Abstract
Progressive multifocal leukoencephalopathy (PML) is a debilitating demyelinating disease of the CNS caused by the infection and destruction of glial cells by JC virus (JCV) and is an AIDS-defining disease. Infection with JCV is common and most people acquire antibodies early in life. After initial infection, JCV remains in an asymptomatic persistent state and can be detected by PCR in many tissues including brain. A major question in PML pathogenesis is how the virus reactivates from persistence in HIV-1/AIDS. Our studies with primary cultures of glial cells have implicated transcription factors NF-κB and NFAT4, which bind to a unique site in the JCV noncoding control region and stimulate viral gene expression. Furthermore, these transcription factors are controlled by pathways downstream of proinflammatory cytokines, e.g., TNF-α activates NF-κB and stimulates JCV transcription. Thus, we hypothesize that HIV-1/PML initiation may involve reactivation of JCV by cytokine disturbances in the brain such as occur in HIV-1/AIDS. In this study, we evaluated HIV-1/PML clinical samples and non-PML controls for expression of TNF-α and its receptors and subcellular localization of NF-κB p65 and NFAT4. Consistent with our hypothesis, HIV-1/PML tissue has high levels of TNF-α and TNFR1 expression and NF-κB and NFAT4 were preferentially localized to the nucleus.
Keywords: Progressive multifocal leukoencephalopathy, Human polyomavirus JC, Tumor necrosis factor-α, NF-κB, NFAT4, proinflammatory cytokines, viral reactivation
INTRODUCTION
The CNS demyelinating disease progressive multifocal leukoencephalopathy (PML) is characterized by a triad of histopathological features: demyelination, bizarre astrocytes, and enlarged oligodendrocytes with nuclear inclusion bodies [1, 2]. PML is manifested by motor deficits, gait ataxia, cognitive and behavioral changes, language disturbances, weakness, or visual deficits with symptoms depending on the location and size of the lesions. It is caused by the ubiquitous polyomavirus JC (JCV), which infects most people in childhood as indicated by seroprevalence studies but thereafter is controlled by the immune system and becomes restricted to a persistent asymptomatic infection. However, PML is rare and seen predominantly in individuals with underlying immune dysfunction, most notably HIV-1/AIDS and in patients receiving immunomodulatory drugs such as natalizumab, an α4β1 integrin inhibitor, used to treat multiple sclerosis and Crohn's disease [3]. Since the number of individuals that constitute the at-risk population is large, PML has high public health significance.
While seroprevalence studies show that most people are infected with JCV, only very rarely and almost always under conditions of severe immune compromise does the virus reactivate from the persistent state and actively replicate causing cytolytic cell destruction. Replication of the virus occurs in the glia of the CNS PML, i.e., astrocytes and oligodendrocytes, thus leading to the generation of expanding demyelinated lesions and the associated pathologies of PML [4]. While the mechanism of reactivation remains unresolved, our molecular and virological studies of JCV in primary human glial cultures have implicated transcription factors NF-κB [5] and NFAT4 [6]. The genome of JCV is a circular double-stranded DNA divided into three regions, the early region encoding the viral early proteins (large and small T/t-antigens), late region encoding the late proteins (VP1, VP2, VP3 and agnoprotein) and the noncoding control region (NCCR) that controls transcription of both coding regions [7]. The NCCR binds multiple transcription factors that regulate JCV [8]. NF-κB [5] and NFAT4 [6] bind to a unique site in the NCCR and activate transcription of viral early and late genes. In turn, these transcription factors are regulated by signal transduction pathways that lie downstream of pro-inflammatory cytokines, which may be dysregulated in conditions that predispose to PML, e.g., cytokine storms in HIV-1/AIDS. In experiments with cultured human glia, we have found that TNF-α stimulates JCV transcription and that this effect is mediated through the same unique site in the JCV NCCR [9]. In addition, epigenetic changes in the acetylation status of NF-κB can also activate JCV transcription [10, 11]. If the mechanisms that we have demonstrated in culture, such as cytokine (TNF-α) stimulation of transcription factors (NF-κB and NFAT4), are at play during the pathogenesis of HIV-1/PML, we would expect to detect these changes in cytokines and transcription factors in HIV-1/PML tissue compared to non-PML controls. In this context, we evaluated brain tissues from HIV patients with and without PML for expression of TNF-α and its receptors and the subcellular localization of NF-κB p65 and NFAT4. If our hypothesis regarding the importance of TNF-α is correct, we would expect to detect increased TNF-α in PML clinical samples and subcellular localization of NF-κB and NFAT4 to the nucleus.
MATERIALS AND METHODS
Clinical Samples
Two sets of brain clinical samples were used for Western blot analysis and immunohistochemistry (IHC). Set 1, which was used in the first experiment (Fig. 1A) consisted of age-matched clinical samples of frozen portions of parieto-occipital lobe were obtained from Dr. Susan Morgello at the Manhattan HIV Brain Bank from three patients with HIV-1/PML (one 41 year-old female, 2 males ages 45 and 51; postmortem intervals 5–7.5 h), three HIV-positive patients with no CNS pathology (all males, ages 43–51; postmortem intervals 5–6 h), and two HIV-negative controls (male, ages 44 and 52; postmortem intervals 17.5 and 21.5 respectively). Set 2, which was used in the second experiment (Fig. 1B) and for IHC were obtained from the National NeuroAIDS Tissue Consortium (NNTC) and consisted of frontal cortex tissues from three patients with HIV-1/PML (1 female, age 35, 2 males, ages 28 and 37, postmortem time to autopsy < 24 h), three patients with HIV-encephalitis (HIVE; 3 males, ages 31, 42, 36, postmortem time to autopsy < 24 h) and 1 HIV-negative control (male, age 49, post-mortem time to autopsy < 24 h). All samples were obtained and utilized in accordance with Temple University Human Subjects Protections and the Institutional Review Board.
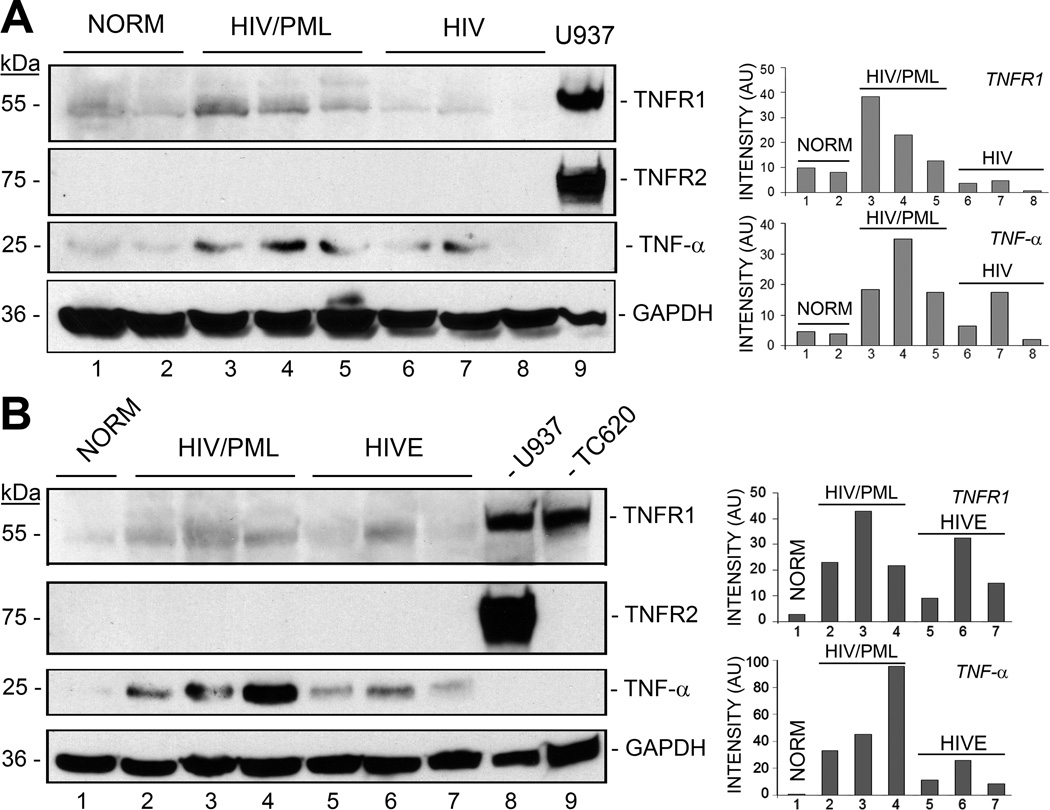
Figure 1. Analysis of expression of TNF-α and its receptors in HIV-1/PML clinical samples and controls by Western blot.
A. Brain tissues from HIV-negative control (NORM, n=2), HIV-1/PML (n=3), and HIV-positive patients with no CNS pathology (n=3) were analyzed for expression levels of TNF-α, TNFR1 and TNFR2 by Western blotting. The loading control was GAPDH. U937 human monocytic leukemia cell line lysate was used as a positive control for TNFR1 and TNFR2. Quantitation of the Western blot was performed and is shown in the histogram on the right with band intensities given in arbitrary units (AU). B. Brain tissues from a second set of clinical samples were analyzed: HIV-negative control (NORM, n=1), HIV-1/PML (n=3), HIV encephalitis (HIVE, n=3). U937 human monocytic leukemia cell line lysate was used as the positive control for TNFR1 and TNFR2and TC620 oligodendroglioma cell line lysate was used as a positive control for TNFR1.
Cell culture
The TC620 human oligodendroglioma and the U937 human monocytic cell lines were cultured at 37°C, 5% CO2 in DMEM + 10% FBS + 1% penicillin/streptomycin + 1% L-glutamine or RPMI 1640 + 10% FBS + 1% penicillin/streptomycin + 1% L-glutamine as previously described [9].
Antibodies
Mouse monoclonal antibodies to TNFR1 and GAPDH and rabbit polyclonal antibodies to TNFR2 and NF-κB p65 were obtained from Santa Cruz Biotechnology, Inc. (Santa Cruz, CA). Mouse monoclonal antibody to TNF-α was obtained from Chemicon international (Temecula CA, clone 195). Mouse monoclonal antibody (Ab587) against JCV capsid protein VP1 was kindly provided by Dr. Walter Atwood, Brown University, Providence, RI. Rabbit polyclonal anti-NFAT4, mouse monoclonal anti-GFAP and mouse monoclonal anti-CC-1 (APC) were from Abcam, Cambridge, MA. Rabbit anti-GFAP was from Proteintech group, Chicago, IL. Rabbit anti-MBP was from EMD Millipore, Billerica MA.
Analysis of clinical samples by Western blot
Frozen brain tissues were homogenized on ice in RIPA buffer (50 mM Tris (pH 8.0), 150 mM NaCl, 1% NP40, 5 mM EDTA, 0.5% sodium deoxycholate, 0.1% sodium dodecyl sulfate) containing protease inhibitors, sonicated, triturated and cleared by centrifugation. Equal amounts of protein per sample were separated by electrophoresis and levels of proteins were analyzed by Western blot as previously described [12]. Western blot data were scanned and quantitated using the Quantity One software (Bio-Rad, Hercules CA).
Immunohistochemistry (IHC)
Double immunofluorescence labeling and deconvolution and confocal microscopy were performed as follows. Formalin-fixed, paraffin-embedded frontal cortex brain tissues from HIV infected patients with either HIVE (n=3 different cases) or with HIV-1/PML (n=3 different cases) were obtained from the NNTC tissue repository. Five micron thick serial sections were mounted on slides rehydrated through ethanol to water, and incubated in citrate buffer for antigen retrieval at 95°C for 30 min. Tissue sections were blocked for 2 h in 5% normal goat serum and 0.1% BSA in 1× PBS, and incubated with combinations of two different primary antibodies overnight in a humidified chamber at room temperature. The following primary antibodies were utilized: mouse anti-TNF-α (1:200, Chemicon, Temecula, CA), mouse anti-TNFR1 (1:200, Santa Cruz, Dallas TX), rabbit anti-NFAT4 (1:200, Abcam, Cambridge, MA), mouse anti-CC-1 (1:200, Abcam), rabbit anti-MBP (1:200, Millipore, Billerica MA), rabbit anti-NF-κB p65 (1:200, Santa Cruz), mouse anti-GFAP (1:200, Abcam) and mouse anti-VP1 (1:200, Dr. Walter Atwood, Brown University). Slides were rinsed three times with 1× PBS, then incubated with fluorescein isothiocyanate (FITC)-conjugated secondary antibody (1:500) and Texas-red isothiocyanate (TRITC)-tagged secondary antibody (1:500) (Thermo-Scientific, Waltham, MA) for 1h at room temperature in the dark. Sections were cover-slipped with an aqueous based mounting media containing DAPI for nuclear labeling (Vector Laboratories, Burlingame, CA), and visualized with a Leica DMI6000B with 3D-deconvolution software.
RESULTS
Levels of TNF-α and TNFR1 are elevated in HIV-1/PML clinical samples
We analyzed two different sets of brain tissue samples by Western blot for changes in the levels of TNF-α and its receptors, TNFR1 and TNFR2 (Fig. 1). Cell lysates used as positive controls were TC620 oligodendroglioma cells, which express high levels of TNFR1, and U937 human monocytic leukemia cells, which express high levels of TNFR1 and TNFR2. In the first set of samples (Fig. 1A), TNF-α and TNFR1 levels were higher to varying degrees in the HIV-1/PML samples (lanes 3–5) than in the HIV-negative (lanes 1 and 2) and HIV-positive samples with no neuropathology (lanes 6–8) except for one HIV-positive sample where TNF-α was increased (lane 7). TNFR2 expression was not detected in any of the samples from this set. In the second set of samples (Fig. 1B), TNF-α and TNFR1 levels were also higher in the HIV-1/PML samples (lanes 2–4) than in the HIV-negative (lane 1) and HIV encephalitis samples (lanes 5–7) except for one HIV encephalitis sample where TNF-α and TNFR1 were increased (lane 6). As in set 1, TNFR2 expression was not detected in samples from set 2. Thus the levels of TNF-α and TNFR1 are elevated in HIV-1/PML.
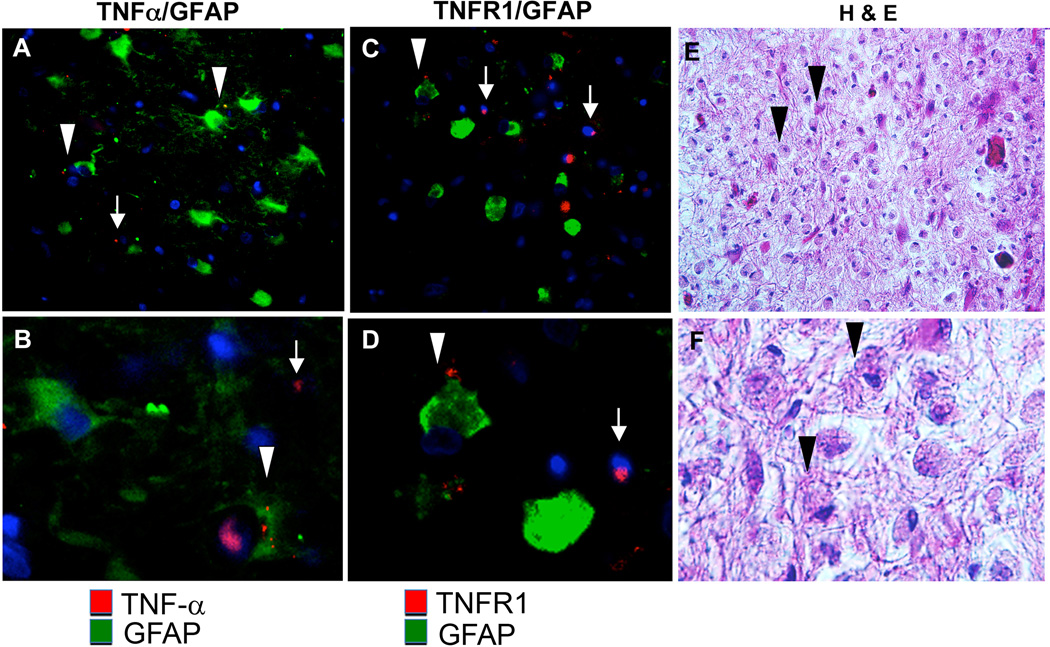
Next, we examined the expression of TNF-α and TNFR1 in HIV-1/PML by immunohistochemistry (IHC). Tissues from a HIV-1/PML patients were immunolabeled for expression patterns of TNF-α and TNFR1. Immunolabeling with the astrocyte marker GFAP indicated reactive gliosis with bizarre cellular architecture associated with PML (Figure 2, A & B). TNF-α was detected in both in GFAP+ bizarre astrocytes (Fig. 2, A, B, arrowheads) and in GFAP-negative cells (arrows). Likewise, TNFR1 was detected in GFAP+ bizarre astrocytes (Fig. 2, C, D, arrowheads) and in GFAP-negative cells (arrows). Nuclear inclusions were observed in numerous cells in a white matter lesion stained with H&E (Fig. 2, E, F, arrowheads). These data are in agreement with results from a previous study in which we performed a single labeling for TNF-α or TNFR1 but no cell lineage-specific labeling for a case of HIV-1/PML [9].
Figure 2. Immunohistochemical analysis of expression of TNF-α and TNFR1 in HIV-1/PML brain tissues.
Double immunofluorescence labeling of HIV-1/PML with (A, B) anti-TNF-α (red) and anti-GFAP (green), (C, D) anti-TNFR1 (red) and anti-GFAP (green). (A–D) Arrowheads indicate astrocyte immunoreactivity with TNR-α or TNFR1, arrows indicate GFAP negative, TNFR1 and TNF-α positive cells. (E, F) Hematoxylin and Eosin (H&E) stain, arrowheads indicate nuclear inclusions in bizarre astrocytes. (A, C, E) 400× magnification, (B, D, F) 400× magnification enlarged to show detail.
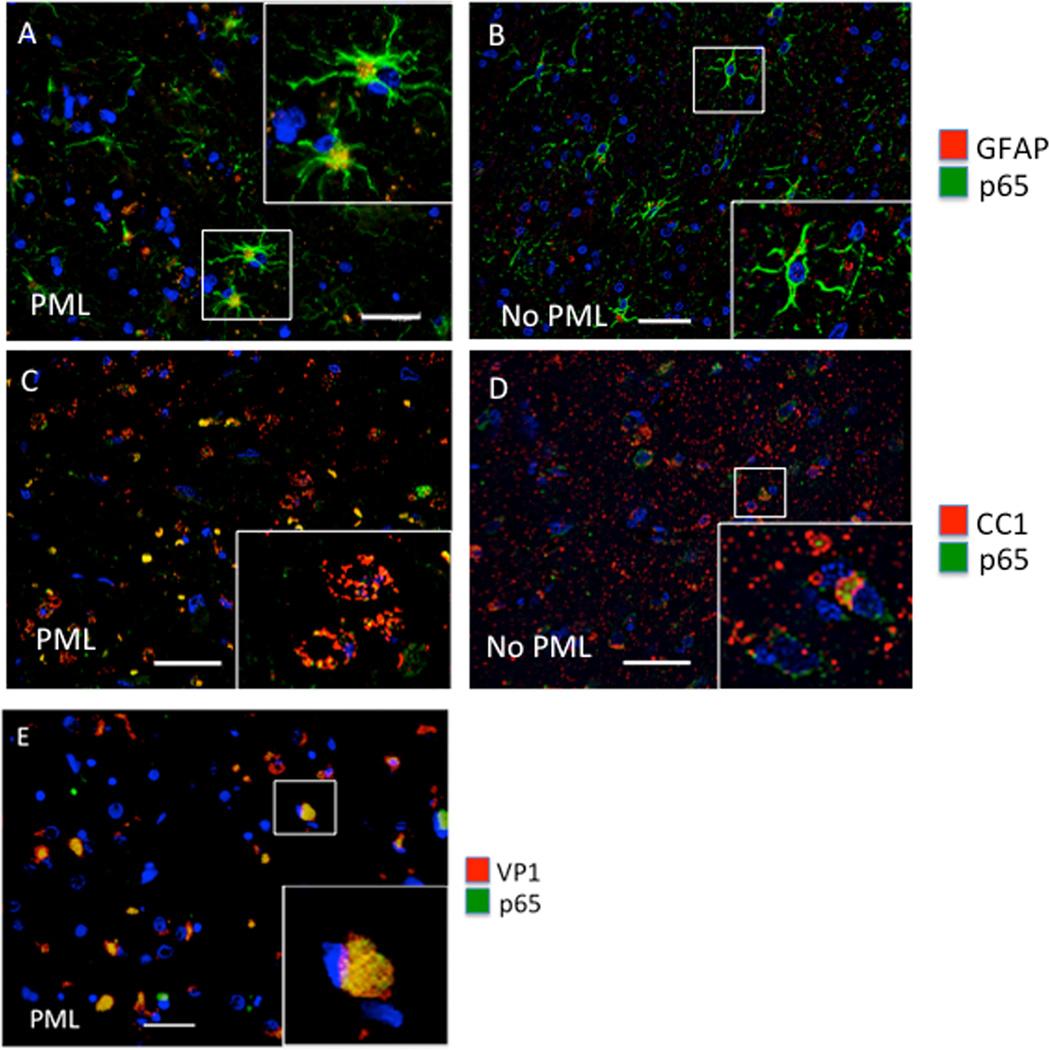
In the next set of experiments, we assessed expression and subcellular localization of NF-κB p65. In our previous cell culture studies, we reported that TNF-α stimulates JCV transcription and that this involves the stimulation of the JCV NCCR via its NF-κB binding site [9]. The regulation of NF-κB activity occurs at several levels including controlled cytoplasmic-nuclear shuttling and modulation of its transcriptional activity [13]. Cytokines such as TNF-α activate downstream signaling pathways that release NF-κB from an inactive cytoplasmic complex with IκB to enable it to enter the nucleus and activate transcription [14]. As shown in Figure 3, NF-κB p65 is largely localized to the nucleus of GFAP positive cells and nearly completely obscures DAPI stain (Fig. 3A, inset). GFAP+ astrocytes in the HIV-1/PML cases are bizarre in morphology, which is characteristic of HIV-1/PML unlike the more ramified astrocytes in the HIV+ cases without PML (Fig. 3B, inset). Likewise, NF-κB p65 is largely localized to the nucleus (Fig. 3C, inset) and colocalizes with oligodendrocytes in PML visualized using the oligodendrocyte marker CC1, but not as robustly in the HIV+ cases without PML, where p65 remains cytoplasmic (Fig. 3D). Note that the oligodendrocyte shown in the inset of Fig. 3C shows a significant cytopathic effect consistent with the cytolytic effects on oligodendrocytes elicited by JCV in PML. Figure 3E indicates NF-κB p65 and JCV VP1 co-localization in the nucleus in infected cells in HIV-1/PML.
Figure 3. Analysis of NF-κB p65 in HIV-1/PML clinical samples by IHC.
Double immunofluorescence labeling of frontal cortex tissues from representative cases with HIV and HIV-1/PML (PML) (A, C), and HIV without PML (no PML) (B, D). Sections are labeled with anti-GFAP for astrocytes (red) and anti-p65 (green) (A, B), or anti-CC1 (red) for oligodendrocytes and anti-p65 (green) (C, D). Panel E is from an HIV+PML case labeled for VP1 (red) and p65 (green). The magnification of all images is 400× and the bar is ~ 40 µm. Insets in each panel are enlarged to show detail.
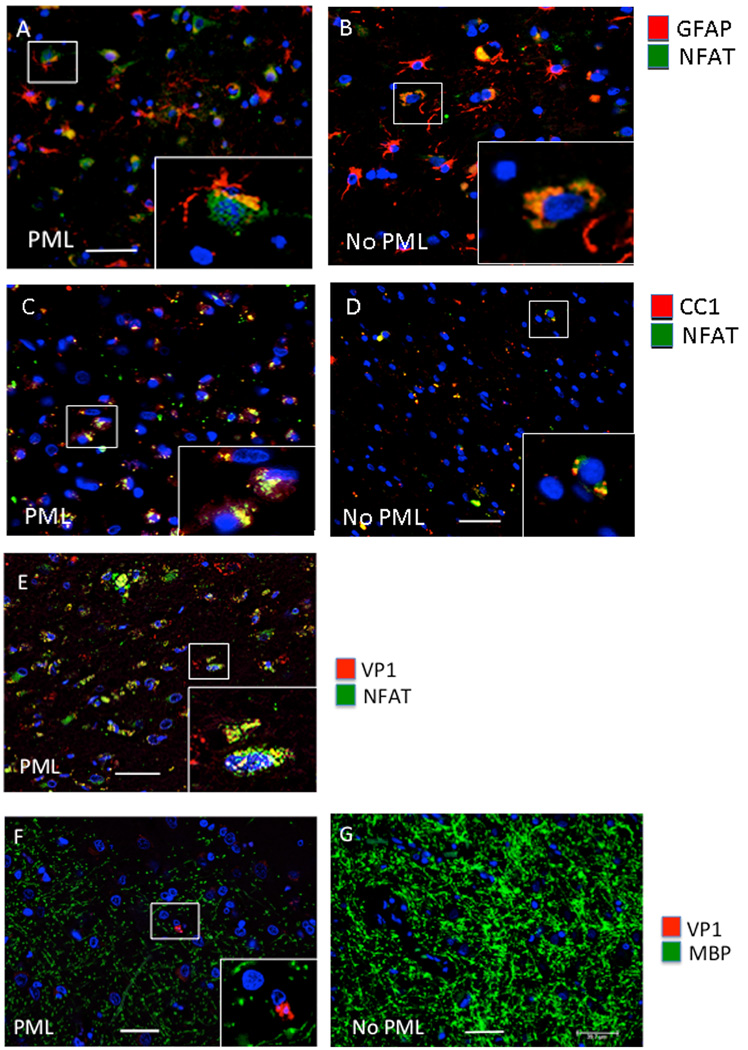
Cell culture studies in our lab [6] and others [15] showed that the transcription factor NFAT4 also regulates JCV and acts in co-operation with NF-κB p65 at the same binding site in the JCV NCCR to activate viral transcription [6]. NFAT4 is a member of the nuclear factor of activated T cells (NFAT) group of transcription factors and is retained in the cytoplasm of quiescent cells [16, 17]. NFAT activation is mediated in part by nuclear import, which requires calcium-dependent dephosphorylation of NFAT by the phosphatase calcineurin [16, 17]). As shown in Figure 4, NFAT4 is largely localized to the nucleus (Fig. 4A) of GFAP+ cells in PML but not in the HIV+ cases without PML where it is largely cytoplasmic (Fig. 4B). NFAT4 is also partially localized to the nucleus (Fig. 4C) of CC1-postive oligodendrocytes in HIV-1/PML but not in the HIV+ cases without PML where it is largely undetected (Fig. 4D). NFAT4 is colocalized with VP1 in the nucleus of JCV-infected cells in HIV-1/PML (Fig. 4E), and as in Fig. 3C, the infected cell shown in the inset of Fig. 4E shows a pronounced cytopathic effect. Infected cells also co-label with VP1 and myelin basic protein (Fig. 4F), as expected. For comparison, the HIV+ case without PML shows intact myelin and no VP1 positive cells (Fig. 4G).
Figure 4. Analysis of NFAT4 in HIV-1/PML clinical samples by IHC.
Double immunofluorescence labeling of frontal cortex tissues from representative cases with HIV and HIV-1/PML (PML) (A, C), and HIV without PML (no PML) (B, D). Sections are labeled with anti-GFAP for astrocytes (red) and anti-NFAT4 (green) (A, B) or anti-CC1 (red) for oligodendrocytes and anti-NFAT4 (green). Panels E and F are from a HIV+PML case labeled for VP1 (red) and either NFAT4 (green) (E) or MBP for myelin (green) (F). Panel F is from the gray/white matter junction in the frontal cortex. Panel G is from an HIV+ no PML representative case showing no loss of MBP (green) and no VP1 (red). The magnification of all images is 400× and the bar is 40 µm. Insets in each panel are enlarged to show detail.
DISCUSSION
The development of primary human glial cell culture systems of both astrocytic [18, 19] and oligodendrocytic [20, 21] lineages has allowed the study of many aspects of the life cycle of JC virus. Studies in these cells and human glial cell lines have defined some of the factors that regulate gene expression and may potentially be involved in regulating the activity of the virus and pathogenesis of PML [8, 22]. This is important because JCV can switch from an asymptomatic persistent state, which is found in the vast majority of infected individuals to a pathogenic replicative state in the progressive multifocal brain lesions of patients with HIV-1/PML (reviewed in [23, 24]). The mechanisms of viral reactivation and the emergence of HIV-1/PML remain unresolved. Potentially important factors that we have identified from our cell culture studies include the transcription factors NF-κB and NFAT4 [5, 6] and pro-inflammatory extracellular cytokines such as TNF-α and IL-1β [9] that can become dysregulated in HIV-1/AIDS [25, 26].
One approach to further investigating the roles of cytokines and transcription factors in the pathology of PML would be to create an animal model. JCV has a strict host range, which is dictated by cellular species-specific factors required for viral replication [27] and involves a region of the JCV genome that has been mapped to the origin of viral DNA replication using JCV/SV40 hybrids in viral tropism experiments [28]. Since JCV replication involves interaction among the JCV early protein T-Ag, the JC viral origin of DNA replication and the host DNA synthesis machinery to initiate viral DNA replication, the inability of JCV to replicate in non-human cells may be due to a requirement for a species-specific component of DNA polymerase for JCV DNA replication [27]. The lack of an animal model for PML due to the failure of JC virus to productively replicate in non-human hosts has proved to be a major obstacle in JCV research. However several laboratories have worked on the problem and some progress has been made using animals that have been engrafted with human cells [29].
The data from glial cell culture studies support the hypothesis that pro-inflammatory cytokines in the brain may be a trigger in the reactivation of latent/persistent JCV and the initiation of PML pathogenesis. Also consistent with this hypothesis is the observation that JCV can be present in the brain of normal individuals without PML as reported by our laboratory [30] and others (reviewed in [23]). Our data described here evaluates HIV-1/PML clinical samples and HIV+, non-PML controls for expression of TNF-α and its receptors and the subcellular localization of NF-κB p65 and NFAT4. Consistent with our hypothesis, HIV-1/PML tissue was found to have higher levels of TNF-α and TNFR1 expression and a distribution of NF-κB and NFAT4 showing preferential localization to the nucleus than HIV+, non-PML cases. These data supports our hypothesis for the involvement of TNF-α, TNFR1, NF-κB and NFAT4 proteins in JCV reactivation in PML.
It is important to note that while inflammatory events may be necessary for the initiation of PML, the importance of such events for the initiation of PML is still debatable and it is very unlikely that alone they are sufficient for disease onset. Infection by JCV occurs in most of the population and yet PML is exceedingly rare implying that there are multiple barriers preventing the development of PML after JCV infection [31]. Chief amongst these barriers is immunosurveillance by the cellular immune system that can act by to destroy infected cells expressing viral antigens and thereby stall infections at an early stage [32]. This highlights the importance of immune system dysfunction in the etiology of HIV-1/PML.
ACKNOWLEDGEMENTS
We thank past and present members of the Center for Neurovirology for their insightful discussion and sharing of ideas and reagents. This study utilized services offered by the Temple University Comprehensive NeuroAIDS Center supported by NIH grant P30MH092177. This work was supported by grant AI077460 awarded by NIH to MKW.
ABBREVIATIONS
- GFAP
Glial cell fibrilliary protein
- NFAT4
Nuclear factor of activated T-cells 4
- TNF-α
Tumor necrosis factor-α
- TNFR1
Tumor necrosis factor-α receptor 1
Footnotes
CONFLICT OF INTEREST
The authors confirm that this article content has no conflict of interest.
AUTHOR CONTRIBUTIONS
HSW performed the Western blots and IHC and critically edited the manuscript; RA and BC performed IHC and critically edited the manuscript; DL prepared Figs. 2–4, wrote the legends and respective results sections and critically edited the manuscript; MKW conceived the experiments, wrote the manuscript, made Fig. 1 and critically edited the manuscript.
REFERENCES
- 1.Berger JR, Aksamit AJ, Clifford DB, et al. PML diagnostic criteria: consensus statement from the AAN Neuroinfectious Disease Section. Neurology. 2013;80:1430–1438. doi: 10.1212/WNL.0b013e31828c2fa1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Berger JR. Progressive multifocal leukoencephalopathy. Handb Clin Neurol. 2014;123C:357–376. doi: 10.1016/B978-0-444-53488-0.00017-1. [DOI] [PubMed] [Google Scholar]
- 3.Tavazzi E, White MK, Khalili K. Progressive multifocal leukoencephalopathy: clinical and molecular aspects. Rev Med Virol. 2012;22:18–32. doi: 10.1002/rmv.710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wollebo HS, White MK, Gordon J, et al. Persistence and pathogenesis of the neurotropic polyomavirus JC. Ann Neurol. 2015;77:560–570. doi: 10.1002/ana.24371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Romagnoli L, Wollebo HS, Deshmane SL, et al. Modulation of JC virus transcription by C/EBPbeta. Virus Res. 2009;146:97–106. doi: 10.1016/j.virusres.2009.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wollebo HS, Melis S, Khalili K, et al. Cooperative roles of NF-κB and NFAT4 in polyomavirus JC regulation at the KB control element. Virology. 2012;432:146–154. doi: 10.1016/j.virol.2012.06.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Frisque RJ, Bream GL, Cannella MT. Human polyomavirus JC virus genome. J Virol. 1984;51:458–469. doi: 10.1128/jvi.51.2.458-469.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.White MK, Safak M, Khalili K. Regulation of gene expression in primate polyomaviruses. J Virol. 2009;83:10846–10856. doi: 10.1128/JVI.00542-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wollebo HS, Safak M, Del Valle L, et al. Role for tumor necrosis factor-α in JC virus reactivation and progressive multifocal leukoencephalopathy. J Neuroimmunol. 2011;233:46–53. doi: 10.1016/j.jneuroim.2010.11.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wollebo HS, Woldemichaele B, Khalili K, et al. Epigenetic regulation of polyomavirus JC. Virol J. 2013;10:264. doi: 10.1186/1743-422X-10-264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wollebo HS, Bellizzi A, Cossari DH, et al. Epigenetic regulation of polyomavirus JC involves acetylation of specific lysine residues in NF-κB p65. J Neurovirol. 2015 doi: 10.1007/s13365-015-0326-2. (In Press) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Darbinyan A, Siddiqui KM, Slonina D, et al. Role of JC virus agnoprotein in DNA repair. J Virol. 2004;78:8593–8600. doi: 10.1128/JVI.78.16.8593-8600.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ghosh S, Karin M. Missing pieces in the NF-kappaB puzzle. Cell. 2002;109(Suppl):S81–S96. doi: 10.1016/s0092-8674(02)00703-1. [DOI] [PubMed] [Google Scholar]
- 14.Ghosh S, Baltimore D. Activation in vitro of NF-kappa B by phosphorylation of its inhibitor I kappa B. Nature. 1990;344:678–682. doi: 10.1038/344678a0. [DOI] [PubMed] [Google Scholar]
- 15.Manley K, O'hara BA, Gee GV, et al. NFAT4 is required for JC virus infection of glial cells. J Virol. 2006;80:12079–12085. doi: 10.1128/JVI.01456-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Crabtree GR, Clipstone NA. Signal transmission between the plasma membrane and nucleus of T lymphocytes. Annu Rev Biochem. 1994;63:1045–1083. doi: 10.1146/annurev.bi.63.070194.005145. [DOI] [PubMed] [Google Scholar]
- 17.Chow CW, Rincón M, Cavanagh J, et al. Nuclear accumulation of NFAT4 opposed by the JNK signal transduction pathway. Science. 1997;278:1638–1641. doi: 10.1126/science.278.5343.1638. [DOI] [PubMed] [Google Scholar]
- 18.Radhakrishnan S, Otte J, Enam S, et al. JC virus-induced changes in cellular gene expression in primary human astrocytes. J Virol. 2003;77:10638–10644. doi: 10.1128/JVI.77.19.10638-10644.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Radhakrishnan S, Gordon J, Del Valle L, et al. Intracellular approach for blocking JC virus gene expression by using RNA interference during viral infection. J Virol. 2004;78:7264–7269. doi: 10.1128/JVI.78.13.7264-7269.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Darbinyan A, Kaminski R, White MK, et al. Isolation and propagation of primary human and rodent embryonic neural progenitor cells and cortical neurons. Methods Mol Biol. 2013;1078:45–54. doi: 10.1007/978-1-62703-640-5_5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Darbinyan A, White MK, Akan S, et al. Alterations of DNA damage repair pathways resulting from JCV infection. Virology. 2007;364:73–86. doi: 10.1016/j.virol.2007.02.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Khalili K, Safak M, Del Valle L, White MK. JC virus molecular biology and the human demyelinating disease, progressive multifocal leukoencephalopathy. In: Shoshkes Reiss C, editor. Neurotropic virus infections. Cambridge, UK: Cambridge University Press; 2008. pp. 190–211. [Google Scholar]
- 23.White MK, Khalili K. Pathogenesis of progressive multifocal leukoencephalopathy--revisited. J Infect Dis. 2011;203:578–586. doi: 10.1093/infdis/jiq097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Khalili K, Gordon J, White MK. The polyomavirus, JCV, and its involvement in human disease. Adv Exp Med Biol. 2006;577:274–287. doi: 10.1007/0-387-32957-9_20. [DOI] [PubMed] [Google Scholar]
- 25.Tylor WR, Glass JD, Griffin JW, et al. Cytokine expression in the brain during the acquired immunodeficiency syndrome. Ann Neurol. 1992;31:349–360. doi: 10.1002/ana.410310402. [DOI] [PubMed] [Google Scholar]
- 26.Kaul M, Lipton SA. Mechanisms of neuronal injury and death in HIV-1 associated dementia. Curr HIV Res. 2006;4:307–318. doi: 10.2174/157016206777709384. [DOI] [PubMed] [Google Scholar]
- 27.Feigenbaum L, Khalili K, Major E, Khoury G. Regulation of the host range of human papovavirus JCV. Proc Natl Acad Sci USA. 1987;84:3695–3698. doi: 10.1073/pnas.84.11.3695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lynch KJ, Frisque RJ. Identification of critical elements within the JC virus DNA replication origin. J Virol. 1990;64:5812–5822. doi: 10.1128/jvi.64.12.5812-5822.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.White MK, Gordon J, Berger JR, Khalili K. Animal models for progressive multifocal leukoencephalopathy. Minireview. J Cell Physiol. 2015 doi: 10.1002/jcp.25047. (In Press) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Pérez-Liz G, Del Valle L, Gentilella A, et al. Detection of JC virus DNA fragments but not proteins in normal brain tissue. Ann Neurol. 2008;64:379–387. doi: 10.1002/ana.21443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Berger JR, Houff SA, Major EO. Monoclonal antibodies and progressive multifocal leukoencephalopathy. MAbs. 2009;1:583–589. doi: 10.4161/mabs.1.6.9884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Beltrami S, Gordon J. Immune surveillance and response to JC virus infection and PML. J Neurovirol. 2014;20:137–149. doi: 10.1007/s13365-013-0222-6. [DOI] [PMC free article] [PubMed] [Google Scholar]