Summary
Cells release extracellular vesicles (ECVs) that play important roles in intercellular communication and may mediate a broad range of physiological and pathological processes. Many fundamental aspects of ECV biogenesis and signaling have yet to be determined, with ECV detection being a challenge and obstacle due to their small size (100nm). We developed an in vivo system to visualize the dynamic release of GFP-labeled ECVs. We show here that specific Caenorhabdidits elegans ciliated sensory neurons shed and release ECVs containing GFP-tagged polycystins LOV-1 and PKD-2. These ECVs are also abundant in the lumen surrounding the cilium. Electron tomography and genetic analysis indicate that ECV biogenesis occurs via budding from the plasma membrane at the ciliary base and not via fusion of multivesicular bodies (MVBs). Intraflagellar transport (IFT) and kinesin-3 KLP-6 are required for environmental release of PKD-2::GFP-containing ECVs. ECVs isolated from wild-type animals induce male tail chasing behavior, while ECVs isolated from klp-6 animals and lacking PKD-2::GFP do not. We conclude that environmentally released ECVs play a role in animal communication and mating related behaviors.
Ciliated sensory neurons shed and release polycystin-containing extracellular vesicles (ECVs)
Caenorhabditis elegans ciliated sensory neurons monitor internal and external conditions. The hermaphrodite has 60 ciliated sensory neurons, the male possesses an additional 52 [1, 2]. Six IL2 (inner labial type 2) and 21 male-specific B-type sensory neurons are unique in that their sensory cilia protrude into the environment via a cuticular pore [1–3]. The C. elegans polycystins LOV-1 and PKD-2 are expressed exclusively in 21 male-specific B-type sensory neurons that include four CEM (cephalic male) neurons in the head, and HOB (hook B-type) and bilateral ray B-type neurons (“RnB” where n=1~9 but not 6) in the tail (Figure 1) [4, 5].
Figure 1.
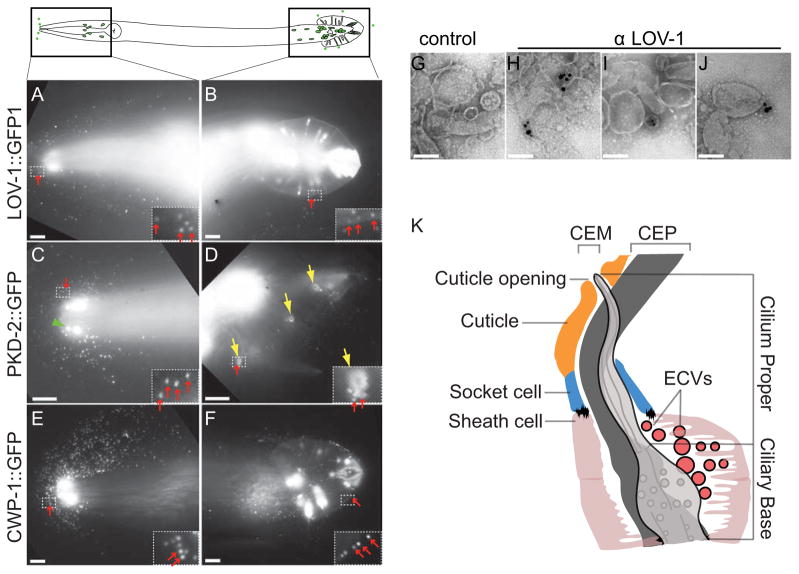
IL2 and male-specific B-type ciliated neurons release GFP-labeled ECVs. Top panel, cartoon of six IL2 and B-type sensory neurons in adult C. elegans male (in the head, four CEM neurons, and in the tail, one HOB and 16 RnB neurons). (A, B) Male head and tail images of LOV-1::GFP reporter (N-terminal extracellular domain of LOV-1 (1–991 aa) fused to GFP). In all panels, red arrows point to ECVs surrounding the head and the tail. Insets show framed area zoomed to 4X, and increased brightness to show GFP-labeled ECVs. (C, D) Male head and tail images of PKD-2::GFP reporter. Green arrow head points to a CEM cilium with PKD-2::GFP enriched at the tip. Yellow arrows point to the cuticular pore of the ray neurons and PKD-2::GFP release around the pore. (E, F) CWP-1::GFP release from the head and the tail. (G–J) Negatively stained ECVs. (G) ECVs with no primary antibody control. (H, I and J) Different images of LOV-1 antibody labeling endogenous LOV-1 on ECVs purified from wild-type hermaphrodites and males without a GFP transgene, detection by 0.8 nm ultrasmall gold, followed by silver enhancement. Scale bar of A–F is 10 μm, of G–J is 100 nm. (K) Model based on electron tomography (ET) of the distal end of the CEM neuron and its surroundings. The glial sheath cell and socket cell form a continuous lumen surrounding the CEM neuron cilium, which is exposed to the environment directly through a cuticular opening. The lumen is shared by CEM and CEP neurons. The CEM neuron is more centrally located in the lumen, while the CEP neuron is closer to the side of the lumen. ECVs are observed in the lumen (161.8 ± 82.8 nm, Average ±SD of vesicles in each lumen).
GFP-tagged LOV-1 and PKD-2 extracellular vesicles (ECVs) are released from the tip of the nose where CEM cilia are exposed, and from the tips of the male tail rays, where the RnB cilia are exposed in late larval L4 and adult males (Fig. 1A–D). PKD-2::GFP labeled ECVs are shed and released by late L4 males and trapped in the L4 molted cuticle (Supplemental Movie 1). Another cilia-enriched protein CWP-1 (co-expressed with polycystin-1, [6]) is abundantly shed and released by male-specific B type sensory neurons (Fig. 1E, F) and from the IL2 neurons in both hermaphrodites and males throughout development (data not shown). We can observe GFP-tagged ECV release from individual RnB ciliated neurons (see inset of Fig. 1B, D, F). Inner labial sensilla, male cephalic sensilla, male ray sensilla, and the male hook sensillum are similar in that each contain two ciliated dendrites, with the tips of the IL2, CEM, RnB, and HOB cilia completely penetrating the cuticle [1] and releasing ECVs (Figure 1, Table 1).
Table 1.
IL2 and male B-type ciliated neurons release specific GFP-labeled ECV cargo
| Reporter | Expression pattern and identity | Animals releasing ECVs (%) | n |
|---|---|---|---|
| PKD-2::GFP | Male B-type neurons, TRP polycystin-2 | 100 | 50 |
| LOV-1::GFP1 | Male B-type neurons, polycystin-1 | 5.6 | 53 |
| CWP-1::GFP | IL2 + male B-type neurons, secreted protein | 100 | 50 |
| Posm-6::LMP-1::GFP | Pan-ciliary, LAMP1 | 13.1 | 38 |
| CHE-11::GFP | Pan-ciliary IFT complex A | 0 | 20 |
| OSM-6::GFP | Pan-ciliary IFT complex B | 0 | 19 |
| OSM-3::GFP | Pan-ciliary kinesin-2 motor | 0 | 20 |
| KAP-1::GFP | Pan-ciliary kinesin-II associated | 0 | 20 |
| KLP-6::GFP | IL2 + male B-type neurons, kinesin-3 | 0 | 31 |
| TBB-4::GFP | Pan-ciliary axonemal tubulin | 0 | 20 |
| ODR-10::GFP | AWA, GPCR | 0 | 50 |
| Ppkd-2::GFP | PKD, Soluble GFP | 0 | 20 |
All reporters use their own promoter except where noted. Transgenic animals express LOV-1::GFP1 (a fusion between the extracellular domain of LOV-1 (1–991 amino acids and GFP) at low levels and in a mosaic pattern, which is reflected in the lower number of animals releasing ECVs. Adult males and hermaphrodites were examined.
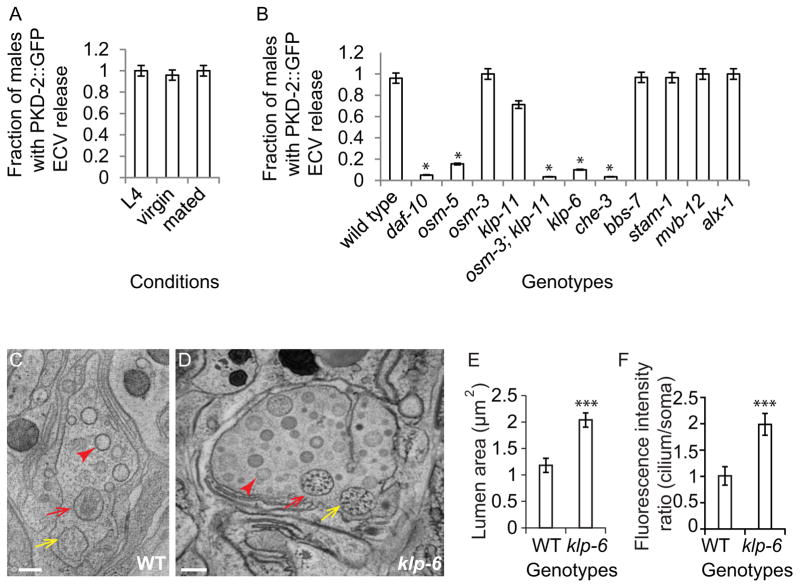
pkd-2 is required for male mating behavior, therefore we asked if PKD-2::GFP containing ECVs are produced in a hermaphrodite-dependent manner. Adult males shed and release PKD-2::GFP ECVs whether cultured as single males (virgin) or in mixed populations (mated), suggesting that ECV production is constitutive in these conditions (Fig. 2A).
Figure 2.
ECV release is constitutive, independent of ESCRT-0 and -I components, and dependent on IFT and the kinesin-3 klp-6. (A) Wild-type L4 larvae, virgin males and mated males equally shed and release PKD-2::GFP ECVs. (B) PKD-2::GFP ECV release is impaired in daf-10 (IFT-A), osm-5 (IFT-B), osm-3;klp-11 (redundant anterograde kinesin-2 motors), klp-6 (kinesin-3) and che-3 (dynein heavy chain, retrograde motor). osm-3, klp-11, and bbs-7 and MVB biogenesis pathway single mutants are not defective in PKD-2::GFP release. Error bar indicates 95% confident intervals, * Different from wild type, Fisher’s exact test, Bonferroni-Holm corrected P < 0.05). (C–D) Cross sections of the cephalic sensillum at CEM transition zone level in wild type and klp-6 mutant. Yellow arrows point to the CEP neuron; red arrows point to the CEM neuron; red arrowheads point to the ECVs. Scale bar is 200 nm. (E) Quantification of the lumen cross-sectional area in wild-type and klp-6 mutant males. (F) klp-6 mutants accumulate PKD-2::GFP at the ciliary region, and the ratio of total fluorescence intensity of cilium to soma is significantly greater than wild type (in E–F, error bar indicates Stdev, *** p<0.001 by Mann-Whitney test).
ECVs were harvested by washing off from culture plates and purified by repeated centrifugations and filtering, as described in Supplemental Methods. Supplemental Movie 1 shows isolated PKD-2::GFP containing vesicles. Negative staining of the ECV preparation reveals vesicle sizes averaging 91.7± 92.5 nm (average ± standard deviation; n=329). By staining with a monoclonal LOV-1 antibody and using ultra-small (<0.8nm) gold labeled secondary antibody detection followed by silver enhancement [7], we observe endogenous LOV-1 containing ECVs (Fig. 1G–J) that are 111.2± 31.1 nm in diameter (n=210; see Supplemental Table 1 for ECV size comparison). These results confirm that C. elegans ECVs contain endogenous LOV-1, and that ECV shedding is not a consequence of overexpressed GFP-tagged proteins.
To test for cargo specificity of the shed vesicles, we examined GFP-tagged reporters of known ciliary components (Table 1). We do not observe environmental release of GFP-tagged β-tubulin TBB-4, IFT-A polypeptide IFT140/CHE-11, IFT-B polypeptide IFT52/OSM-6, motors (kinesin-II, kinesin-2, and kinesin-3 KLP-6), or soluble GFP from B-type, IL2, or any other ciliated sensory neurons. Therefore, in contrast to the polycystins LOV-1 and PKD-2, cilium structural components, intraflagellar transport (IFT) polypeptides, and ciliary motors are not ECV cargo. Likewise, a GFP-labeled GPCR ODR-10::GFP that is expressed in AWA (amphid wing A) neurons is not shed.
Lysosome-associated membrane protein 1 (LAMP1) is a marker of both exosomes and microvesicles, types of ECVs [8, 9]. LMP-1::GFP is shed and released from male B-type ciliated neurons but not other ciliated sensory neurons. Hence, ECV shedding and release is selective, constitutive, and abundant in IL2 and male-specific B-type ciliated sensory neurons, and not a consequence of simply breakage from the cilium.
MVB biogenesis components are not essential for ECV release of PKD-2::GFP
Exosomes and microvesicles/ectosomes are two main types of ECVs that differ in size and origin [10, 11]. Exosomes are 40–100 nm in diameter and released by fusing of the multivesicular body (MVB) with the plasma membrane. Microvesicles (also known as ectosomes) are 100–1000 nm in diameter and generated by pinching off of the plasma membrane. To investigate if components of the MVB pathway regulate PKD-2::GFP ECV biogenesis, we examined PKD-2::GFP shedding and release in animals lacking the function of MVB biogenesis components. The endosomal sorting complexes required for transport (ESCRT-0, I, II and III), are vital components for MVB biogenesis [12]. The signal transducing adaptor molecule STAM-1 is a component of ESCRT-0, binds ubiquitinated PKD-2 and is required for PKD-2 downregulation [13]. MVB-12 is a conserved core component of ESCRT-I [14]. ALX-1 is required for endosomal intralumenal vesicle formation [15]. PKD-2::GFP ECV shedding and release is not reduced in stam-1, mvb-12 or alx-1 mutants as determined by imaging PKD-2::GFP release from individual males, or by visualizing PKD-2::GFP in isolated ECV preparations (Fig. 2B, Supplemental Fig. 1, and data not shown).
IFT and kinesin-3 KLP-6 are required for PKD-2::GFP ECV release
In MDCK cell lines, IFT88 is required for polycystin 2 apical secretion [16]. In C. elegans B-type neurons, IFT regulates PKD-2 ciliary abundance [17, 18]. IFT-B IFT88/osm-5 and IFT-A IFT122/daf-10 are required for PKD-2::GFP ECV release into the environment (Fig. 2C). In contrast, Bardet-Biedl-Syndrome gene 7 homolog bbs-7 is not required for PKD-2::GFP release. These results indicate that polycystin release in both mammalian kidney cells and C. elegans B-type ciliated neurons relies on IFT88 and intact cilia.
In C. elegans amphid channel and phasmid cilia, two IFT motors, homodimeric kinesin-2 OSM-3 and the heterotrimeric Kinesin II, encoded by klp-11, klp-20 and kap-1, act redundantly [19]. The B-type neurons employ the kinesin-3 KLP-6 to regulate IFT and PKD-2 ciliary localization, but klp-6, osm-3, or kinesin-II alone is not essential for CEM ciliogenesis [20, 21]. Neither osm-3 nor klp-11 is required for shedding and release of PKD-2::GFP containing ECVs (Fig. 2B). However, the osm-3;klp-11 double mutant is defective in PKD-2::GFP release (Fig. 2B), indicating that the two anterograde IFT motors act redundantly in this process (Fig. 2B). klp-6 mutant males do not release PKD-2::GFP ECVs to the environment (Fig. 2B, C), suggesting that PKD-2::GFP accumulation at the ciliary base [21] may reflect an ECV shedding or environmental release defect. We conclude that intact cilia and the kinesin-3 klp-6 are required for PKD-2::GFP-ECV shedding and/or release.
ECVs are abundant in the cephalic lumen surrounding the CEM cilium
We performed electron tomography (ET) of the wild-type cephalic sense organ, or sensillum, that houses the CEM neuron (Fig. 1K). The CEM cilium and ciliary base are surrounded by an extracellular lumen that is formed by the glial sheath cell, socket cell and the cuticle, with the ciliary tip exposed through the cuticular opening. ECVs are observed in the lumen surrounding the CEM cilium and cilium base (Fig. 2C, Supplemental Movie 2). We observed one ECV connected to the ciliary base membrane with a long stalk, indicating that this ECV was either budding off or fusing with the membrane (data not shown). Consistent with our finding that MVB components are not required for PKD-2::GFP ECV shedding or release (Fig. 2B, Supplemental Fig. 1), we did not observe MVB structures spanning the CEM neuron from the distal dendrite to the ciliary tip (Supplemental Movie 3 and data not shown).
In the four CEM neurons, we observed a range of 62 to 259 vesicles per lumen (Fig. 2C, Supplemental Movie 2). The average ECV size was 104.7±46.7 nm (average±SD; Supplemental Table 1, Fig. 2C). Therefore, C. elegans ECVs are similar in size to both microvesicles/ectosomes (100 – 1000 nm) and exosomes (40–100nm) [22]. In males, the cephalic lumen surrounds the ciliated endings of both the CEM and CEP neurons. In hermaphrodites, the cephalic lumen contains only the CEP neuronal cilium and lacks ECVs [23], suggesting that luminal ECVs are produced by the male-specific CEM neurons.
To determine if ECVs are native to other ciliated sensilla, we examine the amphid sensilla (major sensory organs in the anterior of the nematode) and the outer labial sensilla. Each amphid sensillum contains 12 cilia surrounded by a lumen that formed by a sheath cell, a socket cell, and the cuticle [23]. Although extensive TEM analysis has been done on the amphids, no luminal ECVs have been reported. We performed ET on the amphid sensillum of adult males. From two tomograms, we found only one vesicle in one and nine vesicles in the other amphid channel lumen (Supplemental Figure 2). By contrast, we observed an average of 162 ECVs in the male-specific cephalic lumen. Furthermore, the location of the ECVs in the cephalic and amphid lumen was different. In the cephalic sensillum, ECVs are found near the ciliary base of CEM neurons (Fig. 1K, 2C) whereas in the amphid sensillum, rare ECVs are in close proximity to amphid channel cilia distal segments (Supplemental Fig. 2). In the outer labial sensillum, the OLQ cilium is surrounded by an extracellular lumen that lacks ECVs (data not shown). Combined, these data suggest that the abundant ECVs found in the cephalic lumen are shed by male-specific CEM ciliated sensory neurons.
klp-6 is required for PKD-2::GFP release
klp-6 mutant males are defective in PKD::GFP ECV release (Fig 2B) and accumulate PKD-2::GFP in ciliary regions (Fig. 2F) [21], which may correlate with defects in ECV biogenesis, shedding into the lumen, or release from the lumen to the exterior of the animal. We therefore examined the cephalic sensillum of the klp-6 mutant by serial thin section TEM. klp-6 mutant males accumulate a large number of luminal ECVs (Fig. 2D) and possess an extracellular lumen doubled in volume compared to wild type (Fig. 2E). ECVs in the lumen surrounding the CEM cilia may be the source of the ECVs released outside of the worm. These results also indicate that klp-6 is not required for ECV biogenesis or shedding. Rather, klp-6 may regulate ECV environmental release from the lumen. We can isolate ECVs from klp-6 mutants via ultracentrifugation. These ECVs contain endogenous LOV-1 but not PKD-2::GFP (data not shown), indicating that klp-6 may play a role in ECV cargo selection.
Isolated ECVs induce male tail chasing behavior
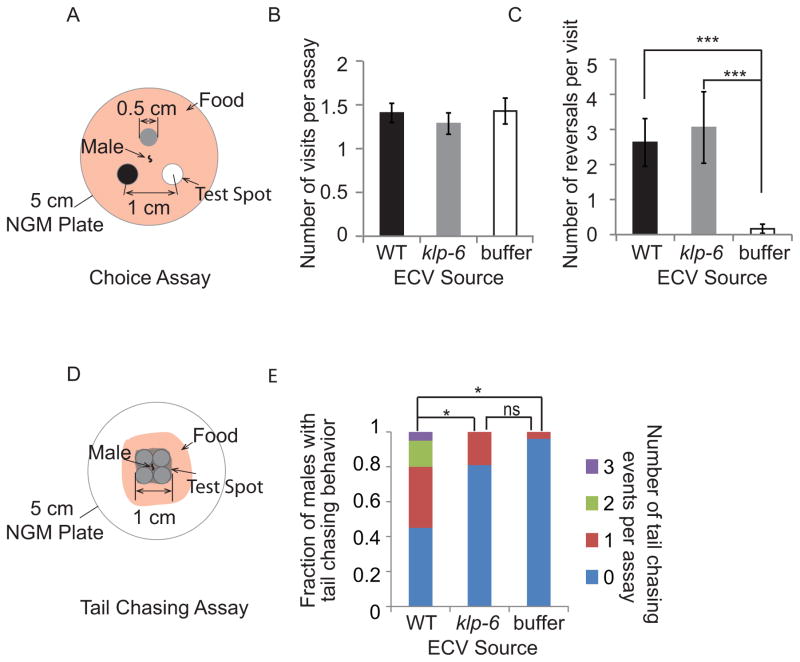
Functions of ECVs include protein disposal and cell-cell communication. As IL2 and B-type neurons release ECVs to the environment, we hypothesized that ECVs may play a role in animal-animal communication. To test this, we developed two behavioral assays. First, we performed a choice assay, to determine if males can distinguish between ECVs versus M9 buffer alone (Fig. 3A). Males display no chemotactic preference for spots containing ECVs from wild-type worms, ECVs from klp-6 worms, or M9 buffer (Fig. 3B). We conclude that ECVs do not act as a long-range chemotactic cue. However, males did alter locomotion by increasing reversal frequency in ECV spots but not M9 spots (Fig. 3C). Males exhibit similar reversal frequency on both wild-type and the klp-6 ECV preparations. We noticed three instances of tail chasing behavior (in 27 recordings) in the wild-type ECV spot, in which the male tail curls to contact its own head and moves in a backward circle. Tail curling enables the male to contact and circle around the hermaphrodite body during mating [24]. Tail curling behavior may also culminate in male-self or male-male contact [25]. Male-male contact is called clumping behavior [25], in which pkd-2 males are defective [26]. Clumping is a population-based behavior and assays would be confounded by ECVs produced by the population versus purified ECVs. We therefore developed an assay to measure individual male-self contact or tail chasing behavior.
Figure 3.
Purified ECVs promote adult male-specific behaviors. (A) Diagram of choice assay plate. Three spots of M9 (negative control), wild-type ECV suspension, and klp-6 ECV suspension were placed equidistance apart on a 5 cm seeded NGM plate. A male was placed in the center of the plate and his behavior recorded for five minutes. (B) The number of times the male visited each test spot is not significantly different. (C) Males exhibit more reversals on either the wild-type or the klp-6 ECV spot than on the M9 buffer (minus ECV) control spot (p<0.001). The number of reversals is not different between the wild-type and klp-6 ECV samples. Statistical analysis was done by Kruskal-Wallis test, six independent trials with 10 assays per trials. (D) To measure tail chasing behavior, the male was placed in a bigger spot comprised of four smaller drops of the ECV preparation, with small food lawn to restrict the male from wandering too far away from the spot. (E) Males displayed tail chasing behavior only on wild-type ECVs (Supplemental Movie 3) but not on klp-6 ECVs or M9 buffer control. Six independent trials with 20, 21, and 25 individual male assays for wild-type ECV, klp-6 ECV and M9 buffer control respectively were performed. * Different from wild type, Fisher’s exact test, Bonferroni-Holm corrected P < 0.05).
In the “tail chasing assay,” a large spot of ECVs from wild-type animals, klp-6 mutants, or M9 buffer was placed and dried on a seeded agar plate (Fig. 3D). A single male was placed on each spot directly. On wild-type ECV spots, males exhibited a high frequency of tail chasing behavior (Fig. 3E, Supplemental Movie 3). In contrast, klp-6 ECV spots were not significantly different than M9 control spots in inducing tail chasing behavior (Fig. 3E). We conclude that environmentally released ECVs and ECV-cargo play a role in animal communication and mating behavior.
Discussion
Here, we use C. elegans as a new model system to visualize ECVs release in vivo. We identify ECV cargo and demonstrate animal behavior changes due to purified ECVs and ECV cargo alone. ECV shedding from ciliated cells is an evolutionarily conserved phenomenon. Membrane bound ECVs containing ciliary proteins including PKD2 have been reported in Chlamydomonas and human urine [7, 16, 27].
From where are ciliary released ECVs derived? Recently Rosenbaum and colleagues showed that Chlamydomonas releases ectosomes from the flagellar tip, and that ectosomes contain an enzyme required to digest the mother cell wall and release newly born daughter cells free [7]. In rat biliary epithelium, polycystin containing exosome-like vesicles may be extruded by the MVB at the base of the cilium [27]. In earlier work, Bloodgood showed that antibody crosslinked flagellar membrane glycoproteins are enriched at the flagellar tip, transported back to the cell body, and released from the base of the Chlamydomonas flagella [28]. In C. elegans cephalic sensillia, ECVs are abundant at the CEM ciliary base of wild-type and klp-6 males (Figure 2C, D, Supplemental Figure 1). In amphid sensilla, we observe rare ECVs at the distal ciliary region of amphid sensillum (Supplemental Figure 2). Combined, these results indicate that ciliary-derived ECVs may be generated and released by multiple pathways.
ECVs are released from most mammalian cell types and carry specific protein and RNA cargo that may be transferred between donor and recipient cells without requiring direct contact. ECVs function in protein degradation and intercellular communication, and mediate a broad range of physiological and pathological processes [22, 29]. Here we show that C. elegans ECVs act as a local sensory cue by promoting male reversal behavior and tail chasing behavior (Figure 3 and Supplemental Movie 4). The cue(s) provided by ECVs are different than ascaroside cues, which were removed by washing in the ECV centrifugation and isolation procedure [30]. Moreover, specific ECV cargo are required for tail chasing behavior: ECVs isolated from klp-6 animals and lacking PKD-2::GFP do not trigger this male-specific locomotory behavior. In natural populations, males are rare and capable of sensing and responding to numerous cues from potential mates (reviewed by [31]). We speculate that ECV-triggered reversals and tail chasing behavior may represent components of a male behavioral repertoire aimed optimizing reproductive success with self-fertilizing hermaphrodites. Consistent with the idea of sexual arms race, C. elegans males secrete unidentified compounds that shorten the life span of hermaphrodites [32]. Whether male-released ECVs play a role in male-induced demise of hermaphrodites is not known. We propose that ECVs and ECV-cargo composition modulates animal-to-animal communication, which to our knowledge, is a new radically function for ECVs.
An important and unresolved question is how ECVs interact with recipient cells. ECVs are closely associated with mammalian cilia [27, 33, 34], yet the physiological relevance remains elusive. Bodily fluids such as breast milk, urine, and seminal fluid are rich in ECVs [27, 29] and may play a role animal communication. For example, breast milk plays a role in development of newborn immunity [35]. We propose that ECVs play important and unexplored roles in animal communication and behavior.
Experimental procedures
All nematodes were grown under standard conditions [36]. The following strain was used to image ECV release and considered wild-type: PT621 him-5(e1490) myIs4 [PKD-2::GFP+Punc-122::GFP]V [18]. Additional methods are provided in Supplemental Experimental methods and strains in Supplemental Table 2.
Supplementary Material
ECVs trapped in Late L4 male tail and purified ECV preparation. First 4 second of the movie shows the Z-stacks of wild type late L4 male tail, note the moving ECVs trapped in L4 cuticle (300mSec per frame). 5–9 second of the movie shows the stream of wild type PKD-2::GFP ECV preparation from ultracentrifugation (125mSec per frame). Scale bar is 10 μm.
Model of CEM neuronal cilium (green) and surrounding ECVs (multiple color) inside the lumen formed by glial sheath cell (pink), socket cell (blue), and cuticle (orange). CEP neuronal cilium (silver) is also included. 0–9 sec of the movie shows the rotating model, 10–13 sec shows a closer view looking into the ciliary pore. Note that most ECVs lie close to the base of the CEM cilium. Scale bar is 100 nm.
Tail chasing behavior of male on wild-type ECV spot. Movie is played at 5X of actual speed. In the video, male tail chasing behavior is observed at 21–22 sec. Scale bar is 0.5 cm.
Highlights.
In vivo imaging reveals that C. elegans ciliated neurons release ECVs.
The polycystins LOV-1 and PKD-2 are ECV cargo.
IFT and kinesin-3 KLP-6 are required for PKD-2::GFP ECV release.
Isolated ECVs trigger cargo-dependent behavioral changes in males.
Acknowledgments
We are grateful to Joel Rosenbaum for ongoing encouragement and constant challenge to decipher ECV roles, and Christopher Wood for protocol for ultra-small gold immuno-EM labeling. We thank Christopher Ward, Barth Grant, Monica Driscoll, and Barr lab members for ongoing discussions, and to Robert O’Hagan, Nathan Schroeder, and Simon Warburton-Pitt for constructive criticism of the manuscript. This work was supported by NIH RO1DK059418 and NIH R01DK074746 to MBB, and by NIH OD010943 to DHH. We thank William Rice at the New York Structural Biology Center for ET help, and Valentine Starovoytov (Rutgers) for TEM assistance. Access to equipment at the NYSBC was supported by funds from Albert Einstein College of Medicine. Some nematode strains were provided by the Caenorhabditis Genetics Center, which is funded by the NIH National Center for Research Resources (P40 OD010440), the C. elegans Gene Knockout Consortium, and the National Bioresource Project for the Nematode.
Footnotes
Supplemental information includes supplemental experimental procedures, two supplemental figures, four supplemental movies, and two supplemental tables.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Perkins LA, Hedgecock EM, Thomson JN, Culotti JG. Mutant sensory cilia in the nematode Caenorhabditis elegans. Dev Biol. 1986;117:456–487. doi: 10.1016/0012-1606(86)90314-3. [DOI] [PubMed] [Google Scholar]
- 2.Sulston JE, Albertson DG, Thomson JN. The Caenorhabditis elegans male: postembryonic development of nongonadal structures. Dev Biol. 1980;78:542–576. doi: 10.1016/0012-1606(80)90352-8. [DOI] [PubMed] [Google Scholar]
- 3.Ward S, Thomson N, White JG, Brenner S. Electron microscopical reconstruction of the anterior sensory anatomy of the nematode Caenorhabditis elegans. J Comp Neurol. 1975;160:313–337. doi: 10.1002/cne.901600305. [DOI] [PubMed] [Google Scholar]
- 4.Barr MM, DeModena J, Braun D, Nguyen CQ, Hall DH, Sternberg PW. The Caenorhabditis elegans autosomal dominant polycystic kidney disease gene homologs lov-1 and pkd-2 act in the same pathway. Curr Biol. 2001;11:1341–1346. doi: 10.1016/s0960-9822(01)00423-7. [DOI] [PubMed] [Google Scholar]
- 5.Barr MM, Sternberg PW. A polycystic kidney-disease gene homologue required for male mating behaviour in C. elegans. Nature. 1999;401:386–389. doi: 10.1038/43913. [DOI] [PubMed] [Google Scholar]
- 6.Miller RM, Portman DS. A latent capacity of the C. elegans polycystins to disrupt sensory transduction is repressed by the single-pass ciliary membrane protein CWP-5. Dis Model Mech. 2010;3:441–450. doi: 10.1242/dmm.002816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wood CR, Huang K, Diener DR, Rosenbaum JL. The cilium secretes bioactive ectosomes. Curr Biol. 2013;23:906–911. doi: 10.1016/j.cub.2013.04.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Logozzi M, De Milito A, Lugini L, Borghi M, Calabro L, Spada M, Perdicchio M, Marino ML, Federici C, Iessi E, Brambilla D, Venturi G, Lozupone F, Santinami M, Huber V, Maio M, Rivoltini L, Fais S. High levels of exosomes expressing CD63 and caveolin-1 in plasma of melanoma patients. PLoS One. 2009;4:e5219. doi: 10.1371/journal.pone.0005219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Flaumenhaft R, Dilks JR, Richardson J, Alden E, Patel-Hett SR, Battinelli E, Klement GL, Sola-Visner M, Italiano JE., Jr Megakaryocyte-derived microparticles: direct visualization and distinction from platelet-derived microparticles. Blood. 2009;113:1112–1121. doi: 10.1182/blood-2008-06-163832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Mathivanan S, Ji H, Simpson RJ. Exosomes: extracellular organelles important in intercellular communication. J Proteomics. 2010;73:1907–1920. doi: 10.1016/j.jprot.2010.06.006. [DOI] [PubMed] [Google Scholar]
- 11.Raposo G, Stoorvogel W. Extracellular vesicles: exosomes, microvesicles, and friends. J Cell Biol. 2013;200:373–383. doi: 10.1083/jcb.201211138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Henne WM, Buchkovich NJ, Emr SD. The ESCRT pathway. Dev Cell. 2011;21:77–91. doi: 10.1016/j.devcel.2011.05.015. [DOI] [PubMed] [Google Scholar]
- 13.Hu J, Wittekind SG, Barr MM. STAM and Hrs Down-Regulate Ciliary TRP Receptors. Mol Biol Cell. 2007;18:3277–3289. doi: 10.1091/mbc.E07-03-0239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Audhya A, McLeod IX, Yates JR, Oegema K. MVB-12, a fourth subunit of metazoan ESCRT-I, functions in receptor downregulation. PLoS One. 2007;2:e956. doi: 10.1371/journal.pone.0000956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Shi A, Pant S, Balklava Z, Chen CC, Figueroa V, Grant BD. A novel requirement for C. elegans Alix/ALX-1 in RME-1-mediated membrane transport. Curr Biol. 2007;17:1913–1924. doi: 10.1016/j.cub.2007.10.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hurd T, Zhou W, Jenkins P, Liu CJ, Swaroop A, Khanna H, Martens J, Hildebrandt F, Margolis B. The retinitis pigmentosa protein RP2 interacts with polycystin 2 and regulates cilia-mediated vertebrate development. Hum Mol Genet. 2010;19:4330–4344. doi: 10.1093/hmg/ddq355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Qin H, Rosenbaum JL, Barr MM. An autosomal recessive polycystic kidney disease gene homolog is involved in intraflagellar transport in C. elegans ciliated sensory neurons. Curr Biol. 2001;11:457–461. doi: 10.1016/s0960-9822(01)00122-1. [DOI] [PubMed] [Google Scholar]
- 18.Bae YK, Qin H, Knobel KM, Hu J, Rosenbaum JL, Barr MM. General and cell-type specific mechanisms target TRPP2/PKD-2 to cilia. Development. 2006;133:3859–3870. doi: 10.1242/dev.02555. [DOI] [PubMed] [Google Scholar]
- 19.Scholey JM. Intraflagellar transport motors in cilia: moving along the cell’s antenna. J Cell Biol. 2008;180:23–29. doi: 10.1083/jcb.200709133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Morsci NS, Barr MM. Kinesin-3 KLP-6 regulates intraflagellar transport in male-specific cilia of Caenorhabditis elegans. Curr Biol. 2011;21:1239–1244. doi: 10.1016/j.cub.2011.06.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Peden EM, Barr MM. The KLP-6 Kinesin Is Required for Male Mating Behaviors and Polycystin Localization in Caenorhabditis elegans. Current Biology. 2005;15:394–404. doi: 10.1016/j.cub.2004.12.073. [DOI] [PubMed] [Google Scholar]
- 22.Gyorgy B, Szabo TG, Pasztoi M, Pal Z, Misjak P, Aradi B, Laszlo V, Pallinger E, Pap E, Kittel A, Nagy G, Falus A, Buzas EI. Membrane vesicles, current state-of-the-art: emerging role of extracellular vesicles. Cell Mol Life Sci. 2011;68:2667–2688. doi: 10.1007/s00018-011-0689-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ward S, Thomson N, White JG, Brenner S. Electron microscopical reconstruction of the anterior sensory anatomy of the nematode Caenorhabditis elegans. J Comp Neurol. 1975;160:313–337. doi: 10.1002/cne.901600305. [DOI] [PubMed] [Google Scholar]
- 24.Sherlekar AL, Janssen A, Siehr MS, Koo PK, Caflisch L, Boggess M, Lints R. The C. elegans male exercises directional control during mating through cholinergic regulation of sex-shared command interneurons. PLoS One. 2013;8:e60597. doi: 10.1371/journal.pone.0060597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Gems D, Riddle DL. Genetic, behavioral and environmental determinants of male longevity in Caenorhabditis elegans. Genetics. 2000;154:1597–1610. doi: 10.1093/genetics/154.4.1597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kaletta T, Van Der Craen M, Van Geel A, Dewulf N, Bogaert T, Branden M, King KV, Buechner M, Barstead R, Hyink D, Li HP, Geng L, Burrow C, Wilson P. Towards understanding the polycystins. Nephron. 2003;93:E9–E17. doi: 10.1159/000066650. [DOI] [PubMed] [Google Scholar]
- 27.Hogan MC, Manganelli L, Woollard JR, Masyuk AI, Masyuk TV, Tammachote R, Huang BQ, Leontovich AA, Beito TG, Madden BJ, Charlesworth MC, Torres VE, LaRusso NF, Harris PC, Ward CJ. Characterization of PKD protein-positive exosome-like vesicles. J Am Soc Nephrol. 2009;20:278–288. doi: 10.1681/ASN.2008060564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Bloodgood RA, Woodward MP, Salomonsky NL. Redistribution and shedding of flagellar membrane glycoproteins visualized using an anti-carbohydrate monoclonal antibody and concanavalin A. J Cell Biol. 1986;102:1797–1812. doi: 10.1083/jcb.102.5.1797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Cocucci E, Racchetti G, Meldolesi J. Shedding microvesicles: artefacts no more. Trends Cell Biol. 2009;19:43–51. doi: 10.1016/j.tcb.2008.11.003. [DOI] [PubMed] [Google Scholar]
- 30.Ludewig AH, Schroeder FC. Ascaroside signaling in C. elegans. WormBook. 2013:1–22. doi: 10.1895/wormbook.1.155.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Chasnov JR. The evolutionary role of males in C. elegans. Worm. 2013;2:e21146. doi: 10.4161/worm.21146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Maures TJ, Booth LN, Benayoun BA, Izrayelit Y, Schroeder FC, Brunet A. Males Shorten the Life Span of C. elegans Hermaphrodites via Secreted Compounds. Science. 2013 doi: 10.1126/science.1244160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Masyuk AI, Huang BQ, Ward CJ, Gradilone SA, Banales JM, Masyuk TV, Radtke B, Splinter PL, LaRusso NF. Biliary exosomes influence cholangiocyte regulatory mechanisms and proliferation through interaction with primary cilia. Am J Physiol Gastrointest Liver Physiol. 2010;299:G990–999. doi: 10.1152/ajpgi.00093.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Pampliega O, Orhon I, Patel B, Sridhar S, Diaz-Carretero A, Beau I, Codogno P, Satir BH, Satir P, Cuervo AM. Functional interaction between autophagy and ciliogenesis. Nature. 2013;502:194–200. doi: 10.1038/nature12639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Admyre C, Johansson SM, Qazi KR, Filen JJ, Lahesmaa R, Norman M, Neve EP, Scheynius A, Gabrielsson S. Exosomes with immune modulatory features are present in human breast milk. J Immunol. 2007;179:1969–1978. doi: 10.4049/jimmunol.179.3.1969. [DOI] [PubMed] [Google Scholar]
- 36.Brenner S. The genetics of Caenorhabditis elegans. Genetics. 1974;77:71–94. doi: 10.1093/genetics/77.1.71. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
ECVs trapped in Late L4 male tail and purified ECV preparation. First 4 second of the movie shows the Z-stacks of wild type late L4 male tail, note the moving ECVs trapped in L4 cuticle (300mSec per frame). 5–9 second of the movie shows the stream of wild type PKD-2::GFP ECV preparation from ultracentrifugation (125mSec per frame). Scale bar is 10 μm.
Model of CEM neuronal cilium (green) and surrounding ECVs (multiple color) inside the lumen formed by glial sheath cell (pink), socket cell (blue), and cuticle (orange). CEP neuronal cilium (silver) is also included. 0–9 sec of the movie shows the rotating model, 10–13 sec shows a closer view looking into the ciliary pore. Note that most ECVs lie close to the base of the CEM cilium. Scale bar is 100 nm.
Tail chasing behavior of male on wild-type ECV spot. Movie is played at 5X of actual speed. In the video, male tail chasing behavior is observed at 21–22 sec. Scale bar is 0.5 cm.