Abstract
Nephropathy associated with type 2 diabetes is the single most common cause of end-stage renal disease. The aim of the present study was to evaluate the preventive effect of ethanolic extract of Embelia ribes fruit (EER) against high fat diet (HFD) and low dose streptozotocin (STZ)-induced diabetic nephrotoxicity in Wistar rats. HFD-fed and low dose STZ (35 mg/kg, i.p)-induced diabetic rats were treated with EER (100 and 200 mg/kg/day) for 21 days while continuing on HFD. Preventive effects of EER were demonstrated by significant reduction (p< 0.01) in body weight gain, fasting blood glucose, blood pressure, serum lactate dehydrogenase (LDH), creatinine, alkaline phosphatase (ALP), total cholesterol and triglyceride levels, while elevation in serum albumin and total protein levels. Insulin sensitizing effects were seen during oral glucose tolerance testing. Further, EER treatment significantly (p< 0.01) decreased the kidney thiobarbituric acid-reactive substance (TBARS) levels, while increasing the superoxide dismutase (SOD), catalase (CAT) and glutathione (GSH) levels in diabetic rats. Histological studies of kidney also supported the experimental findings. Taken together, our data suggest that EER attenuates renal injury in type 2 diabetic rats, possibly by improvement in glucose and lipid metabolism, enhancement of insulin sensitivity, blood pressure lowering, and inhibition of lipid peroxidation process.
Keywords: type 2 diabetes, nephropathy, creatinine, streptozotocin, insulin, high fat diet
Introduction
Nephropathy is one of the major complications of both type 1 and type 2 diabetes mellitus, and the morbidity and mortality due to diabetic nephropathy (DN) continues to increase in industrialized nations (Balakumar et al., 2009[4]). Nephropathy associated with type 2 diabetes is much more widespread and account for about one third of all patients requiring kidney replacement therapy in western countries (Ruggenenti and Remuzzi, 2000[27]). DN is characterized by a progressive increase in albuminuria, proteinuria, reduction in glomerular filtration rate (GFR), hypertension, and a high risk of cardiovascular morbidity and mortality (Balakumar et al., 2009[4]). Risk factors for DN include hyperglycemia, insulin resistance, hypertension, genetic predisposition, glomerular hyperfiltration, proteinuria, advanced glycation end-products, reduced nephron number and lipid disorders (Cooper, 2001[11]; Hall, 2006[15]). Hyperglycemia generates more reactive oxygen species (ROS) and promotes oxidative stress, which is known to play crucial role in the pathogenesis of DN (Alhaider et al., 2011[1]). Further, obesity and insulin resistance contribute to the progression of renal disease (Knight and Imig, 2007[17]). Thus, metabolic and hemodynamic factors should be controlled in order to prevent the progression of DN.
Currently available therapeutic options to treat patient with DN includes agents like angiotensin converting enzyme inhibitors, angiotensin AT1 receptor blocker and few antioxidants, which have been shown to improve the function of diabetic kidney to some extent (Balakumar et al., 2009[4]). Therefore, efforts are being made to explore new promising therapeutic interventions to treat DN. Recently, herbs and/or natural products have been highlighted as an alternative to the current management of DN (Shafi et al., 2012[29]).
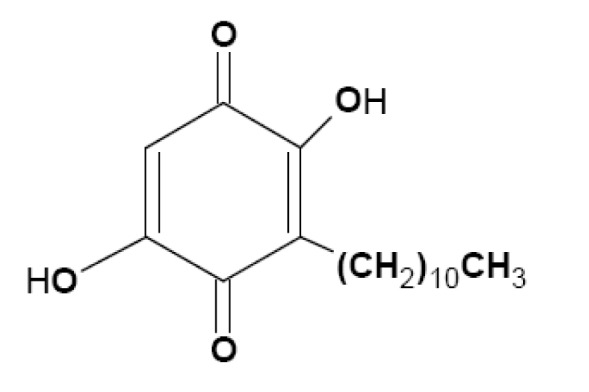
Embelia ribes Burm. (family Myrsinaceae), a large climbing shrub with long slender brittle stem, long internodes, terete branches, is sparsely distributed in the moist deciduous forests of the Western Ghats in India, Sri Lanka, Malaysia, South China and Kenya. Embelin (2,5-dihydroxy-3-undecyl-1,4-benzoquinone, Figure 1(Fig. 1)) is a major bioactive constituent and marker compound in E. ribes fruit (Madhavan et al., 2011[22]). The fruit is bitter in taste, and has been used to treat fever, inflammatory diseases, and a variety of gastrointestinal ailments for thousands of years. The plant is popularly known as 'vidanga' in the Indian system of medicine 'Ayurveda' and it is an ingredient in many Ayurvedic preparations. Earlier studies in our laboratory showed that ethanolic fruit extract of E. ribes possesses antihyperglycemic, lipid lowering and antioxidant properties in insulin-deficient diabetes in rat models, induced by high dose of streptozotocin (Bhandari et al., 2002[6], 2007[5]). However, therapeutic potential of E. ribes for its preventive effect on diabetic nephrotoxicity in type 2 diabetic rats is unclear. Several animal models have been used to assess the pathogenesis of diabetes and associated complications. However, rats fed a high-fat diet (HFD) and given a low dose of streptozotocin (STZ) develop type 2 diabetes with insulin resistance, hyperinsulinemia, hyperglycemia, hyperlipidemia, hypertension, proteinuria and kidney lesions, similar to human type 2 diabetes and nephropathy where multiple factors appear to act synergistically to accelerate kidney damage (Danda et al., 2005[12]). Hence, in the present work, we investigated the preventive role of ethanolic extract of E. ribes (EER) fruits on diabetic nephrotoxicity using a model of type 2 diabetes induced by HFD and low dose STZ in Wistar rats.
Figure 1. Chemical structure of embelin (2,5-dihydroxy-3-undecyl-1,4-benzoquinone).
Materials and Methods
Chemicals
STZ was purchased from Sigma Aldrich, St. Louis, MO, USA. Commercially available standard kits were used for the determination of insulin (ultra sensitive rat insulin ELISA kit, Crystal Chem Inc., IL, USA), total cholesterol, triglycerides, alkaline phosphatise (ALP), creatinine, albumin and total protein (Span Diagnostics Ltd., Surat, India), and lactate dehydrogenase (LDH; Crest Biosystems, Goa, India). All other chemicals were commercial products of analytical reagent grade.
Plant material and preparation of extraction
The berries of E. ribes were purchased from North Eastern India Ayurveda Research Institute, Barsjoi, Guwahati, Assam, India, and authenticated by Dr. H. B. Singh, Head, Raw Materials and Herbarium, National Institute of Science Communication and Information Resources (NISCAIR), New Delhi, India, where a voucher specimen has been deposited (No. NISCAIR/RHMD/Consult/-2010-11/1500/ 98, dated 26 August, 2010).
The dried and powdered fruits of E. ribes were Soxhlet extracted with 70 % ethanol in a Soxhlet apparatus for 72 h. The solvent was removed under reduced pressure to give a dry extract, 8.4 % w/w yield with respect to the crude material and stored at -20 °C until the experiment.
HPTLC and HPLC analysis of extract
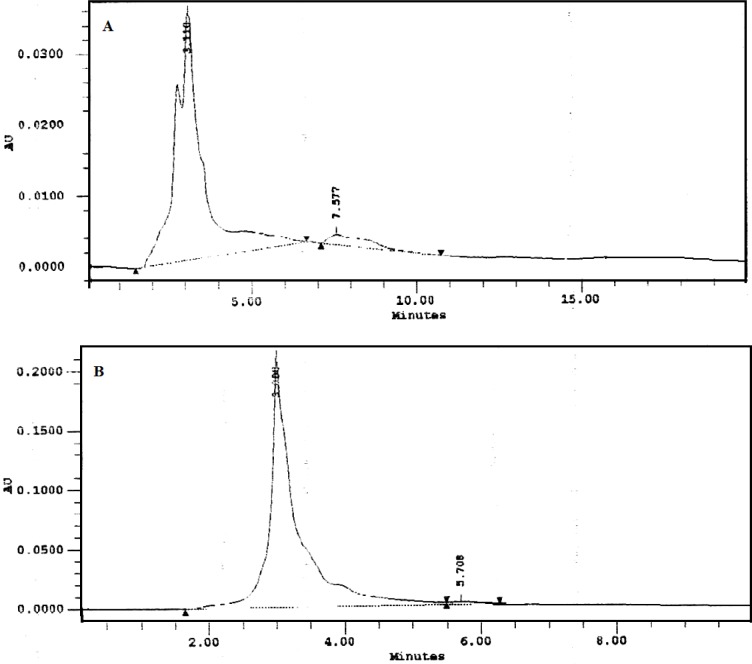
Phytochemical analysis and high performance thin layer chromatography (HPTLC) finger printing data of EER has been previously published (Ansari and Bhandari, 2008[2]). The HPLC analysis of EER was carried out by the methodology of Shelar et al. (2009[30]) on HPLC system (Waters Corporation, USA) equipped with Millennium Chromatography software version 2.15 and the chromatographic separations were performed using a Hyperchrome Column (250 mm×4.6 mm), with a flow rate of 1 ml/min and a sample size of 10 µl. The mobile phase used was methanol (A) and 0.1 % trifluoroacetic acid (B) with a ratio of 88:12 (A:B (v/v)). The flow rate was set 1 ml/min and sample analysis performed at wavelength 288 nm using photodiode array (PDA) detector at ambient temperature. The chromatographic peaks of the EER were confirmed by comparing its retention time and UV spectra with corresponding reference standard embelin. The result was obtained by comparison of peak area (at 288 nm) of EER with that of embelin.
Experimental animals
The male Wistar rats (150-200 g) were procured from central animal house facility of Hamdard University, New Delhi, India. Rats were housed in clean solid bottom, stainless steel mesh polypropylene rat cages and maintained under controlled room temperature (22 ± 2 °C) and humidity (55 ± 5 %) with 12:12 h light and dark cycle. All the rats had free access to standard rat feed and water ad libitum. The study was approved by the Institutional Animal Ethics Committee of Jamia Hamdard, New Delhi, India, (Approval No. 316) and performed as per the guidelines of Committee for the Purpose of Control and Supervision of Experiments on Animals (CPCSEA), India. Rats were allowed to acclimate to the experimental environment for 1 week before dietary intervention was initiated.
High fat diet and low dose streptozotocin-induced type 2 diabetes
The rats were allocated into two dietary regimens by feeding either standard rat feed or HFD (from National Institute of Nutrition (NIN), Hyderabad, India) ad libitum, respectively, for the initial period of 2 weeks (Srinivasan et al., 2005[31]). The composition of HFD was described elsewhere (Chaudhari et al., 2012[10]). After the 2 weeks of dietary manipulation, the group of rats fed by HFD was injected intraperitoneally (i.p.) with low dose of STZ (35 mg/kg), while the respective control rats were given vehicle citrate buffer (pH 4.4) in a dose volume of 1 ml/kg, i.p. The fasting blood glucose was measured 3 days after the vehicle or STZ injection. The rats with the fasting blood glucose ≥200 mg/dl were considered diabetic and selected for further pharmacological studies. The rats were allowed to continue to feed on their respective diets until the end of the study.
In this experiment, a total of 40 rats (10 normal; 30 HFD+STZ-induced diabetic rats) were used and were divided into four groups of 10 rats in each as follows:
Group I: normal control rats administered 1 % gum acacia in saline daily (NC)
Group II: diabetic control rats administered 1 % gum acacia daily (DC)
Group III: diabetic rats administered ethanolic extract of E.ribes [100 mg/kg, body weight (bw)] (EER100)
Group IV: diabetic rats administered ethanolic extract of E. ribes (200 mg/kg, bw) (EER200).
All the drugs were administered orally via a standard orogastric cannula everyday for the period of 21 days. Diet and water were provided ad libitum. Body weight was measured weekly. At the end of 21 days, the diets were removed from the cages 12 h before the animals were euthanized. Blood samples were collected by the retro-orbital method and centrifuged at 1500 g for 15 min to obtain serum. After the collection of blood, the rats were euthanized, kidney was excised immediately, rinsed with phosphate buffer saline, and weighed. The serum and kidney samples were stored at -70 °C until analysis.
Measurement of blood pressure
Mean arterial blood pressure was measured by Non-Invasive Blood Pressure Recorder on last day of the study using rat tail-cuff method (Kent Scientific Corporation, USA).
Fasting blood glucose measurements
Fasting blood glucose levels were measured weekly on lateral tail vein blood samples using an ACCU-Check glucose meter (Roche Diagnostics GmbH, Mann-heim, Germany) throughout the treatment period.
Oral glucose tolerance test (OGTT)
OGTT was performed 3 weeks after treatment. Rats were orally gavaged with 2 g/kg body weight of glucose dissolved in water (40 % w/v) after overnight fasting. Blood samples were collected from tail veins after 0, 15, 30, 60 and 120 min. Serum insulin levels were measured at the indicated time points using ultra sensitive rat insulin ELISA kit (Crystal Chem Inc., IL, USA). The results were expressed as area under the curve (AUC0-120 min).
Determination of the serum biochemical parameters
Serum levels of insulin, total cholesterol, triglycerides, LDH, ALT, creatinine, albumin and total protein were measured using the commercially available standard kits and according to manufacturer's instructions.
Insulin sensitivity was assessed by computing the homeostatic model assessment (HOMA) by following formula:
HOMA= Insulin (µU/ml) × glucose (mM) / 22.5
Determination of lipid peroxidation and free radical scavenging activity in kidney tissue
A portion of the kidney was minced and homogenized (10 % w/v) for determination of lipid peroxidation by thiobarbituric acid reactive substances (TBARS) (Ohkawa et al., 1979[24]), reduced glutathione (GSH) (Calberg and Mannerviek, 1975[8]), superoxide dismutase (SOD) (Marklund and Marklund, 1974[23]), and catalase (CAT) (Caliborne, 1985[9]) activities. The protein content of the samples was measured to the method of Lowry et al. (1951[21]).
Histopathological examinations
For histological examination, the kidney was collected and fixed in 10 % neutral buffered formalin, embedded in paraffin. Standard sections of 5 μm thickness were cut, which were then stained with hematoxylin and eosin (H&E), and examined by light microscopy.
Statistical analysis
Data are expressed as the mean ± standard error of the mean (SEM). The statistical significance of difference between the mean values for the treatment groups was analyzed by ANOVA (analysis of variance) followed by Dunnett's t-test using Graph pad InStat® version 3.06 (Graph Pad Software, San Diego, CA, USA). Values of p< 0.05 were considered significant.
Results
HPLC analysis of EER extract
The presence of embelin in EER was established by HPLC and quantified as 3.3 % (Figure 2(Fig. 2)).
Figure 2. HPLC chromatograms of Embelia ribes ethanol extract (A) and the standard embelin (B).
Effect of EER on body weight gain, kidney weight and kidney weight/body weight ratio
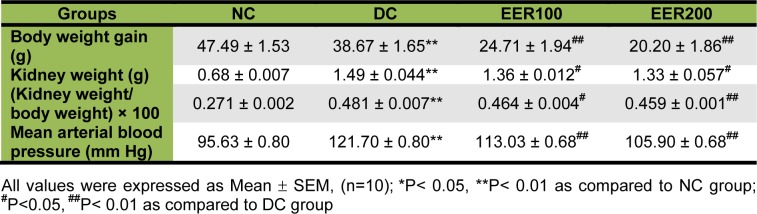
A statistically significant (p< 0.01) decrease in body weight gain of HFD-fed and low dose STZ treated group (i.e. DC group) was observed as compared to NC group. Treatment with EER for 21 days at 100 and 200 mg/kg/day of body weight by the oral route significantly (p< 0.01) decreased the body weight gain compared to the body weight gain of DC group. Moreover, the kidney weight evaluation at the end of the study revealed that there was remarkable elevation in kidney weights in DC group as compared to NC group. Kidney weight body weight ratio was significantly (p< 0.01) higher in DC group compared with the NC group. In contrast, kidney weights and kidney weight/body weight ratio were significantly (p< 0.05) lower in the EER100 and EER200 treated groups compared with the DC group (Table 1(Tab. 1)).
Table 1. Effect of EER on body weight gain, kidney weight and mean arterial blood pressure in HFD-fed and low dose STZ-induced type 2 diabetes in Wistar rats.
Effect of EER on blood pressure
A statistically significant (p< 0.01) increase in mean arterial blood pressure was observed in DC group compared to the NC group by 3 weeks after induction of diabetes. EER (100 and 200 mg/kg) restored the elevated mean arterial blood pressure in DC group (Table 1(Tab. 1)).
Effect of EER on fasting blood glucose levels
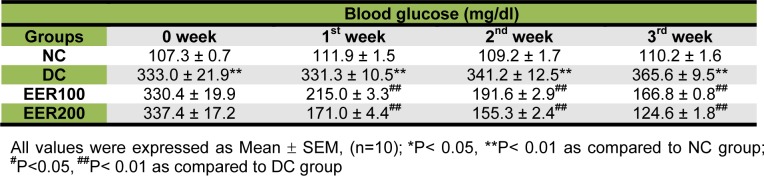
Before treatment, fasting blood glucose levels of EER100 and EER200 were comparable with DC group (Table 2(Tab. 2), week 0). The levels of fasting blood glucose in EER100 and EER200 treated groups reduced by 35 % and 48 % after 1 week, by 43 % and 54 % after 2 weeks, and by 54 % and 65 % after 3 weeks, respectively when compared with DC group (p< 0.01).
Table 2. Effect of EER on fasting blood glucose levels in HFD-fed and low dose STZ-induced type 2 diabetes in Wistar rats.
Effect of EER on insulin release in response to OGTT
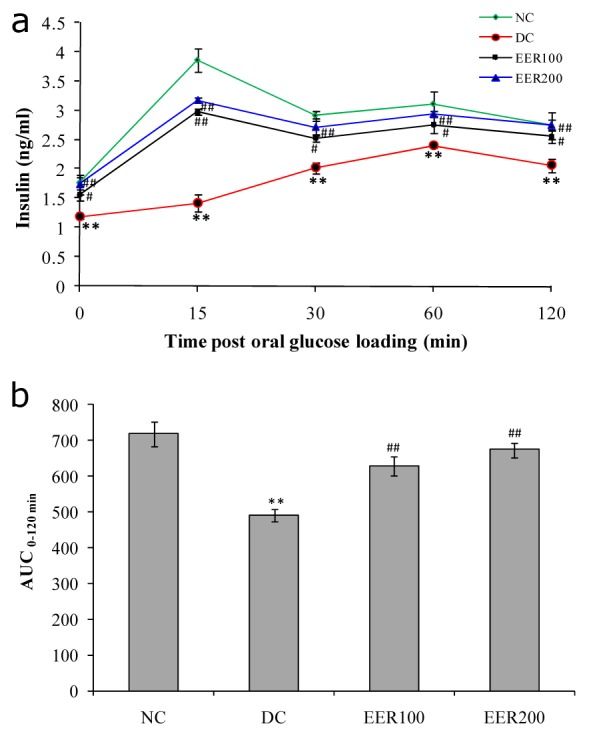
Rats in diabetic control group (DC) had significantly lower (P< 0.01) serum insulin levels at all the time points i.e. 0, 15, 30, 60 and 120 minutes post-oral glucose loading as compared to normal control group (NC). Compared to the diabetic control (DC), treatment with EER100 and EER200 resulted in a significant increase (p< 0.05 and p< 0.01, respectively) in the serum insulin levels in Wistar rats at all the time points (Figure 3A(Fig. 3)). The AUC0-120 min of insulin during the OGTT was significantly lower (p< 0.01) in the DC group as compared to NC, EER100 and EER200 group (Figure 3B(Fig. 3)).
Figure 3. a) Effect EER treatment on serum insulin levels in response to an oral glucose challenge in HFD-fed and low dose STZ-induced type 2 diabetes in Wistar rats, b) Effect EER treatment on area under curve (AUC 0-120 min) of serum insulin levels in response to an oral glucose challenge in HFD-fed and low dose STZ-induced type 2 diabetes in Wistar rats.
All values were expressed as mean SEM, (n=10); *P< 0.05, **P< 0.01 as compared to NC group; #P<0.05, ##P< 0.01 as compared to DC group
Effect of EER on biochemical parameters
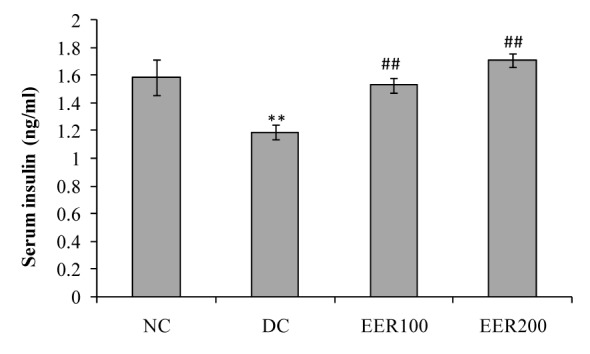
Serum insulin levels at the end of the study period are shown in Figure 4(Fig. 4). In comparison to NC group, HFD-fed and low dose STZ treated diabetic rats showed significantly (p< 0.01) decreased serum insulin levels. EER treatment in diabetic rats at both doses showed significant (p< 0.01) increase in serum insulin levels. Insulin resistance in diabetic rats (DC group) was indicated by higher HOMA values as compared to normal control rats (NC group). Rats treated with EER100 and EER200 showed improved insulin sensitivity as compared to DC group (Figure 5(Fig. 5)).
Figure 4. Effect EER treatment on serum insulin levels in HFD-fed and low dose STZ-induced type 2 diabetes in Wistar rats.
All values were expressed as Mean SEM, (n=10); *P< 0.05, **P< 0.01 as compared to NC group; #P<0.05, ##P< 0.01 as compared to DC group
Figure 5. Effect EER treatment on homeostasis model assessment of insulin resistance (HOMA-IR) in HFD-fed and low dose STZ-induced type 2 diabetes in Wistar rats.
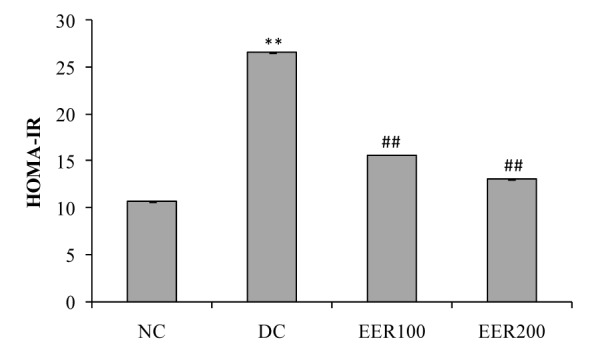
All values were expressed as mean SEM, (n=10); *P< 0.05, **P< 0.01 as compared to NC group; #P<0.05, ##P< 0.01 as compared to DC group
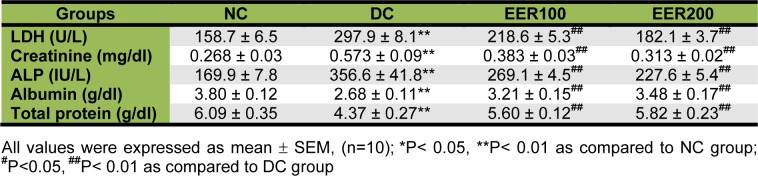
Oral administration of EER at 100 and 200 mg/kg dose for 21 days in diabetic rats significantly (p< 0.01) reduced serum triglycerides by 30 and 44 %, and total cholesterol by 15 and 24 %, respectively as compared to DC rats (Figure 6(Fig. 6)). The HFD-fed and low dose STZ-induced type 2 diabetic rats showed a significant (p< 0.01) increase in the levels of serum LDH, creatinine, ALP and a significant (p< 0.01) decrease in the levels of total protein and albumin when compared to NC rats. Treatment with EER at both the doses caused a significant reversal of all these changes towards the normal (Table 3(Tab. 3)).
Figure 6. Effect EER treatment on serum total cholesterol and triglyceride levels in HFD-fed and low dose STZ-induced type 2 diabetes in Wistar rats.
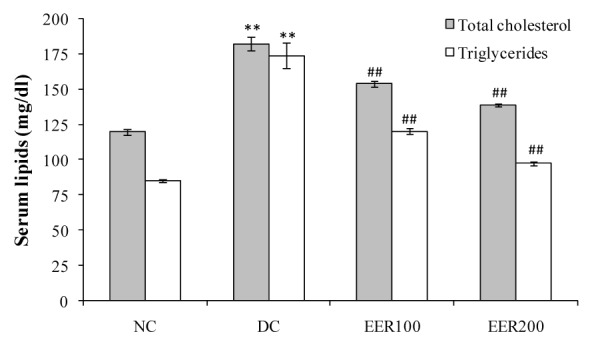
All values were expressed as mean SEM, (n=10); *P< 0.05, **P< 0.01 as compared to NC group; #P<0.05, ##P< 0.01 as compared to DC group
Table 3. Effect of EER on serum biochemical parameters in HFD-fed and low dose STZ-induced type 2 diabetes in Wistar rats.
Effect of EER on kidney oxidative stress biomarkers
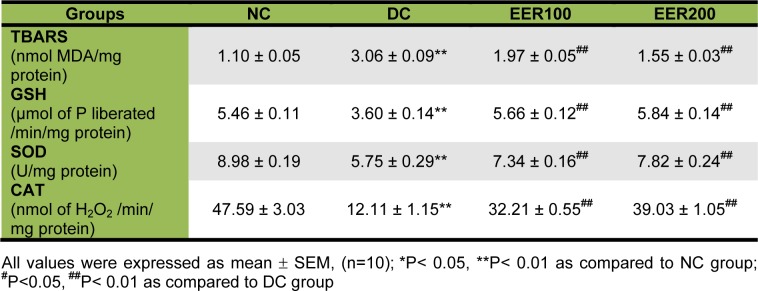
TBARS levels were determined by evaluating kidney malondialdehyde (MDA) content. Rats in DC group showed a significant (p< 0.01) increase in kidney TBARS content by 2.7-fold as compared with those in NC group. Administration of EER at 100 mg/kg dose caused a significant decrease in kidney TBARS content by 1.5-fold while 200 mg/kg dose showed 1.9-fold reduction as compared to DC group. As shown in Table 4(Tab. 4), the levels of kidney GSH, SOD, and CAT in DC group were decreased by 1.52-, 1.56, and 3.93-fold, respectively as compared to the NC group. Administration of EER at 100- and 200 mg/kg dose for 21 days significantly (p< 0.01) increased in kidney GSH (1.57- and 1.62-fold), SOD (1.28- and 1.36-fold), and CAT (2.66- and 3.22-fold) levels, respectively, when compared with DC group.
Table 4. Effect of ethanolic E. ribes extract on kidney oxidative stress parameters in HFD-fed and low dose STZ-induced type 2 diabetes in Wistar rats.
Effect of EER on histopathological analysis of kidney
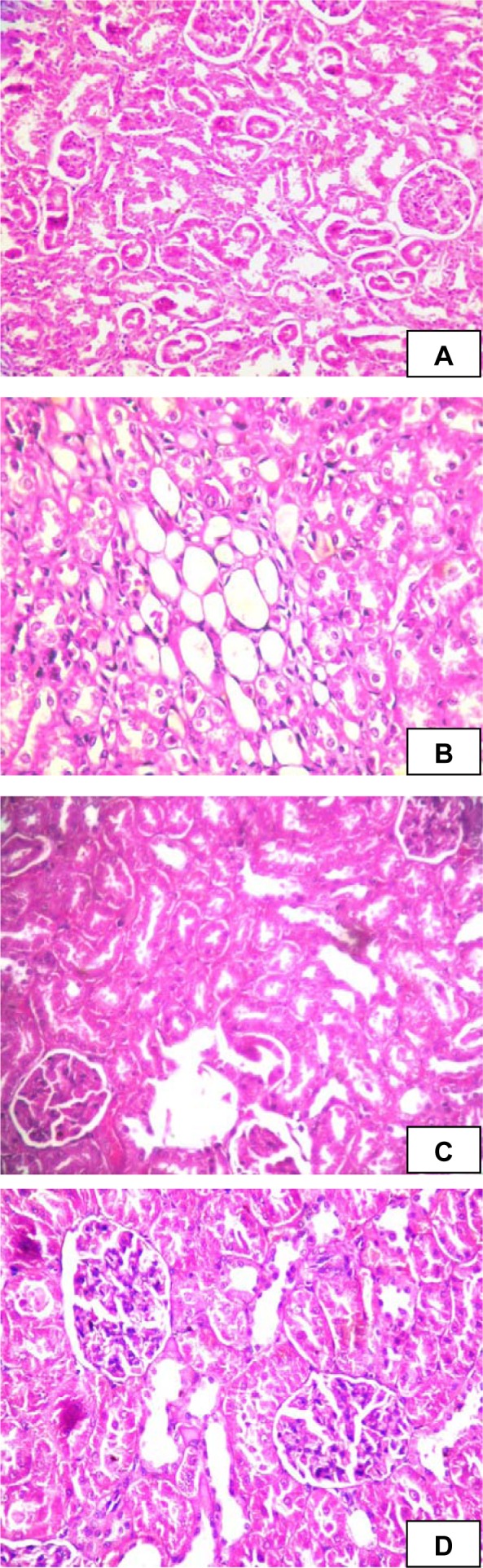
In the histopathological studies, kidney sections in NC group showed glomeruli and proximal convoluted tubules. The kidney sections of diabetic rat showed congestion, proteinuria, haemorrhage, and tubular degeneration. However, EER at 100 and 200 mg/kg treated diabetic rats kidney showed glomeruli and tubules without proteinuria and haemorrhage (Figure 7(Fig. 7)).
Figure 7. Histological changes in kidney of experimental rats. (A) Normal control group (NC), kidney shows glomeruli and proximal convoluted tubules; (B) Diabetic control group (DC), kidney shows tubular degeneration and congestion of capillaries; (C) Ethanolic extract of E. ribes (100 mg/kg) treated group (EER100), shows normal histological structure; (D) Ethanolic extract of E. ribes (200 mg/kg) treated group (EER200), shows normal architecture of kidney.
Discussion
In this study, the effects of EER on diabetic nephrotoxicity were assessed using a HFD and low dose STZ-induced type 2 diabetic rat model. This rat model exhibits hyperglycemia, hyperlipidemia and insulin resistance that would closely reflect the natural history and metabolic characteristics of human type 2 diabetes (Srinivasan et al., 2005[31]). Further, Danda et al. (2005[12]) showed that type 2 diabetic rats (induced by feeding HFD and low dose STZ) developed more pronounced kidney lesions than type 1 diabetic (induced by high dose STZ injection) rats. Hence, this is an appropriate experimental model to examine the effect of various therapeutic agents on diabetic nephrotoxicity.
The presence of embelin in EER was established by HPLC and quantified as 3.3 % (Figure 1(Fig. 1)). Embelin, a naturally occurring alkyl substituted hydroxy benzoquinone, is considered one of the major bioactive constituents and marker compounds in E. ribes fruits (Madhavan et al., 2011[22]).
As overweight or obesity in type 2 diabetes can exacerbate metabolic abnormalities, there is need for weight loss (Krentz, 2007[19]). In fact, lifestyle interventions to promote weight loss are generally considered first-line therapy for type 2 diabetes. In our study, diabetic rats treated with EER at 100 and 200 mg/kg showed a significant decline in body weight gain, kidney weight, and kidney weight/body weight ratio, suggesting a weight reducing effect of extract. Herein, we have corroborated previous finding showing that embelin (active constituent of E. ribes) decreased body weight in HFD-fed obese rats (Chaudhari et al., 2012[10]).
Hypertension has been reported to aggravate DN through its additive glomerular hypertension (Hostetter et al., 1982[16]). Moreover, hypertension is also associated with an elevation of ROS and reducing antioxidant levels (Tomohiro et al., 2007[32]). EER (100 and 200 mg/kg) treatment for 21 days produced a significant reduction in mean arterial blood pressure in diabetic rats. Thus, EER may be beneficial in patients with DN as optimal control and maintenance of normal sugar and blood pressure level are vital to prevent the progression of DN (Balakumar et al., 2009[4]).
Hyperglycemia is an important causal factor in mediating the development and progression of DN (Roy et al., 2010[26]). It also activates multiple signalling pathways that leads to increased generation of reactive oxygen species (ROS), which can damage the kidney, pancreatic β-cells, and induce insulin resistance (Brownlee, 2001[7]; Aragno et al., 2004[3]). In order to clarify the antihyperglycemic effect of EER in type 2 diabetic rats, we have measured fasting blood glucose levels at weekly interval for the period of 3 weeks. The present fasting blood glucose data of diabetic rats receiving EER (100 and 200 mg/kg) daily for the period of 21 days clearly demonstrates that EER at both the doses could control hyperglycemia, the key feature of diabetes mellitus. The observed antihyperglycemic effect of EER is supported by our previous reports (Bhandari et al., 2002[6], 2007[5]). It appears likely that EER effect on diabetic renal injury is due to improvement in glycemic control.
Insulin plays a homeostatic role in normal kidney function. However, insulin-resistance has been associated with alterations in glomerular hemodynamics and renal damage (Knight and Imig, 2007[17]). Hence, insulin sensitizing drugs may have preventive effects on renal injury in obesity and insulin resistance. We found that HFD-fed and low dose STZ-treated diabetic rats had frank hyperglycemia in the presence of comparable amount of serum insulin concentrations, indicated the persistence of insulin resistance. The presence of insulin resistance is further indicated by higher values of HOMA in diabetic control group. The HOMA values were significantly (p< 0.01) decreased in EER100 and EER200 treated groups when compared with diabetic control (DC) group. Insulin sensitizing capability of EER was further confirmed by serum insulin concentration-time curve for 120 min (AUC0-120 min) in OGTT. Compared to diabetic control, EER at 100 and 200 mg/kg treated group showed a clear increase in the early-phase serum insulin in Wistar rats. Serum insulin levels at all the time points and AUC0-120 min were significantly (p< 0.05) higher in EER treated group compared to diabetic control (DC) group, indicating the insulin sensitizing potential of EER. Furthermore, the circulating serum insulin levels observed in the present study revealed significant effect of EER on insulin secretion in HFD-fed and low dose STZ-induced diabetic rats during the 3 weeks of treatment period. These diabetic rats had significantly lower circulating serum insulin levels, whereas EER treated diabetic rats had significantly increased serum insulin levels. Thus, an important benefit of EER in treating diabetic renal injury is to improve glycemic control by attenuating insulin resistance.
The present study also showed that EER treatment effectively improved lipid metabolites including total cholesterol and triglycerides levels in diabetic rats. It is well known that impaired insulin action results in hyperlipidemia (Reaven, 2005[25]). Studies have shown that DN is resulted from elevated renal local lipid accumulation (Yokoyama et al., 2010[34]). Regulation of serum or tissue lipid levels leads to decrease in the risk of lipid-induced glomerular injury (Dominguez et al., 2000[13]). It is possible that EER improved lipid metabolites by enhancement of insulin sensitivity in the present study.
Diabetes is the leading cause of chronic kidney disease (CKD), which makes estimation of renal function crucial. To demonstrate an alteration of renal function in diabetic rats, LDH, creatinine, ALP, albumin and total protein levels were measured in serum. Increased levels of LDH are found in renal, hepatic, pulmonary and cardiovascular diseases. Currently, measuring urine albumin excretion and creatinine (for estimation of GFR) remains the most effective screening method for the early detection of DN (Kramer, 2004[18]). ALP levels are significantly increased in human patients suffering from renal insufficiency (Sanchez Navarro et al., 2002[28]). Further, it has been shown that ALP-enzyme-regulating gene is expressed not only in the liver but also at high level in the kidneys too (Lindblom et al., 2007[20]). The decrease in total protein and albumin levels in diabetic rats may be due to microproteinuria and albuminuria and/or may be due to increased protein catabolism. We have shown that treatment with EER significantly prevented enhanced levels of serum LDH, creatinine and ALP as well as alteration in serum albumin and total protein, valuable markers of renal disease. The biochemical changes were correlated with a histopathological examination, which revealed that HFD- and low dose STZ caused a marked damage in renal structure showing congestion, proteinuria, haemorrhage, and tubular degeneration (Figure 7B(Fig. 7)). This finding is supported by previous pathological study on the pathological feature of nephrotoxicity (Danda et al., 2005[12]). Treatment with EER prevented such alterations and protected the histological aspects of kidney.
Studies have shown that there is a close relationship between hyperglycemia, increase of ROS, and lipid peroxidation (LPO) in the progression of DN (Ha and Kim, 1999[14]). Kidney is one of the organs that are vulnerable to oxidative damage caused by free radicals. ROS could react with polyunsaturated fatty acids which lead to lipid peroxidation. Malondialdehyde (MDA) is a by-product of LPO and reflects the degree of oxidation in the body (Ohkawa et al., 1979[24]). In the present study, kidney TBARS level determined by evaluating MDA content were decreased after 21 days of EER treatment at both the doses 100 mg/kg and 200 mg/kg dose in HFD and low dose STZ-induced diabetic rats. Further, in our study, the activities of GSH (a major endogenous antioxidant), SOD and CAT (scavenger of free radicals) were significantly decreased in HFD fed and low dose STZ-induced diabetic rats as reported earlier (Zhang et al., 2010[35]; Veerapur et al., 2010[33]). Treatment of diabetic rats with EER at both the doses had reversed the activities of these enzymatic antioxidants. Therefore, it is reasonable to assume that EER treatment at both the doses improves the oxidative balance of kidney in diabetic rats, because EER treatment was able to reduce the level of TBARS and free radical generation.
In conclusion, the present study demonstrates that EER can be used to prevent progression of diabetic nephrotoxicity for the first time. Administration of EER improves renal function and ameliorates renal histopathological changes in HFD fed and low dose STZ-induced type 2 diabetic rat model; possibly by improvement in glucose and lipid metabolism, enhancement of insulin sensitivity, blood pressure lowering, and inhibition of lipid peroxidation process. Further investigation of this preventive effect of EER against diabetic nephrotoxicity may have a considerable impact on developing clinically feasible strategies to prevent or delay in progression of diabetic kidney injury.
Acknowledgements
We would like to thank Mr. Uday Vegad, Aum Research Laboratories, Ahmedabad, India for HPLC analysis of the extract.
References
- 1.Alhaider AA, Korashy HM, Ahmed MS, Mobark M, Kfoury H, Mansour MA. Metormin attenuates streptozotocin-induced diabetic nephropathy in rats through modulation of oxidative stress genes expression. Chem Biol Interact. 2011;192:233–242. doi: 10.1016/j.cbi.2011.03.014. [DOI] [PubMed] [Google Scholar]
- 2.Ansari MN, Bhandari U. High Performance Thin Layer Chromatographic method for quantification of embelin from Embelia Ribes Burm fruits. Indian Drugs. 2008;45:908–910. [Google Scholar]
- 3.Aragno M, Mastrocola R, Catalano MG, Brignardello E, Danni O, Boccuzzi G. Oxidative stress impairs skeletal muscle repair in diabetic rats. Diabetes. 2004;53:1082–1088. doi: 10.2337/diabetes.53.4.1082. [DOI] [PubMed] [Google Scholar]
- 4.Balakumar P, Arora MK, Ganti SS, Reddy J, Singh M. Recent advances in pharmacotherapy for diabetic nephropathy: Current perspectives and future directions. Pharmacol Res. 2009;60:24–32. doi: 10.1016/j.phrs.2009.02.002. [DOI] [PubMed] [Google Scholar]
- 5.Bhandari U, Jain N, Pillai KK. Further studies on antioxidant potential and protection of pancreatic β-cells by Embelia ribes in experimental diabetes. Exp Diab Res. 2007;2007:1–6. doi: 10.1155/2007/15803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bhandari U, Kanojia R, Pillai KK. Effect of ethanolic extract of Embelia ribes on dyslipidemia in diabetic rats. Int J Exp Diab Res. 2002;3:159–162. doi: 10.1080/15604280214278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Brownlee M. Biochemistry and molecular cell biology of diabetic complications. Nature. 2001;414:813–820. doi: 10.1038/414813a. [DOI] [PubMed] [Google Scholar]
- 8.Calberg I, Mannerviek B. Glutathione reductase levels in rat brain. J Biol Chem. 1975;250:5475–5480. [PubMed] [Google Scholar]
- 9.Caliborne AL. Assay of catalase. In: Greenwald RA, editor. Handbook of methods of oxygen radical research. Boca Raton, FL: CRC Press; 1985. pp. 283–285. [Google Scholar]
- 10.Chaudhari HS, Bhandari U, Khanna G. Preventive effect of embelin from Embelia ribes on lipid metabolism and oxidative stress in high-fat diet-induced obesity in rats. Planta Med. 2012;78:651–657. doi: 10.1055/s-0031-1298379. [DOI] [PubMed] [Google Scholar]
- 11.Cooper ME. Interaction metabolic and hemodynamic factors in mediating experimental diabetic nephropathy. Diabetologia. 2001;44:1957–1972. doi: 10.1007/s001250100000. [DOI] [PubMed] [Google Scholar]
- 12.Danda RS, Habiba NM, Rincon-Choles H, Bhandari BK, Barnes JL, Abboud HE, et al. Kidney involvement in a nongenetic rat model of type 2 diabetes. Kidney Int. 2005;68:2562–2571. doi: 10.1111/j.1523-1755.2005.00727.x. [DOI] [PubMed] [Google Scholar]
- 13.Dominguez JH, Tang N, Xu W, Evan AP, Siakotos AN, Agarwal R, et al. Studies of renal injury III: Lipid-induced nephropathy in type II diabetes. Kidney Int. 2000;57:92–104. doi: 10.1046/j.1523-1755.2000.00814.x. [DOI] [PubMed] [Google Scholar]
- 14.Ha H, Kim KH. Pathogenesis of diabetic nephropathy: the role of oxidative stress and protein kinase C. Diabetes Res Clin Pract. 1999;45:147–151. doi: 10.1016/s0168-8227(99)00044-3. [DOI] [PubMed] [Google Scholar]
- 15.Hall PM. Prevention of progression in diabetic nephropathy. Diabetes Spectr. 2006:18–24. [Google Scholar]
- 16.Hostetter TH, Rennke HG, Brenner BM. The case for intrarenal hypertension in the initiation and progression of diabetic and other glomerulopathies. Am J Med. 1982;72:375–380. doi: 10.1016/0002-9343(82)90490-9. [DOI] [PubMed] [Google Scholar]
- 17.Knight SF, Imig JD. Obesity, insulin resistance, and renal function. Microcirculation. 2007;14:349–362. doi: 10.1080/10739680701283018. [DOI] [PubMed] [Google Scholar]
- 18.Kramer H. Screening for kidney disease in adults with diabetes mellitus: don’t forget serum creatinine. Am J Kidney Dis. 2004;44:921–923. [PubMed] [Google Scholar]
- 19.Krentz AJ. Antidiabetic treatment in obese patients with type 2 diabetes-effects of medication on bodyweight. Eur Endocrin Dis. 2007:32–36. [Google Scholar]
- 20.Lindblom P, Rafter I, Copley C, Andersson U, Hedberg JJ, Berg AL, et al. Isoforms of alanine aminotransferases in human tissues and serum differential tissue expression using novel antibodies. Arch Biochem Biophys. 2007;466:66–77. doi: 10.1016/j.abb.2007.07.023. [DOI] [PubMed] [Google Scholar]
- 21.Lowry OH, Rosebrough NJ, Farr AL, Randall RJ. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951;193:265–275. [PubMed] [Google Scholar]
- 22.Madhavan SN, Arimboor R, Arumughan C. RP-HPLC-DAD method for the estimation of embelin as marker in Embelia ribes and its polyherbal formulations. Biomed Chromatogr. 2011;25:600–605. doi: 10.1002/bmc.1489. [DOI] [PubMed] [Google Scholar]
- 23.Marklund S, Marklund G. Involvement of the superoxide anion radical in the autooxidation of pyrogallol and a convenient assay for superoxide dismutase. Eur J Biochem. 1974;47:469–474. doi: 10.1111/j.1432-1033.1974.tb03714.x. [DOI] [PubMed] [Google Scholar]
- 24.Ohkawa H, Ohishi N, Yagi K. Assay for lipid peroxides in animal tissues by thiobarbituric acid. Anal Biochem. 1979;95:359–364. doi: 10.1016/0003-2697(79)90738-3. [DOI] [PubMed] [Google Scholar]
- 25.Reaven GM. Why Syndrome X? From Harold Himsworth to the insulin resistance syndrome. Cell Metab. 2005;1:9–14. doi: 10.1016/j.cmet.2004.12.001. [DOI] [PubMed] [Google Scholar]
- 26.Roy S, Trudeau K, Roy S, Behl Y, Dhar S, Chronopoulos A. New insights into hyperglycemia-induced molecular changes in microvascular cells. J Dent Res. 2010;89:116–128. doi: 10.1177/0022034509355765. [DOI] [PubMed] [Google Scholar]
- 27.Ruggenenti P, Remuzzi G. Nephropathy of type 1 and type 2 diabetes: diverse pathophysiology, same treatment? Nephrol Dial Transplant. 2000;15:1900–1902. doi: 10.1093/ndt/15.12.1900. [DOI] [PubMed] [Google Scholar]
- 28.Sanchez Navarro MR, Fernandez-Conde ME, Martin SB, Samaniego C. Alkaline phosphatase isoenzymes in the serum of patient with renal insufficiency. An Med Int. 2002;19:449–452. [PubMed] [Google Scholar]
- 29.Shafi S, Tabassum N, Ahmad F. Diabetic nephropathy and herbal medicines. Int J Phytopharmacol. 2012;3:10–17. [Google Scholar]
- 30.Shelar R, Maurya C, Tekale P, Katkar K, Naik V, Suthar A, et al. Embelin: An HPLC method for quantitative estimation in Embelia ribes Burm. F. Int J Pharm Clin Res. 2009;1:146–149. [Google Scholar]
- 31.Srinivasan K, Viswanad B, Asrat L, Kaul CL, Ramarao P. Combination of high-fat diet-fed and low-dose streptozotocin-treated rat: A model for type 2 diabetes and pharmacological screening. Pharmacol Res. 2005;52:313–320. doi: 10.1016/j.phrs.2005.05.004. [DOI] [PubMed] [Google Scholar]
- 32.Tomohiro T, Kumai T, Sato T, Takeba Y, Kobayashi S, Kimura K. Hypertension aggravates glomerular dysfunction with oxidative stress in a rat model of diabetic nephropathy. Life Sciences. 2007;80:1364–1372. doi: 10.1016/j.lfs.2006.11.054. [DOI] [PubMed] [Google Scholar]
- 33.Veerapur VP, Prabhakar KR, Kandadi MR, Srinivasan KK, Unnikrishnan MK. Antidiabetic effect of Dodonaea viscosa aerial parts in high fat diet and low dose streptozotocin-induced type 2 diabetic rats: A mechanistic approach. Pharm Biol. 2010;48:1137–1148. doi: 10.3109/13880200903527736. [DOI] [PubMed] [Google Scholar]
- 34.Yokoyama M, Tanigawa K, Murata T, Kobayashi Y, Tada E, Suzuki I, et al. Dietary polyunsaturated fatty acids slow the progression of diabetic nephropathy in streptozotocin-induced diabetic rats. Nutr Res. 2010;30:217–225. doi: 10.1016/j.nutres.2010.03.002. [DOI] [PubMed] [Google Scholar]
- 35.Zhang L, Yang J, Chen X, Zan K, Wen X, Chen H, et al. Antidiabetic and antioxidant effects of extracts from Potentilla discolour Bunge on diabetic rats induced by high fat diet and streptozotocin. J Ethnopharmacol. 2010;132:518–524. doi: 10.1016/j.jep.2010.08.053. [DOI] [PubMed] [Google Scholar]