Abstract
Background-AIMS: Little is known about the prognostic significance of elevated serum relaxin in acute myocardial infarction (AMI) patients. The present study is designed to investigate the potential association between serum relaxin levels and the risk of AMI. Materials and methods: We measured circulating relaxin levels in 80 patients (median age 62.3 years) who presented with first-time AMI 8 hours after the incident. The circulating relaxin-2 levels in 80 healthy volunteers (median age 61.5 years) was also measured. Relaxin-2 was detected using enzyme immunoassay in both groups. Results: Serum relaxin levels were significantly higher in patients with AMI (27.4 ± 6.3 ng/ml) compared to controls (9.2 ± 2.3 ng/ml) (P < 0.01). We found that a relaxin level > 13.8 ng/ml had a sensitivity of 79% and a specificity of 86% for predicting AMI. Relaxin revealed the higher sensitivity and specificity for diagnosing AMI. Conclusions: Elevated relaxin in plasma may be a novel biomarker for early detection of AMI.
Keywords: Acute myocardial infarction, biomarker, relaxin
Introduction
Acute myocardial infarction (AMI) is the world’s leading cause of morbidity and mortality. An early and correct diagnosis may warrant immediate initiation of reperfusion therapy to potentially reduce the mortality rate [1]. Biomarkers, used to establish a diagnosis in patients with AMI, have emerged largely from targeted analyses of known myocardial proteins and become more and more important for diagnosis of AMI [2,3]. Current biomarkers such as creatine kinase-MB isoenzymes, cardiac myoglobin, and troponins have been widely applied in clinical diagnosis [4]. Among these, cardiac troponins are currently considered as the ‘gold standard’ for AMI diagnosis [5]. However, the exploration of new biomarkers with high sensitivity and specificity in early diagnosis of AMI never stop.
The relaxin peptide family in humans consists of seven members, relaxin-1, -2 and -3 and insulin-like (INSL) peptides 3, 4, 5 and 6 [6,7]. Since human relaxin-2 (H2 relaxin) is the equivalent of relaxin-1 in non-primate species, both will be simply referred to as relaxin [7].
Relaxin is mainly known as a reproductive hormone which is produced by the corpus luteum and/or placenta in many species. There are varying effects of relaxin on the cervix, mammary glands, nipples, pubic symphysis and uterus of different species [8]. Relaxin mediates various physiological processes of normal pregnancy and parturition, for example, in the relaxin knockout (KO) mouse [9] and relaxin immunoneutralized rat [10], pup delivery was prolonged and difficult thus significantly reducing their survival. It is also crucial for the remodeling and growth of the uterus, cervix and vagina throughout pregnancy. It has recently found human recombinant relaxin significant reduction of plasma histamine, increase in cardiac histamine content and decrease in cardiac mast cell degranulation [11]. Furthermore, human relaxin could counteract reperfusion-induced cardiac injury, afford a clear-cut protection to the heart of swine with induced myocardial infarction [12].
Recent reports show that relaxin are also present in various biological fluids, including blood, and the levels of relaxin are linked to the diagnosis and prognosis for diseases [13-15]. We hypothesized that the relaxin might release into the circulation during AMI and the elevated relaxin in plasma from AMI patients could be potential biomarkers for the diagnosis of AMI.
Materials and methods
Study population
Our case-control study included 80 patients who presented with AMI for the first time and were assessed 4 hours after the incident. Controls consisted of 80 healthy individuals matched by the “frequency matching method” for age, sex, and body mass index (BMI). Cases presented with AMI from Oct. 2010 through Sep. 2012 to the Department of internal medicine-cardiovascular, the people’s hospital of weifang and controls were randomly selected from participants of the center of physical examination of the people’s hospital of weifang. Patients with inflammatory diseases, infectious diseases, renal or liver problems, diabetic patients, pregnancy and those with any history of myocardial infarction were excluded. The study was approved by the people’s hospital of weifang. All participants formally consented to participate in all stages of the study.
Anthropometric measurements and clinical assessments
Elevation of myocardial necrotic markers in the serum and ST segment elevation on electrocardiogram was diagnosed with AIM. BMI was calculated using the international standard equation (weight/height2) and recorded as kg/m2 [16]. Waist circumference was defined as the measurement around the narrowest diameter between the lower costal margin and iliac creat [16]. Hip circumference was defined by measuring around the widest diameter over the greater trochanters [16]. These findings were used to calculate the waist-to-hip ratio (WHR). Blood pressure was measured at least 10 minutes before blood sampling in both groups.
Laboratory procedures
The patients of two groups were given 10-12 hours of fasting, then the blood samples were collected from them. Blood samples were then centrifuged, coded and stored at -70°C until analyzed. Serum levels of triglycerides, high density lipoprotein (HDL), total cholesterol (TCH), low density lipoprotein (LDL), and fasting blood sugar (FBS) were checked with enzymatic methods. A commercially available ELISA Kit (Guangzhou, China) was used to checked serum relaxin levels. The mean inter- and intra-assay coefficients of variation were < 10% for all assays.
Statistical analysis
The results were reported as mean ± standard deviation and all statistical analyses were performed with the use of computer software (SPSS, version 11, SPSS Institute, Chicago, IL, USA). Baseline variables were compared between two groups using the independent student’s t- and chi-square tests. Distributions of continuous variables were analyzed using the Shapiro-Wilks test for normality. As distribution of variables such as relaxin levels, systolic (SBP) and diastolic blood pressure (DBP), HDL and triglyceride levels, and WHR were little-to-mild skewed toward the right, their log-transformed values were used for analysis. Correlations between serum relaxin levels and independent variables were analyzed using Pearson’s correlation coefficient. The Receiver Operating Characteristic (ROC) curve was used to describe relaxin concentrations as a potential diagnostic factor and the optimal cut-off point was estimated. P-values less than 0.05 were considered statistically significant.
Results
Relaxin levels in AMI and healthy individuals
A total of 80 patients with new onset AMI and no histories of any such prior incident as well as 80 healthy individuals (control group) were recruited for the study. There were 100 male and 60 female participants within the age range of 25-83 years (mean = 61.2, SD = 9.76). Serum relaxin levels were significantly higher in patients with AMI (27.4 ± 6.3 ng/ml) compared to controls (9.2 ± 2.3 ng/ml) (P < 0.01, Table 1). Pearson correlation coefficients showed that no relation was found between plasma relaxin and other variables in study subjects (Table 2).
Table 1.
Baseline characteristics of study subjects
| Factor | AMI group | Control group | P-value |
|---|---|---|---|
| Number | 80 | 80 | -- |
| Age (years) | 62.3 ± 12.4 | 61.5 ± 12.2 | 0.53 |
| Sex, male (%) | 63 (78.7%) | 61 (76.2%) | 0.48 |
| BMI (kg/m2) | 27.2 ± 5.36 | 26.9 ± 5.18 | 0.96 |
| Weight (kg) | 71.8 ± 12.4 | 69.3 ± 13.3 | 0.27 |
| Waist (cm) | 105.7 ± 9.2 | 106.4 ± 10.6 | 0.93 |
| SBP* (mmHg) | 140 ± 22.8 | 118.4 ± 19.3 | 0.000 |
| DBP* (mmHg) | 88.2 ± 14.7 | 75.9 ± 11.3 | 0.000 |
| Cholesterol (mg/dL) | 184.5 ± 38.2 | 182.0 ± 24.8 | 0.73 |
| TG (mg/dL) | 137.6 ± 78.3 | 127.3 ± 45.0 | 0.74 |
| HDL* (mg/dL) | 42.2 ± 9.76 | 46.3 ± 11.8 | 0.016 |
| LDL* (mg/dL) | 110.5 ± 37.8 | 118.3 ± 25.3 | 0.24 |
| FBS (mg/dL) | 112.4 ± 15.6 | 97.4 ± 16.5 | 0.000 |
| Relaxin (ng/mL) | 27.4 ± 6.3 | 9.2 ± 2.3 | 0.001 |
Mean ± SD was reported: BMI: Body mass index; SBP: Systolic blood pressure; DBP: Diastolic blood pressure; FBS: Fasting blood sugar; HDL: High-density lipoprotein; LDL: Low density lipoprotein;
Log transformed data were used in t-test.
Table 2.
Pearson correlation coefficients between plasma relaxin and other variables in study subjects
| Variables | Correlation coefficient | P-value |
|---|---|---|
| Waist | -0.146 | 0.36 |
| SBP | 0.130 | 0.24 |
| DBP | 0.053 | 0.652 |
| Cholesterol | -0.076 | 0.309 |
| TG | -0.007 | 0.942 |
| HDL | -0.062 | 0.53 |
| LDL | -0.038 | 0.838 |
| FBS | 0.005 | 0.92 |
Correlation of relaxin with other clinical characteristics
Sensitivity of relaxin to detect AMI
The ability of log relaxin to detect patients with AMI was explored using a ROC curve. The area under the ROC curve was 0.82 (95% CI: 0.7-0.91). A relaxin value > 13.8 ng/ml (log relaxin > 0.92) had a sensitivity of 79% and a specificity of 86% for detecting individuals with AMI (Figure 1).
Figure 1.
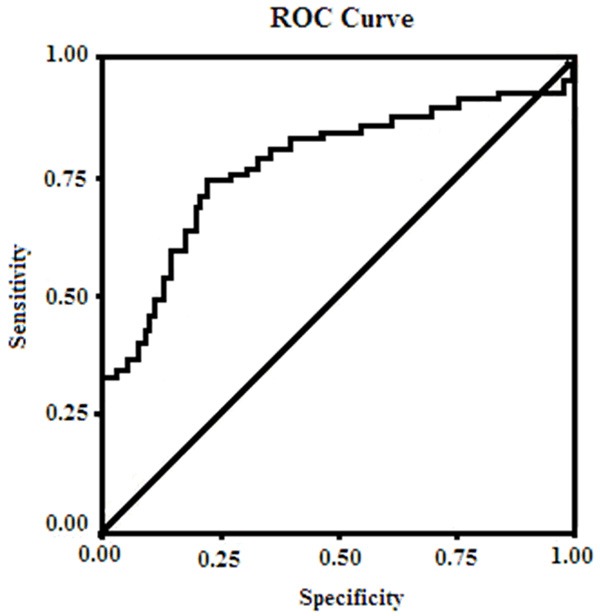
ROC curve used for the definition of the cut-off value of relaxin that best characterizes AMI and control groups.
Discussion
Relaxin is produced by the heart and as the specific heart receptors [17-20]. It has been recently validated as a cardiotropic hormone. The significant function of relaxin in heart is to increase coronary blood flow and positive chronotropism and inotropism [21,22]. It could also be able to counteract the pathophysiological mechanisms of ischemic heart disease [23]. Furthermore, relaxin could result in systemic vasodilation and extracellular body fluid expansion [22,23], and convert the cardiocirculatory apparatus to the needs of pregnancy [22].
Our study showed that serum relaxin levels were significantly high in AIM patients compared to controls. This finding was consistent with other studies in cancer [13]. Furthermore, a relaxin level > 13.8 ng/ml had a sensitivity of 79% and a specificity of 86% for detecting individuals with AMI. We believe that relaxin may be considered as a biomarker for predicting the probability of AMI in the future. Elevated relaxin in AMI patients might be a protective mechanism. Our finding support existing literature regarding the role of relaxin in the process of AMI.
In the present study, we did not find significant relation between relaxin and other biochemical parameters.
The case-control design limited our ability to infer a causal relationship between increased serum relaxin levels and AMI.
In conclusion, this report has showed elevated serum relaxin concentrations in Chinese patients with AMI. Relaxin may be considered as a biomarker for predicting the probability of AMI. However, a large-scale prospective cohort study is necessary to resolve the potential causal relationship between relaxin and AMI.
Disclosure of conflict of interest
None.
References
- 1.White HD, Chew DP. Acute myocardial infarction. Lancet. 2008;372:570–584. doi: 10.1016/S0140-6736(08)61237-4. [DOI] [PubMed] [Google Scholar]
- 2.Thygesen K, Alpert JS, White HD. Universal definition of myocardial infarction. Eur Heart J. 2007;28:2525–2538. doi: 10.1093/eurheartj/ehm355. [DOI] [PubMed] [Google Scholar]
- 3.Jaffe AS, Babuin L, Apple FS. Biomarkers in acute cardiac disease: the present and the future. J Am Coll Cardiol. 2006;48:1–11. doi: 10.1016/j.jacc.2006.02.056. [DOI] [PubMed] [Google Scholar]
- 4.de Winter RJ, Koster RW, Sturk A, Sanders GT. Value of myoglobin, troponin T, and CK-MB mass in ruling out an acute myocardial infarction in the emergency room. Circulation. 1995;92:3401–3407. doi: 10.1161/01.cir.92.12.3401. [DOI] [PubMed] [Google Scholar]
- 5.Jaffe AS, Ravkilde J, Roberts R, Naslund U, Apple FS, Galvani M, Katus H. It’s time for a change to a troponin standard. Circulation. 2000;102:1216–1220. doi: 10.1161/01.cir.102.11.1216. [DOI] [PubMed] [Google Scholar]
- 6.Park J, Chang C, Hsu S. New Insights into biological roles of relaxin and relaxin-related peptides. Rev Endocr Metab Disorders. 2005;6:291–296. doi: 10.1007/s11154-005-6187-x. [DOI] [PubMed] [Google Scholar]
- 7.Kong RC, Shiling PJ, Lobb DK, Gooley PR, Bathgate RAD. Membrane receptors: structure and function of the relaxin family peptide receptors. Mol Cell Endocrinol. 2010;320:1–15. doi: 10.1016/j.mce.2010.02.003. [DOI] [PubMed] [Google Scholar]
- 8.Sherwood OD. Relaxin’s physiological roles and other diverse actions. Endocr Rev. 2004;25:205–234. doi: 10.1210/er.2003-0013. [DOI] [PubMed] [Google Scholar]
- 9.Zhao L, Roche PJ, Gunnersen JM, Hammond VE, Tregear GW, Wintour EM, Beck F. Mice without a functional relaxin gene are unable to deliver milk to their pups. Endocrinology. 1999;140:445–453. doi: 10.1210/endo.140.1.6404. [DOI] [PubMed] [Google Scholar]
- 10.Guico-Lamm ML, Sherwood OD. Monoclonal antibodies specific for rat relaxin. II. Passive immunization with monoclonal antibodies throughout the second half of pregnancy disrupts birth in intact rats. Endocrinology. 1988;123:2479–2485. doi: 10.1210/endo-123-5-2479. [DOI] [PubMed] [Google Scholar]
- 11.Nistri S, Cinci L, Perna AM, Masini E, Mastroianni R, Bani D. Relaxin induces mast cell inhibition and reduces ventricular arrhythmias in a swine model of acute myocardial infarction. Pharmacol Res. 2008;57:43–8. doi: 10.1016/j.phrs.2007.11.001. [DOI] [PubMed] [Google Scholar]
- 12.Perna AM, Masini E, Nistri S, Briganti V, Chiappini L, Stefano P, Bigazzi M, Pieroni C, Bani Sacchi T, Bani D. Novel drug development opportunity for relaxin in acute myocardial infarction: evidences from a swine model. FASEB J. 2005;19:1525–7. doi: 10.1096/fj.04-3664fje. [DOI] [PubMed] [Google Scholar]
- 13.Ren P, Yu ZT, Xiu L, Wang M, Liu HM. Elevated serum levels of human relaxin-2 in patients with esophageal squamous cell carcinoma. World J Gastroenterol. 2013;19:2412–8. doi: 10.3748/wjg.v19.i15.2412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wolf JM, Williams AE, Delaronde S, Leger R, Clifton KB, King KB. Relationship of serum relaxin to generalized and trapezial-metacarpal joint laxity. J Hand Surg Am. 2013;38:721–8. doi: 10.1016/j.jhsa.2013.01.019. [DOI] [PubMed] [Google Scholar]
- 15.Ma J, Niu M, Yang W, Zang L, Xi Y. Role of relaxin-2 in human primary osteosarcoma. Cancer Cell Int. 2013;13:59. doi: 10.1186/1475-2867-13-59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Senter S, Francis GS. A new, precise definition of acute myocardial infarction. Cleve Clin J Med. 2009;76:159–66. doi: 10.3949/ccjm.75a.08092. [DOI] [PubMed] [Google Scholar]
- 17.Taylor MJ, Clark CL. Evidence for a novel source of relaxin: atrial cardiocytes. J Endocrinol. 1994;143:R5–R8. doi: 10.1677/joe.0.143r005. [DOI] [PubMed] [Google Scholar]
- 18.Dschietzig T, Richter C, Bartsch C, Laule M, Armbruster FP, Baumann G, Stangl K. The pregnancy hormone relaxin is a player in human heart failure. FASEB J. 2001;15:2187–2195. doi: 10.1096/fj.01-0070com. [DOI] [PubMed] [Google Scholar]
- 19.Osheroff PL, Ho W. Expression of relaxin mRNA and relaxin receptors in postnatal and adult rat brain and hearts. J Biol Chem. 1993;268:15193–15199. [PubMed] [Google Scholar]
- 20.Hsu SY, Nakabayashi K, Nishi S, Kumagai J, Kudo M, Sherwood OD, Hsueh AJ. Activation of orphan receptors by the hormone relaxin. Science. 2002;295:671–673. doi: 10.1126/science.1065654. [DOI] [PubMed] [Google Scholar]
- 21.Bani Sacchi T, Bigazzi M, Bani D, Mannaioni PF, Masini E. Relaxininduced increased coronary flow through stimulation of nitric oxide production. Br J Pharmacol. 1995;116:1589–1594. doi: 10.1111/j.1476-5381.1995.tb16377.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Dschietzig T, Stangl K. Relaxin: a pregnancy hormone as a central player of body fluid and circulation homeostasis. Cell Mol Life Sci. 2003;60:688–700. doi: 10.1007/s00018-003-2169-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Samuel C, Parry LJ, Summers RJ. Physiological or pathological-a role for relaxin in the cardiovascular system? Curr Opin Pharmacol. 2003;3:152–158. doi: 10.1016/s1471-4892(03)00011-0. [DOI] [PubMed] [Google Scholar]


