Abstract
Objective
The objective of this study was to evaluate pretransplant sinus computed tomography (CT) as predictor of post–hematopoietic stem cell transplant sinusitis.
Methods
We evaluated pretransplant and posttransplant CT findings in 100 children using the Lund-Mackay system and “common-practice” radiology reporting and correlated these with the presence of acute sinusitis.
Results
Fourteen percent of patients with normal screening CT developed posttransplant sinusitis, compared with 23%with radiographic abnormalities and 22% with clinical sinusitis alone, not statistically significant. Sensitivity of CT findings for clinical sinusitis ranged between 19% and 56%. Except for mucosal thickening (71% specificity), other findings had high specificity between 92% and 97%, particularly when combined. Lund-Mackay score change of 10 or greater from baseline was associated with a 2.8-fold increased likelihood of having sinusitis (P < 0.001).
Conclusions
Screening CT can serve as a baseline, with a Lund-Mackay score change of 10 or greater constituting a significant threshold. The strongest correlation with the presence of acute sinusitis was seen with combined CT findings.
Keywords: sinusitis, hematopoietic stem cell transplantation, multidetector computed tomography
Rhinitis and sinusitis occur frequently in patients undergoing hematopoietic stem cell transplant (HSCT), with a reported prevalence of 30% to 51%.1, 2 Widely cited research by Billings and colleagues3 has suggested that radiographic findings of severe sinusitis on pre-HSCT screening computed tomography (CT) in children correlate with the incidence of clinical sinusitis following transplantation. However, their study otherwise largely failed to reach statistical significance, and its conclusions have been questioned in light of subsequent investigations of adult HSCT patients where such a correlation was not found.4, 5 While the relationship of screening sinus CT findings and post-HSCT sinusitis remains uncertain, many patients receive medical or surgical treatment prior to and after the transplant.5, 6 Reliable identification of sinusitis is further complicated in the setting of immunosuppression, where radiographic abnormalities may appear relatively nonspecific without significant mucosal thickening or air-fluid levels.7 Therefore, comparison with pre-HSCT to assess for baseline changes may be important in the workup for children with neutropenic fever. In addition, in current practice, acute sinusitis is typically a clinical diagnosis, and unlike chronic sinusitis, it is rarely imaged. To the best of our knowledge, the specific CT findings associated with the clinical diagnosis of acute sinusitis in this immunocompromised pediatric population have not been described.
The purpose of our study was to evaluate the role of screening sinus CT prior to HSCT as a predictor for the development of post-HSCT sinusitis and as a baseline examination from which to compare post-HSCT scans. Furthermore, we correlate CT findings with the presence of acute clinical sinusitis in this unique patient population.
MATERIALS AND METHODS
Study Population
This study is compliant with the Health Insurance Portability and Accountability Act and was approved by our institutional review board, which waived the requirement for informed consent. We retrospectively evaluated the electronic medical records and CT scans from 100 consecutive pediatric patients who underwent screening paranasal sinus CT prior to HSCT between 2006 and 2010.
Image Acquisition
Computed tomography studies were retrieved from our picture archiving and communication system (Advanced Visualization; Emageon, Birmingham, Ala) and obtained using different multi–detector-row scanners (Siemens Somatom Sensation, Definition AS, and Definition Flash; Siemens Medical Solutions, Malvern, Pa). Images were obtained through the paranasal sinuses without intravenous contrast material using our institution’s pediatric low-dose protocols, which vary depending on the particular scanner. Typical parameters are as follows: 100 kV, 40 to 90 mAs (adjusted by age), 0.5– to 0.8-second rotation time, pitch value of 1, average acquisition time of 6 to 8 seconds, and 64 × 0.6-mm collimation, with estimated effective doses ranging between 0.07 and 0.2 mSv. Data were reconstructed at 0.75-mm section thickness at 0.5-mm intervals for multiplanar reformation. For diagnostic reading, 3– or 5-mm section thickness and 3- or 5-mm reconstruction intervals were used in bone and soft-tissue algorithms in axial, sagittal, and coronal planes.
CT Scoring
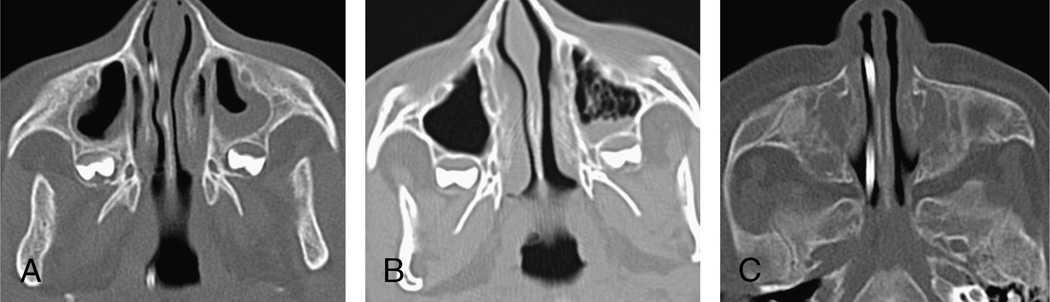
Pre- and post-HSCT sinus disease was evaluated using 2 methods: a quantitative scoring system described by Lund and Mackay 8 and “common-practice” radiology reporting of acute sinusitis based on the presence of air-fluid levels, frothy secretions, or total to near-total opacification of any individual sinus (Fig. 1). The Lund-Mackay system assigns a numerical score to radiographic findings on a sinus CT scan. The right and left maxillary, anterior ethmoid, posterior ethmoid, sphenoid, and frontal sinuses were evaluated for degree of aeration. A score of 0 to 2 was applied to each sinus, with 0 for no sinus disease, 1 for partial sinus opacification, and 2 for complete opacification. Sinuses not yet developed did not receive a score. The osteomeatal units were evaluated for patency and assigned a score of either 0 (patent) or 2 (occluded). To correct for variation in the number of developed sinuses at different ages, the percentage of total sinus opacification was calculated for each patient, and the total score of sinus opacification was divided by the total number of developed sinuses. Based on this result, the severity of sinusitis on CT was categorized into 4 groups: 0% for no evidence of sinusitis, less than 25% for mild sinusitis, 25% to 50% for moderate sinusitis, and greater than 50% for severe sinusitis. The severity of sinus opacification on CT was compared between the pre- and post-HSCT scans and correlated with the presence of clinical sinusitis based on symptoms at the time of scanning, as reported by the primary care team (rhinorrhea, nasal congestion, and headache).
FIGURE 1.
Axial CT images through the maxillary sinuses. A, Fluid level with a characteristic meniscus in the left maxillary sinus, with background mucosal thickening. B, Frothy secretions in the left maxillary sinus. C, Bilateral opacification of the maxillary sinuses.
Statistical Analysis
Data were tested for normality of distribution using the Shapiro-Wilk test. Fisher exact test with Katz’s approximation was used to separately evaluate the risk of developing sinusitis based on pre-HSCT CT findings, severity of sinusitis, and Lund-Mackay score. T tests were used to assess for differences in mean post-HSCT Lund-Mackay raw scores as well as mean and absolute score change between the sinusitis and nonsinusitis groups. The data were sorted and processed using commercially available software packages (Excel version 14 [Microsoft, Redmond, Wash] and SPSS version 20 [SPSS, Chicago, Ill]). P < 0.05 was considered statistically significant.
RESULTS
Average patient age at the time of transplant was 10.7 years (range, 8 months to 22 years). There were 37 females and 63 males. Indications for transplant included acute myeloid leukemia (n = 21), acute lymphoblastic leukemia (n = 13), biphenotypic leukemia (n = 3), myelodysplastic syndrome (n = 7), chronicmyeloid leukemia (n = 3), aplastic anemia (n = 13), lymphoma (n = 9), neuroblastoma (n = 7), Ewing sarcoma (n = 3), brain tumors (n = 6), and others (n = 15). Seventy of the 100 patients who had a screening CT prior to transplant also underwent post-HSCT CT. Overall, 9 patients had clinical sinusitis prior to HSCT, whereas 18 patients developed sinusitis after HSCT (Table 1). Eight of 56 asymptomatic patients (14%) with a normal sinus CT prior to HSCT developed clinical sinusitis following transplant, compared with 8 (23%) of 35 asymptomatic patients with radiographic abnormalities and 2 (22%) of 9 patients who were symptomatic but had a normal CT scan (Table 2). None of these differences were statistically significant (P = 0.20). Furthermore, subgroup analysis of patients with abnormal pre-HSCT scans stratified by the Lund-Mackay score (mild vs moderate/severe) was also not found to be significantly different for the development of clinical sinusitis after HSCT (P = 0.58; Table 2).
TABLE 1.
Evidence of Sinusitis Before and After HSCT
| Pre-HSCT, n (%) | Post-HSCT, n (%) | |
|---|---|---|
| No clinical or radiographic evidence of sinusitis | 56 (56) | 22 (31) |
| Radiological evidence of sinusitis alone | 35 (35) | 30 (43) |
| Clinical evidence of sinusitis | 9 (9) | 18 (26) |
| Total | 100 | 70 |
TABLE 2.
Development of Clinical Sinusitis After HSCT
| Pre-HSCT Findings | No. Patients | Post-HSCT Clinical Sinusitis | % | P |
|---|---|---|---|---|
| No clinical or radiographic abnormalities | 56 | 8 | 14 | |
| Radiographic abnormality alone | ||||
| Total | 35 | 8 | 23 | 0.20 |
| Mild Lund-Mackay | 25 | 6 | 24 | |
| Moderate/severe Lund-Mackay | 10 | 2 | 20 | |
| Mucosal disease alone | 19 | 5 | 26 | |
| Fluid level/frothy secretion/total–near-total opacification | 16 | 3 | 19 | |
| Clinical sinusitis alone | 9 | 2 | 22 | 0.20 |
| Total | 100 | 18 | 18 | |
The sensitivity of any individual radiographic finding (mucosal thickening, fluid level, frothy secretions, or total/near-total sinus opacification) or multiple abnormalities for the presence of clinical sinusitis ranged between 19% and 56% (Table 3). On the other hand, the overall specificity was high, except for mucosal thickening alone (71%), with the greatest specificity in the presence of multiple abnormalities (97%). The positive predictive value of having acute clinical sinusitis for a given radiographic abnormality was highest for total sinus opacification (56%), frothy secretions (53%), and fluid levels (47%) and lowest for mucosal thickening alone (13%). The positive predictive value was greatest (67%) with the combined presence of at least 2 abnormalities (Table 3). Conversely, negative predictive values were highest for frothy secretions and total or near-total sinus opacification (89% and 92%, respectively).
TABLE 3.
Correlation of Post-HSCT CT Findings With the Presence of Clinical Sinusitis
| CT Finding | Sensitivity, % | Specificity, % | PPV, % | NPV, % |
|---|---|---|---|---|
| Mucosal thickening | 19 | 71 | 13 | 78 |
| Fluid level | 26 | 94 | 47 | 87 |
| Frothy secretions | 37 | 94 | 53 | 89 |
| Total–near-total opacification | 56 | 92 | 56 | 92 |
| Multiple abnormalities* | 37 | 97 | 67 | 89 |
Combined presence of at least 2 of 3 abnormalities (fluid level, frothy secretions, or total–near-total sinus opacification).
Using the screening sinus CT as a baseline examination, a significant difference was found in Lund-Mackay score change from baseline between patients who did and did not develop post-HSCT clinical sinusitis (10.4 and 4.2, respectively; P < 0.0001; Table 4). Furthermore, patients with a change in the Lund-Mackay score of 10 or greater were 2.8 times more likely to have clinical sinusitis (P < 0.001; confidence interval, 1.32–5.81;Table 4).
TABLE 4.
Comparison of Pre- and Post-HSCT Lund-Mackay Score and Development of Clinical Sinusitis
| No Clinical Sinusitis | Clinical Sinusitis | P | |
|---|---|---|---|
| Average post-HSCT Lund-Mackay score | 6.83 | 13 | 0.0002 |
| Average change in Lund-Mackay score | 4.2 | 10.3 | <0.0001 |
| Lund-Mackay score change <10 | 26 | 4 | 0.0002* |
| Lund-Mackay score change ≥10 | 5 | 11 |
Relative risk = 2.773; 95% confidence interval, 1.32 to 5.81.
DISCUSSION
The diagnosis of acute sinusitis in HSCT patients may be challenging. Clinical manifestations and radiographic findings can have a more variable and inconsistent presentation in this group compared with immunocompetent individuals, because post-HSCT patients may not be able to mount an adequate immunologic response. Despite this limitation, current practice warrants the application of traditional imaging and standard symptoms because of the lack of data in the immunocompromised population. Therefore, in the absence of more specific measures, utilizing standard immunocompetent clinical and imaging criteria for sinusitis is important to increase our understanding of their predictive power in immunocompromised patients. Recent research by Arulrajah and colleagues9 has shown significant differences in the severity of radiographic findings and the number of symptoms between pediatric post-HSCT patients and immunocompetent children. Therefore, the evaluation of sinusitis in this particular post-HSCT population is important. To date, despite the widespread use of screening CT, there are very few studies in the literature assessing the utility of this modality in children undergoing HSCT,3, 7 and there is no clear consensus.
Billings and colleagues3 retrospectively evaluated the correlation of pre-HSCT screening CT findings with the development of sinusitis after transplant in 51 children. While they concluded that the severity of radiographic sinus disease on screening CT using the Lund-Mackay system correlated well with the subsequent development of clinical sinusitis after transplant, such results were based on a very small sample size and were significant only for severe radiographic sinusitis. That study also found a correlation between the presence of radiographic sinusitis on screening CT and the presence of radiographic sinusitis in the posttransplant period. In contrast, we found that neither the presence nor the severity of pre-HSCT radiographic sinus disease correlated significantly with the development of clinical sinusitis in the posttransplant period. This is concordant with subsequent research in adult patients that did not identify an increased risk based on pre-HSCT radiographic abnormalities.4, 5 Our results include both formal scoring using the Lund-Mackay system and more “common-practice” findings, which appear to be widely utilized in radiology reporting. However, we did notice a statistically significant difference in Lund-Mackay score change from baseline in patients who developed post-HSCT clinical sinusitis. Furthermore, those patients with a score change equal to or greater than 10 were almost 3 times as likely to have clinical sinusitis, which may be valuable particularly when the clinical picture is not clear. This suggests that the Lund-Mackay scoring system may have some utility in the evaluation of acute sinusitis in this group of patients, despite the fact that it was originally developed as an assessment tool for chronic rhinosinusitis.8 This potential use may be rendered even more relevant by the fact that approximately 60% of posttransplant CT scans demonstrated 1 or more radiographic abnormalities.
Regarding the relationship between radiographic abnormalities and clinical disease, the sensitivity of CT abnormalities was low. On the other hand, with the exception of mucosal thickening alone, specificity was very high, particularly in the presence of 2 or more radiographic abnormalities. While our study identified that mucosal thickening alone had moderate specificity, it is worth mentioning that in the general pediatric population there is a high prevalence of mucosal thickening as an incidental finding, in the absence of upper respiratory tract symptoms, as reported on brain magnetic resonance imaging studies performed for reasons other than sinusitis.10, 11 Thus, the value of mucosal thickening per se is relatively low, and its isolated use as a marker for sinusitis should probably be discouraged. We found that total or near-total sinus opacification, frothy secretions, and fluid levels had the strongest correlation with the presence of acute clinical sinusitis, but their individual positive predictive values were low. Correlation was strongest in the combined presence of at least 2 findings. Negative predictive values for frothy secretions and total or near-total sinus opacification were relatively high, suggesting that the absence of these findings may be reassuring and could represent a useful piece of information to be included in the report. Notably, frothy secretions may be obscured or entirely invisible in regular soft tissue or bone windows, and this finding can be easily missed. In our experience, these become visible with larger window widths and can be made most conspicuous while visually adjusting bone algorithm reconstructions to window width settings between bone and lung.
Although the American College of Radiology does not recommend routine imaging of the paranasal sinuses in children with uncomplicated sinusitis, it gives its highest appropriateness score to performing unenhanced CT in cases of persistent, recurrent, or chronic sinusitis or when intracranial or intraorbital extension is a concern.12 Radiation dose can be substantially reduced by parameter modifications such as decreasing kVp and mAs and increasing pitch. While our current protocols allow diagnostic-quality images at reasonably low doses, these can be further reduced to levels comparable to those of radiography, albeit at the cost of increasing image noise and potentially rendering small bony septations such as the ethmoid sinuses difficult to evaluate.13 As we continue to optimize our pediatric CT protocols, we maintain a low threshold for imaging the paranasal sinuses in this particular group of high-risk immunocompromised patients because of the high morbidity and mortality associated with invasive infections.
Our study has inherent limitations derived from its retrospective design. All patients undergoing a CT study were either being screened for or clinically suspected of having sinusitis, which confers a certain degree of selection bias. There are also design limitations related to the use of ionizing radiation in the pediatric population. For obvious reasons, a prospective study comparing CT findings between immunocompromised, post-HSCT patients, and normal, asymptomatic individuals would be hard to justify. Nevertheless, we believe that within the frame of these limitations, the results of this study increase our understanding regarding the utility of a screening examination that is already widely performed and that can potentially influence medical or surgical management decisions.
In summary, pre-HSCT CT can serve as a baseline for comparison with subsequent scans, where a Lund-Mackay score change of 10 or greater may constitute a significant threshold. None of the pre-HSCT radiographic findings correlated with the subsequent development of clinical sinusitis after transplant. The strongest correlation with the presence of acute clinical sinusitis was seen with the combined findings of total sinus opacification, frothy secretions, and fluid levels, which may prompt further clinical workup. Finally, the absence of frothy secretions and extensive sinus opacification may be reassuring.
Footnotes
The authors declare no conflict of interest.
REFERENCES
- 1.Shibuya TY, Momin F, Abella E, et al. Sinus disease in the bone marrow transplant population: incidence, risk factors, and complications. Otolaryngol Head Neck Surg. 1995;113:705–711. doi: 10.1016/s0194-5998(95)70009-9. [DOI] [PubMed] [Google Scholar]
- 2.Savage DG, Taylor P, Blackwell J, et al. Paranasal sinusitis following allogeneic bone marrow transplant. Bone Marrow Transplant. 1997;19:55–59. doi: 10.1038/sj.bmt.1700601. [DOI] [PubMed] [Google Scholar]
- 3.Billings KR, Lowe LH, Aquino VM, et al. Screening sinus CT scans in pediatric bone marrow transplant patients. Int J Pediatr Otorhinolaryngol. 2000;52:253–260. doi: 10.1016/s0165-5876(00)00296-2. [DOI] [PubMed] [Google Scholar]
- 4.Thompson AM, Couch M, Zahurak ML, et al. Risk factors for post-stem cell transplant sinusitis. Bone Marrow Transplant. 2002;29:257–261. doi: 10.1038/sj.bmt.1703353. [DOI] [PubMed] [Google Scholar]
- 5.Won YW, Yi SY, Jang JH, et al. Retrospective analysis of paranasal sinusitis in patients receiving hematopoietic stem cell transplantation. Int J Hematol. 2011;93:383–388. doi: 10.1007/s12185-011-0797-8. [DOI] [PubMed] [Google Scholar]
- 6.Moeller CW, Martin J, Welch KC. Sinonasal evaluation preceding hematopoietic transplantation. Otolaryngol Head Neck Surg. 2011;144:796–801. doi: 10.1177/0194599810395089. [DOI] [PubMed] [Google Scholar]
- 7.Sekine L, Manica D, Piltcher O, et al. Rhinosinusitis in autologous and allogeneic bone marrow transplantation: a retrospective study on the performance of imaging studies on severity and prognostic evaluation. Rev Bras Hematol Hemoter. 2010;32:29–33. [Google Scholar]
- 8.Lund VJ, Mackay IS. Staging in rhinosinusitus. Rhinology. 1993;31:183–184. [PubMed] [Google Scholar]
- 9.Arulrajah S, Symons H, Cahoon EK, et al. Relationship between clinical sinusitis symptoms and sinus CT severity in pediatric post bone marrow transplant and immunocompetent patients. Eur J Pediatr. 2012;171:375–381. doi: 10.1007/s00431-011-1560-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.von Kalle T, Fabig-Moritz C, Heumann H, et al. Incidental findings in paranasal sinuses and mastoid cells: a cross-sectional magnetic resonance imaging (MRI) study in a pediatric radiology department. Rofo. 2012;184:629–634. doi: 10.1055/s-0032-1312861. [DOI] [PubMed] [Google Scholar]
- 11.Rak KM, Newell JD2, Yakes WF, et al. Paranasal sinuses on MR images of the brain: significance of mucosal thickening. AJR Am J Roentgenol. 1991;156:381–384. doi: 10.2214/ajr.156.2.1898819. [DOI] [PubMed] [Google Scholar]
- 12.American College of Radiology. [Accessed September 26, 2014];ACR Appropriateness Criteria. 2014 May; Available at: http://www.acr.org/Quality-Safety/Appropriateness-Criteria.
- 13.Mulkens TH, Broers C, Fieuws S, et al. Comparison of effective doses for low-dose MDCT and radiographic examination of sinuses in children. AJR Am J Roentgenol. 2005;184:1611–1618. doi: 10.2214/ajr.184.5.01841611. [DOI] [PubMed] [Google Scholar]