SUMMARY
Objective
Since 2006, human papillomavirus (HPV) vaccination has been routinely recommended for adolescent females in the USA. The quadrivalent vaccine induces long-term seropositivity to HPV 6/11/16, which may be useful as a marker for HPV vaccine coverage.
Methods
We evaluated vaccine type seropositivity (i.e., seropositivity to HPV 6/11/16 with or without HPV18) among females aged 14–59 years participating in the 2003–2010 National Health and Nutrition Examination Survey (cross-sectional, nationally representative surveys). We compared pre-vaccine era (2003–2006) to vaccine era (2007–2010) seropositivity and assessed agreement between vaccine era seropositivity and reported vaccination by kappa statistic.
Results
Seropositivity was 1.0% among 2151 females in the pre-vaccine era and 22.1% among 1420 females in the vaccine era (p < 0.001); 23.1% of vaccine era females reported receipt of one or more HPV vaccine dose. Seropositivity and reported vaccination had high agreement (kappa = 0.79; 95% confidence interval 0.74–0.84). Among seropositive females, 14.5% reported no vaccination.
Conclusion
The increase in vaccine era seropositivity likely reflects vaccination uptake. Our study suggests seropositivity to HPV 6/11/16 may be a useful marker for vaccination coverage in adolescent and young adult females. Discordance between seropositivity and reported vaccination may be explained by inaccurate reporting and/or natural exposure to HPV.
Keywords: Human papillomavirus vaccine, National Health and Nutrition Examination, Survey, Seropositivity
1. Introduction
Since 2006, the Advisory Committee on Immunization Practices (ACIP) has recommended routine vaccination of females in the USA aged 11 or 12 years, and for those aged 13–26 years not previously vaccinated, with three doses of quadrivalent human papillomavirus (HPV) vaccine;1 this recommendation was updated in 2009 to include either quadrivalent or bivalent vaccine.2 Since 2011, ACIP has recommended routine vaccination of males with quadrivalent vaccine.3 Both vaccines have demonstrated >93% efficacy in preventing cervical precancers associated with HPV 16 and 18,4,5 which cause 70% of cervical cancers.6 The quadrivalent vaccine also prevents HPV 6 and 11, which cause 90% of genital warts.7 In the USA, almost all HPV vaccine used is the quadrivalent vaccine.8 HPV vaccine uptake remains low compared to other adolescent vaccines;9 in 2013, only 37.6% of females aged 13–17 years had received all three doses of the HPV vaccine.10
Accurate estimates of HPV vaccination coverage are needed to monitor vaccination programs, identify under-vaccinated populations, and inform efforts to improve coverage. Adolescent vaccination coverage in the USA is measured by annual national surveys involving verification with provider vaccination records.9 Data from provider-verified vaccination are considered the most accurate sources of information, but collection may not be feasible in many settings.11 Other sources of information include self-report of vaccination and state-based vaccine registries. Self-report of vaccination relies on memory and/or household-retained vaccination cards and may be subject to recall bias.12,13 Vaccine registries are often incomplete or non-existent for adolescent immunizations.14 An alternative method for estimating vaccine coverage may be serological assessment of antibodies to HPV vaccine types.
The serological response to HPV infection differs from the serological response to vaccination. Natural infection does not always result in seroconversion.15 In one study, only 69% of females seroconverted after infection with HPV6, and seroconversion rates were lower for HPV 16 and 18.16 Prior to implementation of the HPV vaccination program, seropositivity to at least three of the four HPV vaccine types was only 2.8% among females aged 14–59 years in the USA.17 In contrast, quadrivalent HPV vaccination has been shown to induce seroconversion to all four vaccine types (HPV 6/11/16/18) with geometric mean antibody titers much higher than natural infection.18 However, up to 40% of participants in clinical trials had undetectable antibody to HPV18 at 4 years after vaccination by one serological assay,19,20 although there was continued efficacy against disease outcomes. Given this serological response to HPV vaccination, we focused our assessment on what is likely to be a sensitive and specific serological marker of vaccination, seropositivity to HPV 6, 11, and 16 (with or without seropositivity to HPV 18), referred to as HPV 6/11/16. This marker would detect vaccinated persons who may have lost detectable antibody to HPV18.
To evaluate the use of serology as a marker for vaccination coverage, we describe HPV vaccine type seropositivity in the pre-vaccine and vaccine eras and compare seropositivity to HPV 6/11/16 with report of HPV vaccination in females using data from the National Health and Nutrition Examination Survey (NHANES). We restricted our assessment to females because few males were vaccinated during the NHANES cycles we analyzed.
2. Methods
2.1. Survey design and study population
NHANES is an ongoing series of cross-sectional surveys conducted by the National Center for Health Statistics (NCHS) of the Centers for Disease Control and Prevention (CDC). The survey is nationally representative of the civilian, non-institutionalized US population. NHANES oversamples certain subpopulations to increase the precision of estimates.21 Participants have a household interview followed by a physical examination in a mobile examination center (MEC). Written informed consent was obtained from all participants and parental permission for participants aged <18 years. This survey was approved by the NCHS/CDC Research Ethics Review Board.
We analyzed NHANES 2003–2010 data and considered years 2003–2006 the pre-vaccine era and years 2007–2010 the vaccine era. All female subjects aged 14–59 years selected for NHANES 2003–2010 were eligible for serological testing. In the pre-vaccine era, 5178 females aged 14–59 years were interviewed; 4990 (96.4%) received a physical examination and 4531 (87.5%) serum samples were collected and analyzed. In the vaccine era, 4988 females aged 14–59 years were interviewed; 4860 (97.8%) received a physical examination and 4457 (89.7%) serum samples were collected and analyzed.
2.2. Sociodemographic, behavioral, and HPV vaccination information
Sociodemographic information was ascertained during the household interview. Race/ethnicity categories in NHANES 2003–2010 were self-reported and included non-Hispanic Black, non-Hispanic White, and Mexican American. Health insurance categories included public/government, private, or no insurance. Report of HPV vaccination was collected from 2007 to 2010. Participants aged ≥ 16 years and emancipated minors were interviewed directly; for those aged <16 years, parents or guardians were interviewed. The question to assess vaccination was “Human papillomavirus (HPV) vaccine is given to prevent cervical cancer in girls and women. It is given in 3 separate doses over 6 months and has been recommended for girls and women since June 2006. {Have you/has participant} ever received one or more doses of the HPV vaccine?” Answers included “Yes”, “No”, and “Don’t know”. Participants who responded “Yes” were asked “How many doses {have you/has participant} received?” Answers included “1 dose”, “2 doses”, “3 doses”, or “Don’t know”.
2.3. Serological testing
Serum samples from NHANES participants were heat-inactivated and blindly tested in duplicate using a multiplexed, competitive Luminex immunoassay (cLIA) performed by Merck Research Laboratories (Wayne, PA, USA) or Pharmaceutical Product Development Inc. (Wayne, PA, USA). This assay was used in the quadrivalent vaccine trials and measures antibodies to a type-specific neutralizing epitope on virus-like particle 6/11/16/18, as described elsewhere.22 Antibody levels for each HPV type were reported as positive or negative on the basis of serostatus cutoff values expressed as arbitrary units, established by Merck (i.e., milli-Merck units per milliliter).22 Seropositivity for individual HPV types was defined as anti-HPV antibody titers ≥20 mMU/ml for HPV6, ≥16 mMU/ml for HPV11, ≥20 mMU/ml for HPV16, and ≥24 mMU/ml for HPV18.22
2.4. Statistical analysis
Statistical analyses were conducted using SAS 9.3 (SAS, Inc., Cary, NC, USA) and SAS-callable SUDAAN 11.0 (Research Triangle Institute, Research Triangle Park, NC, USA) to calculate standard errors that account for the complex survey design. Estimates were weighted using the MEC weights provided by NCHS for survey years 2003–2010 to be nationally representative and to account for non-response to the interview and medical examination and oversampling of certain populations.21
We report seropositivity to HPV vaccine types in the pre-vaccine era (2003–2006) and vaccine era (2007–2010) in females aged 14–59 years by age group. In the vaccine era, we determined receipt of at least one HPV vaccine dose (i.e., vaccine initiation) in females aged 14–59 years and examined factors associated with seropositivity to HPV vaccine types in females aged 14–26 years, the age group in NHANES eligible for HPV vaccination. We measured the agreement between report of vaccination and seropositivity to HPV 6/11/16 using the kappa statistic.23 We evaluated seropositivity in the vaccine era by selected demographic characteristics and examined bivariate statistical associations using the adjusted Wald F-test. Estimates were considered unreliable if the relative standard error (RSE) was >30%. Two-sided p-values <0.05 were considered significant.
3. Results
3.1. Seropositivity in the pre-vaccine era (2003–2006) and vaccine era (2007–2010) and report of HPV vaccination in the vaccine era (2007–2010) in females aged 14–59 years
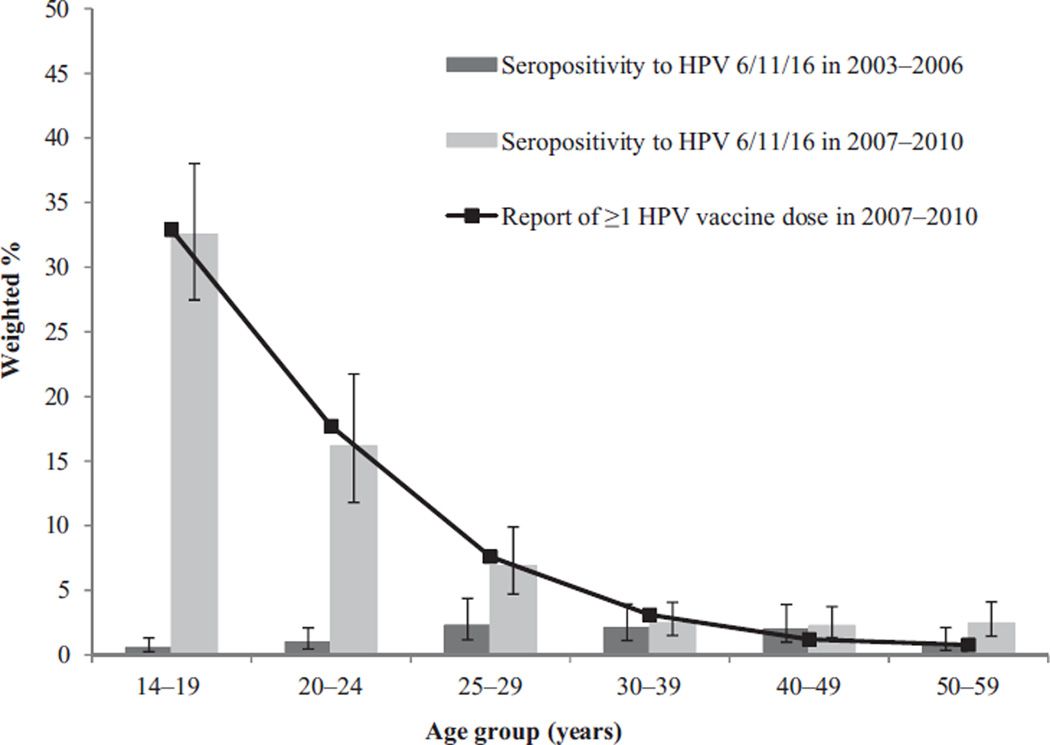
Seropositivity to HPV 6/11/16 in the pre-vaccine and vaccine eras by age group is shown in Figure 1. Seropositivity to HPV 6/11/16 increased significantly from the pre-vaccine to vaccine eras in the younger age groups, from 0.6% (95% confidence interval (CI) 0.2–1.3) to 32.5% (95% CI 27.5–38.0) in females aged 14–19 years, from 1.0% (95% CI 0.5–2.1) to 16.2% (95% CI 11.8–21.7) in females aged 20–24 years, and from 2.3% (95% CI 1.2–4.4) to 6.9% (95% CI 4.7–9.9) in females aged 25–29 years. In the older age groups (>30 years of age), there were no statistically significant differences between the two eras.
Figure 1.
Seropositivity to HPV 6/11/16a by age group in the pre-vaccine era (2003–2006) and vaccine era (2007–2010) and reported receipt of one or more dose of HPV vaccine in the vaccine era in females aged 14–59 yearsb; National Health and Nutrition Examination Survey, 2003–2010.
HPV, human papillomavirus.
aWith or without seropositivity to HPV18.
bAt the time of the survey.
A total of 4782 females aged 14–59 years responded to the HPV vaccination question. Vaccine initiation varied significantly by age group (p < 0.001) and was reported by 32.9% (95% CI 27.9–38.2%) of females aged 14–19 years, 17.7% (95% CI 12.5–24.4%) of females aged 20–24 years, and 7.6% (95% CI 5.4–10.7%) of females aged 25–29 years (Figure 1). In the older age groups, report of vaccine initiation ranged from 0.8% to 3.1%.
3.2. HPV seropositivity in the vaccine era (2007–2010) in females aged 14–26 years
A total of 1420 females aged 14–26 years had valid serology results (Table 1). Overall, 44.1% were seropositive for any HPV vaccine type and 17.1% were seropositive for all four HPV vaccine types. Seropositivity to individual HPV types 6, 11, 16, and 18 were 34.9%, 25.6%, 32.9%, and 21.1%, respectively. Seropositivity to HPV 6/11/16 was 22.1%. Seropositivity to any HPV vaccine type was higher in females aged 20–26 years compared to females aged 14–19 years (47.4% vs. 40.1%; p < 0.05). In contrast, seropositivity to all four HPV vaccine types was higher in the younger age group compared to the older age group (25.9% vs. 9.7%; p < 0.01), as was seropositivity to HPV 6/11/16 (32.5% vs. 13.5%; p < 0.01).
Table 1.
Seropositivity to HPV 6, 11, 16, and 18 in the vaccine era in females aged 14–26 yearsa; National Health and Nutrition Examination Survey, 2007–2010.
| Group | No. subjects | Weighted % (95% CI) | ||||||
|---|---|---|---|---|---|---|---|---|
| HPV6 | HPV11 | HPV16 | HPV18 | Any HPV | HPV 6/11/16b | All 4 HPV types | ||
| Overallc | 1420 | 34.9 (31.0–38.9) | 25.6 (22.1–29.4) | 32.9 (29.4–36.8) | 21.1 (17.9–24.7) | 44.1 (40.2–48.1) | 22.1 (18.7–25.9) | 17.1 (14.0–20.6) |
| ** | ** | * | ** | ** | ||||
| Age, years | ||||||||
| 14–19 | 779 | 36.2 (31.6–41.1) | 35.2 (30.4–40.2) | 35.6 (31.5–42.0) | 26.6 (21.8–32.0) | 40.1 (35.4–44.9) | 32.5 (27.5–38.0) | 25.9 (21.2–31.3) |
| 20–26 | 641 | 33.8 (29.2–38.7) | 17.6 (13.6–22.5) | 30.0 (25.5–34.8) | 16.6 (12.9–21.1) | 47.4 (42.4–52.5) | 13.5 (9.8–18.2) | 9.7 (6.5–14.3) |
| ** | ** | ** | ** | * | ||||
| Race/ethnicity | ||||||||
| White, non-Hispanic | 499 | 36.4 (30.5–42.8) | 28.6 (23.2–34.6) | 36.1 (30.7–41.8) | 22.9 (18.5–27.9) | 45.3 (39.6–51.0) | 25.5 (20.3–31.5) | 19.8 (15.5–24.9) |
| Black, non-Hispanic | 301 | 40.4 (35.5–45.6) | 21.7 (17.8–26.1) | 34.4 (30.3–38.7) | 24.2 (19.5–29.6) | 56.0 (50.3–61.6) | 16.1 (12.7–20.2) | 12.4 (8.3–18.0) |
| Mexican American | 353 | 27.2 (21.8–33.3) | 19.8 (14.4–26.6) | 23.0 (17.6–29.4) | 12.2 (8.2–17.7) | 33.7 (26.9–41.2) | 16.0 (11.2–22.4) | 10.9 (7.2–16.1) |
| ** | ** | ** | ** | ** | ** | ** | ||
| Report of vaccinationd | ||||||||
| ≥1 HPV vaccine | 324 | 84.9 (81.0–88.1) | 88.3 (85.3–90.8) | 89.9 (86.7–92.5) | 65.8 (59.3–71.8) | 92.3 (88.7–94.9) | 82.5 (78.7–85.8) | 64.0 (57.4–70.0) |
| No HPV vaccine | 1067 | 20.2 (17.0–23.7) | 6.9 (5.2–9.2) | 16.1 (13.3–19.3) | 7.9 (6.2–10.0) | 30.0 (26.2–34.1) | 4.2 (2.7–6.52) | 3.1 (1.9–4.9) |
HPV, human papillomavirus; CI, confidence interval.
Age at the time of the survey.
With or without seropositivity to HPV18.
Includes females aged 14–26 years with valid HPV serology results.
Includes females aged 14–26 years with valid HPV serology results and who responded to the HPV vaccination question with “Yes” or “No”.
P <0.05.
P <0.01.
When examined by race/ethnicity, seropositivity to any HPV vaccine type was highest in non-Hispanic Blacks (56.0%), followed by non-Hispanic Whites (45.3%) and Mexican Americans (33.7%) (p < 0.01), whereas seropositivity to all four HPV vaccine types was highest in non-Hispanic Whites (19.8%), followed by non-Hispanic Blacks (12.4%) and Mexican Americans (10.9%) (p < 0.05). There was no statistically significant difference by race/ethnicity for seropositivity to HPV 6/11/16.
Of 1391 females with valid serology results and vaccination data, 23.1% reported vaccine initiation. Of 1382 females who reported the number of doses received, 6.3% reported receipt of only one dose, 5.4% only two doses, 14.6% all three vaccine doses; the remaining 73.7% reported no HPV vaccination. In those who reported vaccine initiation, 92.3% were seropositive for any HPV vaccine type; 84.9%, 88.3%, 89.9%, and 65.8% were seropositive for HPV 6, 11, 16, and 18, respectively. In those reporting no HPV vaccination, seropositivity to any HPV vaccine type was 30.0% and seropositivity to individual HPV types ranged from 6.9% to 20.2%. Seropositivity to HPV 6/11/16 types was 82.5% for those reporting vaccine initiation and 4.2% for those reporting no vaccination. Seropositivity to all four HPV vaccine types was 64.0% for those reporting vaccine initiation and 3.1% for those reporting no vaccination.
3.3. Seropositivity to HPV 6/11/16 and report of HPV vaccination in the vaccine era (2007–2010) in females aged 14–26 years
There were 309 females seropositive for HPV 6/11/16; of these, 85.5% reported HPV vaccine initiation. Of the 1082 females who were not seropositive for HPV 6/11/16, 94.8% reported no HPV vaccination and 5.2% reported HPV vaccine initiation. The overall kappa for seropositivity to HPV 6/11/16 and HPV vaccine initiation was 0.79 (95% CI 0.74–0.84). The kappa for seropositivity to HPV 6/11/16 and vaccination increased with increasing number of doses; from 0.49 (95% CI 0.34–0.63) for report of one dose to 0.62 (95% CI 0.59–0.74) for report of two doses and 0.84 (95% CI 0.78–0.89) for report of three doses.
We further examined those with discrepant findings between seropositivity and report of vaccination. Among females seropositive for HPV 6/11/16, 14.5% reported no HPV vaccination (Table 2); there were no significant differences in the percentage of females reporting no vaccination by age, race/ethnicity, or health insurance.
Table 2.
Report of no HPV vaccination among those seropositive for HPV 6/11/16a in females aged 14–26 yearsa, by selected characteristics; National Health and Nutrition Examination Survey, 2007–2010
| Characteristics | No. subjects | Seropositive for HPV 6/11/16a | ||
|---|---|---|---|---|
| Weighted % reporting no vaccination (95% CI) |
p-value | |||
| Overall | 309 | 14.5 | (9.3–21.9) | |
| Age, years | 0.58 | |||
| 14–19 | 235 | 13.4 | (8.6–20.4) | |
| 20–26 | 74 | 16.7c | (7.9–31.9) | |
| Race/ethnicity | 0.09 | |||
| White, non-Hispanic | 134 | 12.7c | (6.2–24.2) | |
| Black, non-Hispanic | 54 | 33.4 | (18.5–52.5) | |
| Mexican American | 65 | 13.7 | (7.4–24.2) | |
| Health insurance | 0.38 | |||
| Public/government | 107 | 15.5 | (9.6–23.9) | |
| Private | 157 | 12.9c | (6.6–24.0) | |
| None | 41 | 23.4 | (12.3–39.8) | |
HPV, human papillomavirus; CI, confidence interval.
With or without seropositivity to HPV18.
Includes females aged 14–26 years at the time of the survey, seen in the mobile examination center (MEC) with valid HPV serology who were seropositive for HPV 6/11/16 (with or without seropositivity to HPV18).
Relative standard error (RSE) >30%.
4. Discussion
In this nationally representative survey, we found seropositivity to HPV 6/11/16 was significantly higher in the vaccine era (2007–2010) compared to the pre-vaccine era (2003–2006) in adolescent and young adult females. We also found high agreement between seropositivity to HPV 6/11/16 and report of HPV vaccination. Our study suggests seropositivity to HPV 6/11/16 (with or without seropositivity to HPV 18) may be a useful marker for quadrivalent HPV vaccination coverage.
HPV vaccination results in high seroconversion for all vaccine types with higher antibody titers than natural infection.18 This differs from some other vaccines, which produce antibody titers lower than natural infection.24–26 Efficacy has been very high in all vaccine trials to date and a serological correlate of immunity has not been established.27 Previous studies of HPV-vaccinated individuals suggest seropositivity to HPV18, when measured by the cLIA, may decline over time,19,20 although antibodies were detected by other assays.28 Consistent with those studies, we found seropositivity to HPV18 in females reporting vaccination to be lower than other vaccine types using the cLIA (65.8% vs. 84.9– 89.9%).
The high agreement between seropositivity to HPV 6/11/16 and report of vaccination in females aged 14–26 years increased with increasing vaccine dose, which may indicate incomplete serological detection of HPV vaccination or more accurate reporting of vaccination with receipt of an increasing number of vaccine doses. Parent-reported vaccination has been shown to have high agreement with provider-verified vaccination records for receipt of at least one HPV vaccine dose (kappa = 0.76) and all three doses (kappa = 0.74) in females aged 13–17 years,29 but this might vary over time and in different demographic groups. Although we were unable to validate report of HPV vaccination with provider verification, our results are similar to vaccination coverage estimates from national immunization surveys.30
Although we found high agreement between seropositivity to HPV 6/11/16 and report of vaccination, some females aged 14–26 years were seropositive for HPV 6/11/16 and reported no vaccination. It is unclear if this is due to under-reporting of vaccination, naturally acquired HPV 6/11/16 infection,17 or both. In one study comparing parent-report of HPV vaccination with provider record verification for females aged 13–17 years, certain demographic groups had a higher probability of false-negative parental report.31 Also, age may confound analyses, as older age is associated with a higher likelihood of exposure to HPV. Future assessments should compare seropositivity to HPV 6/11/16 with provider report of vaccination to evaluate discrepant findings.
This study is subject to several limitations. First, misclassification of HPV vaccination could have occurred with report of vaccination and seropositivity to at least three HPV vaccine types; either measure could have over- or under-estimated actual HPV vaccination. However, for report of vaccination, the short duration between licensure of the HPV vaccine and the NHANES cycles we analyzed would minimize recall bias. Second, the small sample size in some groups limited our ability to conduct stratified sub-analyses; we were unable to further examine females who were not seropositive for HPV 6/11/16 but reported HPV vaccination. Third, our findings are specific for the quadrivalent HPV vaccine, seropositivity as measured by the cLIA, and females in the USA. Future assessments should evaluate serological markers in global settings with different serological assays and for bivalent vaccine and second-generation vaccines that target additional HPV vaccine types.32
Population-based serological studies for vaccine type HPV provide a biological marker not subject to reporting bias and may be useful in settings where assessment of vaccination through provider or patient report is not feasible. However, while serological studies may provide information on vaccine uptake, such evaluations cannot determine number of doses or age at vaccination.
In conclusion, this is the first study to compare report of HPV vaccination and HPV seropositivity. Seropositivity to HPV 6/11/16 increased in adolescent and young adult females in the vaccine era. Our findings suggest seropositivity to HPV 6/11/16 (with or without seropositivity to HPV18) may be used to assess quadrivalent HPV vaccine initiation in the general population, especially in the young age groups targeted by the HPV vaccine.
Acknowledgments
Funding: None.
Footnotes
Disclaimer: The findings and conclusions in this report are those of the authors and do not necessarily represent the official position of the United States Centers for Disease Control and Prevention (CDC). Mention of company names or products does not imply endorsement by CDC.
Ethics approval: The authors have read and complied with the policy of the journal on ethical consent. Approval was not required.
Conflict of interest: The authors have no conflicts of interest to disclose.
References
- 1.Markowitz LE, Dunne EF, Saraiya M, Lawson HW, Chesson H, Unger ER, et al. Quadrivalent human papillomavirus vaccine: recommendations of the Advisory Committee on Immunization Practices (ACIP) MMWR Recomm Rep. 2007;56:1–24. [PubMed] [Google Scholar]
- 2.Centers for Disease Control and Prevention. FDA licensure of bivalent human papillomavirus vaccine (HPV2, Cervarix) for use in females and updated HPV vaccination recommendations from the Advisory Committee on Immunization Practices (ACIP) MMWR Morb Mortal Wkly Rep. 2010;59:626–629. [PubMed] [Google Scholar]
- 3.Centers for Disease Control and Prevention. FDA licensure of quadrivalent human papillomavirus vaccine (HPV4, Gardasil) for use in males and guidance from the Advisory Committee on Immunization Practices (ACIP) MMWR Morb Mortal Wkly Rep. 2010;59:630–632. [PubMed] [Google Scholar]
- 4.Paavonen J, Naud P, Salmerón J, Wheeler CM, Chow SN, Apter D, et al. Efficacy of human papillomavirus (HPV)-16/18 AS04-adjuvanted vaccine against cervical infection and precancer caused by oncogenic HPV types (PATRICIA): final analysis of a double-blind, randomised study in young women. Lancet. 2009;374:301–314. doi: 10.1016/S0140-6736(09)61248-4. [DOI] [PubMed] [Google Scholar]
- 5.The Future, II., Study Group. Quadrivalent vaccine against human papillomavirus to prevent high-grade cervical lesions. N Engl J Med. 2007;356:1915–1927. doi: 10.1056/NEJMoa061741. [DOI] [PubMed] [Google Scholar]
- 6.De Sanjosé S, Quint WG, Alemany L, Geraets DT, Klaustermeier JE, Lloveras B, et al. Human papillomavirus genotype attribution in invasive cervical cancer: a retrospective cross-sectional worldwide study. Lancet Oncol. 2010;11:1048–1056. doi: 10.1016/S1470-2045(10)70230-8. [DOI] [PubMed] [Google Scholar]
- 7.Lacey CJ, Lowndes CM, Shah KV. Chapter 4: burden and management of noncancerous HPV-related conditions: HPV-6/11 disease. Vaccine. 2006;24:S35–S41. doi: 10.1016/j.vaccine.2006.06.015. [DOI] [PubMed] [Google Scholar]
- 8.Centers for Disease Control and Prevention. Human papillomavirus vaccination coverage in adolescent girls, 2007–2012, and postlicensure vaccine safety monitoring, 2006–2013––United States. MMWR Morb Mortal Wkly Rep. 2013;62:591–595. [PMC free article] [PubMed] [Google Scholar]
- 9.Centers for Disease Control and Prevention. National and state vaccination coverage in adolescents aged 13–17 years––United States, 2013. MMWR Morb Mortal Wkly Rep. 2014;63:625–633. [PMC free article] [PubMed] [Google Scholar]
- 10.Centers for Disease Control and Prevention. Human papillomavirus vaccination coverage among adolescents, 2007–2013, and postlicensure vaccine safety monitoring, 2006–2014—United States. MMWR Morb Mortal Wkly Rep. 2014;63:620–624. [PMC free article] [PubMed] [Google Scholar]
- 11.Centers for Disease Control and Prevention. General recommendations on immunization––recommendations of the Advisory Committee on Immunization Practices (ACIP) MMWR Recomm Rep. 2011;60:1–64. [PubMed] [Google Scholar]
- 12.Luman ET, Ryman TK, Sablan M. Estimating vaccination coverage: validity of household-retained vaccination cards and parental recall. Vaccine. 2009;27:2534–2539. doi: 10.1016/j.vaccine.2008.10.002. [DOI] [PubMed] [Google Scholar]
- 13.Goldstein KP, Kviz FJ, Daum RS. Accuracy of immunization histories provided by adults accompanying preschool children to a pediatric emergency department. JAMA. 1993;270:2190–2194. [PubMed] [Google Scholar]
- 14.Centers for Disease Control and Prevention. Percentage of U.S. adolescents 11–17 years with 2+ adolescent immunizations in IIS––United States, 2012. Atlanta, GA: CDC; 2013. [accessed December 2014]. Immunization Information Systems. Available at: http://www.cdc.gov/vaccines/programs/iis/annual-report-IISAR/2012-data.html#adolescent. [Google Scholar]
- 15.Doorbar J, Quint W, Banks L, Bravo IG, Stoler M, Broker TR, et al. The biology and life-cycle of human papillomaviruses. Vaccine. 2012;30:F55–F70. doi: 10.1016/j.vaccine.2012.06.083. [DOI] [PubMed] [Google Scholar]
- 16.Carter JJ, Koutsky LA, Hughes JP, Lee SK, Kuypers J, Kiviat N, et al. Comparison of human papillomavirus types 16, 18, and 6 capsid antibody responses following incident infection. J Infect Dis. 2000;181:1911–1919. doi: 10.1086/315498. [DOI] [PubMed] [Google Scholar]
- 17.Introcaso CE, Dunne EF, Hariri SH, Panicker G, Unger ER, Markowitz LE. Pre-vaccine era human papillomavirus type 6, 11, 16, and 18 seropositivity in the United States, National Health and Nutrition Examination Surveys, 2003–2006. Sex Transm Infect. 2013;90:505–508. doi: 10.1136/sextrans-2013-051490. [DOI] [PubMed] [Google Scholar]
- 18.Schiller JT, Castellsague X, Garland SM. A review of clinical trials of human papillomavirus prophylactic vaccines. Vaccine. 2012;30:F123–F138. doi: 10.1016/j.vaccine.2012.04.108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Joura EA, Kjaer SK, Wheeler CM, Sigurdsson K, Iversen OE, Hernandez-Avila M, et al. HPV antibody levels and clinical efficacy following administration of a prophylactic quadrivalent HPV vaccine. Vaccine. 2008;26:6844–6851. doi: 10.1016/j.vaccine.2008.09.073. [DOI] [PubMed] [Google Scholar]
- 20.Villa LL, Ault KA, Giuliano AR, Costa RL, Petta CA, Andrade RP, et al. Immunologic responses following administration of a vaccine targeting human papillomavirus types 6, 11, 16, and 18. Vaccine. 2006;24:5571–5583. doi: 10.1016/j.vaccine.2006.04.068. [DOI] [PubMed] [Google Scholar]
- 21.Johnson CL, Paulose-Ram R, Ogden CL, Carroll MD, Kruszan-Moran D, Sylvia M, et al. National Health and Nutrition Examination Survey: analytic guidelines, 1999–2010. National Center for Health Statistics. Vital Health Stat. 2013;2:1–16. [PubMed] [Google Scholar]
- 22.Dias D, Van Doren J, Schlottmann S, Kelly S, Puchalski D, Ruiz W, et al. Optimization and validation of a multiplexed Luminex assay to quantify antibodies to neutralizing epitopes on human papillomaviruses 6, 11, 16, and 18. Clin Diagn Lab Immunol. 2005;12:959–969. doi: 10.1128/CDLI.12.8.959-969.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Landis JR, Koch GG. The measurement of observer agreement for categorical data. Biometrics. 1977;33:159–174. [PubMed] [Google Scholar]
- 24.Ward BJ, Boulianne N, Ratnam S, Guiot MC, Couillard M, De Serres G. Cellular immunity in measles vaccine failure: demonstration of measles antigen-specific lymphoproliferative responses despite limited serum antibody production after revaccination. J Infect Dis. 1995;172:1591–1595. doi: 10.1093/infdis/172.6.1591. [DOI] [PubMed] [Google Scholar]
- 25.Fedova D, Bruckova M, Plesnik V, Slonim D, Sejda J, Svandova E, et al. Detection of postvaccination mumps virus antibody by neutralization test, enzyme-linked immunosorbent assay and sensitive hemagglutination inhibition test. J Hyg Epidemiol Microbiol Immunol. 1987;31:409–422. [PubMed] [Google Scholar]
- 26.Weibel RE, Buynak EB, McLean AA, Hilleman MR. Long-term follow-up for immunity after monovalent or combined live measles, mumps, and rubella virus vaccines. Pediatrics. 1975;56:380–387. [PubMed] [Google Scholar]
- 27.Schiller JT, Lowy DR. Immunogenicity testing in human papillomavirus-like-particle vaccine trials. J Infect Dis. 2009;200:166–171. doi: 10.1086/599988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Brown DR, Garland SM, Ferris DG, Joura E, Steben M, James M, et al. The humoral response to Gardasil over four years as defined by total IgG and competitive Luminex immunoassay. Hum Vaccines. 2011;7:230–238. doi: 10.4161/hv.7.2.13948. [DOI] [PubMed] [Google Scholar]
- 29.Dorell CG, Jain N, Yankey D. Validity of parent-reported vaccination status for adolescents aged 13–17 years: National Immunization Survey-Teen, 2008. Public Health Rep. 2011;126:60–69. doi: 10.1177/00333549111260S208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Centers for Disease Control and Prevention. National and state vaccination coverage among adolescents aged 13 through 17-United States, 2010. MMWR Morb Mortal Wkly Rep. 2011;60:1117–1123. [PubMed] [Google Scholar]
- 31.Attanasio L, McAlpine D. Accuracy of parental reports of children’s HPV vaccine status: implications for estimates of disparities, 2009–2010. Public Health Rep. 2014;129:237–244. doi: 10.1177/003335491412900305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Joura E on behalf of the V503-001 study team. Efficacy and immunogenicity of a novel 9-valent HPV L1 virus-like particle vaccine in 16- to 26-year old women; EUROGIN Congress 2013; Florence, Italy. Nov 3–6, 2013; Report No SS8-4 (abstr). [Google Scholar]