hashimoto encephalopathy was rare in males, and this was a chronic case. Lesion was located in subcortical white matter, and it could be a malignant lesion because MRI detection showed obvious space occupying effect, obvious midline shift and edema. The symptoms were obviously improved after the steroid treatment, and MRI also showed that midline shift and edema were resolved. According to published reports, the patient in this case was the first one with hashimoto’s encephalopathy that we found malignant lesion with obvious space occupying effect.
Clinical features of Hashimoto’s encephalopathy have been divided into two types [3]: ① blood vessel type: acute stroke-like onset, clinical manifestations include hemiplegia, aphasia, partial anesthesia, ataxia, pyramidal tract symptoms, cerebellum symptoms, and mild cognitive impairment; ② diffusive type: slow-onset, clinical manifestations include drowsiness, myoclonus, stupid, epileptic seizure, megrim, mental disorder and disturbance of consciousness. Other clinical manifestations are presented as: atypical choreic movements, depression, stiff man syndrome, salatal muscles tremor, creutzfeldt-jakob disease, headache and fatigue [2,4,5]. Symptoms are usually periodical, some of them could end up with spontaneous remissions, and some could benefit from the steroid treatment. For some female patients, the onset of symptoms parallels with the menstrual cycles, and symptoms could be ameliorated or even cured if medications are used to disturb menstrual cycles. Clinical manifestations in elder patients lack special features, so it is difficult to diagnose and the prognosis is usually poor. This is particularly true when it comes to the impairment of cognitive functions [6]. Hashimoto’s encephalopathy is also accompanied with other autoimmune diseases. A report including 20 cases demonstrated that about 30% of the patients can be diagnosed with other autoimmune diseases, including type-I diabetes, SLE and Sjogren’s syndrome.
However, it’s still not clear whether the connection between autoimmune thyroid disease and Hashimoto encephalopathy exists or not. For patients with Hashimoto’s encephalopathy, the results of thyroid function tests could turn out to be normal, subclinical hypothyroidism or hypothyroidism, subclinical hyperthyroidism or hyperthyroidism. Of these, patients with subclinical hypothyroidism are most common [4,7]. No significant correlation has been found between improved nervous system symptoms and the improvement of thyroid functions. Although both Grave’s disease and Hashimoto’s encephalopathy are autoimmune thyroid diseases, patients with Hashimoto encephalopathy are rarely diagnosed with subclinical hyperthyroidism or hyperthyroidism. Payer et al. [8] and Chong et al. [9] reported that among 6 patients who were found to be subclinical hyperthyroidism or hyperthyroidism, 2 of them were diagnosed with subclinical hyperthyroidism, and 4 were diagnosed with hyperthyroidism (thyrotoxicosis). Neurological symptoms in one patient with hyperthyroidism would not be remarkably improved after anti-thyroid medication, but the symptoms could be significantly improved with the steroid treatment.
Two types of antibodies, against TSH receptor and thyroid gland [1], play important roles in the pathogenesis of autoimmune thyroid disease. Compared with the first type of antibodies, the second type could be more important in the pathogenesis of Hashimoto’s thyroiditis, including anti-peroxidase antibodies, anti-globulin antibody and anti-sodium-iodine symporter antibodies. Almost all patients with Hashimoto’s encephalopathy can be detected with high titers of anti-peroxidase antibodies in serum [1,2]. However, Anti-peroxidase antibodies could also be detected in about 10% of the healthy population, especially in women. The antibody is also found in many cerebropathy and autoimmune diseases, the anti-thyroid antibodies can be detected in serums from patients with tumor, abnormal hyperplasia of the bone marrow, and patients with hepatitis treated by interferon and interleukin-1 and colony stimulating factor. However, once interferon and the other related medications are stopped, the titers of these antibodies decrease spontaneously [10]. Furthermore, the generation of anti-thyroid antibodys shows correlation with various genotypes. Literature has shown that HLA B8 and DR3 haplotype have correlations with atrophic thyroiditis, and HLA DR5 haplotype in the white people has correlation with autoimmune goiter. Although some autoimmune thyroid diseases are connected to genotypes, no cases of hereditary Hashimoto encephalopathy has been reported [11-14]. Meanwhile, there is no evidence supporting that anti-thyroid antibody promotes the occurrence of Hashimoto’s encephalopathy, but most scholars believed that autoimmune factors contribute to occurrence of Hashimoto’s encephalopathy, while no significant correlation has been detected between degree of antibody and the severity of the disease [4,5,15]. Therefore, it’s proposed that the autoimmune factors participating in the pathogenesis of thyroid diseases may activate the autoimmune factors that can cause Hashimoto’s encephalopathy, In this report, symptoms of the first and last patients were improved after the steroid treatment, and aTPOAb titer decreased significantly. The second patient was diagnosed with optic neuritis the steroid was stopped, and aTPOAb titer increased remarkably. These findings imply that aTPOAb titer may be an indicator reflecting the progress of disease.
Some reports indicated that generation anti-neuron antibodies cause brain atrophy in patients with Hashimoto’s encephalopathy [6,16,17]. Apart from anti-thyroid antibodies, another antibody named anti-α-enolization antibody was found in the serum of patients with Hashimoto’s encephalopathy, but this antibody could not be found healthy people and in patients with other neuronal diseases. Therefore, this antibody may be the one of the diagnostic markers for Hashimoto’s encephalopathy [18,19]. Another report found a anti-neuron antibody that can recognize a 36kDa antigen [20]. Then, anti-myelin basic protein antibodies and anti-bovine cerebrosides antibodies were detected in these patients, and these antibodies showed that pathogenesis of Hashimoto’s encephalopathy may also involve a sery of autoantibodies against the central nervous system.
According to limited cases reported, the pathological characteristics of this disease can be divided into three types [1,4,21]: ① vascular inflammatory lesions: lymphocytes cells infiltrating into the brain tissues and meninges through the blood vessels nearby; ② demyelinating lesions; ③ non-vascular autoimmune meningoencephalitis: complete vascular structure, inflammatory cells infiltration detected in brain and meninges, mild gliosis. Nicoline Schiess et al. collected 9 cases with pathological reports [22]: One of them was found with ventricle enlargement, 5 cases were detected with lymphocyte infiltration, and anti-neuron antibodies were found in only one of them.
Taken together, the pathogenesis of Hashimoto’s encephalopathy is still unclear. Pathological studies indicate abnormal blood vessels are important in the progression of the disease. Vasculopathy leads to cerebral ischemic anoxia and edema that further cause relevant clinical manifestations (blood vessel type or diffusive type), and the fact that symptoms can be improved using steroid treatment indirectly supports the idea [5]. In the report, Case 3 was treated with therapies of antiplatelet, anticoagulation and circulation improvement when the disease was initially diagnosed, symptoms were then improved, which implies that vascular factors may play a role in initial pathogenesis of Hashimoto’s encephalopathy. Demyelinating lesions and the vascular inflammatory autoimmune meningoencephalitis suggest that autoantibodies contribute to occurrence of the disease. Some autoantibodies, including anti-thyroid antibodies, anti-α-enolization antibodies, anti-myelin basic antibodies and anti-bovine cerebrosides antibodies have been detected, but the pathophysiological significance of them remains unclear. Although no significant correlation has been proven between the impairment of nervous system and thyroid gland dysfunction, anti-thyroid antibodies do not directly contribute to the damages to neurons, but excessive secretion of TSH may cause damage to brain tissues in multiple ways [2,3]. Therefore, the underlying mechanisms of thyroid dysfunction and the pathogenesis of Hashimoto’s encephalopathy couldn’t be separated.
Accessory examinations
Indeed, except for the high titers of autoantibodies that can be detected in serum, other auxiliary examinations in Hashimoto’s encephalopathy can also help with the diagnosis and surveillance.
Electroencephalogram
Nonspecific slowing of background activity is a feature of Hashimoto’s encephalopathy, which may reflect severity of lesion and could be used as a predictor for the progression of disease and effects of treatment [1,3,4]. There are 90% patients whose diffusive slow waves are distributed in the frontal and temporal lobes [15,21].
Cerebrospinal fluid tests
Protein concentrations are increased (fluctuation: 0.48-2.98 g/L) in cerebrospinal fluid among 75-80% of the patients with Hashimoto’s encephalopathy, accompanied with mild increase in lymphocyte counts. Synthesis of IgG in cerebrospinal fluid also increases, but oligoclonal bands are detected only in a small number of patients and anti-thyroid antibodies can be found in some of the patients, circulating immune complexes and 14-3-3 protein in cerebrospinal fluid. However, the specificities of these markers have not been satisfying [1,15].
Imaging
No specific features in imaging have been validated for the diagnosis of Hashimoto’s encephalopathy. The results of CT and MRI could be negative for most of the patients, and in some patients, abnormal T2 high signal can be detected in the subcortical white matter. These abnormalities can disappear with the improvement of disease conditions [3,4]. While no abnormalities could be observed in cerebral angiogram and MRA examination, some patients show reduced perfusion in cortex and basal ganglia when examined with SPECT. However, for these patients, the results of PET would usually be negative [1,4].
Diagnosis and differential diagnosis
As no reliable diagnostic criteria for Hashimoto’s encephalopathy has been validated, the diagnosis of this disease should be after the exclusions of infections, metabolic disorders, intoxication and tumor, when faced with patients suffering from diffusive cognitive dysfunctions or regional brain disorders and presented with high titers of anti-thyroid antibodies. According to Chong et al. [23], diagnosis of Hashimoto’s encephalopathy should meet the following three points: cognitive disorder; cerebrospinal fluid analysis that can exclude infectious diseases; and high titers of anti-thyroid antibodies in serum.
Gianluca Tamagno et al. [24] proposed diagnosis criteria: Requirements: ① acute or subacute onset, focal neurological signs and symptoms or diffuse nervous system damage; ② relevant autoimmune thyroid diseases exist; ③ thyroid dysfunctions could not explain occurrence the nervous system symptoms and signs; ④ exclude infection, metabolic disorders, intoxication and other neurologic disorder; ⑤ responsive to the steroid treatment.
Other conditions considered: ① high titers of anti-thyroid antibodies; ② cerebrospinal fluid protein increase but the count of cells is normal; ③ non-specific EEG abnormalities.
“Definite” diagnosis: satisfy all requirements and other conditions; “highly possible” diagnosis: satisfy all requirements and one of the other conditions; “maybe” diagnosis: satisfy all requirements only.
Differential diagnosis of Hashimoto’s encephalopathy includes [1,5,25]: Progressive dementia-Sporadic Creutzfeldt-Jakob disease, Alzheimer’s disease, dementia with Lewy bodies, and corticobasal degeneration. Of these, sporadic Creutzfeldt-Jakob disease is presented with progressive dementia, psychiatric symptoms, myoclonus, and ataxia. 14-3-3 protein can be detected in the cerebrospinal fluid. These symptoms are similar to Hashimoto’s encephalopathy, but characteristic 3 Hz spike and ware complex could only be found in the electroencephalogram for sporadic Creutzfeldt-Jakob disease. Meanwhile, it doesn’t respond to the steroid treatment and progresses rapidly.
Central nervous system vasculitis-stroke-like symptoms, some patients have no obvious abnormity shown in the results of MRI and cerebrovascular examination, and the disease is sensitive to steroid and other immunosuppression treatments. However, the titer of anti-thyroid antibodies is always normal.
Autoimmune pituitary gland inflammation-autoimmune pituitary gland inflammation has correlations with Hashimoto’s thyroiditis, and high titers of anti-peroxidase antibodies can also found in serum. However, the disease is accompanied with disturbance of water and electrolyte, ACTH deficiency, abnormal MRI signals in sellar. The anti-pituitary antibody is positive in some of the patients.
Limbic encephalitis-similar symptoms and signs of Hashimoto’s thyroiditis can also be found in limbic encephalitis, and the latter can be divided into limbic encephalitis associated with paraneoplastic syndrome and limbic encephalitis associated with none paraneoplastic syndrome. Limbic encephalitis associated with paraneoplastic syndrome can be presented with tumor lesions, and tumor cells can generate antibodies against neurons that contributes to occurrence of the disease. Anti-peroxidase antibodies are always negative, and patients with this disease respond poorly to the steroid treatment. Limbic encephalitis associated with none paraneoplastic syndrome is always presented with complex partial seizures and parahypnosis, which is related to the antibodies against the voltage-gated potassium channels in serum, but not anti-peroxidase antibodies. Abnormally high T2 signal could be found in the medial temporal lobe, and the disease is sensitive to steroid treatment.
Moreover, other factors, including the central nervous system infection, toxic encephalopathy, autoimmune vasculitis, migraine coma and acute disseminated encephalomyelitis should be differentiated with Hashimoto’s thyroiditis.
Treatment and prognosis
High-dose prednisone treatment (1-2 mg/kg) is the first choice, but the dose should be slowly reduced to avoid recurrence. Most patients need to maintain steroid treatment for a long time (4-10 years). After using prednisone for 4-6 weeks, symptoms are improved and the dose can be slowly reduced; Another choice could be prednisone (1000 mg) for 3-5 days and then reducing the dose, and then orally taking small dose of steroid for a long time [1,15]. For those patients who are not sensitive to the steroid treatment, azathioprine combined with steroid, IVIG/plasma exchange, cyclophosphamide or methotrexate can be considered. TYSABRI [5,24] is the ideal medication for treating Hashimoto’s thyroiditis. For some patients with seizure, the utilization of steroid prior to antiepileptic therapy may be effective, and simple antiepileptic treatment may aggravate the symptoms of epilepsy. And the abnormalities shown in electroencephalogram will also be more obvious [5,15]. For those patients with hypothyroidism or subclinical hypothyroidism, no correlation has been found between thyroxine therapy and outcomes of the disease. However, it’s believed that thyroxine therapy should be provided for those patients with thyroid dysfunction (for example, oral thyroxine or anti-thyroid drugs), although these treatments won’t change the prognosis significantly [4,21,26-28].
Clinic symptoms of most patients can be improved with steroid treatment, with electroencephalogram results returning to normal. But serum thyroid antibody is still high even after the treatment. Compared with adults, children with Hashimoto’s encephalopathy may suffer from sequel, including cognitive defects, despite of early diagnosis and treatment [3]. Meanwhile, the prognosis of diffuse Hashimoto’s encephalopathy is better than the vascular type [21].
Acknowledgements
This work was supported by grants from the Key research project of medical science of Health and family Planning Commission of Hebei Province (Funding No. 20100084).
Disclosure of conflict of interest
None.
References
- 1.Payer J, Petrovic T, Baqi L, Lisy L, Langer P. Hashimoto’s encephalopathy and rare cases of hyperthyroidism (review and case report) Endocr Regul. 2009;43:169–178. [PubMed] [Google Scholar]
- 2.Oide T, Tokuda T, Yazaki M, Watarai M, Mitsuhashi S, Kaneko K, Hashimoto T, Ohara S, Ikeda S. Anti-neuronal autoantibody in Hashimoto’s encephalopathy: neuropathological, immunohistochemical, and biochemical analysis of two patients. J Neurol Sci. 2004;217:7–12. doi: 10.1016/j.jns.2003.08.005. [DOI] [PubMed] [Google Scholar]
- 3.Ochi H, Horiuchi I, Araki N, Toda T, Araki T, Sato K, Murai H, Osoegawa M, Yamada T, Okamura K, Ogino T, Mizumoto K, Yamashita H, Saya H, Kira J. Proteomic analysis of human brain identifies alpha-enolase as a novel autoantigen in Hashimoto’s encephalopathy. FEBS Lett. 2002;528:197–202. doi: 10.1016/s0014-5793(02)03307-0. [DOI] [PubMed] [Google Scholar]
- 4.Thieben MJ, Lennon VA, Boeve BF, Aksamit AJ, Keegan M, Vernino S. Potentially reversible autoimmune limbic encephalitis with neuronal potassium channel antibody. Neurology. 2004;62:1177–1182. doi: 10.1212/01.wnl.0000122648.19196.02. [DOI] [PubMed] [Google Scholar]
- 5.Sauter NP, Atkins MB, Mier JW, Lechan RM. Transient thyrotoxicosis and persistent hypothyroidism due to acute autoimmune thyroiditis after interleukin-2 and interferon-alpha therapy for metastatic carcinoma: a case report. Am J Med. 1992;92:441–444. doi: 10.1016/0002-9343(92)90278-j. [DOI] [PubMed] [Google Scholar]
- 6.Weetman AP, McGregor AM. Autoimmune thyroid disease: further developments in our understanding. Endocr Rev. 1994;15:788–830. doi: 10.1210/edrv-15-6-788. [DOI] [PubMed] [Google Scholar]
- 7.Huang X, Yu Y, Zhang H, Liu J, Sun Y, Chang M, Cui C. Hashimoto encephalopathy associated with hyperthyroidism: A case report. Exp Ther Med. 2014;8:515–518. doi: 10.3892/etm.2014.1761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Vasconcellos E, Pina-Garza JE, Fakhoury T, Fenichel GM. Pediatric manifestations of Hashimoto’s encephalopathy. Pediatr Neurol. 1999;20:394–398. doi: 10.1016/s0887-8994(99)00006-5. [DOI] [PubMed] [Google Scholar]
- 9.Chong JY, Rowland LP, Utiger RD. Hashimoto encephalopathy: syndrome or myth? Arch Neurol. 2003;60:164–171. doi: 10.1001/archneur.60.2.164. [DOI] [PubMed] [Google Scholar]
- 10.Abrams P, De Leeuw I, Vertommen J. In new-onset insulin-dependent diabetic patients the presence of anti-thyroid peroxidase antibodies is associated with islet cell autoimmunity and the high risk haplotype HLA DQA1*0301-DQB1*0302. Belgian Diabetes Registry. Diabet Med. 1996;13:415–419. doi: 10.1002/(SICI)1096-9136(199605)13:5<415::AID-DIA96>3.0.CO;2-X. [DOI] [PubMed] [Google Scholar]
- 11.Liu CY, Tseng MC, Lin PH. Encephalopathy associated with autoimmune thyroid disease (Hashimoto’s thyroiditis) presenting as depression: a case report. Gen Hosp Psychiatry. 2011;33:641, e647–649. doi: 10.1016/j.genhosppsych.2011.05.019. [DOI] [PubMed] [Google Scholar]
- 12.Tamagno G, Celik Y, Simo R, Dihne M, Kimura K, Gelosa G, Lee BI, Hommet C, Murialdo G. Encephalopathy associated with autoimmune thyroid disease in patients with Graves’ disease: clinical manifestations, follow-up, and outcomes. BMC Neurol. 2010;10:27. doi: 10.1186/1471-2377-10-27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Tamagno G, Federspil G, Murialdo G. Clinical and diagnostic aspects of encephalopathy associated with autoimmune thyroid disease (or Hashimoto’s encephalopathy) Intern Emerg Med. 2006;1:15–23. doi: 10.1007/BF02934715. [DOI] [PubMed] [Google Scholar]
- 14.Canton A, de Fabregas O, Tintore M, Mesa J, Codina A, Simo R. Encephalopathy associated to autoimmune thyroid disease: a more appropriate term for an underestimated condition? J Neurol Sci. 2000;176:65–69. doi: 10.1016/s0022-510x(00)00302-6. [DOI] [PubMed] [Google Scholar]
- 15.Ban Y, Tomer Y. Genetic susceptibility in thyroid autoimmunity. Pediatr Endocrinol Rev. 2005;3:20–32. [PubMed] [Google Scholar]
- 16.Ghoreishi E, Shahidi GA, Rohani M, Nabavi M, Aghaei M, Akhoundi FH. Palatal-Myoclonus as a Presentation of Hashimoto Encephalopathy: an interesting case report. Iran J Psychiatry. 2013;8:149–151. [PMC free article] [PubMed] [Google Scholar]
- 17.Singh H, Ray S, Agarwal S, Verma RP, Talapatra P, Gupta V. Spectroscopic correlation and role of Azathioprine in long-term remission in patients of Hashimoto encephalopathy. Ann Indian Acad Neurol. 2013;16:443–446. doi: 10.4103/0972-2327.116936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ceylan I, Yener S, Bayraktar F, Secil ML. Roles of ultrasound and power Doppler ultrasound for diagnosis of Hashimoto thyroiditis in anti-thyroid marker-positive euthyroid subjects. Quant Imaging Med Surg. 2014;4:232–238. doi: 10.3978/j.issn.2223-4292.2014.07.13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Shinkov AD, Borisova AM, Kovacheva RD, Vlahov YD, Dakovska LN, Atanassova ID, Petkova PL, Aslanova NL, Vukov MI. Influence of serum levels of thyroid-stimulating hormone and anti-thyroid peroxidase antibodies, age and gender on depression as measured by the Zung Self-Rating Depression Scale. Folia Med (Plovdiv) 2014;56:24–31. doi: 10.2478/folmed-2014-0004. [DOI] [PubMed] [Google Scholar]
- 20.Puzdrowski RL. Anti-Zebrin II immunopositivity in the cerebellum and octavolateral nuclei in two species of stingrays. Brain Behav Evol. 1997;50:358–36. doi: 10.1159/000113346. [DOI] [PubMed] [Google Scholar]
- 21.Burman P, Totterman TH, Oberg K, Karlsson FA. Thyroid autoimmunity in patients on long term therapy with leukocyte-derived interferon. J Clin Endocrinol Metab. 1986;63:1086–1090. doi: 10.1210/jcem-63-5-1086. [DOI] [PubMed] [Google Scholar]
- 22.Chaudhuri A, Behan PO. The clinical spectrum, diagnosis, pathogenesis and treatment of Hashimoto’s encephalopathy (recurrent acute disseminated encephalomyelitis) Curr Med Chem. 2003;10:1945–1953. doi: 10.2174/0929867033456945. [DOI] [PubMed] [Google Scholar]
- 23.Peschen-Rosin R, Schabet M, Dichgans J. Manifestation of Hashimoto’s encephalopathy years before onset of thyroid disease. Eur Neurol. 1999;41:79–84. doi: 10.1159/000008007. [DOI] [PubMed] [Google Scholar]
- 24.Hollowell JG, Staehling NW, Flanders WD, Hannon WH, Gunter EW, Spencer CA, Braverman LE. Serum TSH, T(4), and thyroid antibodies in the United States population (1988 to 1994): National Health and Nutrition Examination Survey (NHANES III) J Clin Endocrinol Metab. 2002;87:489–499. doi: 10.1210/jcem.87.2.8182. [DOI] [PubMed] [Google Scholar]
- 25.Jang JW, Park S, Park Y, Kim JE, Kim S. Symptomatic aggravation after corticosteroid pulse therapy in definite sporadic Creutzfeldt-Jakob disease with the feature of Hashimoto inverted question marks encephalopathy. BMC Neurol. 2014;14:179. doi: 10.1186/s12883-014-0179-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Yu HJ, Lee J, Seo DW, Lee M. Clinical Manifestations and Treatment Response of Steroid in Pediatric Hashimoto Encephalopathy. J Child Neurol. 2013;29:938–942. doi: 10.1177/0883073813499823. [DOI] [PubMed] [Google Scholar]
- 27.Pocsay G, Gazdag A, Engelhardt J, Szaniszlo I, Szolnoki Z, Forczek G, Miklo L. [Hashimoto encephalopathy] . Orv Hetil. 2013;154:1312–1316. doi: 10.1556/OH.2013.29684. [DOI] [PubMed] [Google Scholar]
- 28.Arya R, Anand V, Chansoria M. Hashimoto encephalopathy presenting as progressive myoclonus epilepsy syndrome. Eur J Paediatr Neurol. 2013;17:102–104. doi: 10.1016/j.ejpn.2012.07.001. [DOI] [PubMed] [Google Scholar]


