Abstract
The outcome of patients with metastatic renal cell carcinoma has been substantially improved with administration of the currently available molecularly targeted therapies. However, proper selection of therapy and management of toxicities remain challenging. NCCN convened a multidisciplinary task force panel to address the clinical issues associated with these therapies in attempt to help practicing oncologists optimize patient outcomes. This report summarizes the background data presented at the task force meeting and the ensuing discussion.
Keywords: NCCN, renal cell carcinoma, molecular targeted therapy, VEGF, mTOR, TKI, patient outcomes
Background
Cancers of the kidney and renal pelvis account for approximately 3% of all cancers in the United States.1 An estimated 58,240 new cases of kidney cancer and 13,040 deaths from this malignancy are expected in 2010.1 Most of these cases consist of renal cell carcinomas (RCCs), which are genetically and histologically distinct from cancers of the renal pelvis. An increased understanding of RCC biology coupled with new clinical trials data have resulted in the availability of several new therapeutic options for patients with advanced RCC. Consequently, the landscape of renal cancer treatment has changed dramatically over recent years and continues to change rapidly. The NCCN Clinical Practice Guidelines in Oncology (NCCN Guidelines) provide up-to-date treatment recommendations for clinicians and are updated continually to include the newly available therapies.2
The rapid addition of new treatment options has made the treatment algorithm increasingly complex and created a significant challenge for practicing oncologists. The biggest challenges for physicians treating RCC are in selecting from a growing list of agents the optimal treatment for a given patient, and how to effectively manage toxicities. The molecular targeted therapies are associated with toxicity profiles that are distinct from those associated with conventional cytotoxic therapies. In addition, safety profile and effectiveness of the targeted agents often change when these drugs are used in a wider, less carefully selected population than in patients enrolled in clinical trials. If not recognized and managed appropriately, these side effects may interfere with a patient’s ability to continue on a treatment regimen, and may lead to poor outcomes or premature termination of an effective treatment choice.
NCCN convened a multidisciplinary task force meeting to address the clinical issues associated with currently available therapies to help practicing oncologists optimize patient outcomes. The panel consisted of 11 members, including physicians and nurse practitioners with expertise in the field genitourinary medical oncology and kidney cancer. All panel members were from NCCN Member Institutions and were identified and invited solely by NCCN. During a day-long meeting held on October 20, 2010, in Philadelphia, the task force members provided didactic presentations on topics such as molecular biology of RCC; mechanism of action of individual therapeutic agents (including cytokines and targeted agents); sequencing strategies; safety profiles of the targeted agents; strategies to manage the toxicities of targeted agents; current standards and recent trials that may change the standard of care; and promising agents in development. The presentations were followed by extensive discussions. This report summarizes the background data presented at the meeting and the ensuing discussions.
Molecular Biology of RCC
It is now well known that RCC is not a single disease, but rather comprises several histologic subtypes that differ in clinical course and response to therapy. Even among patients with the same histologic subtype and clinical characteristics, growing evidence shows heterogeneity in prognosis and response defined by the molecular profile of the tumor.3 Identifying and understanding the genetic basis of each different subtype has significant impact on diagnosis and the development of molecular therapeutics. As acknowledged by the 2004 WHO classification of renal cell neoplasms, which is based on histology and genetic alterations, recognizing the various subtypes is of great value in making the correct diagnosis and providing optimal treatment.4,5
The different types of RCC are caused by aberrant expression of different genes.6 The molecular and genetic changes seen in different histologies in the hereditary syndromes are often recapitulated in the sporadic cases. Clear cell RCC is characterized by the presence of inactivating mutations in the von Hippel-Lindau (VHL) gene. The VHL protein forms a complex that targets the hypoxia inducible factors (HIFs) for ubiquitin-mediated degradation. Mutation of the proto-oncogene MET, which codes for the hepatocyte growth factor receptor, is found in the hereditary form of type 1 papillary RCC and in a subset of sporadic type 1 papillary kidney cancers. Hereditary leiomyomatosis RCC, a hereditary form of type 2 papillary RCC, is caused by inactivating mutation of a Krebs cycle enzyme called fumarate hydratase. A familial form of chromophobe renal carcinoma and oncocytoma are both associated with the Birt Hogg Dube (BHD) syndrome and mutations of the BHD gene.
Of the molecular and genetic changes identified, the loss of DNA in the short arm of chromosome 3 (3p) and gain in the long arm of chromosome 5 (5q) are the most frequent genetic alterations in clear cell RCC. Chromosomal region 3p25.3 is the site of the VHL gene. Molecular and genetic methodologies have been highly successful in subclassifying renal tumors. However, further molecular studies with newer technologies are underway to unfold the mechanisms through which each type of renal tumor develops and to define previously unrecognized entities.
For the past decade, DNA copy number analysis, high-density single nucleotide polymorphism (SNP) array, and genome-wide expression profiling have emerged as useful tools for identifying RCC. Recently, Pei et al.7 compared classical cytogenetics to SNP-based microarrays to detect recurrent sites of genomic imbalance in a series of 20 sporadic clear cell RCC samples. Their findings show frequent loss of 3p and gain of 5q in clear cell RCC and are consistent with those of previous investigators.8–11 This study confirmed that genomic copy number analysis is a useful detection tool that has the potential to replace or at least complement classic karyotypic analysis of tumors. This technology is moving forward rapidly and has recently entered the clinic setting to help diagnose RCC. The power of applying molecular analysis in clinical practice has not yet been fully realized.
Most clear cell RCC have either somatic or germline inactivating mutations in the VHL gene, which are largely absent in other cancers. However, VHL inactivation alone induces senescence, suggesting that other mutations are required to further drive clear cell RCC development in individuals carrying the VHL mutant gene.12 Analysis of the genomic abnormalities show 2 major patterns: gross genomic loss, and gene-specific insertions and deletions.13 SNP and comparative genome hybridization analyses have shown that RCCs are characterized by gross chromosomal losses and gains, which can identify histologically and prognostically relevant subsets. For example, clear cell RCCs may show loss of chromosomes 3p, 9p, and 14q, and gains of 5q. Loss of 9p and 14q has been associated with worse outcome, whereas 5q gain seems to impart a more favorable outcome.8–10,14–16 Papillary RCC is characterized by gains in chromosomes 7 and 17.17 An increase or decrease in the expression of specific genes within these chromosomal regions likely has prognostic implications, but these genes have yet to be elucidated. To search for additional genetic changes in clear cell RCC, Dalgliesh et al.13 sequenced the coding exons of 3544 genes in 101 clear cell RCCs (see Author Note, page S-24). They also performed DNA copy number analyses using high-density SNP array, and genome-wide expression array analyses. Their results showed that 82% of the clear cell RCCs had upregulation of genes associated with cellular hypoxia, with 65% carrying VHL-inactivating point mutations. Losses of 3p, in which VHL resides, were the most frequent copy number changes, seen in 87% using SNP array analyses. Sequencing data identified inactivating mutations in 2 genes encoding enzymes involved in histone modification in the hypoxia expression phenotype, suggesting a mechanism for altered expression of other genes that may be involved in tumor development. Mutations in known oncogenes and other tumor suppressor genes were not, or rarely, observed. New targets for therapy may emerge from further study of the somatic genetic changes in RCC using a larger proportion of genes than was probed in the study by Dalgliesh et al.13
Insight regarding how currently available targeted agents are effective against clear cell RCC derives from identification of the VHL gene and its regulation of the hypoxic response. Mutation of the VHL gene results in accumulation of HIF-1α and HIF-2α and upregulation of several hypoxia-responsive genes. Some of these genes are involved in angiogenesis and enhance new vasculature (e.g., vascular endothelial growth factor [VEGF]), some are growth factors and stimulate cell growth (e.g., platelet-derived growth factor [PDGF] or tumor growth factor α), and some are involved in glucose transportation and increase the energy supply to the cells (e.g., glucose transporter–1). Therefore, inhibition of these pathways is a rational therapeutic strategy for preventing tumor growth. As outlined later, some of the antiangiogenic agents developed for RCC therapy predominantly target receptors and ligands on the tumor endothelium, and may be less likely to have a direct effect on the epithelial tumor cell itself.
Although HIF-1α and HIF-2α proteins share significant homology and regulate overlapping hypoxia inducible target genes, they have differing effects on RCC cell growth. They have distinct effects on cell metabolism and proliferation but overlap in their effects on angiogenesis. HIF-1α stimulates glycolytic enzyme expression and has been shown to block cell-cycle progression in vitro through posttranslationally inhibiting the c-Myc oncoprotein.18 However, HIF-2α does not regulate glycolytic gene expression but regulates the expression of a transcription factor, OCT-4, and promotes cell-cycle progression.18
VHL genotype and HIF-α expression status were analyzed in a study of 160 primary clear cell RCCs. Tumors with intact VHL and low HIF expression and those with mutated VHL and increased expression of both HIF-1α and HIF-2α exhibited enhanced Akt/mTOR and ERK/MAPK signaling. By contrast, VHL-deficient tumors expressing only HIF-2α showed reduced activation of these growth factor signaling pathways but had elevated c-myc activity, resulting in enhanced proliferation and resistance to replication stress. These differences in VHL and HIF status and their downstream effects on tumor cell proliferation and stress response may provide molecular criteria for selecting tumor-specific targeted therapies, and led to the hypothesis that reduced or absent HIF-1α may be a marker of resistance to VEGF-directed therapies and mTOR inhibitors. HIF-1α/HIF-2α expression should be studied in clinical trials of VEGF targeted therapies to validate this hypothesis.
In addition to VHL, other molecules in the cell regulate HIF expression. The mammalian target of rapamycin (mTOR) is a serine threonine kinase that acts as a homeostatic sensor to coordinate growth factor signals with signals that reflect the status of the cell environment, such as oxygen and nutrient availability. As described later, mTOR exists in 2 multiprotein complexes known as mTOR complex 1 (mTORC1) and mTOR complex 2 (mTORC2). Although mTORC1 relays signals after PI3K-Akt activation and nutrient availability, mTORC2 contributes to Akt activation by phosphorylating Akt on serine 473.19 Activation of mTORC1 results in increased protein synthesis through regulation of ribosomal translation of mRNA of critical proteins involved in cell growth and angiogenesis. Activation of mTORC1 is associated with elevation of HIF-1α, whereas mTORC2 activation is associated with higher HIF-2α levels.20 Accordingly, abnormal signaling that results in mTOR activation may contribute to increased HIF expression and abnormal angiogenesis. The implications of upstream and downstream signaling by mTOR, the differing functions of mTORC1 and mTORC2, and the specificity of currently available mTOR inhibitors for mTORC1, are discussed later.
Unlike colorectal cancer, for which a clear multistep model of tumor progression exists,21 the series of events leading to RCC remains unknown. Further molecular studies will likely elucidate mechanisms that lead to RCC and provide an improved classification that exceeds histology alone in predicting clinical outcomes. The hope is also that molecular technologies will be increasingly useful in solving difficult diagnostic challenges, predicting clinical outcomes, and guiding the development of more effective treatments for kidney cancer.
Conclusions
RCCs exhibit a wide spectrum of molecular characteristics that are associated with their diverse morphologies and clinical behaviors. Each histologic type of RCC is characterized by specific genetic alterations, unique clinical behavior, and possibly unique responses to therapeutic modalities. The currently accepted classification is based on histologic findings, but molecular characteristics of the tumors will likely become increasingly important in assessing prognosis and response to therapy.
Although loss-of-function VHL mutation is the hallmark of clear cell RCC, several molecular studies indicate that this alone is not sufficient for tumorigenesis, and that additional genes are involved. Upregulation of the HIFs is a major contributor to increased angiogenesis. Multiple molecules are involved in HIF regulation. Considerable effort has been put into the search for additional biomarkers of RCC that may elucidate mechanisms of tumor progression.
Currently, the gap between molecular findings and clinical outcomes is large. An improved understanding of the molecular factors involved in the development and progression of RCC will help reduce this gap. Correlation of molecular changes with patient outcomes will be an essential component of this research, and is possible through clinical trials. Therefore, participation in clinical trials is very important.
Improved knowledge of the biology of RCC has provided the foundation for the development of novel therapeutic approaches currently approved for treatment of this disease. Going forward, additional alterations of molecules and pathways need to be identified that may subcategorize renal tumors, predict their clinical outcomes, and define new therapeutic targets.
Mechanism of Action of Individual Therapeutic Agents
Metastatic RCC is considered refractory to conventional radiation and cytotoxic therapies, and susceptible to immune modulation only in a small proportion of patients. Greater understanding of the biology of RCC has led to the development of novel targeted therapies. The development of targeted therapy has revolutionized the treatment of metastatic RCC, with improvement in progression-free survival (PFS) and overall survival along with new challenges in the management of toxicity. To maximize the treatment benefit of these new agents, detailed knowledge of their modes of action is important.
Recognition that loss of VHL function leads to unregulated angiogenesis in clear cell RCC has played a major role in drug development. Since December 2005, 6 new drugs have been approved by the FDA for the treatment of metastatic RCC. The currently available targeted agents can be classified as VEGF-pathway targeting agents (bevacizumab, sunitinib, sorafenib, and pazopanib) and mTOR-pathway targeting agents (temsirolimus and everolimus). Immunotherapeutic agents (interleukin-2 and interferon-α) are playing a much smaller role, and cytotoxic agents (e.g., gemcitabine and doxorubicin) are used on rare occasions.
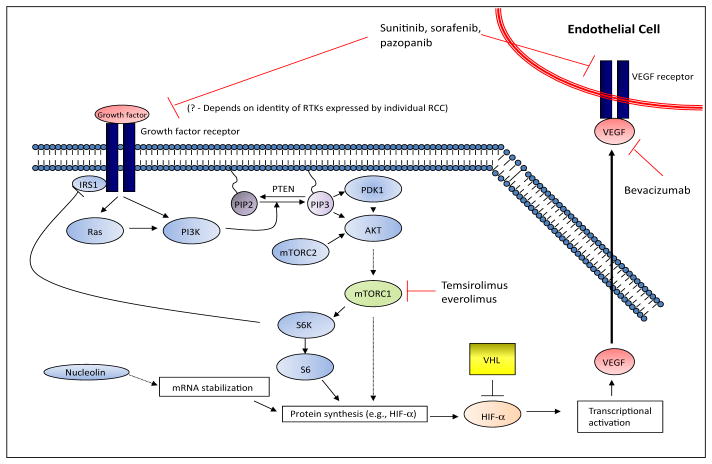
VHL mutation, HIF upregulation, and VEGF overexpression are important contributors to RCC. The first studies to establish the therapeutic value of targeting the downstream consequences of VHL loss of function used a VEGF-directed monoclonal antibody, bevacizumab.22–24 Encouraged by the initial observations with bevacizumab, 3 small molecule inhibitors of VEGF receptor (VEGFR) tyrosine kinase were successfully developed for the treatment of RCC: sunitinib, sorafenib, and pazopanib (see Figure 1).
Figure 1.
Therapeutic targets in renal cell carcinoma.
Abbreviations: HIF, hypoxia-induced factor; mTORC1/2, mammalian target of rapamycin complexes 1 and 2; PDK1, phosphoinositide-dependant kinase-1; PI3K, phosphatidylinositol 3-kinase; RCC, renal cell carcinoma; RTKs, receptor tyrosine kinase; VEGF, vascular endothelial growth factor; VHL, von Hippel-Lindau.
Modified from Courtney KD, Choueiri TK. Optimizing recent advances in metastatic renal cell carcinoma. Curr Oncol Rep 2009;11:218–226; with kind permission from Springer Science+Business Media.
VEGF Pathway Components as Molecular Targets and their Mechanism of Action
Typically, ligand binding induces dimerization of the tyrosine kinase receptor (e.g., VEGFR). This results in activation of the cytoplasmic tyrosine kinase domain and autophosphorylation of tyrosines, stimulating multiple downstream signaling pathways and leading to cell growth, proliferation, and angiogenesis. The tyrosine kinase inhibitors (TKIs) block the downstream signaling of the receptor tyrosine kinases.
The VEGF pathway of angiogenesis is complex, and VEGF receptors are also implicated in lymphangiogenesis. Several VEGF ligands and receptor inhibitors have been evaluated preclinically and clinically, and several are being used in clinical practice; 4 of these were approved for the treatment of advanced RCC.
Bevacizumab is a 93% humanized monoclonal antibody that binds and neutralizes all biologically active forms of VEGF-A. Bevacizumab inhibits angiogenesis in experimental models, and inhibits growth of human tumor xenografts in vivo.25,26 Bevacizumab selectively binds the VEGF-A ligand but has no effect on the other isoforms of VEGF or on the VEGFRs.
The TKIs, in addition to inhibiting the tyrosine kinase domain of VEGFRs, also inhibit other tyrosine kinase–dependent receptors, including PDGF receptors (PDGFRs), FMS-like tyrosine kinase 3 (FLT-3), and stem-cell growth factor. In a study evaluating the binding of more than 20 kinase inhibitors (including sunitinib and sorafenib) with a panel of 119 protein kinases, specificity varied widely and was not strongly correlated with chemical structure or the identity of the intended target.27 In addition to specificity, the relative potency with which these TKIs block the activity of specific tyrosine kinase also varied.28,29 Because each of the VEGFR-TKIs may inhibit one or more other kinases, they are often referred to as multitargeted kinase inhibitors.
Sorafenib is an oral TKI that targets several receptor tyrosine kinases,30–32 including the VEGFRs, PDGFR-β, FLT-3, and c-KIT. Sorafenib also blocks the activity of the Raf family of serine-threonine kinases.33 Raf is a key molecule in the mitogen-activated protein kinase (MAPK) pathway. MAPK signals are instrumental in facilitating cell proliferation, and are overactive in tumors.34 Sorafenib, therefore, may target both tumor angiogenesis and proliferation through blocking Raf activity in tumor endothelial cells and epithelial cells. Sunitinib is an oral TKI of VEGFR types 1 and 2, PDGFRs (-α and -β), colony- stimulating factor 1, and the FLT-3 and RET kinases.35,36 Pazopanib is another oral TKI that targets several VEGF and PDGF receptors with a slightly different kinase profile than sorafenib and sunitinib.37
mTOR Pathway Components as Molecular Targets and Their Mechanism of Action
Another established therapeutic target in RCC is mTOR, a central component of multiple converging cell signaling pathways, including one that senses levels of key amino acids; the tuberous sclerosis complex (TSC) proteins 1– and 2–dependent nutrient-sensing adenosine monophosphate kinase; and growth factor–activated phosphatidylinositol-3 kinase (PI3K) pathways.38–40 mTOR is a serine/threonine kinase that exists in 2 main multiprotein complexes known as mTORC1 and mTORC2.41 mTORC1 is composed of mTOR, RAPTOR (regulatory associated protein of mTOR), PRAS 40, and mLST8, and is inhibited by the natural product rapamycin (also known as sirolimus). mTORC1 phosphorylates 2 proteins, p70 ribosomal protein S6 kinase (p70S6K) and the binding protein for eukaryotic initiation factor 4E (4E-BP1). Through p70S6K and 4E-BP1, mTORC1 regulates the translation of key proteins such as c-Myc, cyclin D, and HIF that are involved in tumor cell growth, proliferation, and response to hypoxic stress.39,40 mTORC2 is composed of mTOR, mLST8, mSIN1, and Proctor-1, and is resistant to inhibition by rapamycin. mTORC2 phosphorylates and activates Akt19 to control cell proliferation and survival. Immunohistologic evidence of mTOR activation is found in 60% of RCC primary tumors42 and 66% of metastatic lesions.43
Two analogs of sirolimus (rapamycin), temsirolimus and everolimus, are FDA-approved inhibitors of mTOR. Sirolimus, temsirolimus, and everolimus are allosteric inhibitors of mTORC1. Although it is metabolized to sirolimus, temsirolimus is a potent mTOR inhibitor with similar activity to sirolimus. Sirolimus and its analogs bind to FK-506 binding protein-12 (FKBP-12), resulting in a drug FKBP-12 moiety that binds to the rapamycin-binding domain on mTOR within mTORC1. By contrast, mTORC2 function is resistant to inhibition by temsirolimus, everolimus, and sirolimus.44 mTORC1 controls the translation of key proteins involved in cell growth, proliferation, and angiogenesis. mTOR inhibition by temsirolimus reduces HIF1-α and VEGF, causing a reduction in angiogenesis, a common mechanism shared by all of the approved targeted agents for RCC (see Figure 1). In addition to effects on HIF and angiogenesis, inhibition of mTOR kinase leads to reduced translation of several key proteins involved in cell growth and proliferation. The mTOR inhibitors approved for RCC differ in route of administration and schedule. Temsirolimus is administered weekly intravenously, whereas everolimus is given orally as a daily dosage.
Immunomodulating Agents
Until recently, systemic therapies for advanced RCC were limited to cytokines, including IL-2 and IFN-α.45–47 Although these agents have been helpful for some patients, in most cases the clinical benefit is modest and achieved at the expense of significant toxicity. IL-2 is a T-cell growth factor that stimulates immune cells. IL-2 was shown to have potent antitumor activity initially in several murine tumor models,48 and subsequently in patients with RCC.45–47 Type I IFNs, such as IFN-α, increase the survival of activated T-cell subsets and increase antigen presentation on the surface of cancer cells, thereby increasing these cells’ susceptibility to attack by the immune system. In addition, IFNs have direct antiproliferative and proapoptotic effects in some mammalian tumor cells. The biologic responses to IFN have great variability, a consequence of complex signaling from the IFN receptor through JAK1, TYK2, and PI3K to multiple signal transducers and activators of transcription and other transcription factors that vary in expression in normal and cancer cells.49 The complexity of IFN signaling and cellular response likely contributes to the variable clinical response to IFNs in RCC and other tumors. The current efficacy of these agents in carefully selected patients is discussed in Cytokine Therapy in Metastatic RCCs, page S-7.
Chemotherapy
RCCs are resistant to conventional cytotoxic therapies. Rates of response to conventional chemotherapy alone are very low (4%–6%).50,51 Increased expression of multidrug resistance genes is suspected to be one of the main causes of chemotherapy failure in kidney cancer.52
Chemotherapy may be more efficacious in those who have tumors with significant sarcomatoid differentiation. Agents such as gemcitabine and doxorubicin have shown moderate activity.53,54 Gemcitabine (2′-deoxy-2′,2′-difluorocytidine) is a nucleoside analog that is phosphorylated by intracellular nucleoside kinases to the active di- and triphosphates dFdCDP and dFdCTP, inhibiting DNA synthesis. Doxorubicin is an anthracycline antibiotic that inhibits DNA/RNA synthesis through direct binding (intercalation) or through inhibiting Topo II (DNA repair). The most recent version of the NCCN Clinical Practice Guidelines in Oncology (NCCN Guidelines) for Kidney Cancer recommend both gemcitabine and doxorubicin for sarcomatoid-dominant tumors, with a category 3 designation (to view the most recent version of these guidelines, visit the NCCN Web site at www.NCCN.org).2
Conclusions
Increased angiogenesis is the hallmark of advanced RCC, and increased VEGF and VEGFR signaling and mTOR activity are important factors underlying dysregulated angiogenesis in RCC. The treatment of RCC has changed considerably with the development of VEGF/VEGFR and mTOR-targeted therapies, which has been facilitated by knowledge of molecular mechanisms involved in the pathogenesis of the disease. To maximize the treatment benefit and manage toxicities of VEGF/VEGFR and mTOR inhibitors, detailed understanding of the mode of action of these agents is important.
Cytokine Therapy in Metastatic RCCs
For years before targeted agents became available the only systemic therapies with activity against metastatic RCC were those involving IL-2 or IFN-α.45–47 With both IFN-α and IL-2, objective response rates between 5% and 27% have been reported.46,55,56 In patients treated with IFN-α, durable complete responses are rare. The frequency and durability of response seem slightly better with high-dose IL-2, although direct comparison of IFN-α and high-dose intravenous bolus IL-2 (HD IL-2), as approved by the FDA, has not been performed. HD IL-2 is associated with substantial toxicity.57–59 Randomized studies comparing IFN-α plus IL-2 with use of a single cytokine have failed to show improved survival associated with combination therapy.55,60 The NCCN Guidelines for Kidney Cancer include therapy with HD IL-2 as a first-line treatment option for selected patients with metastatic RCC as a category 2A recommendation.2
IL-2 administration induces cytokines and other vasoactive and inflammatory mediators that lead to increased capillary permeability and decreased vascular resistance. This results in a shift of fluid from the bloodstream into the extravascular space, which can be exacerbated by the liberal use of fluid resuscitation and the universal occurrence of hypoalbuminemia. Further consequences include pulmonary congestion, pleural effusions, ascites, and peripheral edema, and can contribute to other organ effects, such as cardiac, hepatic, renal, and central nervous system dysfunction. Therefore, safe and effective administration of HD IL-2 requires an experienced and knowledgeable health care team in an intensive care unit setting.
How Treatment is Selected for Individual Patients
Before 2005, the only FDA-approved drug for treating metastatic RCC was IL-2. However, 6 targeted agents are now approved based on a series of randomized controlled trials showing a benefit in survival or at least PFS over IFN-α or best supportive care. These therapies are safe at approved doses for a broader range of patients, rather than being limited to patients with excellent organ function and no comorbidities. This leaves practicing oncologists with the challenge of selecting the optimal therapy for each patient with advanced RCC from several effective therapies, as listed in the NCCN Guidelines.2 IFN-α is now rarely used as a single agent or in combination with IL-2, but rather is combined with bevacizumab in a regimen that has shown longer PFS than IFN-α alone.22 The remainder of this section focuses on considerations in selecting patients for treatment with HD IL-2 versus one or more of the newer targeted agents for advanced RCC.
The challenge of choosing optimal first-line therapy among many options can be reduced to a series of steps, including an initial decision about whether the patient is believed to have a substantial likelihood of benefit (achievement of a durable complete response) and a high probability of tolerating the reversible but severe toxicities of HD IL-2. The targeted agents, although never compared directly with HD IL-2, rarely have provided long-term unmaintained responses or complete responses. The best outcomes generally consist of moderately durable partial responses or disease stabilization lasting 12 to 18 months and requiring continuous treatment. Furthermore, the limited experience with patients who received HD IL-2 after initial exposure to small molecule therapies targeting VEGFR (e.g., sunitinib, sorafenib) suggests a poor therapeutic index. A higher rate of life-threatening or fatal IL-2 toxicities and a lack of antitumor activity have been reported.61 Thus, the initial consideration should be whether the patient is a good candidate for HD IL-2 and should be referred for this treatment. Because much of the published data for the targeted agents include patients previously treated with cytokine therapy, no reason exists to expect inferior benefit from any of these agents in patients previously treated with IL-2.
Possibility of Determining Potential Responders Before Beginning IL-2 Therapy
Although using a set of reliable biologically or immunologically based criteria to select therapies for advanced RCC would be preferable, the existing biomarker data are insufficient to adopt in routine clinical practice. Several retrospective analyses suggest clinical characteristics and tumor features that could predict for benefit of cytokine therapy, including a favorable score on the University of California Los Angeles (UCLA) Survival After Nephrectomy and Immunotherapy (SANI) scale; clear cell histology; and carbonic anhydrase-9 (CA-9) expression by the tumor.62–66 Based on scores obtained from regional lymph node status, constitutional symptoms, location of metastases, sarcomatoid histology, and thyroid-stimulating hormone levels (TSH), the UCLA SANI scale stratifies the survival of patients who present with metastatic RCC into low- (score, 0), intermediate- (score, 1–5), and high-risk (score, > 5) groups. The UCLA SANI score is limited strictly to predicting survival for patients presenting with metastases and who undergo nephrectomy and IL-2–based immunotherapy.
In an attempt to refine the identification of predictive factors, Upton et al.65 examined pathology from patients with RCC responding to IL-2 in a pooled cohort from the Beth Israel Deaconess Medical Center, with some treated in Cytokine Working Group (CGW) trials. For clear cell RCC, response to IL-2 was associated with certain histologic features, such as the presence of alveolar features and the absence of papillary and granular features. Non–clear cell RCC or clear cell RCC with papillary, no alveolar, and/or more than 50% granular features responded poorly to IL-2. A strong association was also present between CA-9 expression and response to HD IL-2. This retrospective study involved a limited patient cohort and classified tumors using histologic subtypes that are not widely used by pathologists.
More recently, the CGW completed the SELECT clinical trial, prospectively evaluating patient and tumor factors associated with favorable and unfavorable outcomes from HD IL-2 therapy.67 In addition to the parameters analyzed by Upton et al.,65 ongoing analyses include other molecular parameters of angiogenesis and hypoxic pathways, and resistance to immunotherapy. Initial analysis of the 120 patients treated in this trial showed a higher-than-expected response rate of 25% (95% CI, 18%–34%)68 and a lack of association between objective response and the favorable constellation of pathology or CA-9 expression, as reported in the earlier retrospective study. Not surprisingly, the prognostic UCLA SANI score validated the frequently reported association between favorable condition and better outcome.
Conclusions
HD IL-2–based immunotherapy is currently the only treatment that can produce long-lasting un-maintained complete or excellent partial remissions. However, the longstanding criticisms of HD IL-2, including its limited efficacy, the inability to prospectively identify patients most likely to experience durable response, its high cost, and the severe toxicity concerns, remain valid and limit wider application of this therapy. Although imperfect, the best criteria for selecting patients for IL-2 therapy include their performance status, overall clinical condition, medical comorbidities, tumor histology, Memorial Sloan-Kettering Cancer Center (MSKCC) or UCLA SANI risk scores, and attitude toward risk. Until its value as a predictive marker can be confirmed, the use of CA-9 expression in selecting patients for IL-2 therapy cannot be recommended. Importantly, safe and effective administration of HD IL-2 requires an experienced and knowledgeable health care team.
Sequencing Strategies
Since 2005, 6 targeted agents have been approved by the FDA for the treatment of metastatic RCC. Sorafenib and sunitinib, approved in 2005 and 2006, respectively, were the first targeted therapies for advanced RCC. They were followed by temsirolimus in August 2007. Everolimus gained approval in March 2009, and the combination of bevacizumab with IFN-α was approved for RCC in August 2009. Pazopanib was approved in October 2009. Additional compounds in late development are expected to be approved in the near future.
For treatment-naïve patients with metastatic RCC, the NCCN Guidelines for Kidney Cancer list several options based on evidence from large randomized phase III studies (to view the most recent version of these guidelines, visit the NCCN Web site at www.NCCN.org).2 The treatment guidelines consider tumor histology and patient prognosis (good, intermediate, or poor) according to the MSKCC prognostic model for patients with metastatic RCC. The MSKCC model classifies patients according to the presence or absence of 5 adverse prognostic factors: Karnofsky performance status of 70 or less, serum lactate dehydrogenase level greater than 1.5 times the upper limit of normal (ULN), hemoglobin level below normal, corrected serum calcium level higher than the ULN, and time from diagnosis to therapy less than* 1 year. Patients with none of these factors are considered low-risk or good prognosis, those with 1 or 2 factors are considered intermediate-risk, and patients with 3 or more factors are considered poor-risk based on shorter survival compared with the good- and intermediate-risk patients.
Sunitinib, pazopanib, and bevacizumab plus IFN-α are all listed with a category 1 designation for treatment-naïve patients with clear cell metastatic RCC. Temsirolimus is listed with a category 1 designation for patients with poor prognosis (for both clear cell and non–clear cell RCC). Both high-dose IL-2 and sorafenib may be alternatives for selected patients with clear cell RCC. In the pivotal phase III trials on which the recommendations are based, most patients benefiting from therapy with sorafenib, sunitinib, or bevacizumab plus IFN-α had an ECOG score of 0 to 1 or Karnofsky score greater than 70%, and/or MSKCC low or intermediate risk status.69 By contrast, most patients enrolled in the Global Advanced Renal Cell Carcinoma (ARCC) trial evaluating temsirolimus had MSKCC poor risk status.44 Except for the temsirolimus ARCC trial, all of the phase III clinical trials of targeted therapies have enrolled patients with predominantly clear cell RCC, and excluded those with predominantly non–clear cell RCC. Approximately 20% of patients had non–clear cell RCC in the temsirolimus ARCC trial. A planned subset analysis suggested that the patients with non–clear cell RCC may have experienced the greatest benefit from temsirolimus relative to IFN-α.44,70
In clinical practice, the treatment plan and therapeutic goal are defined using patient-specific criteria (e.g., overall health status, MSKCC risk status, tumor histology) and based on tumor burden and rate of growth. In selecting treatment, the potential therapeutic sequence must be considered for each individual patient to optimize the use of multiple available drugs.
In terms of objective response, PFS, and overall survival, combining 2 or more agents in RCC has not proved to be more efficacious than treatment with a single agent. Table 1 summarizes studies with various combinations of VEGFR inhibition, VEGF ligand inhibition, and mTOR inhibition. The table shows that many combinations have been either intolerable or difficult to administer, requiring substantial modification of doses. From a toxicity standpoint, perhaps the best combinatorial approach has been VEGF ligand inhibition together with an mTOR inhibitor. The combination of bevacizumab and temsirolimus was found to be tolerable at full doses of each drug, with early studies showing a favorable efficacy profile. Similarly, the combination of bevacizumab and everolimus was deemed worthy of further investigation. Both of these combinations are the experimental arms in contemporary phase III trials for metastatic RCC.
Table 1.
Phase I/II Studies Using Various Combinations of Therapy
| VEGFR Inhibitor | VEGF Inhibitor | mTOR Inhibitor | Dose/Toxicity | Reference |
|---|---|---|---|---|
| Sunitinib | — | Temsirolimus | Sunitinib: 25 mg Temsirolimus: 15 mg Intolerable |
Patel et al.109 |
| Sunitinib | — | Everolimus | Sunitinib: 37.5 mg Everolimus: 20 mg |
Kroog et al.161 |
| Sunitinib | Bevacizumab | Intolerable | Feldman et al.162 | |
| — | Bevacizumab | Temsirolimus | Bevacizumab: 10 mg Temsirolimus: 25 mg |
Merchan et al.163 |
| — | Bevacizumab | Everolimus | Bevacizumab: 10 mg Everolimus: 10 mg |
Hainsworth et al.164 |
| Sorafenib | Bevacizumab | — | Sorafenib: 200 mg Bevacizumab: 5 mg |
Sosman et al.165 |
| Sorafenib | Temsirolimus | Sorafenib: 200 mg Temsirolimus: 25 mg |
Patnaik et al.166 | |
| Sorafenib | Everolimus | Sorafenib: 400 mg Everolimus: 5 mg |
Cen et al.167 |
Abbreviations: mTOR, mammalian target of rapamycin; VEGF, vascular endothelial growth factor; VEGFR, vascular endothelial growth factor receptor.
The targeted agents have provided life span and quality of life improvement for patients with metastatic RCC. However, for most patients no cure exists. In the absence of curative therapy, sequential treatment with the available targeted therapies can be used to reduce tumor burden or slow the rate of tumor progression, with the hope of converting RCC to a more chronic condition rather than a rapidly fatal disease. Additionally, in the absence of curative therapy, quality of life considerations are of great importance. Conceivably, sequential monotherapy would better preserve quality of life than a more toxic combination therapy without compromising overall survival. Another advantage to sequential monotherapy is the likelihood that single agents can be administered at higher doses and for longer duration, whereas the doses of each agent given in combination are usually lowered to avoid additive toxic effects. These dose reductions may negate the potential benefit of combination treatment. However, deciding on the best sequence for targeted therapy remains a significant challenge.
Optimal Sequence
Ideally, the choice and sequencing of a treatment should be driven by an understanding of the mechanisms of tumor progression, the mechanisms of action of the targeted agents, and the mechanisms of resistance to treatment. Most of the available sequencing information is derived from empiric clinical observations.
TKI After Cytokine Therapy
Evidence from prospective randomized trials shows that TKIs, including sorafenib,33 sunitinib,35, 71 and pazopanib,72 can be used effectively after cytokine therapy. The NCCN Guidelines list sorafenib, sunitinib, and pazopanib as category 1 recommendations for patients who have undergone therapy with a cytokine.2 Although this is a proven sequencing strategy, the diminishing use of cytokines in general for RCC makes this sequence less relevant to current practice. Low-dose IFN-α and IL-2 alone are not FDA-approved and should not typically be used. HD IL-2 remains an important initial treatment for select patients, and those patients should proceed to VEGF targeted therapy if disease progression occurs after IL-2.
TKI After Another TKI Therapy or Prior mTOR
Studies investigating the sequential use of sunitinib and sorafenib are mostly retrospective. The limited prospective data available suggest that minimal cross-resistance occurs between TKIs, either sorafenib followed by sunitinib failures or vice versa.73–81 Axitinib is another potent TKI, currently in phase III evaluation, that has shown promising activity after failure of initial TKI therapy with sorafenib or sunitinib.82
The reasons that a second VEGF pathway–targeted therapy may have efficacy after failure of a first TKI are not fully clarified. Because endothelial cells, the presumed target of anti-VEGF therapy, do not share the clonal abnormalities of tumor cells, the mechanisms of resistance to VEGF therapy are not well understood. Available VEGF pathway–targeted therapies have important distinctions, including differences in target specificity, the relative potency against specific tyrosine kinases, and pharmacokinetic/pharmacodynamic differences.28,29
mTOR Inhibitor After TKI Therapy or Prior mTOR Therapy
The randomized phase III RECORD-1 trial established that inhibition of mTOR with everolimus is beneficial in patients previously treated with 1 or 2 TKIs (sunitinib or sorafenib).83 The study also showed that everolimus was comparable in efficacy regardless of which TKI had been received. Based on this study, everolimus is considered a standard option and listed with a category 1 designation in the NCCN Guidelines2 for treatment of advanced RCC after failure of one or more VEGF pathway–targeted therapies. A recently initiated phase III NCI-sponsored study will examine treatment with everolimus alone versus everolimus plus bevacizumab in this patient population. A retrospective analysis of patients with advanced RCC who received temsirolimus after disease progression on sunitinib therapy found the sequence to be feasible in terms of tolerability and safety.84 Further studies are needed to determine the clinical benefit of this sequence.
No data are available regarding the benefit of administering a second rapamycin analog mTOR inhibitor (temsirolimus or everolimus) in the clinical setting of disease progression after treatment with the other approved mTOR inhibitor.
Conclusions
Sequential therapy is the current standard of care for patients with metastatic RCC. The optimal sequence of targeted therapies is unknown. In metastatic RCC, several clinical studies currently underway and outlined in the following section will help determine the sequences of single-agent therapies that will maximize the duration of tumor control, quality of life, and overall survival for patients with metastatic RCC.
Current Standards and Recent Trials That May Change the Standard of Care
Academia and industry have shown an unprecedented level of interest in further improving the therapeutics for metastatic RCC, which remains predominantly incurable. This interest is inspired by the gains in treating advanced disease with novel VEGF pathway inhibitors and the mTOR inhibitors, and advances in understanding of the underlying biology of RCC.
The rapid addition of new treatment options for metastatic RCC has made the treatment algorithm more complex. Challenges facing physicians treating these patients include selecting first-line treatment according to prognostic category and tumor histology; choosing subsequent treatment when disease progresses; and identifying and incorporating safer and more effective agents into practice. A further challenge is to find biomarkers that more precisely define individual prognosis and predict response to a specific therapeutic agent; in short, to develop a more personalized approach to treatment.
Randomized trials comparing the efficacy and tolerability of currently available agents are needed to further improve patient outcomes. Current clinical trials that may further impact standard of care are highlighted in this section.
Clinical Trials With Currently Available Targeted Agents in the First-Line Setting
All of the approved targeted agents for metastatic RCC were shown to be superior to either IFN or best supportive care in randomized clinical trials. How each of the approved therapies compare in terms of efficacy and tolerability remains to be determined in all clinical settings—first-line and subsequent lines of treatment—and in all RCC populations—those with clear cell RCC, non–clear cell RCC, poor prognosis, and good to intermediate prognosis.
COMPARZ is a phase III trial comparing pazopanib with sunitinib as first-line treatment for patients with locally advanced or metastatic RCC with clear cell histology, no prior systemic therapy, measurable disease, and a Karnofsky performance status of 70% or greater (ID number: NCT00720941).85 The primary objective of COMPARZ is to determine if the PFS of patients receiving pazopanib is similar (i.e., noninferior) to that of patients receiving sunitinib. Secondary end points include comparisons of overall survival, overall response rate, safety, and quality of life. The trial has completed accrual and the results are awaited.
Clinical Trials to Determine Optimal Sequence
Most patients with metastatic RCC require second, third, or further lines of therapy. Results of several ongoing trials should help clinicians select second-line and later therapy to maximize the length of disease control and survival.
The RECORD-3 trial is an open-label, multi-center phase II study comparing the efficacy and safety of everolimus as first-line treatment followed by second-line sunitinib, and sunitinib as first-line treatment followed by second-line everolimus.86 The primary end point of this trial is to assess whether first-line of treatment with everolimus is associated with a noninferior PFS compared with first-line treatment with sunitinib. This is a randomized phase II study with a planned accrual of approximately 400 patients, and will include patients with metastatic RCC unsuitable for cytokine therapy, ECOG performance status of 0 or 1, and MSKCC risk score of good or intermediate. A secondary end point of this study is to compare the PFS after second-line treatment with everolimus or sunitinib. Because patients with non–clear cell RCC will be included, RECORD-3 will help determine which sequence of mTOR inhibitor and VEGFR-TKI is optimal for patients with all RCC histologies.
Another international, randomized, multicenter study is comparing PFS for a second VEGFR-inhibitor, sorafenib, with that for an mTOR inhibitor, temsirolimus, given as second-line therapy after disease progression on first-line therapy with sunitinib.87 The safety and tolerability of temsirolimus and sorafenib when used as single agents for second-line treatment also will be determined and compared. Eligible patients have progressive disease according to RECIST criteria while receiving sunitinib as first-line therapy, and at least one measureable lesion. The subjects will be randomly assigned to either weekly intravenous infusions of temsirolimus or sorafenib orally 2 times a day.
A recently completed phase III trial (AXIS) compared axitinib, a specific inhibitor of VEGFR 1, 2, and 3, with sorafenib, a VEGFR and Raf kinase inhibitor, as second-line treatment after sunitinib or other first-line therapies. Patients treated with axitinib had significantly longer PFS than those receiving sorafenib.88 Additional details of this study are expected shortly.
The Sequential Two-Agent Assessment in Renal Cell Carcinoma Therapy (START) trial is randomizing previously untreated patients with meta-static RCC to either everolimus, bevacizumab, or pazopanib.89 At first progression, patients will be re-randomized to 1 of the remaining 2 agents. This 240-patient study is designed to determine which of 6 potential sequences of 2 agents provides the longest overall time to progression.
Clinical Trials to Determine Optimal Combinations of Targeted Agents
Combining 2 or more agents has not proved to be more effective than a single agent for treatment of RCC in terms of proportion of patients obtaining objective response, PFS, and overall survival. Many of these combinations have been intolerable or difficult to administer and require substantial reduction of doses because of greater toxicity.
The BeST (Bevacizumab, Sorafenib, and Temsirolimus) trial is a randomized phase II study of different combinations of bevacizumab, sorafenib, and temsirolimus for treating advanced RCC.90 Subjects with metastatic clear cell RCC and who have had no prior systemic therapy are randomly assigned to receive either bevacizumab only; temsirolimus and bevacizumab; bevacizumab and sorafenib; or temsirolimus and sorafenib. The primary efficacy measure is PFS, and approximately 400 subjects will be enrolled in this NCI-sponsored cooperative group study. This phase II trial will determine 2 important issues: 1) the clinical benefit of single-agent bevacizumab therapy in a larger group of patients with RCC than has been studied previously; and 2) whether one or more of the combinations of targeted agents will produce a PFS that is significantly better than expected for the best single-agent therapy, an improvement that would justify a larger randomized study comparing single-agent sequential therapy with combination therapy.
The clinical efficacy of combining VEGF inhibition and mTOR inhibition is being studied in 2 separate ongoing trials with 2 different mTOR inhibitors. The first is an international randomized, phase IIIb, open-label study (INTORACT) comparing PFS among patients treated with bevacizumab plus temsirolimus and those treated with bevacizumab plus INF-α as first-line treatment.91 The secondary end points are safety and overall survival. The second is a phase II trial comparing PFS among patients treated with bevacizumab plus everolimus versus bevacizumab plus IFN-α as first-line treatment.92
Clinical Trials in the Neoadjuvant Setting
Targeted agents stop the growth of RCC by blocking blood vessel development (angiogenesis) and inhibiting the function of proteins critical for cell growth and survival. Systemic treatment with targeted therapy before surgery offers the potential advantage of reducing tumor size and invasion of normal structures to facilitate tumor resection.
In patients known to be at high risk of RCC recurrence based on high tumor stage and grade, the administration of targeted therapy before (neo-adjuvant) or after (adjuvant) surgery may inhibit or prevent the growth and spread of remaining tumor cells. The role of systemic targeted therapy after surgery for patients without metastatic disease is being addressed in several adjuvant therapy studies. The benefit of systemic therapy before surgery for resectable localized tumors has not been established, and remains a question for future research.
Clinical Trials to Determine the Role of Cytoreductive Nephrectomy Prior to Targeted Therapy
Randomized trials conducted by SWOG (SWOG 8949) and EORTC showed significantly prolonged survival for selected patients with metastatic RCC who had their primary renal tumor surgically removed before starting IFN therapy, compared with those who started IFN without nephrectomy.93–95 The importance of this cytoreductive nephrectomy has not been established for patients who present with metastatic RCC and an intact primary tumor, and for whom targeted therapy with a VEGFR-TKI or mTOR inhibitor is planned. The CARMENA trial is a phase III study96 evaluating initial (cytoreductive) nephrectomy before sunitinib treatment compared with starting sunitinib without nephrectomy for patients with metastatic RCC. The primary end point of this trial is overall survival. Another phase III study will compare immediate surgery followed by sunitinib to sunitinib therapy followed by surgery.97 The primary end point of this study is PFS.
Clinical Trials in the Adjuvant Setting
The prognosis of patients after surgical resection of clinically localized RCC is variable and depends on factors such as tumor stage, grade, and histology. For locally advanced RCC, surgical excision either through radical or partial nephrectomy is the cornerstone of management. The role of targeted therapy in the adjuvant setting to prevent or delay tumor recurrence is unknown. Three large global studies using targeted agents, the ASSURE (Adjuvant Sorafenib or Sunitinib for Unfavorable Renal Carcinoma) trial (ECOG E2805), SORCE, and S-TRAC (Sunitinib Treatment of Renal Adjuvant Cancer), are addressing the role of adjuvant treatment for patients at risk for RCC recurrence after surgical resection of the primary tumor.
In the phase III ASSURE trial, patients are randomized to sunitinib, sorafenib, or placebo after nephrectomy.98 The S-TRAC is a randomized, double-blind, phase III study comparing sunitinib therapy for 1 year with placebo in patients with high risk for RCC recurrence after nephrectomy.99 The SORCE trial is a phase III, double-blind, placebo controlled study comparing placebo with sorafenib therapy for 1 or 3 years in patients who undergo nephrectomy and are considered to be at high or intermediate risk for relapse.100 The primary end point for all 3 of these adjuvant trials is disease-free survival.
Conclusions
Numerous ongoing randomized trials will address important unresolved questions regarding the use of targeted therapy in RCC at all stages, including 1) whether one potent VEGFR-TKI is better than another in the first-line treatment of metastatic RCC, 2) what second-line treatments are the most effective after initial therapy with a VEGFR-TKI, 3) whether combinations of 2 targeted agents are more effective than single-agent therapies or just more toxic, and 4) whether early use of targeted therapy after nephrectomy for localized cancer prevents or delays tumor recurrence in patients known to be at high risk for recurrence after surgery alone. Encouraging patients to participate in clinical trials that will answer important questions such as these is strongly recommended to advance the management of RCC.
Safety Profiles of Targeted Agents
All the targeted agents approved for metastatic RCC are associated with toxicities. Some of the toxicities are class effects characteristic of VEGF pathway or mTOR inhibition (summarized in Table 2). These side effects occur at different times during therapy, and not all patients develop all toxicities. Side effects of the individual agents vary in intensity, possibly because of varying potencies against the intended kinase or drug target as measured by the IC50 or half maximal inhibitory concentration values. IC50 is the concentration of a drug that is required for 50% inhibition of a biologic process in vitro. For instance, a higher incidence of hypertension is associated with the more potent VEGFR-TKIs.101 Some of the toxicities are considered “off-target” effects and are not obviously related to target inhibition or the intended mechanism of action of the drug. This section addresses the selected drug-related toxicities seen in patients with advanced RCC.
Table 2.
Toxicities Commonly Associated With VEGF, VEGFR, and mTOR Inhibition
| Toxicities/Agents | HFSR | Hypertension | Cytopenia | Proteinuria | GI | Hyperlipildemia and Hyperglycemia |
|---|---|---|---|---|---|---|
| Sunitinib | Yes | Yes | Yes | Yes | Yes | No |
| Sorafenib | Yes | Yes | Yes | Yes | Yes | No |
| Pazopanib | No | Yes | No | Yes | Yes | No |
| Bevacizumab | No | Yes | No | Yes | Yes | No |
| Temsirolimus | No | No | Yes | No | Yes | Yes |
| Everolimus | No | No | Yes | No | Yes | Yes |
Abbreviations: GI, gastrointestinal; HFSR, hand and foot syndrome reaction; mTOR, mammalian target of rapamycin; VEGF, vascular endothelial growth factor; VEGFR, vascular endothelial growth factor receptor.
Safety Profile of VEGF Pathway Inhibitors
The drug safety profiles are derived from data from pivotal phase III trials and meta-analyses. The most reliable safety analyses are based on randomized studies of patients with similar entry level characteristics and uniform monitoring and data collection methods.
Hypertension
Hypertension is common with the VEGFR-TKIs and the monoclonal antibody, bevacizumab. The degree of hypertension varies with the potency of inhibition (IC50) of the drugs. For example, pazopanib is a more potent VEGFR-TKI than sorafenib and is associated with a higher incidence of hypertension than sorafenib. Among all VEGFR-TKIs, the incidence of hypertension varies from 8% to 45%, with grade 3 or 4 hypertension in 4% to 16% of patients.
A possible mechanism for the hypertension associated with anti-VEGF/VEGFR inhibition may be related to inhibition of nitric oxide.102 Inhibition of VEGF in arterial endothelial cells decreases the release of nitric oxide, which acts on arterial smooth muscle cells to cause vasodilation.103 Similarly, through inhibiting VEGF signaling, VEGFR-TKIs may have the direct effect of reducing nitric oxide release by vascular endothelial cells, thereby inducing hypertension.104,105
Proteinuria
Proteinuria and hypertension are toxic effects shared by all known therapies targeting the VEGF pathway.104,105 Proteinuria is abnormally high protein excretion in the urine denoting structural damage to the glomerular filtration barrier. Bevacizumab therapy has been associated with the development of proteinuria in 23% to 38% of patients with colorectal cancer, and in up to 64% of patients with RCC, among whom 6.5% experienced a grade 3 to 4 proteinuria.24,106 In the AVOREN study, grade 3 or 4 proteinuria and hypertension were reported in 7% and 3%, respectively, of patients treated with bevacizumab plus IFN-2α.22 In the CALGB 90206 trial, the incidence of grade 3/4 proteinuria and hypertension in patients receiving bevacizumab plus IFN-α was slightly higher, at 15% and 10%, respectively.23 A meta-analysis was performed to determine the risk of developing proteinuria among patients with and without RCC receiving bevacizumab.107 In patients receiving bevacizumab, the incidence was 19.3% (95% CI, 11.9%–29.6%) for all-grade proteinuria, and 2.3% (95% CI, 1.2%–4.1%) for grade 3 to 4 proteinuria. The analysis also showed that the risk of high-grade proteinuria depended on tumor type. Among patients with RCC receiving bevacizumab, the incidence of high-grade proteinuria was 10.0% (95% CI, 4.3%–22.4%) with a relative risk (RR) of 48.7 (95% CI, 9.7–244.3) compared with controls who did not receive bevacizumab. By contrast, the incidence of high-grade proteinuria was 1.7% (95% CI, 0.09%–3.2%) with RR of 5.2 (95% CI, 3.3–8.4) among 5999 patients without RCC.107 Sunitinib therapy is also associated with proteinuria.108 Case reports of proteinuria have been described with sorafenib and pazopanib.109,110
The mechanism for proteinuria caused by VEGF pathway inhibition may be linked to the filtration barrier. The filtration barrier of the renal glomerulus is formed by endothelial cells, basement membrane, and podocytes. VEGF-dependent inhibition of interactions between podocytes and glomerular endothelial cells disrupts the filtration barrier, leading to abnormally high protein excretion in the urine.111 Mild to moderate proteinuria is usually asymptomatic. Periodic monitoring of urinary protein may be necessary in patients on anti-VEGF agents, and those showing nephrotoxicity with impaired glomerular filtration rate require referral to a nephrologist.
Arterial Thrombosis
Among other cardiovascular toxicities, the risk of developing arterial thromboembolic events (ATE) is elevated with bevacizumab. A systematic review and meta-analysis of published randomized controlled trials reported that treatment with bevacizumab may significantly increase the risk of developing ATE and increase cardiac ischemic events in patients with cancer.112 According to the meta-analysis, bevacizumab significantly increased the risk of ATE with an RR of 1.44 (95% CI, 1.08–1.91; P = .013) in patients with a wide variety of advanced solid tumors. The study also showed that the risk of ATE associated with bevacizumab varies with tumor type, with a higher risk seen in patients with RCC (RR, 5.14; 95% CI, 1.35–19.64) than in controls not receiving bevacizumab.112
ATE may be a class effect of angiogenesis inhibitors. Treatment with the VEGFR inhibitors sunitinib and sorafenib is also associated with a significant increase in the risk of ATE. A systematic review and meta-analysis to determine the RR of ATE associated with the use of sunitinib and sorafenib reported the incidence to be 1.4% among 9387 patients. In addition, a significant threefold increase in the risk of ATEs was seen among patients treated with sunitinib and sorafenib compared with controls.113 This risk did not differ with the type of TKI used (sunitinib or sorafenib) or type of malignancy (RCC vs. non-RCC).113
The mechanism of ATE through VEGF inhibition is not fully understood. One hypothesis is that VEGF-signaling inhibitors can disrupt the regenerative capacity of endothelial cells and cause vascular wall defects, leading to thrombosis.111 The risk factors for development of ATE in patients treated with VEGF/VEGFR have not been clearly identified. Therefore, clinicians must be aware of the possibility of increased ATE in select patients and provide continued surveillance.
Other Cardiac Effects
Decreased left ventricular ejection fraction71 and QT-interval prolongation have been described with the use of sunitinib.114 Cardiovascular toxicity, including acute coronary syndromes, is observed in patients treated with sunitinib. Sorafenib is associated with increased incidence of myocardial infarction compared with placebo.33 Schmidinger et al.115 showed that among 86 patients treated with either sunitinib or sorafenib, 33.8% experienced a cardiac event and 40.5% showed electrocardiographic changes.
Reports from small studies suggest risk factors for congestive heart failure in patients on VEGF/VEG-FR therapy, including a history of coronary artery disease, hypertension, and low body mass index.116 VEGFR inhibition, c-kit, and ribosomal protein S6 kinase have been implicated as possible causes of cardiac failure, with hypertension and hypothyroidism as contributing factors.117
Dermatologic Toxicities
Dermatologic toxicities occur commonly with sorafenib and sunitinib but are uncommon with bevacizumab and pazopanib. Rashes are reported with sorafenib. The hand and foot syndrome reaction (HFSR) is a distinct localized cutaneous reaction characterized by erythema, numbness, tingling, and either dysesthesia or paresthesia, particularly on the palms and/or soles.118 It rarely affects the trunk, neck, chest, scalp, and extremities. HFSR is experienced by patients receiving TKIs, and is similar to hand and foot syndrome associated with other chemotherapeutic agents in terms of the areas affected, dose-dependency, and symptoms, but differs in histologic characteristics. HFSR associated with VEGFR-TKI is characterized by thick, well-defined hyperkeratotic lesions frequently affecting digit flexural locations (see Figure 2).
Figure 2.
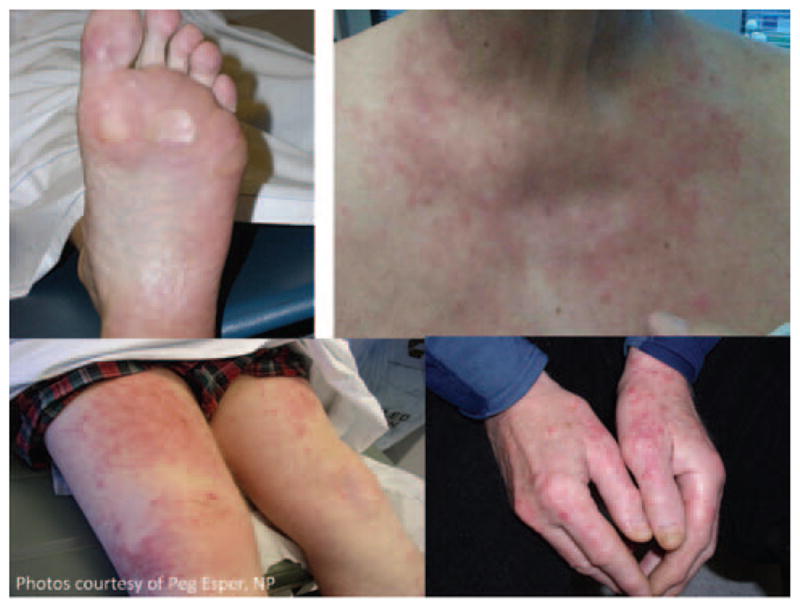
Hand and foot syndrome reaction.
A meta-analysis of clinical trials evaluating the risk of HFSR among patients receiving sorafenib showed that the overall incidence was 33.8% for all-grade HFSR (95% CI, 24.5%–44.7%) and 8.9% for high-grade HFSR (95% CI, 7.3%–10.7%). In addition, sorafenib was associated with a significantly increased risk of HFSR, with a RR of 6.6 (95% CI, 3.7–11.7; P < .001), compared with controls not receiving sorafenib.119
In another systematic review and a meta-analysis to determine risk for HFSR among patients receiving sunitinib, the summary incidences of all-grade and high-grade HFSR were 18.9% (95% CI, 14.1%–24.8%) and 5.5% (95% CI, 3.9%–7.9%), respectively. In addition, patients receiving sunitinib had a significantly increased risk of all-grade HFSR (RR, 9.86; 95% CI, 3.1–31.31; P < .001) compared with controls.120 By contrast with sorafenib,119 patients with RCC receiving sunitinib had significantly decreased risk of HFSR compared with patients with a non-RCC malignancy (RR, 0.56; 95% CI, 0.50–0.64; P < .001).120 The pathogenesis for HFSR remains unknown. HFSR might result from the effect of mechanical pressure to palms and soles. Alternatively, the sensitivity of palms and soles to several TKIs could be partly related to the increased number of eccrine sweat glands in the extremities. Several receptor tyrosine kinases, including PDGF and c-kit, are located in the eccrine sweat glands but not apocrine sweat glands. HFSR might be caused by sunitinib or sorafenib inhibition of these receptors. Because HFSR is seen more commonly with sunitinib and sorafenib and less often with pazopanib, experts have speculated that the common kinases between sunitinib and sorafenib, FLT3 and RET, may be implicated.
Because of the high incidence of HFSR associated with sunitinib and sorafenib, early detection and timely treatment is important.
Gastrointestinal Toxicities
Gastrointestinal side effects of VEGF/VEGFR inhibitors include diarrhea, stomatitis, nausea, changes in taste perception, and anorexia. Table 3 outlines the percentage of grade 3 and 4 gastrointestinal toxicities seen with the VEGF/VEGFR inhibitors in the pivotal phase III trials. The pathophysiologic mechanism of gastrointestinal toxicities caused by VEGF/VEGFR inhibitors is unknown. Because VEGF plays a role in mucosal homeostasis, mucosal damage may be a consequence of VEGF inhibition.121–123
Table 3.
Gastrointestinal Toxicities Seen With mTOR Inhibitor Therapy
| GI Toxicity | Temsirolimus All-Grade/High-Grade (%) | Everolimus All-Grade/High-Grade (%) |
|---|---|---|
| Diarrhea | 27/2 | 15/1 |
| Nausea | 37/2 | 15/0 |
| Vomiting | 19/2 | 12/0 |
| Anorexia | 32/3 | 16/< 1 |
| Mucositis | 20/1 | 14/1 |
| Stomatitis | 20/1 | 44/4 |
Abbreviations: GI, gastrointestinal; mTOR, mammalian target of rapamycin.
Hepatic Toxicities
Hepatic toxicity is seen more frequently with pazopanib than with the other VEGFR-TKIs. Elevated serum levels of the hepatic enzymes alanine aminotransferase (ALT) and aspartate aminotransferase (AST) are the most frequent sign of hepatic toxicity associated with pazopanib. The serum ALT and AST were elevated in 54% and 53% patients with metastatic RCC treated with pazopanib in a large phase II study.119 Isolated elevations of transaminases and bilirubin have also been also observed. The UGT1A1 polymorphism seen in Gilbert’s syndrome is frequently associated with pazopanib-induced hyperbilirubinemia,124 suggesting that some instances of isolated hyperbilirubinemia in patients treated with pazopanib may be benign manifestations of Gilbert’s syndrome.
Clinicians must be aware of the possibility of hepatic toxicity, and perform liver function tests periodically in patients undergoing treatment with any of the VEGFR-TKIs. Although occurring less frequently than with pazopanib, hepatoxicity was associated with sunitinib therapy in an expanded access trial of 4371 patients, leading to fatal hepatic failure in 4 patients and prompting an FDA black box warning regarding the hepatic toxicity of sunitinib.114
Endocrine Dysfunction
Hypothyroidism is the main endocrine toxicity of VEGF- and VEGFR-targeted therapies. The reduced thyroid function associated with these therapies could be caused by regression of capillaries around thyroid follicles. Clinical or laboratory evidence of hypothyroidism has been found in a significant proportion of patients on sunitinib and may contribute to fatigue in these patients.125 Hypothyroidism has also been seen after bevacizumab. Consequently, thyroid function tests should be performed at baseline and then every 3 months in patients receiving VEGFR-TKIs. Symptomatic patients with depressed T3 and T4 levels should undergo thyroid hormone replacement. Subclinical hypothyroidism is defined as an increase in TSH above the upper limit of normal with normal tri-iodothyronine (T3) and thyroxine (T4) levels. Select patients with subclinical hypothyroidism may also benefit from thyroid hormone replacement therapy.
Additional Adverse Events
Patients receiving sunitinib, sorafenib, pazopanib, or bevacizumab also have hematologic (anemia and neutropenia) and metabolic abnormalities (e.g., hypophosphatemia, hypo hyperglycemia, hyperglycemia). Fatigue is a common non-specific adverse event related to the use of all targeted therapies.22,23,33,36,56,72 Gastrointestinal perforation is an infrequent but potentially life-threatening event during anti-VEGF therapy. Patients treated with bevacizumab had a significantly increased risk of gastrointestinal perforation compared with those treated with control medication.126 Risk varied with bevacizumab dose and tumor type; the highest risk was observed in patients with RCC (RR, 5.67; 95% CI, 0.66–48.42).126
Safety Profile of mTOR Inhibitors
The toxicity profiles of the mTOR inhibitors everolimus and temsirolimus are similar (see Table 2 and 3). However, head-to-head trials involving patients with the same entry-level characteristics and using similar patient monitoring and data collection methods are required to compare safety profiles. Careful monitoring and early interventions can minimize the severity of side effects and improve treatment tolerability and efficacy of mTOR inhibitors. The most common adverse effects of mTOR inhibition are gastrointestinal, pulmonary (pneumonitis), and metabolic (hyperlipidemia, hyperglycemia, and hypercholesterolemia) toxicities.
Pulmonary Toxicity
Pulmonary toxicity or pneumonitis is a class effect associated with mTOR inhibitors.127 Pneumonitis caused by mTOR inhibitors is a noninfectious, nonmalignant inflammatory reaction of the lungs that typically appears as ground-glass opacities on radiographic imaging. The physiopathologic mechanism of pulmonary toxicity is still not clearly defined. Hypersensitivity is a possible mechanism. Pneumonitis resolves if the drug is discontinued or reduced in dose, or with the use of steroids. In rare instances, interstitial lung disease may interfere with activities of daily living and require oxygen administration. Asymptomatic interstitial lung disease should be monitored closely with frequent radiographic and physical assessments.
Metabolic Toxicities
mTOR inhibition significantly raises the levels of serum high-density lipoproteins, but also induces significant increases in low-density lipoproteins, cholesterol, and particularly triglycerides. Hyperglycemia, hypercholesterolemia, and hyperlipidemia are seen in patients treated with mTOR inhibitors.
The mTOR/S6K1 pathway is involved in glucose metabolism and insulin signaling pathway; therefore, its inhibition may cause hyperglycemia and exacerbation of preexisting diabetes. Hyperglycemia is commonly associated with temsirolimus and everolimus treatment. Close monitoring of glucose levels is recommended in all patients undergoing mTOR inhibitor therapy.
Temsirolimus and everolimus are metabolized through the cytochrome P450 pathway making food–drug and drug–drug interactions an important consideration because of competition for the metabolic pathway. Temsirolimus or everolimus levels may be increased by cytochrome P450 pathway inhibitors, such as ketoconazole and other azole fungal agents, or decreased by cytochrome P450 pathway inducers, such as corticosteroids, rifampin, and phenobarbital. Interaction with warfarin may lead to prolongation of international normalized ratio and prothrombin time, and therefore patients undergoing this anticoagulant therapy require close monitoring of these parameters. Patients must be educated about these interactions, which may significantly lower the efficacy or increase the toxicity of the agents.
Mucositis and Stomatitis
Mucositis and stomatitis are the most common adverse events associated with mTOR inhibitors. Stomatitis related to mTOR inhibitors is distinct from typical radiation- or chemotherapy-induced stomatitis. Typically, it has a rapid onset (usually within 5 days) and mild to moderate severity (grade 1–2). Mouth sores are usually found on the mucosa of the lips, lateral tongue, buccal mucosa, and soft palate. The exact mechanism of mucositis and stomatitis is unknown.
Additional Adverse Events
Fatigue is a common adverse effect of mTOR inhibitors and all of the targeted therapies for RCC.44,83 Dermatologic reactions ranging from mild rash to severe pruritic dermatitis may occur as a result of mTOR inhibitor therapy. The dermatologic toxicity typically manifests as a maculopapular or acneform rash, and also as dryness, eczema, skin discoloration, and nail dystrophy. mTOR inhibitors may cause gastrointestinal toxicities such as diarrhea, nausea, vomiting, and anorexia.
Conclusions
Although the toxic effect profiles of VEGF-pathway inhibitors and mTOR inhibitors are similar within their classes, the incidence and severity of events vary among agents and individuals. Therefore, clinicians must be aware of the adverse effects of each agent to prevent, recognize, and treat the side effects of treatment and optimize patient outcomes.
Strategies to Manage the Toxicities of Targeted Agents
The toxicities associated with any therapy can lead to decreased quality of life, decreased compliance, treatment interruption, dose reductions, or even discontinuation of treatment, all of which may undermine therapeutic efficacy. Recognition and prompt management of side effects are critical to avoid unnecessary dose reductions. This section outlines available preventive or therapeutic strategies for managing toxicities most commonly seen with VEGF/VEGFR and mTOR inhibitors, including dermatologic toxicities, cardiac toxicities, respiratory toxicities, gastrointestinal toxicities, oral mucositis, and metabolic toxicities.
Dermatologic Toxicities
Dermatologic toxicities associated with targeted therapies for advanced kidney cancer vary, but can include rash, paronychia, pruritus, yellowing of skin, and hair depigmentation.128 An important part of the management plan is educating patients about the likelihood of developing dermatologic toxicity. One toxicity that can significantly affect quality of life is the development of HFSR/acral erythema. Management of HFSR, which is commonly seen in patients receiving sunitinib and sorafenib, should begin before any symptoms occur. Most patients develop signs of HFSR within the first 2 to 6 weeks of therapy.129 All interventions are primarily based on anecdotal evidence. Proactively moisturizing the skin with emollient creams devoid of alcohol and fragrance is helpful. Creams with urea are considered to be better for the feet, especially for patients with severe calluses. Vigorous exercise or activities that place undue stress on the hands and feet should be avoided, especially during the first month of therapy to avoid capillary damage and skin breakdown, because VEGF-pathway inhibition impairs wound healing. To reduce pressure on the feet, shoes with padded insoles or gel inserts may be used during therapy. Management of rashes may include use of emollient creams or topical antihistamine creams or lotions, or antidandruff shampoo. Mild, nondeodorant soaps may be used and hot showers/baths should be avoided. Topical or systemic steroids may be indicated based on the severity of the rash. Topical analgesics, such as lidocaine 2%, may also be used for pain management.
Significant debate exists regarding how best to grade the various dermatologic toxicities. A standard grading tool such as the NCI’s Common Terminology Criteria for Adverse Events (CTCAE130; see Table 4) is used to determine the grade of dermatologic toxicity. This provides a framework for early intervention. The appropriate intervention depends on the severity of the dermatologic toxicity (grade), goals of the treatment, and the individual patient.
Table 4.
Grades of Dermatologic Toxicities Using the Common Terminology Criteria for Adverse Events
| Grade of Rash Maculo-Papular* (based on percentage of BSA covered by macules/papules)
| ||||
|---|---|---|---|---|
| 1 | 2 | 3 | 4 | 5 |
| Macules/papules covering <10% BSA with or without symptoms (e.g., pruritus, burning, tightness) | Macules/papules covering 10%–30% BSA with or without symptoms (e.g., pruritus, burning, tightness); limiting instrumental ADL | Macules/papules covering >30% BSA with or without associated symptoms; limiting self-care ADL | — | — |
Abbreviations: ADL, activities of daily living; BSA, body surface area.
A disorder characterized by the presence of macules (flat) and papules (elevated). Also known as morbilliform rash, it is one of the most common cutaneous adverse events, frequently affecting the upper trunk, spreading centripetally and associated with pruritus.
Data from National Cancer Institute. Common Terminology Criteria for Adverse Events (CTCAE). Version 4.0. Available at: http://evs.nci.nih.gov/ftp1/CTCAE/CTCAE_4.03_2010-06-14_QuickReference_8.5x11.pdf. Accessed January 7, 2011.
In patients experiencing severe dermatologic toxicities, dose reductions or interruptions may be necessary if the side effects become intolerable or are interfering with activities of daily living.
Cardiac Toxicities
Cardiac status and risk factors for developing cardiac complications should be assessed before therapy is initiated. This evaluation includes review of the patient’s baseline blood pressure and current antihypertensive medications. Preexisting hypertension should be effectively controlled before therapy with a VEGF-pathway inhibitor is initiated.
Patients should be monitored for signs and symptoms of heart failure or changes in left ventricular ejection fraction while on therapy. Communicating with and coordinating care with the primary care physician or cardiologist is important to ensure continuity of care. Again, patient education is critical. Patients should be instructed to monitor their blood pressure and maintain a log of readings (the frequency of which varies based on the individual patient’s blood pressure, comedications, and cardiac history). This log should be brought to the clinical appointments for provider review on subsequent visits.
The Investigational Drug Steering Committee of the NCI formed the Cardiovascular Toxicities Panel, which included members from the Angiogenesis Task Force including experts in the management of hypertension and cardiovascular toxic effects in cancer patients. This panel generated consensus recommendations to optimize risk assessment, monitoring, and safe administration of VEGF pathway–targeted agents (Table 5).131
Table 5.
Summary of Recommendations for Assessment, Surveillance, and Management of Blood Pressure in Patients Receiving VEGF Pathway Inhibitors
|
From Maitland ML, Bakris GL, Black HR, et al. Initial assessment, surveillance, and management of blood pressure in patients receiving vascular endothelial growth factor signaling pathway inhibitors. J Natl Cancer Inst 2010;102:596–604; by permission of Oxford University Press.
Respiratory Toxicities
Pneumonitis or interstitial lung disease is a potential complication of treatment with mTOR inhibitors. Patients also can experience dyspnea and cough with any of the agents. Patient education is important regarding the symptoms that they need to watch for and report to their health care provider. Patients must be monitored on an ongoing basis for respiratory symptoms, such as cough, dyspnea, or change in their oxygen saturation. Radiographic tests should be performed as indicated. Differentiating between infectious and noninfectious pneumonitis is important; noninfectious pneumonitis typically appears with ground-glass opacity on radiographic imaging.
Corticosteroids, antibiotics for upper respiratory infections, and dose interruptions may be required in some patients. Asymptomatic patients should be monitored closely with radiographic assessments as indicated. Asymptomatic patients may continue on treatment with mTOR inhibitors, but treatment may need to be discontinued or interrupted if they become symptomatic and some may require dose reduction when restarting therapy.132
Gastrointestinal Toxicities
Patients typically experience a few and occasionally all of the gastrointestinal toxicities at some point during treatment with any of the targeted agents. Therefore, educating patients about the likelihood of these events is an important step in managing any gastrointestinal-related side effects.
Management plans should include standard measures, such as increasing fluid intake and dietary modifications. Careful consideration should be given when treating patients with preexisting conditions, such as irritable bowel syndrome. Patients should be made aware of the over-the-counter agents such as Imodium and Pepto-Bismol. They should be advised that new supportive strategies should be tried only with their health care provider’s approval, because drug interactions could make their treatment either less effective or more toxic.
For patients experiencing diarrhea, maintaining adequate hydration is important, and intravenous fluid administration should be given as necessary. Increasing the use of psyllium products and the need for a prescription antidiarrheal agent should be considered. Patients should be advised to discontinue any supportive strategy that results in worsening symptoms or the development of new side effects, and to contact their heath care providers immediately.
Oral Mucositis
No proven preventive treatments for oral mucositis and ulcerations are available. However, adherence to oral hygiene regimens tends to reduce the duration and severity of mucositis. These include increasing fluid intake, using a soft-bristle toothbrush, brushing at least twice a day for 90 seconds, replacing the toothbrush regularly, using baby toothpaste, rinsing the mouth 4 times daily with a bland rinse such as one containing salt or baking soda, and avoiding any mouthwashes containing alcohol. Periodically, topical treatments such as viscous lidocaine may be used for pain control. Prescribed oral rinses that include antifungal agents such as nystatin are contraindicated in this patient population because they may increase the serum concentration of the targeted agent and increase toxic effects.133,134
Common Laboratory Anomalies
Baseline and ongoing evaluation of bone marrow function should be performed in all patients. Anemia is a common hematologic toxicity for patients being treated with targeted therapy. Neutropenia is a serious adverse event, especially when combined with fever, and should be recognized and treated. Patients should be advised to report new-onset fever or any symptoms of infection to their health care provider for prompt evaluation.
Baseline and ongoing monitoring of chemistries such as creatinine, liver function, and lipids must be carried out. Patients who are known to have diabetes, thyroid dysfunction, or hyperlipidemia before treatment will need to be monitored more closely because treatment may exacerbate these syndromes. If possible, optimal control of comorbid conditions should occur before the start of treatment.
Hyperglycemia is associated with mTOR inhibitor therapy and requires regular monitoring of glucose levels for appropriate intervention. Administration of glucose-lowering agents (metformin or insulin) may be required in some patients. Exposure to sunitinib may decrease blood sugar levels, particularly in patients on insulin therapy.135 Therefore, close monitoring of the blood sugar levels is also recommended for patients undergoing sunitinib therapy. Patient diaries to record fasting blood sugars and parameters for contacting the clinic are essential.133
Thyroid dysfunction has been reported for most targeted treatments now approved for treatment of advanced RCC.136–141 Symptomatic patients with elevated TSH should be treated. TSH levels should ideally be checked before treatment initiation. Routine monitoring of thyroid function is recommended for patients undergoing therapy with targeted agents and after treatment discontinuation to observe for return of normal thyroid function.
Other Adverse Issues
Patients must be educated regarding the potential for delayed wound healing. Patients with other comorbidities, such as diabetes, are at greater risk for experiencing serious issues with all adverse effects and with wound healing.
If patients develop wounds of any type or are scheduled for any surgical procedure, health care providers must be notified immediately. Safety data on the use of VEGF-targeted therapies in the perioperative setting are limited, and therefore no recommendations can be made regarding how long the treatment must be held before or after a surgical procedure, and clinicians should refer to the package insert.114,136–140
Fatigue is a nonspecific side effect seen with all of the targeted agents, and a common symptom for almost all patients in general. Unfortunately, the cause of fatigue is not well understood, and may not always be treatment-related. Anemia, depression, pain, and/or thyroid dysfunction also contribute to fatigue. Every patient must be screened for fatigue. NCCN has guidelines for management of cancer-related fatigue (see NCCN Clinical Practice Guidelines in Oncology [NCCN Guidelines] for Cancer-Related Fatigue; to view the most recent version of these guidelines, visit the NCCN Web site at www.NCCN.org).142 For treatable factors contributing to fatigue, appropriate treatment should be instituted. Some evidence shows that nonpharmacologic interventions, such as exercise, improving sleep patterns, massage, and using relaxation techniques, can also help reduce fatigue.142
If toxicities seem disproportionate to what would be expected, health care providers should confirm whether the patient is taking any concomitant medications, including any over-the-counter drugs or herbal supplements, to ensure that no drug interactions are occurring. Strong or moderate CYP3A4 inducers or inhibitors may have an effect on drug levels of TKIs and mTOR inhibitors because of competition with the metabolic pathway, and concomitant use will require dose adjustments. Potential drug interactions are an important component of patient education and communication with other health care providers. In addition, general instructions should be emphasized regarding whether the medication can be taken with food, what to do if a dose is missed and, in the case of everolimus, the importance of not opening the individual blister pack until the pill is to be taken because of moisture and light sensitivity.
The main goal of any anticancer therapy is to optimize patient outcome. The reduction or interruption of therapy to spare toxicities that may be manageable can adversely affect patient outcomes. The decision must balance effective disease control with the patients’ quality of life. Therefore, being aware of the safety profiles of the targeted agents, maximizing patient education, identifying possible barriers to adherence to therapy, and promptly recognizing symptoms and initiating therapeutic management strategies before withholding therapy are important.142 Establishing a plan with patients at the start of therapy to monitor adherence and evaluate side effects and tailoring patient education individually can help achieve optimal results.
Conclusions
Early and aggressive management of toxicities can help maintain adherence to treatment and optimal dosing levels. Patients and their caregivers must be thoroughly educated about the toxicities and their management, and be provided with emergency contact information of a health care professional to answer any questions or concerns that may arise during treatment.
Promising Agents in Development
Further progress in the treatment of RCC will require elucidation of how tumors become resistant to existing therapies and identification of new molecular targets for drug development. This section reviews promising novel agents in development. As shown in Table 6, these agents can be grouped into 3 categories based on their mechanism of action: angiogenesis inhibitors, signal transduction inhibitors, and immune checkpoint inhibitors.
Table 6.
Emerging Agents in Clinical Trials for Treatment of Renal Cell Carcinomas
| Class | Target/Mechanism of action | Agent | Phase | Trial/Comment |
|---|---|---|---|---|
| Angiogenesis Inhibitors | VEGFR | Axitinib | III | Phase III trial in sunitinib-refractory RCC; head-to-head comparison with sorafenib |
| AZD2171 | II | Phase II single agent in refractory RCC | ||
| AV-951 | III | Phase III randomized, controlled study of AV-951 versus sorafenib in patients with advanced RCC | ||
| IMC-1121B | II | Phase II in patients treated with prior TKI | ||
| VEGFR, FGFR | Dovitinib | III | Phase III randomized, controlled study of dovitinib versus sorafenib in patients treated with a prior TKI and mTOR inhibitor | |
| VEGF/PLGF | VEGF Trap (AVE0005) | II | Phase II in patients treated with a prior TKI and mTOR inhibitor | |
| ANG 1/2 | AMG836 | II | Combination studies planned in first-line setting | |
| Signal Transduction Inhibitors | AKT/PKB | Perifosine | II | Two single-agent trials are ongoing in patients experiencing TKI failure |
| RX-0201 | II | Planned single-agent study | ||
| Cell cycle | Ispinesib | II | Single-agent study in patients receiving second-line therapy | |
| HDAC | Panobinostat Vorinostat Entinostat |
I/II | Studies of single-agent and combination chemotherapy are planned in first-line setting or in patients experiencing TKI failure | |
| HGF/c-MET | GSK1363089 | II | A phase II study of single agent in papillary RCC | |
| AMG102 | II | Single-agent trial in patients unable to receive or previously treated with VEGF/VEGFR agents | ||
| Immune Checkpoint Inhibitors | PDL-1/PD-1 | MDX-1105/1106 | I | Single-agent studies |
Abbreviations: ANG, angiopoietin; FGFR, fibroblast growth factor receptor; HDAC, histone deacetylase; HGF, hepatocyte growth factor; mTOR, mammalian target of rapamycin; PD, programmed death-1; PDL-1, programmed death-ligand 1; PLGF, placental growth factor; RCC, renal cell carcinoma; TKI, tyrosine kinase inhibitor; VEGF, vascular endothelial growth factor; VEGFR, vascular endothelial growth factor receptor.
Novel Angiogenesis Inhibitors
The currently available angiogenesis inhibitors are moderately active agents that rarely provide durable and unmaintained disease control, and are associated with chronic toxic effects. Therefore, a need exists to develop more effective and less-toxic angiogenesis inhibitors.
Axitinib (AG-013736)
Axitinib (AG-013736) is a selective oral inhibitor of VEGFR-1, -2, and -3. In a phase II study of 62 patients with sorafenib-refractory metastatic clear cell RCC, 22.6% of patients treated with axitinib at a starting dose of 5 mg twice daily achieved a RECIST-defined objective response. Median PFS for this group was 7.4 months and overall survival was 13.6 months.82 In another phase II trial, patients with cytokine-refractory RCC who had undergone no prior VEGF-directed therapy were treated with axitinib, 5 mg, twice daily.101 For this group of patients, the objective response rate was 44%, median response duration was 23.0 months, and median PFS was 15.7 months. Based on these encouraging results, a phase III trial (AXIS) was designed to determine whether axitinib is superior to sorafenib in delaying tumor progression in patients with metastatic RCC after failure of a first-line regimen.143 The study has been completed and results are expected shortly.88
Tivozanib (AV-951)
Tivozanib (AV-951) is a highly potent and selective oral inhibitor of VEGFR-1, -2, and -3. After the successful completion of a phase II trial, a global phase III clinical trial called TIVO-1 was initiated to evaluate the efficacy of tivozanib compared with sorafenib in patients with advanced RCC.144 The primary end point of the trial is PFS, and secondary end points include overall survival, objective response rate, safety, and quality of life.
Dovitinib (TKI258)
Dovitinib (TKI258) is an orally administered inhibitor of VEGFR and fibroblast growth factor receptors (FGFR).145 Experts hypothesize that FGF/FGFR signaling may serve as an escape pathway in tumors that are being treated with inhibitors of other cellular signaling components, such as VEGFR.146 A phase I study showed that dovitinib was well tolerated and had encouraging antitumor activity in heavily pretreated patients.147 Subjects had high baseline VEGF and FGF levels, which may have been a reflection of prior anti-VEGF therapy.147 The toxicity profile of dovitinib was considered acceptable, with nausea, diarrhea, vomiting, and asthenia the most common adverse events. The median PFS was 6.1 months in this small study of 20 patients who underwent prior VEGFR-TKI or mTOR inhibitor treatment.148 Based on these preliminary results, a phase III study is planned to compare the efficacy of dovitinib and sorafenib as third-line treatment for patients with metastatic RCC whose disease has progressed after both VEGF-targeted and mTOR inhibitor therapy.149
Ramucirumab (IMC-1121B)
Ramucirumab (IMC-1121B) is a fully human immunoglobulin G1 monoclonal antibody to VEGFR-2. Unlike bevacizumab, which targets the VEGF protein itself, or sunitinib and sorafenib, which target the VEGFR intracellularly, ramucirumab blocks the receptor extracellularly to prevent binding of VEGF. A multicenter phase II study evaluated ramucirumab in 39 patients with metastatic predominantly clear cell RCC with either progressive disease or intolerance to sorafenib and/or sunitinib.150 Among these, 2 patients experienced confirmed partial responses and 19 had stable disease lasting more than 5 months; 4 patients with stable disease underwent treatment without progression for at least 1 year. The median PFS was an encouraging 6 months in this predominantly sunitinib-refractory population. Adverse events occurring in more than 10% of patients included headache, fatigue, and nausea. The preliminary report shows that ramucirumab has an acceptable toxicity profile and is active in patients with metastatic clear cell RCC refractory to TKIs, supporting further study of this agent in RCC.
AMG 386
Angiopoietins are upregulated at sites of tumor angiogenesis and promote new blood vessel growth through interaction with the receptor Tie-2. AMG 386 is a selective angiopoietin-1/-2–neutralizing peptibody that inhibits angiogenesis through preventing interaction of angiopoietins with Tie-2 receptors. Studies are underway to determine whether combination of AMG 386 with either sorafenib or sunitinib would yield improved PFS compared with single agents, especially because AMG 386 has an entirely difference mechanism of action than sorafenib or sunitinib. Preliminary phase I studies in patients who underwent several prior lines of treatment have suggested that combining AMG 386 and VEGFR inhibition (with sunitinib or sorafenib) did not increase toxicity. The objective response rate was 53% with full-dose sunitinib and AMG 386, and 29% with full-dose sorafenib and AMG 386.151 A phase II study with sorafenib and AMG 386 is currently ongoing.152 Further studies with these combinations are planned.
Aflibercept or VEGF Trap (AVE0005)
Aflibercept, also known as VEGF-TRAP, is a fusion protein specifically designed to bind all isoforms of VEGF. Aflibercept was shown to have significant antitumor and antiangiogenesis effects in a murine model of RCC.153 This novel agent also binds and inhibits placental growth factor, which has been implicated in a potential mechanism of resistance to anti-VEGF therapies. A randomized phase II study is currently determining the effect of 2 different doses of aflibercept in patients with metastatic RCC.154
Other Novel Agents
New agents with putative mechanisms of action that differ from those of the angiogenesis inhibitors are in early clinical development for patients with RCC (see Table 6).
Vorinostat
Histone deacetylase inhibitors have shown anti-angiogenesis and antitumor effects in combination with anti-VEGF agents in preclinical models.155 A phase I/II study showed that the combination of 200 mg of vorinostat given orally twice daily with bevacizumab, 15 mg/kg, every 3 weeks is reasonably well tolerated.156,157 Median PFS was 5.4 months and median overall survival was 16.1 months in patients with metastatic RCC who received this combination after prior therapies.158
MDX-1105/MDX-1106
MDX-1105 and MDX-1106 are fully human monoclonal antibodies against human protein PDL-1 (programmed death-ligand 1) and human cell surface receptor, PD-1 (programmed death-1), respectively, with immunopotentiation activity. Activated PD-1 negatively regulates T-cell activation and effector function through the suppression of P13K/Akt pathway activation. MDX-1106 binds to and blocks the activation of PD-1, and results in the activation of T-cells and cell-mediated immune responses against tumor cells.
Blocking the PD-1 immune checkpoint with MDX-1106 has shown antitumor activity and limited toxicity after intermittent dosing.159 The results of a multicenter phase I trial show that MDX-1106 administered biweekly is well tolerated and has antitumor activity at 1 to 10 mg/kg in patients with advanced refractory tumors, including RCC, melanoma, and non–small cell lung cancer.160 Phase II studies of MDX-1105 and MDX-1106 in patients with metastatic RCC are planned.
Conclusions
A high degree of interest has been shown in new targets and treatments for patients with RCC. The development of novel targeted therapies modulating angiogenesis, signal transduction, and immune response will require a new generation of rationally designed clinical trials. The development of new targeted agents and methods to identify patients most likely to benefit from them will lead to more effective and safer treatment of RCC.
Author Note
A recent exome sequencing study identified truncating mutations of PBRM1 in 92 of 227 (41%) cases of clear cell RCC. PBRM1 codes for BAF180 protein, a subunit of the PBAF SWI/SNF chromatin remodeling complex, and is implicated in replication, transcription, and control of cell proliferation and differentiation. This gene, like VHL and SETD2, is located on chromosome 3p21. (Reference: Varela I, Tarpey P, Raine K, et al. Exome sequencing identifies frequent mutation of the SWI/SNF complex gene PBRM1 in renal carcinoma. Nature 2011;469:539–542.)
Individual Disclosures for the NCCN Task Force: Optimizing Treatment of Advanced Renal Cell Carcinoma With Molecular Targeted Therapy Panel Members
| Panel Member | Clinical Research Support | Advisory Boards, Speakers Bureau, Expert Witness, or Consultant | Patent, Equity, or Royalty | Other | Date Completed |
|---|---|---|---|---|---|
| Gary R. Hudes, MD | None | OSI Pharmaceuticals, Inc.; AVEO Pharmaceuticals, Inc.; and Pfizer Inc. | None | None | 9/8/10 |
| Michael A. Carducci, MD | Abbott Laboratories; AstraZeneca Pharmaceuticals LP; Boehringer Ingelheim GmbH; Bristol-Myers Squibb Company; Cephalon, Inc.; Eli Lilly and Company; GlaxoSmithKline; Eastern Cooperative Oncology Group; GenSpera, Inc.; Kinex Pharmaceuticals, LLC; Pfizer Inc.; Roche Laboratories, Inc.; and sanofi-aventis U.S. | Active Biotech AB; Amgen Inc.; AstraZeneca Pharmaceuticals LP; Astellas Pharma Inc.; Centocor, Inc.; GlaxoSmithKline; Onyx Pharmaceuticals, Inc.; Medivation, Inc.; Pfizer Inc.; Genentech, Inc.; Novartis Pharmaceuticals Corporation; Kinex Pharmaceuticals, LLC; sanofi-aventis U.S.; and Takeda Pharmaceuticals North America, Inc. | None | None | 10/1/10 |
| Toni K. Choueiri, MD | None | GlaxoSmithKline; Novartis Pharmaceuticals Corporation; AVEO Pharmaceuticals, Inc.; and Pfizer Inc. | None | None | 12/2/10 |
| Peg Esper, MSN, MSA, RN, ANP-BC, AOCN | None | Genentech, Inc.; Novartis Pharmaceuticals Corporation; Pfizer Inc. | None | None | 10/19/10 |
| Eric Jonasch, MD | None | GlaxoSmithKline; and AVEO Pharmaceuticals, Inc. | None | None | 10/13/10 |
| Rashmi Kumar, PhD | None | None | None | None | 7/15/10 |
| Kim A. Margolin, MD | Pfizer Inc. | None | None | None | 12/2/10 |
| M. Dror Michaelson, MD, PhD | Abbott Laboratories; Eisai Inc.; Genentech, Inc.; Novartis Pharmaceuticals Corporation; and Pfizer Inc. | Genentech, Inc.; and Pfizer Inc. | None | None | 12/1/10 |
| Robert J. Motzer, MD | GlaxoSmithKline; Novartis Pharmaceuticals Corporation; and Pfizer Inc. | AstraZeneca Pharmaceuticals LP | None | None | 12/7/10 |
| Roberto Pili, MD | None | Pfizer Inc.; Genentech, Inc.; and GlaxoSmithKline | None | None | 12/17/10 |
| Susan Roethke, MSN, CRNP, AOCN, ANP-BC | None | Novartis Pharmaceuticals Corporation and Pfizer Inc. | None | None | 10/20/10 |
| Sandy Srinivas, MD | None | Genentech, Inc; GlaxoSmithKline; Novartis Pharmaceuticals Corporation; and Pfizer Inc. | None | None | 12/1/10 |
Footnotes
Corrected from an earlier version that incorrectly stated “greater than.”
References
- 1.Jemal A, Siegel R, Xu J, Ward E. Cancer statistics, 2010. CA Cancer J Clin. 2010;60:277–300. doi: 10.3322/caac.20073. [DOI] [PubMed] [Google Scholar]
- 2.Motzer RJ, Agarwal N, Beard C, et al. [Accessed January 5, 2011];NCCN Clinical Practice Guidelines in Oncology for Kidney Cancer, version 1.2011. Available at: www.NCCN.org/professionals/physician_gls/PDF/kidney.pdf.
- 3.Brannon AR, Reddy A, Seiler M, et al. Molecular stratification of clear cell renal cell carcinoma by consensus clustering reveals distinct subtypes and survival patterns. Genes Cancer. 2010;1:152–163. doi: 10.1177/1947601909359929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Eble J, Sauter G, Epstein JI, Sesterhenn IA. World Health Organization Classification of Tumours: Pathology and Genetics of Tumours of the Urinary System and Male Genital Organs. Lyon, France: IARC Press; 2004. [Google Scholar]
- 5.Lopez-Beltran A, Scarpelli M, Montironi R, Kirkali Z. 2004 WHO classification of the renal tumors of the adults. Eur Urol. 2006;49:798–805. doi: 10.1016/j.eururo.2005.11.035. [DOI] [PubMed] [Google Scholar]
- 6.Linehan WM, Walther MM, Zbar B. The genetic basis of cancer of the kidney. J Urol. 2003;170:2163–2172. doi: 10.1097/01.ju.0000096060.92397.ed. [DOI] [PubMed] [Google Scholar]
- 7.Pei J, Feder MM, Al-Saleem T, et al. Combined classical cytogenetics and microarray-based genomic copy number analysis reveal frequent 3;5 rearrangements in clear cell renal cell carcinoma. Genes Chromosomes Cancer. 2010;49:610–619. doi: 10.1002/gcc.20771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gunawan B, Huber W, Holtrup M, et al. Prognostic impacts of cytogenetic findings in clear cell renal cell carcinoma: gain of 5q31-qter predicts a distinct clinical phenotype with favorable prognosis. Cancer Res. 2001;61:7731–7738. [PubMed] [Google Scholar]
- 9.Kardas I, Mrozek K, Babinska M, et al. Cytogenetic and molecular findings in 75 clear cell renal cell carcinomas. Oncol Rep. 2005;13:949–956. [PubMed] [Google Scholar]
- 10.Klatte T, Rao PN, de Martino M, et al. Cytogenetic profile predicts prognosis of patients with clear cell renal cell carcinoma. J Clin Oncol. 2009;27:746–753. doi: 10.1200/JCO.2007.15.8345. [DOI] [PubMed] [Google Scholar]
- 11.Beroukhim R, Brunet J, Napoli A, et al. Patterns of gene expression and copy number alterations in von-Hippel Lindau disease-associated and sporadic clear cell carcinoma of the kidney. Cancer Res. 2009;69:4674–4681. doi: 10.1158/0008-5472.CAN-09-0146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Young AP, Schlisio S, Minamishima YA, et al. VHL loss actuates a HIF-independent senescence programme mediated by Rb and p400. Nat Cell Biol. 2008;10:361–369. doi: 10.1038/ncb1699. [DOI] [PubMed] [Google Scholar]
- 13.Dalgliesh GL, Furge K, Greenman C, et al. Systematic sequencing of renal carcinoma reveals inactivation of histone modifying genes. Nature. 2010;463:360–363. doi: 10.1038/nature08672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Brunelli M, Eccher A, Gobbo S, et al. Loss of chromosome 9p is an independent prognostic factor in patients with clear cell renal cell carcinoma. Mod Pathol. 2008;21:1–6. doi: 10.1038/modpathol.3800967. [DOI] [PubMed] [Google Scholar]
- 15.Moch H, Presti JC, Jr, Sauter G, et al. Genetic aberrations detected by comparative genomic hybridization are associated with clinical outcome in renal cell carcinoma. Cancer Res. 1996;56:27–30. [PubMed] [Google Scholar]
- 16.Yoshimoto T, Matsuura K, Karnan S, et al. High-resolution analysis of DNA copy number alterations and gene expression in renal clear cell carcinoma. J Pathol. 2007;213:392–401. doi: 10.1002/path.2239. [DOI] [PubMed] [Google Scholar]
- 17.Monzon FA, Hagenkord JM, Lyons-Weiler MA, et al. Whole genome SNP arrays as a potential diagnostic tool for the detection of characteristic chromosomal aberrations in renal epithelial tumors. Mod Pathol. 2008;21:599–608. doi: 10.1038/modpathol.2008.20. [DOI] [PubMed] [Google Scholar]
- 18.Gordan JD, Lal P, Dondeti VR, et al. HIF-alpha effects on c-Myc distinguish two subtypes of sporadic VHL-deficient clear cell renal carcinoma. Cancer Cell. 2008;14:435–446. doi: 10.1016/j.ccr.2008.10.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sarbassov DD, Guertin DA, Ali SM, Sabatini DM. Phosphorylation and regulation of Akt/PKB by the rictor-mTOR complex. Science. 2005;307:1098–1101. doi: 10.1126/science.1106148. [DOI] [PubMed] [Google Scholar]
- 20.Toschi A, Lee E, Gadir N, et al. Differential dependence of hypoxia-inducible factors 1 alpha and 2 alpha on mTORC1 and mTORC2. J Biol Chem. 2008;283:34495–34499. doi: 10.1074/jbc.C800170200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Fearon ER, Vogelstein B. A genetic model for colorectal tumorigenesis. Cell. 1990;61:759–767. doi: 10.1016/0092-8674(90)90186-i. [DOI] [PubMed] [Google Scholar]
- 22.Escudier B, Pluzanska A, Koralewski P, et al. Bevacizumab plus interferon alfa-2a for treatment of metastatic renal cell carcinoma: a randomised, double-blind phase III trial. Lancet. 2007;370:2103–2111. doi: 10.1016/S0140-6736(07)61904-7. [DOI] [PubMed] [Google Scholar]
- 23.Rini BI, Halabi S, Rosenberg JE, et al. Bevacizumab plus interferon alfa compared with interferon alfa monotherapy in patients with metastatic renal cell carcinoma: CALGB 90206. J Clin Oncol. 2008;26:5422–5428. doi: 10.1200/JCO.2008.16.9847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Yang JC, Haworth L, Sherry RM, et al. A randomized trial of bevacizumab, an anti-vascular endothelial growth factor antibody, for metastatic renal cancer. N Engl J Med. 2003;349:427–434. doi: 10.1056/NEJMoa021491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Gordon MS, Margolin K, Talpaz M, et al. Phase I safety and pharmacokinetic study of recombinant human anti-vascular endothelial growth factor in patients with advanced cancer. J Clin Oncol. 2001;19:843–850. doi: 10.1200/JCO.2001.19.3.843. [DOI] [PubMed] [Google Scholar]
- 26.Kim KJ, Li B, Winer J, et al. Inhibition of vascular endothelial growth factor-induced angiogenesis suppresses tumour growth in vivo. Nature. 1993;362:841–844. doi: 10.1038/362841a0. [DOI] [PubMed] [Google Scholar]
- 27.Fabian MA, Biggs WH, III, Treiber DK, et al. A small molecule-kinase interaction map for clinical kinase inhibitors. Nat Biotechnol. 2005;23:329–336. doi: 10.1038/nbt1068. [DOI] [PubMed] [Google Scholar]
- 28.Eskens F, de Jonge M, Esteves B, et al. Updated results from a phase I study of AV-951 (KRN951), a potent and selective VEGFR-1, -2 and -3 tyrosine kinase inhibitor, in patients with advanced solid tumors [abstract]. Presented at 2008 AACR Annual Meeting; April 12–16, 2008; San Diego, California. p. Abstract LB–201. [Google Scholar]
- 29.Chow LQ, Eckhardt SG. Sunitinib: from rational design to clinical efficacy. J Clin Oncol. 2007;25:884–896. doi: 10.1200/JCO.2006.06.3602. [DOI] [PubMed] [Google Scholar]
- 30.Cohen HT, McGovern FJ. Renal-cell carcinoma. N Engl J Med. 2005;353:2477–2490. doi: 10.1056/NEJMra043172. [DOI] [PubMed] [Google Scholar]
- 31.Motzer RJ, Russo P. Systemic therapy for renal cell carcinoma. J Urol. 2000;163:408–417. [PubMed] [Google Scholar]
- 32.Rini BI, Bukowski RM. Targeted therapy for metastatic renal cell carcinoma: a home run or a work in progress? Oncology. 2008;22:388–396. [PubMed] [Google Scholar]
- 33.Escudier B, Eisen T, Stadler WM, et al. Sorafenib in advanced clear-cell renal-cell carcinoma. N Engl J Med. 2007;356:125–134. doi: 10.1056/NEJMoa060655. [DOI] [PubMed] [Google Scholar]
- 34.Dhanasekaran DN, Johnson GL. MAPKs: function, regulation, role in cancer and therapeutic targeting. Oncogene. 2007;26:3097–3099. doi: 10.1038/sj.onc.1210395. [DOI] [PubMed] [Google Scholar]
- 35.Motzer RJ, Michaelson MD, Redman BG, et al. Activity of SU11248, a multitargeted inhibitor of vascular endothelial growth factor receptor and platelet-derived growth factor receptor, in patients with metastatic renal cell carcinoma. J Clin Oncol. 2006;24:16–24. doi: 10.1200/JCO.2005.02.2574. [DOI] [PubMed] [Google Scholar]
- 36.Motzer RJ, Hutson TE, Tomczak P, et al. Sunitinib versus interferon alfa in metastatic renal-cell carcinoma. N Engl J Med. 2007;356:115–124. doi: 10.1056/NEJMoa065044. [DOI] [PubMed] [Google Scholar]
- 37.Schutz FA, Choueiri TK, Sternberg CN. Pazopanib: clinical development of a potent anti-angiogenic drug. Crit Rev Oncol Hematol. 2010 doi: 10.1016/j.critrevonc.2010.02.012. in press. [DOI] [PubMed] [Google Scholar]
- 38.Nicklin P, Bergman P, Zhang B, et al. Bidirectional transport of amino acids regulates mTOR and autophagy. Cell. 2009;136:521–534. doi: 10.1016/j.cell.2008.11.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Seeliger H, Guba M, Kleespies A, et al. Role of mTOR in solid tumor systems: a therapeutical target against primary tumor growth, metastases, and angiogenesis. Cancer Metastasis Rev. 2007;26:611–621. doi: 10.1007/s10555-007-9077-8. [DOI] [PubMed] [Google Scholar]
- 40.Wullschleger S, Loewith R, Hall MN. TOR signaling in growth and metabolism. Cell. 2006;124:471–484. doi: 10.1016/j.cell.2006.01.016. [DOI] [PubMed] [Google Scholar]
- 41.Guertin DA, Sabatini DM. Defining the role of mTOR in cancer. Cancer Cell. 2007;12:9–22. doi: 10.1016/j.ccr.2007.05.008. [DOI] [PubMed] [Google Scholar]
- 42.Robb VA, Karbowniczek M, Klein-Szanto AJ, Henske EP. Activation of the mTOR signaling pathway in renal clear cell carcinoma. J Urol. 2007;177:346–352. doi: 10.1016/j.juro.2006.08.076. [DOI] [PubMed] [Google Scholar]
- 43.Youssif TA, Fahmy MA, Koumakpayi IH, et al. The mammalian target of rapamycin pathway is widely activated without PTEN deletion in renal cell carcinoma metastases. Cancer. 2011;15:290–300. doi: 10.1002/cncr.25402. [DOI] [PubMed] [Google Scholar]
- 44.Hudes G, Carducci M, Tomczak P, et al. Temsirolimus, interferon alfa, or both for advanced renal-cell carcinoma. N Engl J Med. 2007;356:2271–2281. doi: 10.1056/NEJMoa066838. [DOI] [PubMed] [Google Scholar]
- 45.Negrier S, Escudier B, Lasset C, et al. Recombinant human interleukin-2, recombinant human interferon alfa-2a, or both in metastatic renal-cell carcinoma. Groupe Francais d’Immunotherapie. N Engl J Med. 1998;338:1272–1278. doi: 10.1056/NEJM199804303381805. [DOI] [PubMed] [Google Scholar]
- 46.Fyfe G, Fisher RI, Rosenberg SA, et al. Results of treatment of 255 patients with metastatic renal cell carcinoma who received high-dose recombinant interleukin-2 therapy. J Clin Oncol. 1995;13:688–696. doi: 10.1200/JCO.1995.13.3.688. [DOI] [PubMed] [Google Scholar]
- 47.Dutcher JP, Fisher RI, Weiss G, et al. Outpatient subcutaneous interleukin-2 and interferon-alpha for metastatic renal cell cancer: five-year follow-up of the Cytokine Working Group Study. Cancer J Sci Am. 1997;3:157–162. [PubMed] [Google Scholar]
- 48.Rosenberg SA, Mule JJ, Spiess PJ, et al. Regression of established pulmonary metastases and subcutaneous tumor mediated by the systemic administration of high-dose recombinant interleukin 2. J Exp Med. 1985;161:1169–1188. doi: 10.1084/jem.161.5.1169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.van Boxel-Dezaire AH, Rani MR, Stark GR. Complex modulation of cell type-specific signaling in response to type I interferons. Immunity. 2006;25:361–372. doi: 10.1016/j.immuni.2006.08.014. [DOI] [PubMed] [Google Scholar]
- 50.Yagoda A, Abi-Rached B, Petrylak D. Chemotherapy for advanced renal-cell carcinoma: 1983–1993. Semin Oncol. 1995;22:42–60. [PubMed] [Google Scholar]
- 51.Yagoda A, Petrylak D, Thompson S. Cytotoxic chemotherapy for advanced renal cell carcinoma. Urol Clin North Am. 1993;20:303–321. [PubMed] [Google Scholar]
- 52.Mickisch G, Bier H, Bergler W, et al. P-170 glycoprotein, glutathione and associated enzymes in relation to chemoresistance of primary human renal cell carcinomas. Urol Int. 1990;45:170–176. doi: 10.1159/000281701. [DOI] [PubMed] [Google Scholar]
- 53.Dutcher JP, Nanus D. Long-term survival of patients with sarcomatoid renal cell cancer treated with chemotherapy. Med Oncol. 2010 doi: 10.1007/s12032-010-9649-2. in press. [DOI] [PubMed] [Google Scholar]
- 54.Haas N, Manola J, Pins M, et al. ECOG 8802: phase II trial of doxorubicin (Dox) and gemcitabine (Gem) in metastatic renal cell carcinoma (RCC) with sarcomatoid features [abstract]. Presented at the 2009 ASCO Genitourinary Cancers Symposium; February 26–28, 2009; Orlando, Florida. p. Abstract 285. [Google Scholar]
- 55.McDermott DF, Regan MM, Clark JI, et al. Randomized phase III trial of high-dose interleukin-2 versus subcutaneous interleukin-2 and interferon in patients with metastatic renal cell carcinoma. J Clin Oncol. 2005;23:133–141. doi: 10.1200/JCO.2005.03.206. [DOI] [PubMed] [Google Scholar]
- 56.Motzer RJ, Hutson TE, Tomczak P, et al. Overall survival and updated results for sunitinib compared with interferon alfa in patients with metastatic renal cell carcinoma. J Clin Oncol. 2009;27:3584–3590. doi: 10.1200/JCO.2008.20.1293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.McDermott DF. Update on the application of interleukin-2 in the treatment of renal cell carcinoma. Clin Cancer Res. 2007;13:716s–720s. doi: 10.1158/1078-0432.CCR-06-1872. [DOI] [PubMed] [Google Scholar]
- 58.Yang JC, Sherry RM, Steinberg SM, et al. Randomized study of high-dose and low-dose interleukin-2 in patients with metastatic renal cancer. J Clin Oncol. 2003;21:3127–3132. doi: 10.1200/JCO.2003.02.122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Rosenberg SA, Yang JC, Topalian SL, et al. Treatment of 283 consecutive patients with metastatic melanoma or renal cell cancer using high-dose bolus interleukin 2. JAMA. 1994;271:907–913. [PubMed] [Google Scholar]
- 60.Negrier S, Perol D, Ravaud A, et al. Medroxyprogesterone, interferon alfa-2a, interleukin 2, or combination of both cytokines in patients with metastatic renal carcinoma of intermediate prognosis: results of a randomized controlled trial. Cancer. 2007;110:2468–2477. doi: 10.1002/cncr.23056. [DOI] [PubMed] [Google Scholar]
- 61.Cho DC, Puzanov I, Regan MM, et al. Retrospective analysis of the safety and efficacy of interleukin-2 after prior VEGF-targeted therapy in patients with advanced renal cell carcinoma. J Immunother. 2009;32:181–185. doi: 10.1097/CJI.0b013e3181952b1d. [DOI] [PubMed] [Google Scholar]
- 62.Bui MH, Seligson D, Han KR, et al. Carbonic anhydrase IX is an independent predictor of survival in advanced renal clear cell carcinoma: implications for prognosis and therapy. Clin Cancer Res. 2003;9:802–811. [PubMed] [Google Scholar]
- 63.Leibovich BC, Blute ML, Cheville JC, et al. Prediction of progression after radical nephrectomy for patients with clear cell renal cell carcinoma: a stratification tool for prospective clinical trials. Cancer. 2003;97:1663–1671. doi: 10.1002/cncr.11234. [DOI] [PubMed] [Google Scholar]
- 64.Leibovich BC, Han KR, Bui MH, et al. Scoring algorithm to predict survival after nephrectomy and immunotherapy in patients with metastatic renal cell carcinoma: a stratification tool for prospective clinical trials. Cancer. 2003;98:2566–2575. doi: 10.1002/cncr.11851. [DOI] [PubMed] [Google Scholar]
- 65.Upton M, Parker RA, Youmans A, et al. Histologic predictors of renal cell carcinoma response to interleukin-2-based therapy. J Immunother. 2005;28:488–495. doi: 10.1097/01.cji.0000170357.14962.9b. [DOI] [PubMed] [Google Scholar]
- 66.Atkins M, Regan M, McDermott D, et al. Carbonic anhydrase IX expression predicts outcome of interleukin 2 therapy for renal cancer. Clin Cancer Res. 2005;11:3714–3721. doi: 10.1158/1078-0432.CCR-04-2019. [DOI] [PubMed] [Google Scholar]
- 67.National Institutes of Health. The high-dose aldesleukin (IL-2) “SELECT” trial for patients with metastatic renal cell carcinoma (SELECT) [Accessed January 7, 2011];ClinicalTrials.gov. Available at: http://clinicaltrials.gov/ct2/show/NCT00554515.
- 68.McDermott D, Ghebremichael M, Signoretti S, et al. The high-dose aldesleukin (HD IL-2) SELECT trial in patients with meta-static renal cell carcinoma (mRCC): preliminary assessment of clinical benefit [abstract]. Presented at the 2010 ASCO Genitourinary Cancers Symposium; March 5–7, 2010; San Francisco, California. p. Abstract 321. [Google Scholar]
- 69.Motzer RJ, Bacik J, Murphy BA, et al. Interferon-alfa as a comparative treatment for clinical trials of new therapies against advanced renal cell carcinoma. J Clin Oncol. 2002;20:289–296. doi: 10.1200/JCO.2002.20.1.289. [DOI] [PubMed] [Google Scholar]
- 70.Dutcher JP, de Souza P, McDermott D, et al. Effect of temsirolimus versus interferon-alpha on outcome of patients with advanced renal cell carcinoma of different histologies. Med Oncol. 2009;26:202–209. doi: 10.1007/s12032-009-9177-0. [DOI] [PubMed] [Google Scholar]
- 71.Motzer RJ, Rini BI, Bukowski RM, et al. Sunitinib in patients with metastatic renal cell carcinoma. JAMA. 2006;295:2516–2524. doi: 10.1001/jama.295.21.2516. [DOI] [PubMed] [Google Scholar]
- 72.Sternberg CN, Davis ID, Mardiak J, et al. Pazopanib in locally advanced or metastatic renal cell carcinoma: results of a randomized phase III trial. J Clin Oncol. 2010;28:1061–1068. doi: 10.1200/JCO.2009.23.9764. [DOI] [PubMed] [Google Scholar]
- 73.Dudek AZ, Zolnierek J, Dham A, et al. Sequential therapy with sorafenib and sunitinib in renal cell carcinoma. Cancer. 2009;115:61–67. doi: 10.1002/cncr.24009. [DOI] [PubMed] [Google Scholar]
- 74.Heuer R, Eichelberg C, Zacharias M, Heinzer H. Sequential use of the tyrosine kinase inhibitors sorafenib and sunitinib [abstract] Eur Urol. 2009;8(Suppl 4):183. doi: 10.1016/j.eururo.2008.07.051. Abstract 251. [DOI] [PubMed] [Google Scholar]
- 75.Porta C, Procopio G, Sabbatini R, et al. Retrospective analysis of the sequential use of sorafenib and sunitinib in patients with advanced renal cell carcinoma (RCC) [abstract] Eur Urol. 2009;8(Suppl 4):183. Abstract 252. [Google Scholar]
- 76.Sablin MP, Bouaita L, Balleyguier C, et al. Sequential use of sorafenib and sunitinib in renal cancer: retrospective analysis in 90 patients [abstract] J Clin Oncol. 2007;25(Suppl 18S):Abstract 5038. [Google Scholar]
- 77.Shepard DR, Rini BI, Garcia JA, et al. A multicenter prospective trial of sorafenib in patients (pts) with metastatic clear cell renal cell carcinoma (mccRCC) refractory to prior sunitinib or bevacizumab [abstract] J Clin Oncol. 2008;26(Suppl 18S):Abstract 5123. [Google Scholar]
- 78.Tamaskar I, Pili R. Update on novel agents in renal cell carcinoma. Expert Rev Anticancer Ther. 2009;9:1817–1827. doi: 10.1586/era.09.157. [DOI] [PubMed] [Google Scholar]
- 79.Zimmermann K, Schmittel A, Steiner U, et al. Sunitinib treatment for patients with advanced clear-cell renal-cell carcinoma after progression on sorafenib. Oncology. 2009;76:350–354. doi: 10.1159/000209961. [DOI] [PubMed] [Google Scholar]
- 80.Eichelberg C, Heuer R, Chun FK, et al. Sequential use of the tyrosine kinase inhibitors sorafenib and sunitinib in metastatic renal cell carcinoma: a retrospective outcome analysis. Eur Urol. 2008;54:1373–1378. doi: 10.1016/j.eururo.2008.07.051. [DOI] [PubMed] [Google Scholar]
- 81.Sablin MP, Negrier S, Ravaud A, et al. Sequential sorafenib and sunitinib for renal cell carcinoma. J Urol. 2009;182:29–34. doi: 10.1016/j.juro.2009.02.119. [DOI] [PubMed] [Google Scholar]
- 82.Rini BI, Wilding G, Hudes G, et al. Phase II study of axitinib in sorafenib-refractory metastatic renal cell carcinoma. J Clin Oncol. 2009;27:4462–4468. doi: 10.1200/JCO.2008.21.7034. [DOI] [PubMed] [Google Scholar]
- 83.Motzer RJ, Escudier B, Oudard S, et al. Efficacy of everolimus in advanced renal cell carcinoma: a double-blind, randomised, placebo-controlled phase III trial. Lancet. 2008;372:449–456. doi: 10.1016/S0140-6736(08)61039-9. [DOI] [PubMed] [Google Scholar]
- 84.Gerullis H, Bergmann L, Maute L, et al. Feasibility of sequential use of sunitinib and temsirolimus in advanced renal cell carcinoma. Med Oncol. 2010;27:373–378. doi: 10.1007/s12032-009-9220-1. [DOI] [PubMed] [Google Scholar]
- 85.National Institutes of Health. Pazopanib versus sunitinib in the treatment of locally advanced and/or metastatic renal cell carcinoma (COMPARZ) [Accessed January 7, 2011];ClinicalTrials.gov. Available at: www.clinicaltrials.gov/ct2/show/NCT00720941.
- 86.National Institutes of Health. Efficacy and safety comparison of RAD001 versus sunitinib in the first-line and second-line treatment of patients with metastatic renal cell carcinoma (RECORD-3) [Accessed January 7, 2011];ClinicalTrials.gov. Available at: www.clinicaltrials.gov/ct2/show/NCT00903175.
- 87.National Institutes of Health. Temsirolimus versus sorafenib as second-line therapy in patients with advanced RCC who have failed first-line sunitinib. [Accessed January 7, 2011];ClinicalTrials.gov. Available at: www.clinical-trials.gov/ct2/show/NCT00474786.
- 88.New York, NY: PR Newswire; Nov 19, 2010. [Accessed January 7, 2011]. Pfizer announces positive phase 3 trial results for axitinib in patients with previously-treated metastatic renal cell carcinoma (mRCC) [news release] Available at: www.prnewswire.com/news-releases/pfizer-announces-positive-phase-3-trial-results-for-axitinib-in-patients-with-previously-treated-metastatic-renal-cell-carcinoma-mrcc-109184544.html. [Google Scholar]
- 89.National Institutes of Health. Sequential two-agent assessment in renal cell carcinoma therapy. [Accessed January 7, 2011];ClinicalTrials.gov. Available at: www.clinicaltrial.gov/ct2/show/NCT01217931?term=START+trial&rank=2.
- 90.National Institutes of Health. Bevacizumab, sorafenib, and temsirolimus in treating patients with metastatic kidney cancer. [Accessed January 7, 2011];ClinicalTrials.gov. Available at: www.clinicaltrial.gov/ct2/show/NCT00378703.
- 91.National Institutes of Health. Study comparing bevacizumab + temsirolimus vs. bevacizumab + interferon-alfa in advanced renal cell carcinoma subjects (INTORACT) [Accessed January 7, 2011];ClinicalTrials.gov. Available at: www.clinicaltrials.gov/ct2/show/NCT00631371.
- 92.National Institutes of Health. Safety and efficacy of bevacizumab plus RAD001 versus interferon alfa-2a and bevacizumab in adult patients with kidney cancer (L2201) [Accessed January 7, 2011];ClinicalTrials.gov. Available at: www.clinicaltrials.gov/ct2/show/NCT00719264.
- 93.Flanigan RC, Mickisch G, Sylvester R, et al. Cytoreductive nephrectomy in patients with metastatic renal cancer: a combined analysis. J Urol. 2004;171:1071–1076. doi: 10.1097/01.ju.0000110610.61545.ae. [DOI] [PubMed] [Google Scholar]
- 94.Flanigan RC, Salmon SE, Blumenstein BA, et al. Nephrectomy followed by interferon alfa-2b compared with interferon alfa-2b alone for metastatic renal-cell cancer. N Engl J Med. 2001;345:1655–1659. doi: 10.1056/NEJMoa003013. [DOI] [PubMed] [Google Scholar]
- 95.Mickisch GH, Garin A, van Poppel H, et al. Radical nephrectomy plus interferon-alfa-based immunotherapy compared with interferon alfa alone in metastatic renal-cell carcinoma: a randomised trial. Lancet. 2001;358:966–970. doi: 10.1016/s0140-6736(01)06103-7. [DOI] [PubMed] [Google Scholar]
- 96.National Institutes of Health. Clinical trial to assess the importance of nephrectomy (CARMENA) [Accessed January 7, 2011];ClinicalTrials.gov. Available at: www.clinicaltrials.gov/ct2/show/NCT00930033.
- 97.National Institutes of Health. Immediate surgery or surgery after sunitinib malate in treating patients with metastatic kidney cancer. [Accessed January 7, 2011];ClinicalTrials.gov. Available at: www.clinicaltrials.gov/ct2/show/NCT01099423.
- 98.National Institutes of Health. Sunitinib or sorafenib in treating patients with kidney cancer that was removed by surgery. [Accessed January 7, 2011];ClinicalTrials.gov. Available at: www.clinicaltrials.gov/ct2/show/NCT00326898.
- 99.National Institutes of Health. A clinical trial comparing efficacy and safety of sunitinib versus placebo for thetreatment of patients at high risk of recurrent renal cell cancer (S-TRAC) [Accessed January 7, 2011];ClinicalTrials.gov. Available at: www.clinicaltrials.gov/ct2/show/NCT00375674.
- 100.National Institutes of Health. Sorafenib in treating patients at risk of relapse after undergoing surgery to remove kidney cancer. [Accessed January 7, 2011];ClinicalTrials.gov. Available at: www.clinicaltrials.gov/ct2/show/NCT00492258.
- 101.Rixe O, Bukowski RM, Michaelson MD, et al. Axitinib treatment in patients with cytokine-refractory metastatic renal-cell cancer: a phase II study. Lancet Oncol. 2007;8:975–984. doi: 10.1016/S1470-2045(07)70285-1. [DOI] [PubMed] [Google Scholar]
- 102.Sane DC, Anton L, Brosnihan KB. Angiogenic growth factors and hypertension. Angiogenesis. 2004;7:193–201. doi: 10.1007/s10456-004-2699-3. [DOI] [PubMed] [Google Scholar]
- 103.Tang JR, Markham NE, Lin YJ, et al. Inhaled nitric oxide attenuates pulmonary hypertension and improves lung growth in infant rats after neonatal treatment with a VEGF receptor inhibitor. Am J Physiol Lung Cell Mol Physiol. 2004;287:L344–351. doi: 10.1152/ajplung.00291.2003. [DOI] [PubMed] [Google Scholar]
- 104.Izzedine H, Rixe O, Billemont B, et al. Angiogenesis inhibitor therapies: focus on kidney toxicity and hypertension. Am J Kidney Dis. 2007;50:203–218. doi: 10.1053/j.ajkd.2007.04.025. [DOI] [PubMed] [Google Scholar]
- 105.Zhu X, Wu S, Dahut WL, Parikh CR. Risks of proteinuria and hypertension with bevacizumab, an antibody against vascular endothelial growth factor: systematic review and meta-analysis. Am J Kidney Dis. 2007;49:186–193. doi: 10.1053/j.ajkd.2006.11.039. [DOI] [PubMed] [Google Scholar]
- 106.Gordon MS, Cunningham D. Managing patients treated with bevacizumab combination therapy. Oncology. 2005;69(Suppl 3):25–33. doi: 10.1159/000088481. [DOI] [PubMed] [Google Scholar]
- 107.Kim CY, Chu D, Baer L, Wu S. High-grade proteinuria associated with bevacizumab in patients with renal cell cancer and non-renal cell cancer [abstract] J Clin Oncol. 2009;27(Suppl 18S):Abstract e16089. [Google Scholar]
- 108.Billemont B, Thibault F, Ropert S, et al. Association between acute severe renal toxicity (ASRT) and survival in patients (pts) with metastatic renal cell carcinoma (mRCC) receiving sunitinib [abstract]. Presented at the 2009 ASCO Genitourinary Cancers Symposium; February 26–28, 2009; Orlando, Florida. p. Asbtract 307. [Google Scholar]
- 109.Patel PH, Senico PL, Curiel RE, Motzer RJ. Phase I study combining treatment with temsirolimus and sunitinib malate in patients with advanced renal cell carcinoma. Clin Genitourin Cancer. 2009;7:24–27. doi: 10.3816/CGC.2009.n.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Sleijfer S, Ray-Coquard I, Papai Z, et al. Pazopanib, a multikinase angiogenesis inhibitor, in patients with relapsed or refractory advanced soft tissue sarcoma: a phase II study from the European Organisation for Research and Treatment of Cancer-soft tissue and bone sarcoma group (EORTC study 62043) J Clin Oncol. 2009;27:3126–3132. doi: 10.1200/JCO.2008.21.3223. [DOI] [PubMed] [Google Scholar]
- 111.Kamba T, McDonald DM. Mechanisms of adverse effects of anti-VEGF therapy for cancer. Br J Cancer. 2007;96:1788–1795. doi: 10.1038/sj.bjc.6603813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Ranpura V, Hapani S, Chuang J, Wu S. Risk of cardiac ischemia and arterial thromboembolic events with the angiogenesis inhibitor bevacizumab in cancer patients: a meta-analysis of randomized controlled trials. Acta Oncol. 2010;49:287–297. doi: 10.3109/02841860903524396. [DOI] [PubMed] [Google Scholar]
- 113.Choueiri TK, Schutz FA, Je Y, et al. Risk of arterial thromboembolic events with sunitinib and sorafenib: a systematic review and meta-analysis of clinical trials. J Clin Oncol. 2010;28:2280–2285. doi: 10.1200/JCO.2009.27.2757. [DOI] [PubMed] [Google Scholar]
- 114.Sutent [package insert] New York, NY: Pfizer, Inc; 2010. [Google Scholar]
- 115.Schmidinger M, Zielinski CC, Vogl UM, et al. Cardiac toxicity of sunitinib and sorafenib in patients with metastatic renal cell carcinoma. J Clin Oncol. 2008;26:5204–5212. doi: 10.1200/JCO.2007.15.6331. [DOI] [PubMed] [Google Scholar]
- 116.Telli ML, Witteles RM, Fisher GA, Srinivas S. Cardiotoxicity associated with the cancer therapeutic agent sunitinib malate. Ann Oncol. 2008;19:1613–1618. doi: 10.1093/annonc/mdn168. [DOI] [PubMed] [Google Scholar]
- 117.Joensuu H. Cardiac toxicity of sunitinib. Lancet. 2007;370:1978–1980. doi: 10.1016/S0140-6736(07)61840-6. [DOI] [PubMed] [Google Scholar]
- 118.Lacouture ME, Wu S, Robert C, et al. Evolving strategies for the management of hand-foot skin reaction associated with the multitargeted kinase inhibitors sorafenib and sunitinib. Oncologist. 2008;13:1001–1011. doi: 10.1634/theoncologist.2008-0131. [DOI] [PubMed] [Google Scholar]
- 119.Chu D, Lacouture ME, Fillos T, Wu S. Risk of hand-foot skin reaction with sorafenib: a systematic review and meta-analysis. Acta Oncol. 2008;47:176–186. doi: 10.1080/02841860701765675. [DOI] [PubMed] [Google Scholar]
- 120.Chu D, Lacouture ME, Weiner E, Wu S. Risk of hand-foot skin reaction with the multitargeted kinase inhibitor sunitinib in patients with renal cell and non-renal cell carcinoma: a meta-analysis. Clin Genitourin Cancer. 2009;7:11–19. doi: 10.3816/CGC.2009.n.002. [DOI] [PubMed] [Google Scholar]
- 121.Basson MD. Gut mucosal healing: is the science relevant? Am J Pathol. 2002;161:1101–1105. doi: 10.1016/S0002-9440(10)64385-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Baatar D, Jones MK, Tsugawa K, et al. Esophageal ulceration triggers expression of hypoxia-inducible factor-1 alpha and activates vascular endothelial growth factor gene: implications for angiogenesis and ulcer healing. Am J Pathol. 2002;161:1449–1457. doi: 10.1016/s0002-9440(10)64420-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Jones MK, Kawanaka H, Baatar D, et al. Gene therapy for gastric ulcers with single local injection of naked DNA encoding VEGF and angiopoietin-1. Gastroenterology. 2001;121:1040–1047. doi: 10.1053/gast.2001.29308. [DOI] [PubMed] [Google Scholar]
- 124.Xu CF, Reck BH, Xue Z, et al. Pazopanib-induced hyperbilirubinemia is associated with Gilbert’s syndrome UGT1A1 polymorphism. Br J Cancer. 2010;102:1371–1377. doi: 10.1038/sj.bjc.6605653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Rini BI, Tamaskar I, Shaheen P, et al. Hypothyroidism in patients with metastatic renal cell carcinoma treated with sunitinib. J Natl Cancer Inst. 2007;99:81–83. doi: 10.1093/jnci/djk008. [DOI] [PubMed] [Google Scholar]
- 126.Hapani S, Chu D, Wu S. Risk of gastrointestinal perforation in patients with cancer treated with bevacizumab: a meta-analysis. Lancet Oncol. 2009;10:559–568. doi: 10.1016/S1470-2045(09)70112-3. [DOI] [PubMed] [Google Scholar]
- 127.Maroto JP, Hudes G, Dutcher JP, et al. Drug-related pneumonitis in patients with advanced renal cell carcinoma treated with temsirolimus. J Clin Oncol. 2011 doi: 10.1200/JCO.2010.29.2235. in press. [DOI] [PubMed] [Google Scholar]
- 128.Esper P, Gale D, Muehlbauer P. What kind of rash is it: deciphering the dermatologic toxicities of biologic and targeted therapies. Clin J Oncol Nurs. 2007;11:659–666. doi: 10.1188/07.CJON.659-666. [DOI] [PubMed] [Google Scholar]
- 129.Balagula Y, Lacouture ME, Cotliar JA. Dermatologic toxicities of targeted anticancer therapies. J Support Oncol. 2010;8:149–161. [PubMed] [Google Scholar]
- 130.Common Terminology Criteria for Adverse Events (CTCAE), version 4.0. National Cancer Institute; [Accessed January 7, 2011]. Available at: http://evs.nci.nih.gov/ftp1/CTCAE/CTCAE_4.03_2010-06-14_QuickReference_8.5x11.pdf. [Google Scholar]
- 131.Maitland ML, Bakris GL, Black HR, et al. Initial assessment, surveillance, and management of blood pressure in patients receiving vascular endothelial growth factor signaling pathway inhibitors. J Natl Cancer Inst. 2010;102:596–604. doi: 10.1093/jnci/djq091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.White DA, Camus P, Endo M, et al. Noninfectious pneumonitis after everolimus therapy for advanced renal cell carcinoma. Am J Respir Crit Care Med. 2010;182:396–403. doi: 10.1164/rccm.200911-1720OC. [DOI] [PubMed] [Google Scholar]
- 133.Hutson TE, Figlin RA, Kuhn JG, Motzer RJ. Targeted therapies for metastatic renal cell carcinoma: an overview of toxicity and dosing strategies. Oncologist. 2008;13:1084–1096. doi: 10.1634/theoncologist.2008-0120. [DOI] [PubMed] [Google Scholar]
- 134.Ravaud A. How to optimise treatment compliance in meta-static renal cell carcinoma with targeted agents. Ann Oncol. 2009;20(Suppl 1):i7–12. doi: 10.1093/annonc/mdp073. [DOI] [PubMed] [Google Scholar]
- 135.Billemont B, Medioni J, Taillade L, et al. Blood glucose levels in patients with metastatic renal cell carcinoma treated with sunitinib. Br J Cancer. 2008;99:1380–1382. doi: 10.1038/sj.bjc.6604709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Nexavar [package insert] Montville, NJ: Bayer HealthCare Pharmaceuticals Inc; 2010. [Google Scholar]
- 137.Avastin [package insert] South San Francisco, CA: Genentech, Inc; 2009. [Google Scholar]
- 138.Torisel [package insert] Charlotte, NC: Wyeth Pharmaceuticals Inc; 2010. [Google Scholar]
- 139.Votrient [package insert] Research Triangle Park, NC: GlaxoSmithKline; 2009. [Google Scholar]
- 140.Afinitor [package insert] East Hanover, NJ: Novartis Pharmaceuticals Corporation; 2010. [Google Scholar]
- 141.Shepard DR, Garcia JA. Toxicity associated with the long-term use of targeted therapies in patients with advanced renal cell carcinoma. Expert Rev Anticancer Ther. 2009;9:795–805. doi: 10.1586/era.09.29. [DOI] [PubMed] [Google Scholar]
- 142.Berger AM, Abernethy AP, Atkinson A, et al. [Accessed January 7, 2011];NCCN Clinical Practice Guidelines in Oncology: Cancer-Related Fatigue, version 1.2011. Available at: http://www.nccn.org/professionals/physician_gls/PDF/fatigue.pdf.
- 143.National Institutes of Health. Axitinib (AG 013736) as second line therapy for metastatic renal cell cancer. [Accessed January 7, 2011];ClinicalTrials.gov. Available at: http://clinicaltrials.gov/ct2/show/NCT00678392.
- 144.National Institutes of Health. a study to compare tivozanib (AV-951) to sorafenib in subjects with advanced renal cell carcinoma (TIVO-1) [Accessed January 7, 2011];ClinicalTrials.gov. Available at: www.clinicaltrials.gov/ct2/show/NCT01030783.
- 145.Shi M, Kim KB, Chesney J, et al. Effect of TKI258 in plasma biomarkers and pharmacokinetics in patients with advanced melanoma [abstract] J Clin Oncol. 2009;27(Suppl 18S):Abstract 9020. [Google Scholar]
- 146.Casanovas O, Hicklin DJ, Bergers G, Hanahan D. Drug resistance by evasion of antiangiogenic targeting of VEGF signaling in late-stage pancreatic islet tumors. Cancer Cell. 2005;8:299–309. doi: 10.1016/j.ccr.2005.09.005. [DOI] [PubMed] [Google Scholar]
- 147.Angevin E, Lin C, Pande AU, et al. A phase I/II study of dovitinib (TKI258), a FGFR and VEGFR inhibitor, in patients (pts) with advanced or metastatic renal cell cancer: phase I results [abstract] J Clin Oncol. 2010;28(Suppl 18S):Abstract 3057. [Google Scholar]
- 148.Angevin E. A phase I/II study of TKI258 (dovitinib), a FGFR and VEGFR inhibitor, in patients (pts) with advanced or metastatic renal cell cancer (mRCC): preliminary phase II results [abstract]. Presented at the 35th European Society for Medical Oncology (ESMO) Congress; October 8–12, 2010; Milan, Italy. p. Abstract 507P. [Google Scholar]
- 149.National Institutes of Health. Study of TKI258 versus sorafenib in patients with metastatic renal cell carcinoma. [Accessed January 7, 2011];ClinicalTrials.gov. Available at: www.clinicaltrials.gov/ct2/show/NCT01223027.
- 150.Garcia JA, Hudes GR, Choueiri TK, et al. Phase II study of IMC-1121B in patients with metastatic renal cancer (mRCC) following VEGFR-2 tyrosine kinase inhibitor (TKI) therapy (IMCL CP12-0605/ NCT00515697) [abstract]. Presented at the 2010 ASCO Genitourinary Cancers Symposium; March 5–7, 2010; San Francisco, California. p. Abstract 326. [Google Scholar]
- 151.Appleman LJ, Gordan MS, Samlowski W, et al. Open-label phase 1b study of AMG 386, a selective angiopoietin1/2-neutralizing peptibody, in combination with sorafenib or sunitinib in advanced renal cell carcinoma (RCC): interim results [abstract]. Presented at the 35th European Society for Medical Oncology (ESMO) Congress; October 8–12, 2010; Milan, Italy. p. Abstract 505P. [Google Scholar]
- 152.National Institutes of Health. AMG 386, 20060159 phase 2, RCC 1st line in combination with sorafenib. [Accessed January 7, 2011];ClinicalTrials.gov. Available at: www.clinicaltrials.gov/ct2/show/NCT00467025.
- 153.Verheul HM, Hammers H, van Erp K, et al. Vascular endothelial growth factor trap blocks tumor growth, metastasis formation, and vascular leakage in an orthotopic murine renal cell cancer model. Clin Cancer Res. 2007;13:4201–4208. doi: 10.1158/1078-0432.CCR-06-2553. [DOI] [PubMed] [Google Scholar]
- 154.National Institutes of Health. VEGF trap in treating patients with metastatic or unresectable kidney cancer. [Accessed January 7, 2011];ClinicalTrials.gov. Available at: www.clinicaltrials.gov/ct2/show/NCT00357760.
- 155.Qian DZ, Wang X, Kachhap SK, et al. The histone deacetylase inhibitor NVP-LAQ824 inhibits angiogenesis and has a greater antitumor effect in combination with the vascular endothelial growth factor receptor tyrosine kinase inhibitor PTK787/ZK222584. Cancer Res. 2004;64:6626–6634. doi: 10.1158/0008-5472.CAN-04-0540. [DOI] [PubMed] [Google Scholar]
- 156.Hammers HJ, Verheul H, Wilky B, et al. Phase I safety and pharmacokinetic/pharmacodynamic results of the histone deacetylase inhibitor vorinostat in combination with bevacizumab in patients with kidney cancer [abstract] J Clin Oncol. 2008;26(Suppl 18S):Abstract 16094. [Google Scholar]
- 157.National Institutes of Health. Vorinostat and bevacizumab in treating patients with unresectable or metastatic kidney cancer. [Accessed January 7, 2011];ClinicalTrials.gov. Available at: www.clinicaltrials.gov/ct2/show/NCT00324870.
- 158.Pili R, Lodge M, Verheul H, et al. Combination of the histone deacetylase inhibitor vorinostat with bevacizumab in pretreated patients with renal cell carcinoma: safety, efficacy, and pharmacodynamic results [abstract]. Presented at the 2010 ASCO Genitourinary Cancers Symposium; March 5–7, 2010; San Francisco, California. p. Abstract 350. [Google Scholar]
- 159.Brahmer JR, Drake CG, Wollner I, et al. Phase I study of single-agent anti-programmed death-1 (MDX-1106) in refractory solid tumors: safety, clinical activity, pharmacodynamics, and immunologic correlates. J Clin Oncol. 2010;28:3167–3175. doi: 10.1200/JCO.2009.26.7609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Sznol M, Powderly JD, Smith DC, et al. Safety and antitumor activity of biweekly MDX-1106 (Anti-PD-1, BMS-936558/ONO-4538) in patients with advanced refractory malignancies [abstract] J Clin Oncol. 2010;28(Suppl):Abstract 2506. [Google Scholar]
- 161.Kroog GS, Feldman DR, Kondagunta GV, et al. Phase I trial of RAD001 (everolimus) plus sunitinib in patients with metastatic renal cell carcinoma [abstract] J Clin Oncol. 2009;27(Suppl 15S):Abstract 5037. [Google Scholar]
- 162.Feldman DR, Baum MS, Ginsberg MS, et al. Phase I trial of bevacizumab plus escalated doses of sunitinib in patients with meta-static renal cell carcinoma. J Clin Oncol. 2009;27:1432–1439. doi: 10.1200/JCO.2008.19.0108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Merchan JR, Pitot HC, Qin R, et al. Phase I/II trial of CCI 779 and bevacizumab in advanced renal cell carcinoma (RCC): safety and activity in RTKI refractory RCC patients [abstract] J Clin Oncol. 2009;27(Suppl 15S):Abstract 5039. [Google Scholar]
- 164.Hainsworth JD, Spigel DR, Burris HA, III, et al. Phase II trial of bevacizumab and everolimus in patients with advanced renal cell carcinoma. J Clin Oncol. 2010;28:2131–2136. doi: 10.1200/JCO.2009.26.3152. [DOI] [PubMed] [Google Scholar]
- 165.Sosman JA, Flaherty KT, Atkins MB, et al. Updated results of phase I trial of sorafenib (S) and bevacizumab (B) in patients with metastatic renal cell cancer (mRCC) [abstract] J Clin Oncol. 2008;26(Suppl 15S):Abstract 5011. [Google Scholar]
- 166.Patnaik A, Ricart A, Cooper J, et al. A phase I, pharmacokinetic and pharmacodynamic study of sorafenib (S), a multi-targeted kinase inhibitor in combination with temsirolimus (T), an mTOR inhibitor in patients with advanced solid malignancies [abstract] J Clin Oncol. 2007;25(Suppl 18S):Abstract 3512. [Google Scholar]
- 167.Cen P, Daleiden A, Doshi G, Amato R. A phase I study of everolimus plus sorafenib in patients with metastatic renal cell carcinoma (mRCC) [abstract] J Clin Oncol. 2009;27(Suppl 15S):Abstract e16056. [Google Scholar]