Abstract
The study was performed to investigate the relationship between KRT6B and Notch1 in the development and progress of hepatocellular carcinoma. The cell viability was detected by CCK8 assay. The cell apoptosis was assessed by annexin V-PI double-labeling staining on a flow cytometry. Expression of genes and proteins were analyzed by real-time PCR and Western blotting, respectively. KRT6B gene was overexpressed using a lentiviral expression vector in a human hepatoma cell line in vitro, in order to explore the mechanism by which the KRT6B promoted cell growth. The results of CCK8 and immunohistochemistry showed that honokiol induced cell death in a concentration- dependent manner, and suppressed human hepatoma cells’ proliferation. The mRNA and protein expression of Notch1 was significantly lower in human hepatoma cells with honokiol treatment than that in the untreatment group. Activation of Notch-1 by exogenous transfection of Notch1 intracellular domain increased KRT6B expression in human hepatoma cells. Furthermore, cells were transfected with the wild type pLenti-KRT6B vector, the protein expression of KRT6B and NOTCH1 was significantly upregulated in human hepatoma cells with honokiol treatment. Overexpression of KRT6B promoted hepatoma cells’ proliferation and showed anti-apoptosis effect. This study demonstrated that honokiol could induce human hepatoma cells’ apoptosis. KRT6B, a key mediator of Notch signaling, was downregulated in honokiol-induced hepatocellular carcinoma apoptosis, suggesting that KRT6B might be a novel therapeutic target for the treatment of hepatocellular carcinoma.
Keywords: Notch1, Krt6b, honokiol, hepatocellular carcinoma
Introduction
Hepatocellular carcinoma (HCC) is the sixth most common cancer worldwide and leading cause of death among patients with cirrhosis [1]. Several risk factors have been suggested to be involved in the development of HCC, including aflatoxin exposure, alcohol consumption, chronic inflammation associated with viral hepatitis and familial tendency [2]. In China, HCC is now second only to lung cancer in terms of total deaths [3]. The high mortality rate of HCC is due to the fact that no systemic therapy is available for advanced cases of the disease [4]. Therefore, it is extremely urgent to explore a novel and effective adjuvant therapy drug.
The bark and/or seed cones of the Magnolia tree have been used in traditional herbal medicines in China. Individual constituents of Magnolia have been reported by many investigators to have anti-cancer effects [5]. Honokiol, a small molecular weight natural product isolated and purified from the Magnolia officinalis, has been shown to possess potent anti-oxidation [6], anti-inflammatory [7], anti-neoplastic and anti-angiogenic properties [8,9]. Functional studies reveal that honokiol can induce cell apoptosis in human chondrosarcoma cells in vitro and reduce tumor volume in vivo [9]. Moreover, honokiol significantly inhibit cyclosporine A-induced and Ras-mediated survival of renal cancer cells through the downregulations of vascular endothelial growth factor (VEGF) and cytoprotective enzyme HO-1 [10]. Interestingly, honokiol analogs show much higher growth inhibitory activity in A549 human lung cancer cells and significant increase of cell population in the G0-G1 phase [11].
A previous study suggested that Notch signaling played a crucial role in the transformation and neoplastic proliferation of human malignancy [12]. Aberrant expression of Notch has been reported in breast cancer [13], lung cancer [14], acute myeloid leukemia [15], prostate cancer [16,17]. Some recent studies show that Notch signaling is activated in human hepatocellular carcinoma and induces tumor formation in Mice [4,18].
A novel mutation in keratin 6b (KRT6B) can lead to pachyonychia congenita type 2, also known as Jackson-Lawler syndrome [19]. An examination of KRT6 show that expression of this gene is increased in the As3+-transformed cells and their tumor heterotransplants. Moreover, KRT6 expression is a marker for transformed urothelial cells that had undergone squamous differentiation [20]. Although its role remains essentially unclear, it seems that KRT6B is associated with the increased risk of cancer, such as lung cancer [21], breast carcinoma [22], and urothelial cancer [23]. However, there is little evidence to suggest a potential role for KRT6B in HCC. Moreover, the relationship between Notch-1 and KRT6B in human hepatocarcinogenesis is unknown.
Although the effects of honokiol-induced tumor apoptosis have been studied in some cancers [9-11], the role of honokiol in the process of cell apoptosis in human hepatoma cells remains largely unknown. In this study, we identified KRT6B as a novel mediator of dysregulated Notch signaling, and showed that activation of Notch-1 in human hepatoma cells upregulated the expression of KRT6B. We also performed a detailed experimental analysis to investigate the relationship between Notch signaling and KRT6B expression in honokiol-induced hepatocellular carcinoma apoptosis and to identify signaling pathways that may be targeted for the treatment of highly aggressive HCC.
Materials and methods
Cell culture
The human HCC cell lines, HepG2, was obtained from Chinese Academy of Sciences (Institute of Shanghai Cell Biology and Chinese Type Culture Collection, China), and maintained in DMEM (Dulbecco’s modified Eagle’s medium; Invitrogen), supplemented with 10% fetal bovine serum (FBS) (HyClone, Logan, UT), 100 units/ml penicillin, and 100 mg/ml streptomycin (Invitrogen) at 37°C in a humidified, 5% CO2, 95% air atmosphere. The medium was replenished every day. Confluent cells were treated with various concentrations of honokiol (50 μg/ml, 100 μg/ml).
Cell viability detection by CCK8
Human hepatoma cells (1.0 × 103/well) were plated in 96-well plates (three wells per group) and treated with honokiol (0-100 μg/ml) for 24 or 48, respectively. 10 μL of CCK8 (Dojindo, Kumamoto, Japan) was added to the cells, and the viability of the cells was measured at 490 nm using an ELISA reader (BioTek, Winooski, VT, USA) according to the manufacturer’s instructions.
Quantification of apoptosis by flow cytometry
Apoptosis was assessed using annexin V, a protein that binds to phosphatidylserine (PS) residues which are exposed on the cell surface of apoptotic cells. Cells were treated with vehicle or honokiol for the indicated time. After treatment, cells were washed twice with PBS (pH = 7.4), and re-suspended in staining buffer containing 1 μg/ml PI and 0.025 μg/ml annexin V-FITC. Double-labeling was performed at room temperature for 10 min in the dark before the flow cytometric analysis. Cells were immediately analyzed using FACScan and the Cellquest program (Becton Dickinson). Quantitative assessment of apoptotic cells was also performed by the terminal deoxynucleotidyl transferase-mediated deoxyuridine triphosphate nick endlabeling (TUNEL) method, which examines DNA-strand breaks during apoptosis by using BD ApoAlertTM DNA Fragmentation Assay Kit. Briefly, cells were incubated with honokiol for the indicated time. The cells were trypsinized, fixed with 4% paraformaldehyde, and permeabilized with 0.1% Triton-X-100 in 0.1% sodiumcitrate. After being washed, the cells were incubated with the reaction mixture for 60 min at 37°C. The stained cells were then analyzed with flow cytometer.
Real-time polymerase chain reaction
The human hepatoma cells’ RNA extraction was performed according to the TRIzol manufacturer’s protocol (Invitrogen, Carlsbad, CA, USA). RNA integrity was verified by agarose gel electrophoresis. Synthesis of cDNAs was performed by reverse transcription reactions with 2 μg of total RNA using moloney murine leukemia virus reverse transcriptase (Invitrogen) with oligo dT (15) primers (Fermentas) as described by the manufacturer. The first strand cDNAs served as the template for the regular polymerase chain reaction (PCR) performed using a DNA Engine (ABI 7300). PCR with the following primers: Notch1, forward 5’-CTAAGATCTCCTGAGGGCTTCAAAGTGTC-3’, reverse 5’-GCGAATTCCTTGAAGGCCTCCGGAT-3’; KTR6B, forward 5’-TCCTTTTTAGTTCCCGTAT-3’, reverse 5’-TAATGGGCAGGATGGTTAG-3’; GAPGH, forward 5’-GGTGGAGGTCGGGAGTCAACGGA-3’, reverse 5’-GAGGGATCTCGCTCCTGGAGGA-3’. Glyceraldehyde 3-phosphate dehydrogenase (GAPDH) as an internal control was used to normalize the data to determine the relative expression of the target genes. The reaction conditions were set according to the kit instructions. After completion of the reaction, the amplification curve and melting curve were analyzed. Gene expression values are expressed using the 2-ΔΔCt method.
Western blotting
The Human hepatoma cells were homogenized and extracted in NP-40 buffer, followed by 5-10 min boiling and centrifugation to obtain the supernatant. Samples containing 50 μg of protein were separated on 10% SDS-PAGE gel, transferred to nitrocellulose membranes (Bio-Rad Laboratories, Hercules, CA, USA). After saturation with 5% (w/v) non-fat dry milk in TBS and 0.1% (w/v) Tween 20 (TBST), the membranes were incubated with the following antibodies, Notch1 and KRT6B (Santa Cruz Biotechnology, CA, USA), at dilutions ranging from 1:500 to 1:2,000 at 4°C over-night. After washes with TBST thrice, the membranes were incubated with secondary immunoglobulins (Igs) conjugated to IRDye 800CW Infrared Dye (LI-COR), including donkey anti-goat IgG and donkey anti-mouse IgG at a dilution of 1:10,000-1:20,000. After 1 hour of incubation at 37°C, membranes were washed three times with TBST. Blots were visualized by the Odyssey Infrared Imaging System (LI-COR Biotechnology). Signals were densitometrically assessed (Odyssey Application Software version 3.0) and normalized to the GAPDH signals to correct for unequal loading using the mouse monoclonal anti-GAPDH antibody (Bioworld Technology, USA).
Transfection and selection of stable human hepatoma cells
For the transfection of the Human hepatoma cells lines, lentiviral vectors harboring KRT6B were constructed and the Human hepatoma cells were infected. Briefly, the Human hepatoma cells were cultured in McCoy’s 5α medium containing 10% FBS and when they reached the exponential growth phase, 1.0 × 105 cells (per well) were plated in 96 plates. Next, 300 μL complete culture medium, containing recombinant lentiviruses, control lentiviruses or McCoy’s 5α medium (all containing 6 μg/mL polybrene; Sigma) was added into the plates when the cells reached 50-60% confluence. Two days later, the virus-containing medium was replaced with fresh complete medium.
Statistical analysis
The data from these experiments were reported as mean ± standard errors of mean (SEM) for each group. All statistical analyses were performed by using PRISM version 4.0 (GraphPad). Inter-group differences were analyzed by one-way ANOVA, and followed by Tukey’s multiple comparison test as a post-hoc test to compare the group means if overall P < 0.05. Differences with P value of < 0.05 were considered statistically significant.
Results
Honokiol-induced cell apoptosis in human hepatoma cells
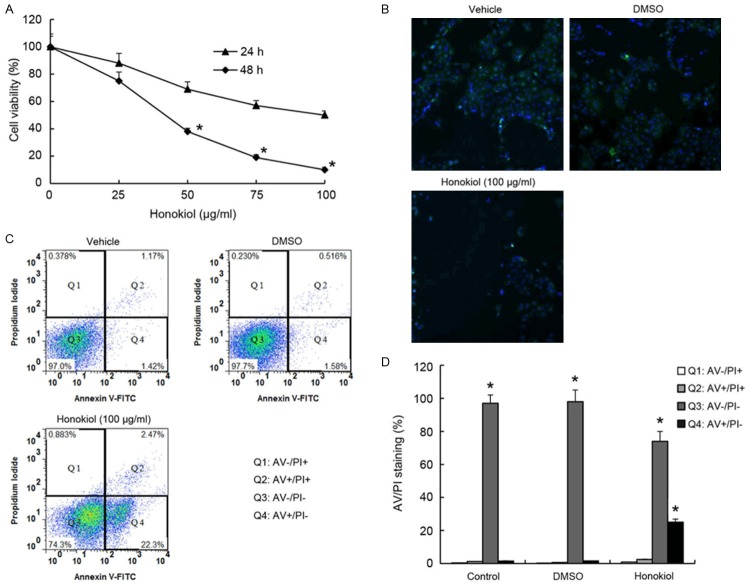
We analyzed the effect of honokiol on cell survival in human hepatoma cells. Treatment of hepatoma cells with honokiol, cell viability was suppressed in a concentration-dependent manner according to CCK8 assay (Figure 1A). In addition, the immunofluorescence staining results showed that honokiol could suppress hepatoma cells proliferation (Figure 1B). We next investigated whether honokiol induced cell death through an apoptotic mechanism. Annexin V-PI double-labeling was used for the detection of PS externalization, a hallmark of early phase of apoptosis. Consistent with the immunofluorescence staining, the results showed significantly (P < 0.05) larger proportion of apoptotic cells at the early phase in honokiol treatment group, compared to untreatment group (Figure 1C and 1D).
Figure 1.
Honokiol induced human hepatoma cell apoptosis. Honokiol-induced the apoptosis of human hepatoma cells are incubated with various concentrations of honokiol for 24 h or 48 h, and the cell viability was examined by CCK8 assay (A). At 24 h post-honokiol (100 μg/ml) treatment, green fluorescent protein visualization of Notch1 in human hepatoma cells by fluorescence microscopy (magnification × 200) (B). Cells are treated with vehicle, DMSO, or honokiol (100 μg/ml) for 24 h, the percentage of apoptotic cells is analyzed by flow cytometric analysis of annexin V/PI double staining (C), the populations of double-positive cells in triplicate in different group were quantified and statistically compared (D). Values are expressed as mean ± SEM, n = 3 in each group. *P < 0.05, versus control group.
MRNA and protein expression of Notch1 in human hepatoma cells
In an attempt to explore the influence of Notch1 on human hepatoma cells when they are prescribed to increase risk of a variety of cancers, to determine whether honokiol induces apoptosis by triggering the Notch apoptotic pathway, we measured the change in the mRNA and protein expressions of Notch1. The present study suggested that Notch1 was associated with proliferation of hepatoma cells. The mRNA and protein expressions of Notch1 were significantly lower in hepatoma cells with honokiol treatment than that in untreatment group (Figure 2). Therefore, our data suggested that suppression the expression of Notch1 was involved in honokiol-mediated cell death.
Figure 2.
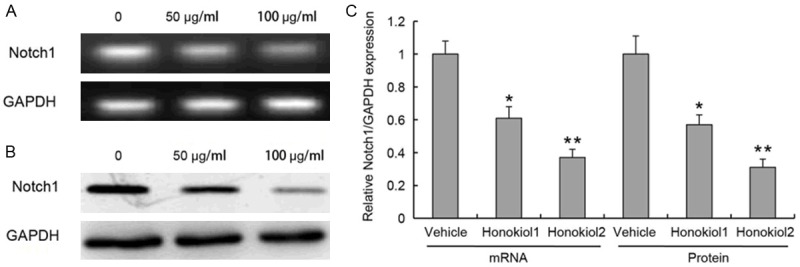
mRNA and protein expression of Notch1 in hepatoma cells. Cells are treated with honokiol in different concentration (50 μg/ml or 100 μg/ml) for 24 h with Western blotting. mRNA (A) and protein (B) expression are measured by PCR and Western blotting, respectively. The quantitative results of real-time PCR and Western blot for each group was statistically compared (C). Values are expressed as mean ± SEM, n = 3 in each group. *P < 0.05, **P < 0.01, versus control group.
Differentially expressed mRNAs in human hepatoma cells
Notch-1 interacts with many downstream effectors that regulate complex cytoplasmic signaling networks. The microarray data of Notch1-none hepatoma cells were treated as control in the selection of differentially expressed genes related to Notch1-transfer. After the removal of redundant and unannotated sequences, with FDR < 1%, 3 genes were found to be significantly downregulated and 47 genes to be significantly upregulated (P < 0.0001) in the Notch1-transfer group compared to that in the Notch1-none group. We found the mRNA expression of KRT6B at the highest levels in Notch1-transger group (Figure 3). Taken together, these results suggested that overexpression of Notch-1 and elevated KRT6B expression play key roles in the pathogenesis of HCC.
Figure 3.
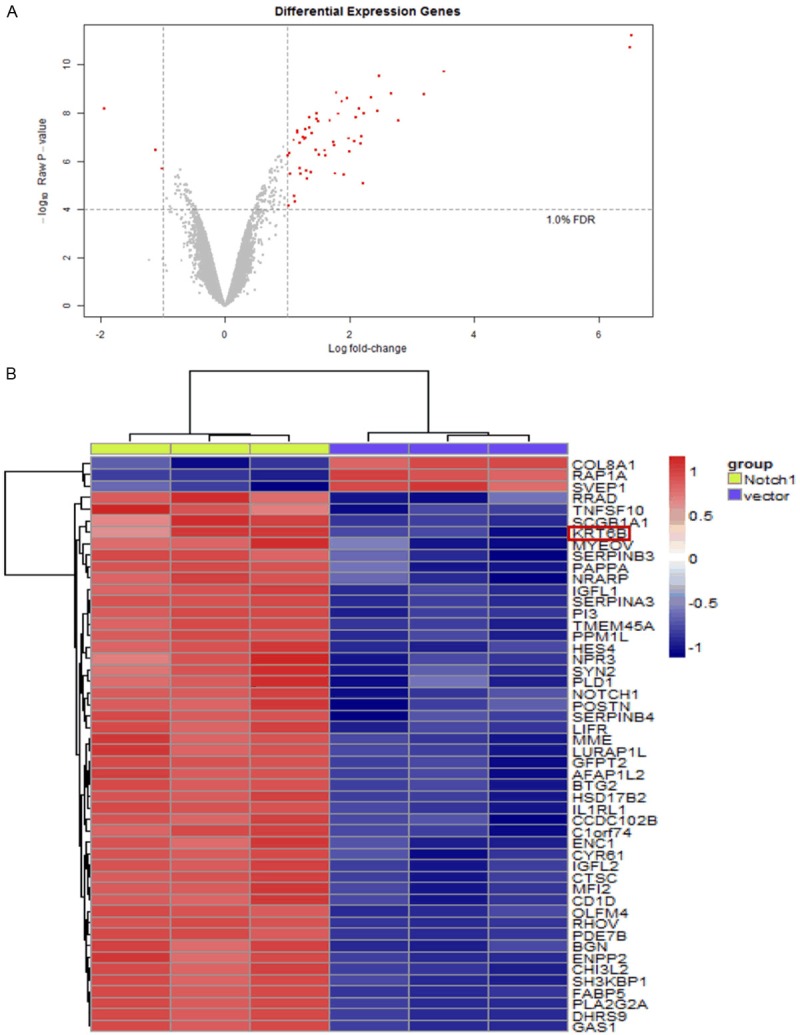
Differentially expressed mRNAs in human hepatoma cells. Differentially expressed mRNAs chosen with FDR < 1%: Volcano plot (A). Hierarchical clustering of differentially express mRNAs in Notch1-none group and Notch1-transger group; red indicates high relative expression and blue indicates low relative expression: heat map (B). The codes are log2-transformed values. Fold changes > 1 and P-values < 0.05 are considered significant.
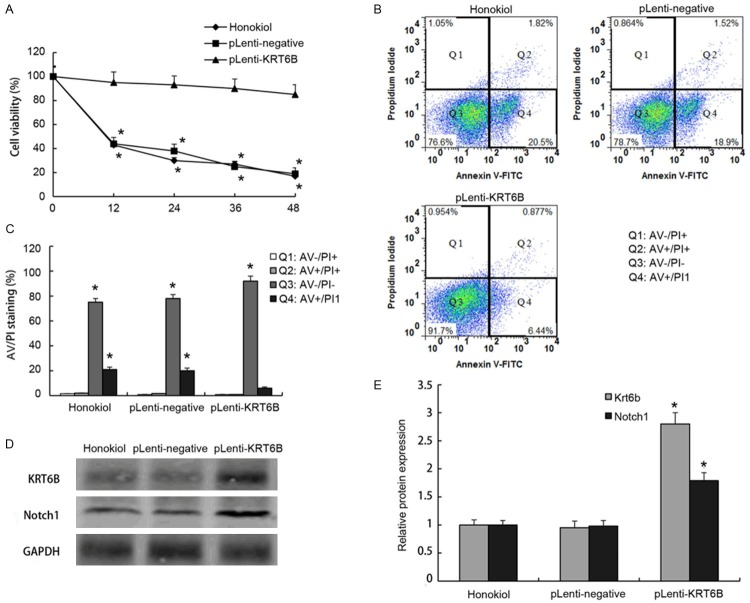
Honokiol-induced human hepatoma cell apoptosis was suppressed by overexpressed KRT6B
In this study, we proposed that KRT6B was involved in honokiol-induced hepatoma cell apoptosis. The CCK8 assay showed that honokiol-induced human hepatoma cell apoptosis was suppressed by overexpressed KRT6B (Figure 4A). The Annexin V-PI double-labeling results showed a large proportion of the early phase of apoptosis cells had treated with honokiol treatment, but overexpressed KRT6B could suppress early phase of apoptosis cells (Figure 4B). Moreover, the protein expression of KRT6B was significantly decreased in hepatoma cells with honokiol treatment (Figure 4C). We next explored the interaction between KRT6B and Notch1 in human hepatoma cells. Western blot analysis of KRT6B and Notch1 expression in human hepatoma cells showed that cells expressing exogenous KRT6B can augment the protein expression of Notch1 (Figure 4C). This suggested that KRT6B was involved in Notch signaling and established a regulatory role for KRT6B in the pathogenesis of HCC.
Figure 4.
Honokiol-induced human hepatoma cell apoptosis was suppressed by overexpressing KRT6B. Honokiol (100 μg/ml)-induced the apoptosis of human hepatoma cells are transfected with pLenti-negative (honokiol + pLenti-negative), or pLenti-KRT6B (honokiol + pLenti-KRT6B) for 24 h, and the cell viability is examined by CCK8 assay (A), cells are treated with honokiol, honokiol + pLenti-negative, or honokiol + pLenti-KRT6B for 24 h, the percentage of apoptotic cells is analyzed by flow cytometric analysis of annexin V/PI double staining (B), and the populations of double-positive cells in triplicate in different group were quantified and statistically compared (C). The protein expression of KRT6B and Notch1 is measured by Western blotting in honokiol group, pLenti-negetive (honokiol + pLenti-negetive) group, or pLenti-KRT6B (honokiol + pLenti-KRT6B) group (D), and gray analysis was performed to semi-quantify the relative protein levels (E). Values are expressed as mean ± SEM, n = 3 in each group. *P < 0.05, versus control group.
Discussion
HCC is a highly aggressive cancer for which there is no currently available effective treatment and it is the third most frequent cause of cancer deaths [4]. Mounting evidence shows that constitutively activated Notch pathway induces human hepatoma cells’ proliferative activity [4,18,24,25]. Notch signaling activated in human HCC samples promotes formation of liver tumors in mice. The Notch signature is a biomarker of response to Notch inhibition in vitro [18]. However, the possible molecular mechanisms underlying the interaction between Notch signaling and KRT6B in the development and progress of human HCC are unknown.
In this study, we utilized a comprehensive and integrative approach to explore the role of Notch signaling in honokiol-induced human hepatoma cell apoptosis. We demonstrated that Notch1 was associated with proliferation of hepatoma cells. Immunohistochemistry of human hepatoma cells showed that Notch1 was heavily expressed and honokiol treatment could curb this negative phenomenon. Moreover, the mRNA and protein expressions of Notch1 were suppressed by honokiol. KRT6B was overexpressed in the human hepatoma cells using pLenti-KRT6B, and then the cells that stably overexpressed KRT6B mRNA and protein were screened. The results indicated that the mRNA and protein levels of KRT6B in the human hepatoma cells were higher than the levels in the vehicle group, and the overexpression of KRT6B significnatly suppressed honokiol-induced human hepatoma cell apoptosis. Furthermore, the present study demonstrated that KRT6B could augment the protein expression of Notch1. Therefore, we proposed that there may be a cross-talk between Notch1 and KRT6B in cell apoptosis progression. Honokiol could induce human hepatoma cell apoptosis, and the underlying mechanism was mediated, at least partially, through downregulation the expression of Notch1 and KRT6B.
According to the CCK8 assay and Annexin V-PI double-labeling staining, honokiol induced cell death through an apoptotic mechanism, and the proportion of apoptotic cells at early phase was significantly larger. Honokiol had been demonstrated to possess the effects of cytoprotective autophagy in prostate cancer cells, induce apoptosis in the human colorectal cancer cells, and reduce cells survival and tumor growth in human chondrosarcoma cells both in vitro and in vivo [9,26,27]. To date, a few studies to our knowledge investigated honokiol on hepatocellular carcinoma. The study on human hepatocellular carcinoma SMMC-7721 cells had found that honokiol-induced cell death was associated with reactive oxygen species production and an increase of Bax/Bcl-2 ratios [28]. Interestingly, honokiol inhibited proliferation and significantly potentiated the apoptotic effects of paclitaxel and doxorubicin in HCC cells [29]. Moreover, honokiol induces apoptosis of HepG2 human hepatocellular carcinoma cells through activation of p38 MAPK pathway, and in turn, activation of caspase-3 [30]. However, we observed a novel phenomenon that the expression of Notch1 increased along with overexpressed KRT6B protein level in hepatocellular carcinoma cells. So, we investigated the relationship between Notch1 and KRT6B, and eventually determined that honokiol induced human hepatocellular carcinoma cell apoptosis through suppressing the expression of Notch1 and KRT6B.
Taken all together, our results identified that honokiol induced cell death and suppressed human hepatocellular carcinoma cell proliferation. The underlying mechanism was mediated, at least partially, through downregulating the expression of Notch1 and KRT6B. Honokiol provided an intriguing explanation of cellular and molecular mechanisms responsible for hepatocellular carcinoma cells’ apoptosis, and maybe an effective adjuvant therapeutic drug for clinical treatment.
Disclosure of conflict of interest
None.
References
- 1.Tabrizian P, Roayaie S, Schwartz ME. Current management of hepatocellular carcinoma. World J Gastroenterol. 2014;20:10223–10237. doi: 10.3748/wjg.v20.i30.10223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hsu CC, Lee HC, Wei YH. Mitochondrial DNA alterations and mitochondrial dysfunction in the progression of hepatocellular carcinoma. World J Gastroenterol. 2013;19:8880–8886. doi: 10.3748/wjg.v19.i47.8880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Zhang Y, Song H, Miao Y, Wang R, Chen L. Frequent transcriptional inactivation of Kallikrein 10 gene by CpG island hypermethylation in non-small cell lung cancer. Cancer Sci. 2010;101:934–940. doi: 10.1111/j.1349-7006.2009.01486.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Fan RH, Li J, Wu N, Chen PS. Late SV40 factor: a key mediator of Notch signaling in human hepatocarcinogenesis. World J Gastroenterol. 2011;17:3420–3430. doi: 10.3748/wjg.v17.i29.3420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lee YJ, Lee YM, Lee CK, Jung JK, Han SB, Hong JT. Therapeutic applications of compounds in the Magnolia family. Pharmacol Ther. 2011;130:157–176. doi: 10.1016/j.pharmthera.2011.01.010. [DOI] [PubMed] [Google Scholar]
- 6.Liou KT, Shen YC, Chen CF, Tsao CM, Tsai SK. Honokiol protects rat brain from focal cerebral ischemia-reperfusion injury by inhibiting neutrophil infiltration and reactive oxygen species production. Brain Res. 2003;992:159–166. doi: 10.1016/j.brainres.2003.08.026. [DOI] [PubMed] [Google Scholar]
- 7.Zhang P, Liu X, Zhu Y, Chen S, Zhou D, Wang Y. Honokiol inhibits the inflammatory reaction during cerebral ischemia reperfusion by suppressing NF-kappaB activation and cytokine production of glial cells. Neurosci Lett. 2013;534:123–127. doi: 10.1016/j.neulet.2012.11.052. [DOI] [PubMed] [Google Scholar]
- 8.Bai X, Cerimele F, Ushio-Fukai M, Waqas M, Campbell PM, Govindarajan B, Der CJ, Battle T, Frank DA, Ye K, Murad E, Dubiel W, Soff G, Arbiser JL. Honokiol, a small molecular weight natural product, inhibits angiogenesis in vitro and tumor growth in vivo. J Biol Chem. 2003;278:35501–35507. doi: 10.1074/jbc.M302967200. [DOI] [PubMed] [Google Scholar]
- 9.Chen YJ, Wu CL, Liu JF, Fong YC, Hsu SF, Li TM, Su YC, Liu SH, Tang CH. Honokiol induces cell apoptosis in human chondrosarcoma cells through mitochondrial dysfunction and endoplasmic reticulum stress. Cancer Lett. 2010;291:20–30. doi: 10.1016/j.canlet.2009.08.032. [DOI] [PubMed] [Google Scholar]
- 10.Banerjee P, Basu A, Arbiser JL, Pal S. The natural product honokiol inhibits calcineurin inhibitor-induced and Ras-mediated tumor promoting pathways. Cancer Lett. 2013;338:292–299. doi: 10.1016/j.canlet.2013.05.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lin JM, Prakasha Gowda AS, Sharma AK, Amin S. In vitro growth inhibition of human cancer cells by novel honokiol analogs. Bioorg Med Chem. 2012;20:3202–3211. doi: 10.1016/j.bmc.2012.03.062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ohnuki H, Jiang K, Wang D, Salvucci O, Kwak H, Sanchez-Martin D, Maric D, Tosato G. Tumor-infiltrating myeloid cells activate Dll4/Notch/TGF-beta signaling to drive malignant progression. Cancer Res. 2014;74:2038–2049. doi: 10.1158/0008-5472.CAN-13-3118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Villa JC, Chiu D, Brandes AH, Escorcia FE, Villa CH, Maguire WF, Hu CJ, de Stanchina E, Simon MC, Sisodia SS, Scheinberg DA, Li YM. Nontranscriptional Role of Hif-1alpha in Activation of gamma-Secretase and Notch Signaling in Breast Cancer. Cell Rep. 2014;8:1077–1092. doi: 10.1016/j.celrep.2014.07.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Chen Y, Li D, Liu H, Xu H, Zheng H, Qian F, Li W, Zhao C, Wang Z, Wang X. Notch-1 signaling facilitates survivin expression in human non-small cell lung cancer cells. Cancer Biol Ther. 2011;11:14–21. doi: 10.4161/cbt.11.1.13730. [DOI] [PubMed] [Google Scholar]
- 15.Chen PM, Yen CC, Wang WS, Lin YJ, Chu CJ, Chiou TJ, Liu JH, Yang MH. Downregulation of Notch-1 expression decreases PU. 1-mediated myeloid differentiation signaling in acute myeloid leukemia. Int J Oncol. 2008;32:1335–1341. doi: 10.3892/ijo_32_6_1335. [DOI] [PubMed] [Google Scholar]
- 16.Kashat M, Azzouz L, Sarkar SH, Kong D, Li Y, Sarkar FH. Inactivation of AR and Notch-1 signaling by miR-34a attenuates prostate cancer aggressiveness. Am J Transl Res. 2012;4:432–442. [PMC free article] [PubMed] [Google Scholar]
- 17.Carvalho FL, Simons BW, Eberhart CG, Berman DM. Notch signaling in prostate cancer: a moving target. Prostate. 2014;74:933–945. doi: 10.1002/pros.22811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Villanueva A, Alsinet C, Yanger K, Hoshida Y, Zong Y, Toffanin S, Rodriguez-Carunchio L, Sole M, Thung S, Stanger BZ, Llovet JM. Notch signaling is activated in human hepatocellular carcinoma and induces tumor formation in mice. Gastroenterology. 2012;143:1660–1669. e1667. doi: 10.1053/j.gastro.2012.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sharma VM, Stein SL. A novel mutation in K6b in pachyonychia congenita type 2. J Invest Dermatol. 2007;127:2060–2062. doi: 10.1038/sj.jid.5700814. [DOI] [PubMed] [Google Scholar]
- 20.Cao L, Zhou XD, Sens MA, Garrett SH, Zheng Y, Dunlevy JR, Sens DA, Somji S. Keratin 6 expression correlates to areas of squamous differentiation in multiple independent isolates of As(+3)-induced bladder cancer. J Appl Toxicol. 2010;30:416–430. doi: 10.1002/jat.1513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Camilo R, Capelozzi VL, Siqueira SA, Del Carlo Bernardi F. Expression of p63, keratin 5/6, keratin 7, and surfactant-A in non-small cell lung carcinomas. Hum Pathol. 2006;37:542–546. doi: 10.1016/j.humpath.2005.12.019. [DOI] [PubMed] [Google Scholar]
- 22.Brogi E, Murphy CG, Johnson ML, Conlin AK, Hsu M, Patil S, Akram M, Nehhozina T, Jhaveri KL, Hudis CA, Seidman AD. Breast carcinoma with brain metastases: clinical analysis and immunoprofile on tissue microarrays. Ann Oncol. 2011;22:2597–2603. doi: 10.1093/annonc/mdr022. [DOI] [PubMed] [Google Scholar]
- 23.Fichtenbaum EJ, Marsh WL Jr, Zynger DL. CK5, CK5/6, and double-stains CK7/CK5 and p53/CK5 discriminate in situ vs invasive urothelial cancer in the prostate. Am J Clin Pathol. 2012;138:190–197. doi: 10.1309/AJCP5ZC4GQVNWTYR. [DOI] [PubMed] [Google Scholar]
- 24.Lin W, Zhao J, Cao Z, Zhuang Q, Zheng L, Zeng J, Hong Z, Peng J. Livistona chinensis seeds inhibit hepatocellular carcinoma angiogenesis in vivo via suppression of the Notch pathway. Oncol Rep. 2014;31:1723–1728. doi: 10.3892/or.2014.3051. [DOI] [PubMed] [Google Scholar]
- 25.Zhang S, Yang Y, Liang Z, Duan W, Yang J, Yan J, Wang N, Feng W, Ding M, Nie Y, Jin Z. Silybin-mediated inhibition of Notch signaling exerts antitumor activity in human hepatocellular carcinoma cells. PLoS One. 2013;8:e83699. doi: 10.1371/journal.pone.0083699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hahm ER, Sakao K, Singh SV. Honokiol activates reactive oxygen species-mediated cytoprotective autophagy in human prostate cancer cells. Prostate. 2014;74:1209–21. doi: 10.1002/pros.22837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lai YJ, Lin CI, Wang CL, Chao JI. Expression of survivin and p53 modulates honokiol-induced apoptosis in colorectal cancer cells. J Cell Biochem. 2014;115:1888–99. doi: 10.1002/jcb.24858. [DOI] [PubMed] [Google Scholar]
- 28.Han LL, Xie LP, Li LH, Zhang XW, Zhang RQ, Wang HZ. Reactive oxygen species production and Bax/Bcl-2 regulation in honokiol-induced apoptosis in human hepatocellular carcinoma SMMC-7721 cells. Environ Toxicol Pharmacol. 2009;28:97–103. doi: 10.1016/j.etap.2009.03.005. [DOI] [PubMed] [Google Scholar]
- 29.Rajendran P, Li F, Shanmugam MK, Vali S, Abbasi T, Kapoor S, Ahn KS, Kumar AP, Sethi G. Honokiol inhibits signal transducer and activator of transcription-3 signaling, proliferation, and survival of hepatocellular carcinoma cells via the protein tyrosine phosphatase SHP-1. J Cell Physiol. 2012;227:2184–2195. doi: 10.1002/jcp.22954. [DOI] [PubMed] [Google Scholar]
- 30.Deng J, Qian Y, Geng L, Chen J, Wang X, Xie H, Yan S, Jiang G, Zhou L, Zheng S. Involvement of p38 mitogen-activated protein kinase pathway in honokiol-induced apoptosis in a human hepatoma cell line (HepG2) Liver Int. 2008;28:1458–1464. doi: 10.1111/j.1478-3231.2008.01767.x. [DOI] [PubMed] [Google Scholar]