Abstract
Resveratrol, an edible polyphenolic phytoalexin obtained primarily from root extracts of the oriental plant, Polygonum cuspidatum and from grapes and red wine, has been reported as an anticancer compound against several types of cancer, the accurate molecular mechanisms of by which it induces apoptosis are limited. In the present study, the molecular mechanisms of resveratrol on human leukemia K562 cells apoptosis was examined. Our results showed that resveratrol significantly decreased cell viability and triggered cell apoptosis in K562 cells. Resveratrol-induced apoptosis of K562 cells was associated with the dissipation of mitochondrial membrane potential (MMP) and the release of cytochrome c into the cytosol. Furthermore, the up-regulation of Bax/Bcl-2 ratio, the activation of caspase-3 and increased cleaved PARP was also observed in K562 cells treated with resveratrol. Thus, we considered that the resveratrol-induced apoptosis of K562 cells might be mediated through the mitochondria pathway, which gives the rationale for in vivo studies on the utilization of resveratrol as a potential cancer therapeutic compound.
Keywords: Resveratrol, human leukemia, mitochondrial signaling pathway, apoptosis
Introduction
Leukemia is a clonal disorder with blocked normal differentiation and cell death of hematopoietic progenitor cells. Chronic myelogenous leukemia (CML), a cancer of the white blood cells characterized by the clonal expansion of myeloid precursors, is a myeloproliferative syndrome linked to a hematopoietic stem cell disorder leading to the increased production of granulocytes at all stages of differentiation [1,2]. The development of tyrosine kinase inhibitors (TKIs) has led to extended lifespans for many patients with chronic myelogenous leukemia. The success of various generations of tyrosine kinase inhibitors in chronic myelogenous leukemia (CML) is well-known, with many patients experiencing long-term benefits from treatment. However, not every patient with CML can tolerate this therapy, shows response to initial treatment, or avoids disease progression or drug resistance, 20% to 30% of patients fail to respond, respond suboptimally, or experience disease relapse after treatment with imatinib, and a key factor is drug resistance [3-6].
A promising source of therapeutic agents is traditional medicine derived from natural compounds. A wide variety of natural compounds derived from medicinal plants have been extensively studied for the treatment of human disease including different types of cancer. Numerous studies have demonstrated that naturally occurring compounds in the human diet may have lower toxicity and less possibility of drug resistance and have long lasting beneficial effects on human health, for example, long-term moderate consumption of red wine is associated with a reduced risk of developing lifestyle-related diseases such as cardiovascular disease and cancer [7-10]. Resveratrol (RSV), trans-3,4’, 5-trihydroxystilbene, is a compound obtained primarily from root extracts of the oriental plant, Polygonum cuspidatum and from red grapes [11]. It has been identified that resveratrol has a strong chemopreventive effect against the development of several cancers [12-14]. It is believed that targeting apoptosis in cancer is feasible. However, the molecular signaling mechanisms by which resveratrol exerts its anti-leukemic effects in CML cell lines remains incompletely understood. The present study attempts to determine the pro-apoptotic effect of resveratrol and to elucidate the effect of resveratrol on apoptosis involving in the collapse of mitochondrial function in the human CML K562 cell line.
Materials and methods
Drugs
Resveratrol (Sigma-Aldrich, Inc., St. Louis, Mo, USA) was dissolved in DMSO at 40 mM as a stock solution. The dilutions of all reagents were freshly prepared before experiment.
Cell lines
The human myeloid leukemia cell line K562 was purchased from Cell Bank, China Academy of Sciences (Shanghai, China). Cancer cells were maintained in RPMI-1640 (Hyclone) supplemented with 10% (v/v) heat-inactivated fetal bovine serum (GIBCO), penicillin-streptomycin (100 IU/ml to 100 μg/ml), 2 mM glutamine, and 10 mM HEPES buffer at 37°C in a humidified atmosphere (5% CO2-95% air).
Growth and cell proliferation analysis
The proliferation of gastric adenocarcinoma cells was evaluated by 3-[4,5-dimethylthiazol-2-yl]-2, 5-diphenyltetrazolium bromide (MTT) assay. K562 cells (5×103 per well) seeded in 96-well plates were incubated with increasing concentrations (10, 20, 40, 80, 160 μM) of resveratrol for 24, 48 and 72 h, respectively. The controls were treated with an equal volume of the drug’s vehicle DMSO, but the applied concentration did not exhibit a modulating effect on cell growth. Thereafter, cell growth inhibition was evaluated by MTT assay.
Hoechst 33258 staining
K562 cells at the logarithmic-growth phase were seeded into 96-well plates (1×104/well). The cells were cultured in normal medium (control group) or with increasing concentrations of resveratrol (20 μM and 40 μM) for 24 h. Then, the cells were fixed with 3.7% paraformaldehyde for 30 min at room temperature, then washed and stained with Hoechst 33258 (Sigma Aldrich) for 30 min at 37°C. Cells were observed under a Nikon 80i fluorescence microscope equipped with a UV filter (Nikon Corporation, Tokyo, Japan).
Annexin V/FITC and 7-AAD staining analysis
K562 cells seeded in 6-well plates (1.5×105 per well) were treated with increasing concentrations of resveratrol for 24 h. Cells were harvested and washed with cold PBS. The cell surface phosphatidylserine in apoptotic cells was quantitatively estimated by using Annexin V/FITC and 7-AAD apoptosis detection kit according to manufacturer’s instructions (Roche, USA). Cell apoptosis was analyzed on a FACScan flow cytometry (Becton Dickinson, USA). Triplicate experiments with triplicate samples were performed.
Mitochondria membrane permeability assay
The mitochondria membrane potential (MMP) was analyzed by using a JC-1 (5, 5’, 6, 6’-tetrachloro-1, 1’, 3, 3-tetraethylbenzimidazolocarbocyanine Iodide) fluorescence probe kit (Beyotime, China). Briefly, K562 cells cultured in six-well plates exposed to 20 and 40 μM resveratrol for 24 h and then were incubated with an equal volume of JC-1 staining solution (5 μg/ml) at 37°C for 20 min and rinsed twice with PBS. Mitochondrial membrane potentials were monitored by determining the relative amounts of dual emissions from mitochondrial JC-1 monomers or aggregates using an Olympus fluorescent microscope under Argon-ion 488 nm laser excitation. Mitochondrial depolarization is indicated by an increase in the green/red fluorescence intensity ratio.
Preparation of total, mitochondria and cytosol proteins
Cells in different groups were lysed for total proteins in lysis buffer containing 50 mM HEPES (pH 7.4), 150 mM NaCl, 0.1% Triton X-100, 1.5 mM MgCl2, 1 mM EDTA, 2 mM sodium orthovanadate, 4 mM sodium pyrophosphate, 100 mM NaF, and 1:500 protease inhibitor mixture (Sigma-Aldrich, USA).
Mitochondria/cytosol kit (Beyotime, China) was used to isolate mitochondria and cytosol according to the manufacture’s protocol. After washing with cold PBS, cancer cells (5×107) were suspended in 500 μl of isolation buffer containing protease inhibitors and lysed on ice for 10 min. Cells were mechanically homogenized with Dunce grinder. The unbroken cells, debris and nuclei were discarded by centrifugation at 800 g for 10 min at 4°C. The supernatants were centrifuged at 12,000 g for 15 min at 4°C. The supernatant cytosol was collected and pellet fraction mitochondria were dissolved in 50 μl of lysis buffer.
Western blotting assay
Western blotting assay was performed to analyze the expressions of apoptotic and related mitochondrial molecules in K562 cells. Briefly, K562 cells (3×105) seeded in 6-well plates were exposed to various concentrations of resveratrol for 72 h. The cells were harvested and lysates (50 μg of protein per lane) were fractionated by 10% SDS-PAGE as described below. The proteins were electro-transferred onto PVDF membranes, and then incubated with primary antibodies overnight including anti-cytochrome c (4280, Cell Signaling), anti-caspase-3 (9662, Cell Signaling), anti-cleaved PARP (9541, Cell Signaling), anti-Bcl-2 (2772, Cell Signaling), anti-Bax (2872, Cell Signaling), and anti-β-actin (ab6276, Abcam). Appropriate horseradish peroxidase-conjugated secondary antibodies were added in TBST containing 5% BSA. The bound antibodies were visualized by using an enhanced chemiluminescence reagent (Millipore, USA) and quantified by densitometry using ChemiDoc XRS + image analyzer (Bio-Rad, USA) adjusted with β-actin as loading control. Triplicate experiments with triplicate samples were performed.
Statistical analysis
All data were described as mean ± S.D., and analyzed by one-way ANOVA using SPSS/Win11.0 software (SPSS Inc., Chicago, IL.). A p value less than 0.05 was considered statistically significant.
Results
Inhibition of human leukemia cell proliferation
K562 cells treated with resveratrol for 24 h, 48 h, and 72 h were subjected to MTT assay. Our results showed that resveratrol effectively inhibited the proliferation of K562 cells. As shown in Figure 1, the inhibition rate increased from 5.2% to 60.9% after treatment with resveratrol for 24 h, from 6.2% to 67.9% for 48 h, from 5.9% to 70.3% for 72 h. The maximum inhibition rate of 70.3% was found with use of 160 μM for 72 h treatment. These results indicated that resveratrol had a dose- and time-dependent antiproliferative effect on K562 cells in the range of 10-160 μM for 24 h, 48 h, and 72 h of exposure.
Figure 1.

The growth inhibition effect of K562 cells induced by resveratrol. K562 cells were exposed to increasing concentrations of resveratrol or an equal volume of the drug’s vehicle DMSO for up to 72 h. Viable cells were evaluated by MTT assay and denoted as a percentage of untreated controls at the concurrent time point. The bars indicate mean ± S.D. (n=3).
Induction of K562 cell apoptosis by resveratrol
To evaluate the resveratrol-induced cell apoptosis of K562 cells, we examined the morphologic changes by Hoechst 33258 staining (Figure 2). The apoptotic morphologic changes in resveratrol-treated groups were observed as compared with the control group. In vehicle control group, nuclei of K562 cells were round and homogeneously stained (Figure 2A), while after treatment of resveratrol the cells revealed significant apoptosis characteristics including cell shrinkage and membrane integrity loss or deformation, nuclear fragmentation and chromatin compaction of late apoptotic appearance (Figure 2B, 2C).
Figure 2.

Resveratrol induced the morphologic changes of K562 cells in vitro. K562 cells treated with resveratrol were subjected to Hoechst 33258 staining and viewed under microscope. A: Vehicle control; B: 20 μM; C: 40 μM.
Then the apoptosis condition of K562 cells were further analyzed by flow cytometry assay. The results showed evident increase of apoptotic cells after exposure to 20 μM and 40 μM of resveratrol for 24 h, and the percentage of apoptotic cells was 24.7% and 49.6%, respectively (Figure 3A-C).
Figure 3.

Detection of apoptotic cells after Annexin V/7-AAD staining by flow cytometric analysis. K562 cells were exposed to increasing concentrations of resveratrol for 24 h. Cells were harvested and stained with AnnexinV/7-AAD. A: Vehicle control; B: 20 μM; C: 40 μM.
Induction of MMP collapse
The lipophilic and cationic fluorescent dye JC-1 is capable of selectively entering mitochondria, where it forms aggregates and emits red fluorescence when MMP is high. At low MMP, JC-1 cannot enter into mitochondria and forms monomers emitting green fluorescence. The ratio of green to red fluorescence provides an estimate of changes in MMP. JC-1 fluorescence probe showed that MMP in K562 cells was significantly decreased after resveratrol treatment. As shown in Figure 4A, the red fluorescence of JC-1 was gradually decreased and the green fluorescence was correspondingly increased after resveratrol treatment. At the concentration of 20 and 40 μM, the ratios of green to red fluorescence were significantly increased (P<0.01 vs. vehicle control, Figure 4B). These results indicated the collapse of MMP in K562 cells after treatment with resveratrol.
Figure 4.
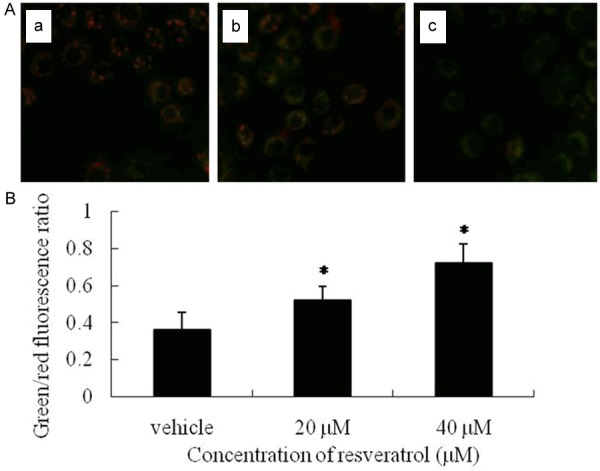
Resveratrol induced mitochondrial membrane potentials collapse in K562 cells. K562 cells were stained with JC-1 probe and imaged by fluorescent microscope. The individual red and green average fluorescence intensities are expressed as the ratio of green to red fluorescence. A. A decrease of red fluorescence ratio indicates a shift correlating with a reduction in mitochondrial depolarization . Representative photographs of JC-1 staining in different groups (a. Control; b. Resveratrol 20 μM; c. Resveratrol 40μM). B. Quantitative analysis of the shift of mitochondrial red fluorescence to green fluorescence among groups. All values are denoted as means ± S.D. from ten independent photographs shot in each group. *, P<0.01 compared with vehicle control cells cultured in complete medium.
Detection of mitochondrial apoptosis related proteins
First, the distribution of cytochrome c before and after resveratrol treatment was examined by western blotting assay. Cytochrome c in K562 cells was redistributed after resveratrol treatment. In K562 cells, the level of cytochrome c in mitochondria was significantly decreased by 42.6% and 65.7%, and the levels of cytochrome c in cytosol were increased to 145.3% and 193.7% of control group, respectively (P<0.01 vs. vehicle control, Figure 5A).
Figure 5.

Effects of resveratrol on apoptosis related proteins. A. Resveratrol induced the redistribution of cytochrome c in K562 cells as estimated by Western blotting assay. B. Resveratrol increased the Bax/Bcl-2 ratio and apoptosis-related protein. C. K562 cells were exposed to various concentrations of resveratrol and the levels of apoptosis-related protein Caspase3 and cleaved PARP were measured by Western blot analysis.
Furthermore, we examined the expressions of Bax and Bcl-2 and then analyzed the ratio of Bax/Bcl-2. As shown in Figure 5B, the level of Bax was significantly increased and Bcl-2 was obviously decreased in resveratrol-treated cancer cells. Statistical analysis showed that resveratrol in the range of 5 μM increased the ratio of Bax/Bcl-2 by 232.5% and 315.3% for 20 μM and 40 μM (P<0.01 vs. vehicle control), respectively.
Additionally, we measured the molecular alteration of apoptosis related proteins in resveratrol-treated cells. Resveratrol was found to activate the caspases cascade pathway as demonstrated by the increases of cleaved caspase-3 and cleaved PARP in K562 cells. As shown in Figure 5C, the levels of caspase-3 and cleaved PARP were significantly increased in K562 cells exposure to resveratrol.
Discussion
A number of studies have revealed that resveratrol hits a variety of target molecules and cellular signaling pathways pertinent to normal human physiology and directly applicable to pathological disease staes, and it has attracted increasing attention in recent years because of its potent chemopreventive and anti-tumor effects involved various signaling mechanisms such as apoptosis induction, suppression of invasion and metastasis, increased antioxidant capacity, and sensitization to chemotherapy-triggered apoptosis [15-18]. In this research, we confirmed that resveratrol inhibited the proliferation of K562 cells in a concentration- and time-dependent manner, and the accurate pro-apoptotic and molecular signaling pathways mediated by resveratrol to induce its complex anti-leukemic effects in cancer cells was investigated.
Programmed cell death or apoptosis is an ordered and orchestrated cellular process which is crucial for all multicellular organisms to control cell proliferation and maintain tissue homeostasis as well as eliminate harmful or unnecessary cells from an organism in physiological and pathological conditions [19,20]. In cancer cells, the programmed cell death is disrupted thus resulting in the overgrowth of malignant cells [21]. There are two signaling pathways identified to be involved in apoptosis induction including mitochondria-mediated intrinsic and death receptor-mediated extrinsic pathways, and both pathways ultimately lead to the activation of the executioner caspases-3 via diverse proapoptotic signals and finally cell death [22,23]. The mitochondria are important and central mediators of both apoptosis and regulated necrosis. In the intrinsic apoptotic pathway, the mitochondrial outer membrane permeabilization occurs and cytochrome c is released from mitochondria into cytosol after apoptosis initiation, followed by activation of caspase-9 and caspase-3 and thereby cleavage of cleavage of poly (-ADP-ribose) polymerase (PARP), which is a specific substrate for caspase-3 [24-26]. The intrinsic apoptotic pathway mediated by mitochondria was mainly triggered by the collapse of mitochondria membrane potential, which process could prompt the release of pro-apoptotic molecules cytochrome-c into the cytoplasm [27,28]. In the present study, 20 μM and 40 μM resveratrol significantly inhibited the proliferation of K562 cells and induced cell apoptosis, molecular analysis found a notable increased levels of caspase-3 and cleaved PARP. Our data suggested that resveratrol could induced the apoptosis of K562 cells. Then the redistribution of cytochrome c in K562 cells was also observed after resveratrol treatment.
In apoptosis, the intrinsic apoptotic pathway is also closely regulated by a group of proteins belonging to the Bcl-2 family, the B-cell leukemia/lymphoma 2 (Bcl-2) family members Bcl-2-associated protein x (Bax) and Bcl-2 homologues antagonist/killer (Bak) undergo oligomerization in the outer mitochondrial membrane resulting in the release of apoptosis inducing substrates and the activation of caspases and nucleases [29-32]. It was previously reported that the release of cytochrome c is associated with the increase of Bax and decrease of Bcl-2 [33]. Our results showed that the expression level of Bcl-2 protein began to decrease and the expression levels of Bax protein was significantly up-regulated simultaneously after resveratrol treatment. The Bax/Bcl-2 ratio was up-regulated dramatically and ultimately induced the occurrence of mitochondria-mediated cell apoptosis. We thus suggested that the induction of apoptosis in K562 cells by resveratrol might be due to the activation of mitochondria mediated intrinsic apoptosis pathway.
In conclusion, our results suggested that resveratrol possessed the activity of anti-proliferation and apoptosis induction in K562 cells. Resveratrol-induced apoptosis of K562 cells might be mediated through the mitochondria pathway. These results support the potential of resveratrol to be developed as a promising agent for treatment of cancers.
Disclosure of conflict of interest
None.
References
- 1.Wang L, Jiang R, Song SD, Hua ZS, Wang JW, Wang YP. Angelica sinensis polysaccharide induces erythroid differentiation of human chronic myelogenous leukemia k562 cells. Asian Pac J Cancer Prev. 2015;16:3715–3721. doi: 10.7314/apjcp.2015.16.9.3715. [DOI] [PubMed] [Google Scholar]
- 2.Taverna S, Giallombardo M, Pucci M, Flugy A, Manno M, Raccosta S, Rolfo C, De Leo G, Alessandro R. Curcumin inhibits in vitro and in vivo chronic myelogenous leukemia cells growth: a possible role for exosomal disposal of miR-21. Oncotarget. 2015;6:21918–33. doi: 10.18632/oncotarget.4204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Jabbour E, Kantarjian H. Chronic myeloid leukemia: 2012 update on diagnosis, monitoring, and management. Am J Hematol. 2012;87:1037–1045. doi: 10.1002/ajh.23282. [DOI] [PubMed] [Google Scholar]
- 4.Jabbour EJ, Cortes JE, Kantarjian HM. Resistance to tyrosine kinase inhibition therapy for chronic myelogenous leukemia: a clinical perspective and emerging treatment options. Clin Lymphoma Myeloma Leuk. 2013;13:515–529. doi: 10.1016/j.clml.2013.03.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Radich JP. Treatment milestones in chronic myelogenous leukemia: stay the course or change therapy? J Natl Compr Canc Netw. 2015;13:697–699. doi: 10.6004/jnccn.2015.0207. [DOI] [PubMed] [Google Scholar]
- 6.Rumjanek VM, Vidal RS, Maia RC. Multidrug resistance in chronic myeloid leukaemia: how much can we learn from MDR-CML cell lines? Biosci Rep. 2013;33:e00081. doi: 10.1042/BSR20130067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Stella C, Kenneth KW T, Ge L. Circumvention of multi-drug resistance of cancer cells by Chinese herbal medicines. Chin Med. 2010;5:26. doi: 10.1186/1749-8546-5-26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Yin SY, Wei WC, Jian FY, Yang NS. Therapeutic Applications of Herbal Medicines for Cancer Patients. Evid Based Complement Alternat Med. 2013;2013:302426. doi: 10.1155/2013/302426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Damery S, Gratus C, Grieve R, Warmington S, Jones J, Routledge P, Greenfield S, Dowswell G, Sherriff J, Wilson S. The use of herbal medicines by people with cancer: a cross-sectional survey. Br J Cancer. 2011;104:927–933. doi: 10.1038/bjc.2011.47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Takizawa Y, Nakata R, Fukuhara K, Yamashita H, Kubodera H, Inoue H. The 4’-hydroxyl group of resveratrol is functionally important for direct activation of PPARα. PLoS One. 2015;10:e0120865. doi: 10.1371/journal.pone.0120865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Huang F, Wu XN, Chen J, Wang WX, Lu ZF. Resveratrol reverses multidrugresistance in human breastcancer doxorubicin-resistant cells. Exp Ther Med. 2014;7:1611–1616. doi: 10.3892/etm.2014.1662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gao Q, Yuan Y, Gan HZ, Peng Q. Resveratrol inhibits the hedgehog signaling pathway and epithelial-mesenchymal transition and suppresses gastric cancer invasion and metastasis. Oncol Lett. 2015;9:2381–2387. doi: 10.3892/ol.2015.2988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Jang M, Cai L, Udeani GO, Slowing KV, Thomas CF, Beecher CW, Fong HH, Farnsworth NR, Kinghorn AD, Mehta RG, Moon RC, Pezzuto JM. Cancer chemopreventive activity of resveratrol, a natural product derived from grapes. Science. 1997;275:218–220. doi: 10.1126/science.275.5297.218. [DOI] [PubMed] [Google Scholar]
- 14.Alexandre P, Guillaume R, Nina F, Frederic L, Cassuto JP, Sophie R, Patrick A. Resveratrol Promotes Autophagic Cell Death in Chronic Myelogenous Leukemia Cells via JNK-Mediated p62/SQSTM1 Expression and AMPK. Cancer Res. 2010;70:1042–1052. doi: 10.1158/0008-5472.CAN-09-3537. [DOI] [PubMed] [Google Scholar]
- 15.Kundu JK, Surh YJ. Cancer chemopreventive and therapeutic potential of resveratrol: mechanistic perspectives. Cancer Lett. 2008;269:243–261. doi: 10.1016/j.canlet.2008.03.057. [DOI] [PubMed] [Google Scholar]
- 16.Huang F, Wu XN, Chen J, Wang WX, Lu ZF. Resveratrol reverses multidrug resistance in human breast cancer doxorubicin-resistant cells. Exp Ther Med. 2014;7:1611–1616. doi: 10.3892/etm.2014.1662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Britton RG, Kovoor C, Brown K. Direct molecular targets of resveratrol: identifying key interactions to unlock complex mechanisms. Ann N Y Acad Sci. 2015;1348:124–33. doi: 10.1111/nyas.12796. [DOI] [PubMed] [Google Scholar]
- 18.Tian H, Yu Z. Resveratrol induces apoptosis of leukemia cell line K562 by modulation of sphingosine kinase-1 pathway. Int J Clin Exp Pathol. 2015;8:2755–2762. [PMC free article] [PubMed] [Google Scholar]
- 19.Goldar S, Khaniani MS, Derakhshan SM, Baradaran B. Molecular mechanisms of apoptosis and roles in cancer development and treatment. Asian Pac J Cancer Prev. 2015;16:2129–2144. doi: 10.7314/apjcp.2015.16.6.2129. [DOI] [PubMed] [Google Scholar]
- 20.Zhang GH, Cai LJ, Wang YF, Zhou YH, An YF, Liu YC. Novel compound PS-101 exhibits selective inhibition in non-small-cell lung cancer cell by blocking the EGFR-driven antiapoptotic pathway. Biochem Pharmacol. 2013;86:1721–1730. doi: 10.1016/j.bcp.2013.10.013. [DOI] [PubMed] [Google Scholar]
- 21.Sharoar MG, Islam MI, Shahnawaz M, Shin SY, Park IS. Amyloid β binds procaspase-9 to inhibit assembly of Apaf-1 apoptosome and intrinsic apoptosis pathway. Biochim Biophys Acta. 2014;1843:685–693. doi: 10.1016/j.bbamcr.2014.01.008. [DOI] [PubMed] [Google Scholar]
- 22.Ma JQ, Ding J, Zhang L, Liu CM. Hepatoprotective properties of sesamin against CCl4 induced oxidative stress-mediated apoptosis in mice via JNK pathway. Food Chem Toxicol. 2014;64:41–48. doi: 10.1016/j.fct.2013.11.017. [DOI] [PubMed] [Google Scholar]
- 23.Shelton SN, Shawgo ME, Matthews SB, Lu Y, Donnelly AC, Szabla K, Tanol M, Vielhauer GA, Rajewski RA, Matts RL, Blagg BS, Robertson JD. KU135, a novel novobiocin-derived C-terminal inhibitor of the 90-kDa heat shock protein, exerts potent antiproliferative effects in human leukemic cells. Mol Pharmacol. 2009;76:1314–1322. doi: 10.1124/mol.109.058545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Tyagi M, Bhattacharyya R, Bauri AK, Patro BS, Chattopadhyay S. DNA damage dependent activation of checkpoint kinase-1 and mitogen-activated protein kinase-p38 are required in malabaricone C-induced mitochondrial cell death. Biochim Biophys Acta. 2014;1840:1014–27. doi: 10.1016/j.bbagen.2013.11.020. [DOI] [PubMed] [Google Scholar]
- 25.Méndez J, Morales Cruz M, Delgado Y, Figueroa CM, Orellano EA, Morales M. Delivery of Chemically Glycosylated Cytochrome c Immobilized in Mesoporous Silica Nanoparticles Induces Apoptosis in HeLa Cancer Cells. Mol Pharm. 2014;11:102–111. doi: 10.1021/mp400400j. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Gajek A, Denel M, Bukowska B, Rogalska A, Marczak A. Pro-apoptotic activity of new analog of anthracyclines - WP 631 in advanced ovarian cancer cell line. Toxicol In Vitro. 2014;28:273–281. doi: 10.1016/j.tiv.2013.11.006. [DOI] [PubMed] [Google Scholar]
- 27.Aporta A, Catalán E, Galán-Malo P, Ramírez-Labrada A, Pérez M, Azaceta G, Palomera L, Naval J, Marzo I, Pardo J, Anel A. Granulysin induces apoptotic cell death and cleavage of the autophagy regulator Atg5 in human hematological tumors. Biochem Pharmacol. 2014;87:410–423. doi: 10.1016/j.bcp.2013.11.004. [DOI] [PubMed] [Google Scholar]
- 28.Zhang C, Yuan XR, Li HY, Zhao ZJ, Liao YW, Wang XY. Downregualtion of dynamin-related protein 1 attenuates glutamate-induced excitotoxicity via regulating mitochondrial function in a calcium dependent manner in HT22 cells. Biochem Biophys Res Commun. 2014;443:138–143. doi: 10.1016/j.bbrc.2013.11.072. [DOI] [PubMed] [Google Scholar]
- 29.Karch J, Molkentin JD. Regulated Necrotic Cell Death: The Passive Aggressive Side of Bax and Bak. Circ Res. 2015;116:1800–1809. doi: 10.1161/CIRCRESAHA.116.305421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Chan SL, Yu VC. Proteins of the bcl-2 family in apoptosis signalling: from mechanistic insights to therapeutic opportunities. Clin Exp Pharmacol Physiol. 2004;31:119–128. doi: 10.1111/j.1440-1681.2004.03975.x. [DOI] [PubMed] [Google Scholar]
- 31.Liu Z, Lu H, Jiang Z, Pastuszyn A, Hu CA. Apolipoprotein l6, a novel proapoptotic Bcl-2 homology 3-only protein, induces mitochondria-mediated apoptosis in cancer cells. Mol Cancer Res. 2005;3:21–31. [PubMed] [Google Scholar]
- 32.Ko JK, Choi KH, Peng J, He F, Zhang Z, Weisleder N, Lin J, Ma J. Amphipathic tail-anchoring peptide and Bcl-2 homology domain-3 (BH3) peptides from Bcl-2 family proteins induce apoptosis through different mechanisms. J Biol Chem. 2011;286:9038–9048. doi: 10.1074/jbc.M110.198457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lucena FR, de Araújo LC, Rodrigues Mdo D, da Silva TG, Pereira VR, Militão GC, Fontes DA, Rolim-Neto PJ, da Silva FF, Nascimento SC. Induction of cancer cell death by apoptosis and slow release of 5-fluoracil from metal-organic frameworks Cu-BTC. Biomed Pharmacother. 2013;67:707–713. doi: 10.1016/j.biopha.2013.06.003. [DOI] [PubMed] [Google Scholar]


