Abstract
The development of the acute respiratory distress syndrome (ARDS) carries significant risk of morbidity and mortality. To date, pharmacologic therapy has been largely ineffective for patients with ARDS. We present our personal review aimed at outlining current and future directions for the pharmacologic prevention of ARDS.
Several available risk-stratification or prediction scores strategies for identification of patients at risk of ARDS have been reported. Although not ready for the clinical everyday use, they are and will be instrumental in the ongoing and future trials of pharmacoprevention of ARDS.
Several systemic medications established the potential role in ARDS prevention based on the preclinical studies and observational data. Due to potential for systemic adverse effects to neutralize any pharmacologic benefits of systemic therapy, inhaled medications appear particularly attractive candidates for ARDS prevention. This is because of their direct delivery to the site of the proposed action (lungs), while pulmonary epithelial surface is still functional.
We postulate that overall morbidity and mortality rates from ARDS in the future will be contingent upon decreasing the overall incidence of ARDS through effective identification of those at risk and early application of proven supportive care and pharmacologic interventions.
Keywords: ARDS, prevention
Introduction
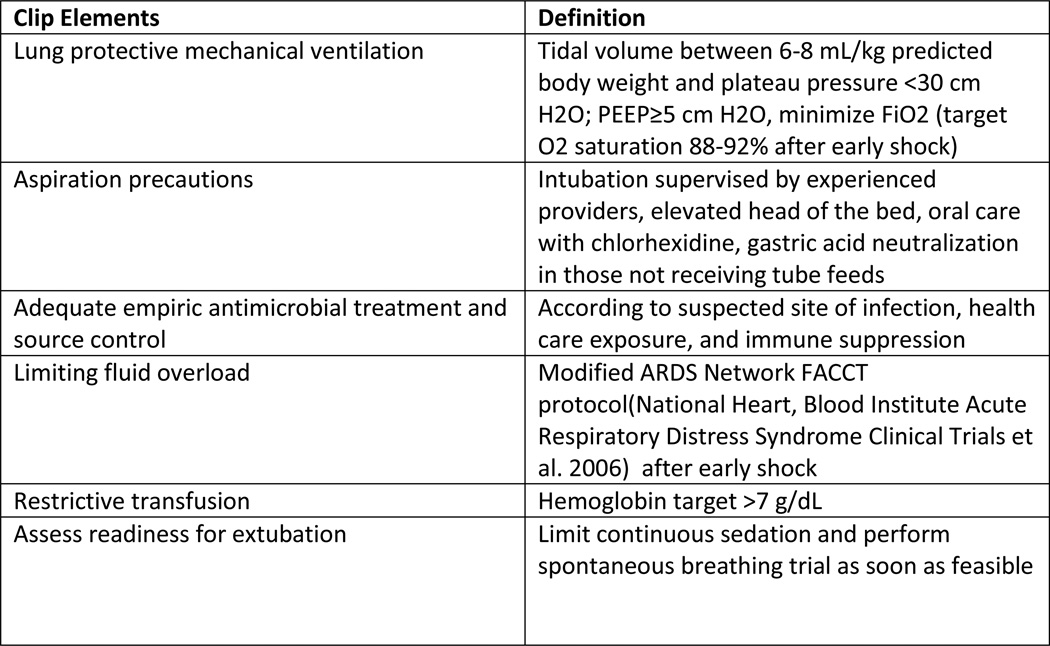
ARDS is a clinicopathologic entity defined by acute onset, hypoxemia and characteristic chest radiographic findings (Ranieri, Rubenfeld et al. 2012). Despite the heterogeneous array of systemic and pulmonary insults that can serve as triggers to this devastating condition, ARDS is considered a distinct condition on the basis of a final common pathway in pathogenesis (Matthay, Ware et al. 2012) and corresponding histopathologic changes (Thompson 2014). Despite decades of research in treatment of ARDS, not a single effective pharmacological intervention emerged. Several common ARDS precipitants were elucidated including ventilator induced lung injury, septic shock, transfusion related lung injury and ventilator associated pneumonia (Estenssoro, Dubin et al. 2002), hence management of ARDS has focused on supportive care measures. These include improvements in recognition and timely management of severe sepsis and septic shock (Rivers, Nguyen et al. 2001; Yealy, Kellum et al. 2014), lung protective ventilation strategies (Acute Respiratory Distress Syndrome Network 2000), restrictive PRBC, platelet and FFP transfusion strategies (Gajic, Rana et al. 2007; Khan, Belsher et al. 2007; Toy, Gajic et al. 2012) the adoption of standardized VAP prevention “bundles” (Rosenthal, Rodrigues et al. 2012) and other institutional protocols such as the proposed Checklist for Lung Injury Prevention (CLIP, Figure 1) (Kor, Talmor et al. 2012). With these improvements in the delivery of supportive care, rates of nosocomial ARDS have been on the decline (Li, Malinchoc et al. 2011). However, mortality associated with ARDS has remained at the similar unsettlingly high ~40% rate for the past approximately 20 years. (Phua, Badia et al. 2009) These stagnant mortality data are certainly not reflective of a lack of interest in finding effective pharmacologic approaches to the treatment of ARDS. The cavalcade of investigational therapies showing promise in preclinical trials failed to demonstrate any meaningful clinical efficacy (Cepkova and Matthay 2006; Bosma, Taneja et al. 2010) when applied after the development of fully established ARDS. As such, a paradigm shift is occurring in how we approach the burden of ARDS, away from treatment and towards prevention of the fully established syndrome. (Ortiz-Diaz, Festic et al. 2013; Beitler, Schoenfeld et al. 2014) Parallels can be drawn to the other critical illness syndromes, of which sepsis have been the most studied and with the best resulting improvement in outcomes following adoption of early identification and goal-directed treatment principles. Since most of the ARDS cases develop 2–5 days after the hospital admission (Gajic, Dabbagh et al. 2011) there is a window of opportunity where early identification of patients at risk and improvements in supportive care, coupled with effective pharmacological interventions could potentially prevent or ameliorate ARDS in patients at risk. Hence, the key question: Can pharmacological intervention administered prior to the fully established ARDS prevent disease development and enhance outcomes in patients at risk? In order to answer this key question, we first have to find answers to the following: who are the patients at risk, when to apply the intervention, and what intervention? The first two questions were answered by the LIPS trial, to a certain degree. The final and perhaps the most important sub-question remains: What are the effective interventions? In order to identify a safe pharmacologic treatment as an effective intervention, the investigators ought to apply systematic translational research directed towards proven mechanistic pathways of ARDS pathophysiology.
Figure 1.
Checklist for Lung Injury Prevention
Preventive Strategies
- Who and when?
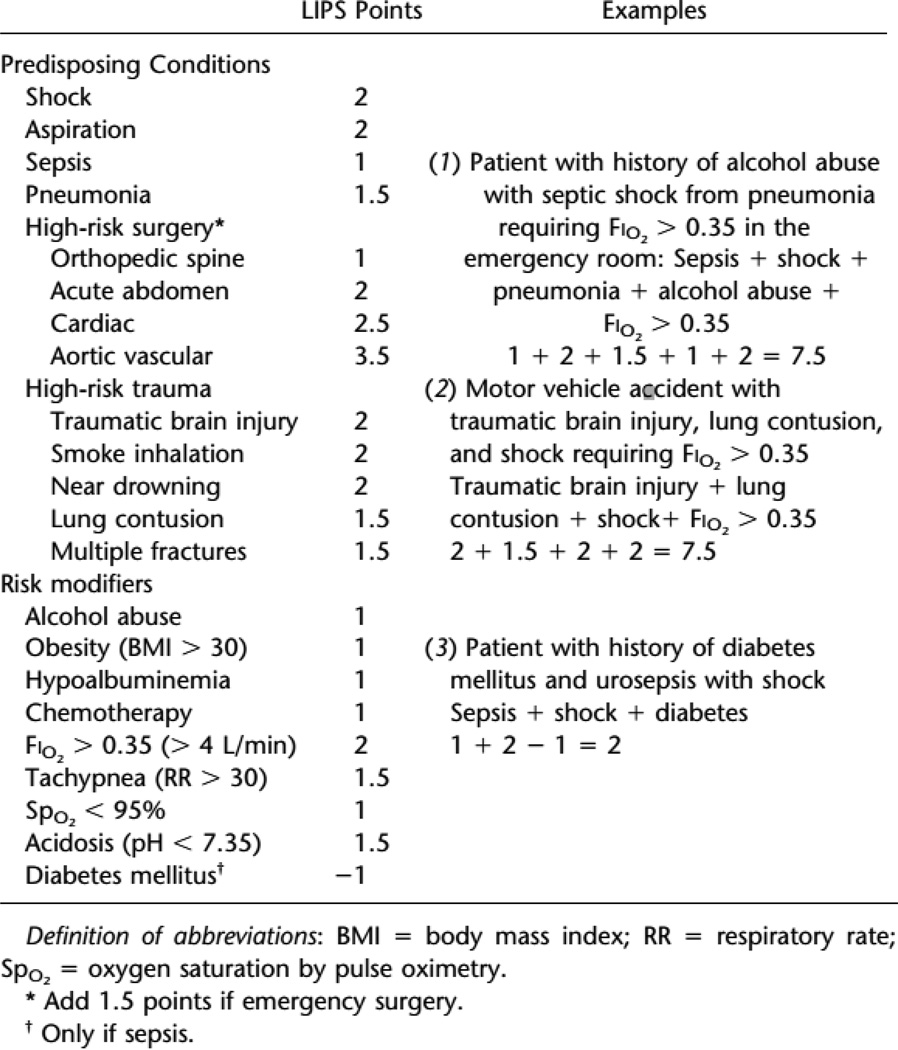
The first critical step in instituting an effective primary prevention program was to identify the population most likely to glean benefit from the prophylactic intervention. The Lung Injury Prediction Score (LIPS) (Gajic, Dabbagh et al. 2011) is currently the best available tool for risk stratification of patients presenting to the hospital without ARDS. To evaluate this prediction model, LIPS investigators prospectively enrolled 5584 patients admitted for sepsis, shock, pancreatitis, pneumonia, aspiration, high-risk trauma, or high-risk surgery and included comorbid conditions such as alcohol abuse, DM, obesity, physical exam and laboratory findings [Figure 2]. Using a cutoff LIPS score of ≥4, the authors found an 18% positive predictive value and 97% negative predictive value with an overall incidence of Acute lung injury (ALI) or ARDS of 6.8%. The patients destined to develop ARDS in the hospital did so most frequently beyond 48 hours of hospitalization so any future intervention should be applied within the first 24 hours after the admission to allow it sufficient time to exhibit its protective effect.
Figure 2.
Lung Injury Prediction Score calculation
A number of other scoring systems for risk stratification with regard to development of ARDS have been proposed. Secondary analysis of the LIPS data have revealed that SpO2/FiO2 ratio, a single (composite) variable from the LIPS, is an independent risk factor for progression to ARDS. (Festic, Bansal et al. 2013). Levitt et Al reported an Early Acute Lung Injury (EALI) score, based on the oxygenation impairment, radiographic abnormalities and presence of concomitant immunosuppression (Levitt, Bedi et al. 2009). This risk score may be more applicable to hospitalized patients already developing early lung injury. Specific to the postoperative population, the Surgical Lung Injury Prediction model, SLIP and refined SLIP-2, have shown similar utility in predicting which surgical patients are at the highest risk for development of ARDS (Kor, Warner et al. 2011), (Kor, Lingineni et al. 2014). While none of these prediction models appear ready for everyday use in clinical practice, they represent useful tools for enrollment in clinical trials investigating pharmacological strategies for ARDS prevention.
Pharmacologic Preventive Strategies
- What interventions?
Key components in the common final pathogenic pathway to ARDS include dysregulated inflammation, maladaptive platelet activation, dysfunction of the coagulation cascade, endothelial and epithelial barrier dysfunction, and failure of T-cell/macrophage mediated clearance of activated neutrophils and necrotic debris [Figure 3]. (Matthay, Ware et al. 2012). As we have begun to elucidate the individual components leading to the full-blown ARDS syndrome, several potential therapeutic targets have emerged [Table 1].
Table 1.
Emerging therapies for the prevention of ARDS and previous studies.
| Medication | Mechanism of action | Animal Studies | Human Studies |
|---|---|---|---|
| Aspirin | Inhibition of platelet mediated cyclooxygenase metabolism involved in platelet-neutrophil-endothelial interactions. | Mice treated with aspirin have less pulmonary platelet and neutrophil sequestration. Also treated animals have improved survival and decreased lung weights. | Observational studies conflicting in terms of their findings. The largest cohort found a non-significant trend toward a protective effect. LIPS-A trial completed, awaiting results. |
| Systemic Corticosteroids | Multi-potent; inhibit inflammatory cytokines; induced apoptosis of macrophages; maintain endothelial cellular barrier. | Majority show improvement of hypoxemia, pulmonary vascular pressure and extra-vascular lung water. | Studies performed in the 1980s in heterogenous groups of patients showed no benefit in administering high-dose steroids. Subgroup analysis of RCT suggest using biomarkers may help with patient selection. |
| Inhaled Heparin | In addition to potentiating anti-thrombin-III, inhibits adhesion of neutrophils to endothelium and degrades intravascular and bronchial fibrin. | Conflicting results with improvement of hypoxemia histology scores and shunt fraction. | Suggest morbidity/mortality benefit in smoke inhalation injury patients; RCT currently enrolling patients. |
| Inhaled Corticosteroids | Same as systemic corticosteroids. In theory, might spare patients from hyperglycemia, myopathy, super-infection, etc. | Most studies conducted in mice indicate that physiological surrogates are improved by treatment prior to or after direct/indirect lung injury. | Subgroup analysis of LIPS-A cohort suggest protective effect of baseline ICS against development of ARDS. LIPS-B recruiting. |
| Inhaled Hypertonic Saline | Inhibition of neutrophil activation/chemotaxis and macrophage cytokine production | Rats with trauma show less pulmonary vascular permeability, neutrophil chemotaxis, matrix metalloprotease activity, IL-8 activity and ARDS at necroscopy. | Intravenous hypertonic saline shown to have no benefit in trauma patients, likely due to effects on coagulation cascade. Prospective study forthcoming from LIPS investigators. |
| Inhaled beta-agonists | Enhanced alveolar fluid clearance and inhibits neutrophil adhesion to the endothelium. | Improved pulmonary mechanics; decrease neutrophil sequestration, inflammatory cytokine concentrations and enhanced surfactant secretion. | LIPS-B ongoing; no difference in ARDS incidence but less pulmonary complications among high risk surgical patients, mainly pneumonia. |
| Statins | Decreases inflammatory cytokine levels, adhesion molecule expression, and neutrophil proliferation. | Improvement in oxygenation, hemodynamic surrogates, neutrophil sequestration and decreased cytokine concentration. | Human observational studies show inconsistent effects of prehospital statin use with regards to development of ARDS. Statin therapy for treatment of established ARDS has been shown to be ineffective. |
| Thrombolytic Therapy | More rapid resolution of pulmonary microthrombi | Rat studies showing mitigation of pulmonary microthrombus formation and decreased neutrophil chemotaxis | No human studies published to date |
| N-Acetyl Cysteine | Repletes intracellular stores of reduced glutathione, decreasing oxidative stress. | In vivo studies elucidating the mechanism of NAC. | Mitigated mortality and lung injury scores in patients with smoke inhalation injury. |
| Renin-angiotensin axis blockers | Angiotensin-2 positively modulates nuclear factor-κβ gene expression. ACE type 2 receptor with angiotensin as its ligand, prevents endothelial damage. | Effective in preventing endothelial damage and inflammatory cytokine expression. | Observational studies showed a protective effect among specific populations including Asians and diabetics |
| Peroxisome Proliferator Receptor agonists | Nuclear receptor superfamily related to the retinoid, steroid and thyroid receptors with three subtypes. They decrease inflammatory cytokine expression, neutrophil and macrophage chemotaxis plus inhibit oxidative burst in neutrophils. | Decreased wet to dry ratios, inflammatory cytokine expression and improved static compliance. | No human studies to date. |
| Curcumin | Up-regulation of PPAR-γ in various inflammatory cells (neutrophils, monocytes, T lymphocytes, endothelial and epithelial cells). Down-regulation of inflammatory transcription factors, enzymes and cytokines. | Decreased wet to dry ratios, and inflammatory cytokine secretion. | No human studies to date. |
Systemic delivery
Aspirin
Several observations regarding the role of activated platelets as ‘instigators’ of endothelial dysfunction (Yiming, Lederer et al. 2008) and neutrophil chemotaxis and degranulation (Zarbock and Ley 2009) in animal models of ARDS have made aspirin an attractive potential agent. Furthermore, animal models specifically investigating the use of aspirin have been promising. In a “two hit” murine model of ARDS that included transfusion related acute lung injury and endotoxinemia, control mice were compared to platelet depleted and aspirin treated mice (Looney, Nguyen et al. 2009). In this study, the platelet-depleted and aspirin treated mice had similarly significant improvements in progression to ARDS and in mortality versus control. Song et al. demonstrated amelioration of the thromboxane-mediated pulmonary hemodynamic changes in aspirin treated mice with endotoxin-induced ARDS (Song, Suzuki et al. 2004). And in an acid-induced “aspiration” murine ARDS model, Zarbock and colleagues demonstrated that aspirin decreased neutrophil chemotaxis across pulmonary endothelium, decreased the degree of pulmonary edema and improved oxygenation in mice (Zarbock, Singbartl et al. 2006).
The results of two observational studies in humans have thus far shown conflicting results with a possible trend towards a benefit of aspirin therapy for the prevention of ARDS. The charts of patients admitted to the medical ICU at Mayo Clinic in Rochester, Minnesota in 2006 with at least one known risk factor for ALI (ARDS) were reviewed. Prehospital use of antiplatelet medication was found in this series to be associated with a lower hazard ratio of ARDS development (HR 0.34, 95% CI 0.13 – 0.88) (Erlich, Talmor et al. 2011). A secondary analysis of 3814 patients (after excluding surgical patients) from the LIPS cohort showed a trend towards a protective effect from aspirin which did not achieve statistical significance after propensity score matching (OR for development of ARDS 0.7, 95% CI = 0.48 – 1.03, p =0.07) (Kor, Erlich et al. 2011). In another recently published propensity score analysis of 1149 patients admitted to the surgical or medical ICU at a tertiary care facility, Chen et al. found prehospital aspirin use to be associated with a significantly lower risk of developing ARDS versus matched controls even after adjusting for propensity scores for prehospital aspirin use (Chen, Janz et al. 2014)
The need for prospective, randomized double-blind human data with regards to aspirin in prevention of ARDS is already being addressed. The Lung Injury Prevention Study with Aspirin (LIPS-A) has been completed (NCT 01504867) with the results forthcoming. This was a phase II double-blind, placebo controlled, multi-center trial comparing aspirin (325mg ×1 day followed by 81 mg ×6 days) to identically appearing placebo. Eligible enrollees were hospitalized adults with a LIPS score of ≥4 at the time of admission. Development of ARDS is the primary outcome and all enrolled patients received CLIP-based support for the standardization of care and avoidance of known nosocomial secondary hits.
Systemic Steroids
Systemic steroids have been shown to possess pleiotropic anti-inflammatory properties; 1) They reduce pro-inflammatory cytokines, chemokines, adhesion molecules and receptors, (Frieri 1999) 2) Increase anti-inflammatory mediators, (Kovalovsky, Refojo et al. 2000) 3) Increase activated protein C (Seam, Meduri et al. 2012), 4) Reduce inducible nitric oxide (iNO) expression (Yu, Ouyang et al. 2009), and 5) Inhibit fibroblast proliferation and collagen deposition (Thompson 2003). The last characteristic specifically may play role in tertiary prevention of excessive fibrosis as complication of fully established ARDS. The main limitation of use of systemic steroids in ARDS is their association with adverse effects, which potentially might offset their beneficial anti-inflammatory effects. Furthermore, studies that looked into role of steroids in the prevention of ARDS were conducted prior to standardization of the lung protective mechanical ventilation; they used different durations of therapy and employed different definitions of ARDS, which further complicated the already heterogeneous characteristics of patients with ARDS. Therefore, it is not surprising that these studies showed conflicting results, hence the equipoise for systemic steroid use in ARDS prevention continue (Brian, Rolf et al.)s. Future studies will have to evaluate which groups of patients at risk and at what point in the course of the disease will favorably respond to systemic steroids. Perhaps, biomarkers will play the important role in attempts to answer these questions. For example, Steinberg et al, in a subgroup analysis of a randomized controlled trial showed that patients randomized to steroids with higher levels of procollagen peptide III, a marker of fibroproliferation, had significantly better survival.
Statins
It has long been appreciated that the biologic effects of statins extend beyond their ability to lower LDL levels. These so-called pleiotropic effects include ischemic stroke prevention, decreased vascular smooth muscle proliferation and reduced reactive oxygen species production; these effects are thought to be mediated by the inhibition of several isoprenylated proteins or isoprenoids (Liao 2002; Arnaud, Veillard et al. 2005; Greenwood and Mason 2007). Murine models have demonstrated a role for statins in the prevention of ARDS (Jacobson, Barnard et al. 2005). Prospective observational human studies have revealed conflicting results; with some suggesting that prehospital statin use is protective against ALI/ARDS in patients with sepsis (Kruger, Fitzsimmons et al. 2006; Falagas, Makris et al. 2008; O'Neal, Koyama et al. 2011) and others showing no clear benefit (Bajwa, Malhotra et al. 2012). A recently complected large, multicenter, randomized, double blinded prospective clinical trial comparing rosuvastatin to placebo in patients with sepsis-associated ARDS failed to show any meaningful benefit of statin therapy in fully established ARDS (Truwit, Bernard et al. 2014).
The discordance between prehospital statin use and a trend toward benefit and the lack of benefit seen with initiation of statins in established ARDS is likely multifactorial. Whether initiation of statins at the time of hospitalization for those at high risk but before the development of ARDS would be beneficial merits further research.
RAAS blockers
There is compelling evidence to suggest that a prominent role in the development of ARDS is played by local pulmonary vascular angiotensin converting enzyme (ACE) activity. For example high levels of ACE have been isolated from broncheoalveolar lavage fluid in subjects with ARDS versus non-ARDS controls (Idell, Kueppers et al. 1987). Additionally, homozygosity for the polymorphism in the gene encoding ACE associated with higher enzyme activity levels has been shown to be an independent risk factor for the development of ARDS (Marshall, Webb et al. 2002). Elegant in vitro studies have linked angiotensin II, the downstream product of ACE activity, to human lung fibroblast collagen production in a bleomycin-toxicity model. This was attenuated by ACE inhibitor or angiotensin receptor blocker therapy (Marshall, Gohlke et al. 2004). As such, inhibition of the renin-angiotensin aldosterone axis has been of interest as a potential target for prevention of ARDS, and there have been encouraging preclinical data in this regard.
For example in a rat model of chemically induced ARDS, captopril therapy markedly improved several surrogate markers of severity of ARDS including circulating endothelial cells, PaO2, and wet to dry lung weight ratio (Liu and Zhao 2002). In another ARDS model using rats subjected to ventilator induced lung injury, captopril significantly reduced markers of inflammation and apoptosis versus in untreated controls. (Wosten-van Asperen, Lutter et al. 2008) In a similarly designed study, Yao showed losartan to be effective at mitigating the ventilator-induced lung injury in rats (Yao, Feng et al. 2008)
Human data are somewhat conflicting but suggest a protective effect of ACE inhibitor or angiotensin receptor blocker (ARB) therapy in selected populations. In a large prospective trial, chronic ACE inhibitor use was associated with lower rates of pneumonia versus placebo, however this benefit was limited only to Asian population (Ohkubo, Chapman et al. 2004). In her review of the interplay between diabetes, hyperglycemia and therapies commonly prescribed for diabetic patients, Honiden noted that the decreased rate of mortality from community-acquired pneumonia noted among Caucasians chronically taking ACEI/ARB therapy only extended to diabetic patients (Honiden and Gong 2009). Interestingly, diabetic patients have been shown to be at decreased risk of developing ARDS compared to non-diabetic patients (Yu, Christiani et al. 2013). A retrospective review of 1423 patients admitted to ICU in Rochester, Minnesota showed a statistically significant reduction in the risk of developing ARDS in those patients taking ACEI/ARB therapy chronically prior to hospitalization, with an odds ratio of 0.49, 95% CI 0.25 – 0.94 (Trillo-Alvarez CA 2009). The LIPS cohort study however did not find any statistically significant decrease in the incidence of ARDS among patients at high risk for developing ARDS taking an ACEI/ARB prior to hospitalization. On the contrary, the data suggested a trend towards worsening mortality outcomes for those patients taking ACEI/ARB therapy who developed ARDS (Watkins TR 2011).
These disparate findings perhaps highlight the interplay between genetic and environmental factors in the pathogenesis of ARDS and the need for ongoing prospective research investigating, which patients could benefit from the use of ACEI/ARB therapy for the prevention of ARDS, if any.
PPAR agonists
Peroxisome proliferator-activated receptors (PPAR) are a family of intracellular receptors which translocate to the nucleus and modulate gene expression in response to ligand binding. These are well known for their effects with regard to glucose and lipid metabolism but are also important mediators of cellular proliferation and inflammation and are expressed in vascular endothelium, smooth muscle cells and various leukocytes (Brown and Plutzky 2007). Animal models have shown PPAR gamma to be implicated in the inflammation associated with asthma, COPD and pulmonary fibrosis (Belvisi and Hele 2008). As such PPAR agonists, particularly the thiazolidinediones have gained attention as having potential utility in the prevention of ARDS. There is ample animal data to suggest a role may exist for thiazolidinedione therapy in the prevention of ARDS. Rosiglitazone has been shown to protect lung tissue in bleomycin toxicity, endotoxemia, pancreatitis, and hyperoxia (Honiden and Gong 2009).
To date no prospective human studies involving the use of PPAR agonists have been performed. The association of thiazolidinedione with worsening congestive heart failure in diabetic patients might have resulted in less interest in investigating this class of drugs.
Inhaled Delivery
The appeal of aerosol or nebulized delivery systems for medications lies in the ability to potentially deliver effective doses of agent while minimizing or wholly avoiding systemic side effects. Parallels can be drawn to inhaled medications already available for an array of chronic pulmonary disorders or bronchopulmonary infections.
Inhaled Corticosteroids
Investigators have examined the preventative and therapeutic roles of inhaled corticosteroids (ICS) in animal models of acute lung injury (ALI) through direct and indirect pulmonary insults. ((Forsgren, Modig et al. 1990; Walther, Jansson et al. 1992; Walther, Jansson et al. 1993; Wang, Zhang et al. 2002; Wang, Zhang et al. 2004; Jansson, Eriksson et al. 2005). Despite heterogeneity in their methodology, these investigators have reported remarkably uniform observations with regard to the ability of ICS to attenuate lung injury. Related to this, ICS are commonly used medications with an excellent safety profile in humans; however, to date no investigators have performed randomized studies of ICS for the prevention or early treatment of ARDS in human subjects. In a secondary analysis of the LIPS cohort, pre-hospitalization ICS exhibited raw protective signal towards ARDS development. Even though signal weakened after comprehensive adjustment with propensity matched analysis, the estimate of effect remained consistent suggesting clinical equipoise and implications for further investigations in role of ICS in ALI/ARDS prevention. (Festic, Ortiz-Diaz et al.)
Beta Agonists
Besides dysregulated inflammation, another hallmark of ARDS involves accumulation of protein-rich exudative edema fluid in the airspaces of the lung. Beta agonists increase the rate of vectorial transport of salt and water across normal epithelium as well as in animal models of ALI. (Matthay, Folkesson et al.) As such, beta agonists may be effective as a preventative therapy due to their ability to enhance resolution of pulmonary edema fluid and maintain endothelial barrier functions under baseline conditions. (Matthay, Folkesson et al.). The first ARDS prevention randomized controlled study of beta agonists have been recently reported. Perkins et al. studied 362 patients undergoing esophagectomy in 12 centers in the United Kingdom over a 3-year period. The incidence of ARDS did not differ between salmeterol and placebo groups, however, postoperative adverse events (primarily pneumonia) were significantly less frequent in the intervention group. Moreover, in a substudy of 53 patients with available plasma samples, salmeterol reduced several biomarkers of alveolar inflammation and epithelial injury. (Schein, Bergman et al. 1987)
Combined inhaled corticosteroids and beta agonists
Given the bronchodilating as well as anti-inflammatory properties of beta agonists, it has been proposed that they may act in synergy when used in combination with ICS. Seventy percent of patients on ICS in the secondary analysis of LIPS cohort (Festic, Ortiz-Diaz et al.) were concomitantly receiving inhaled beta agonists. Based on this experience, investigators initiated a multi-center, double-blind, placebo-controlled, randomized trial evaluating the combined effect of early administered budesonide and formoterol on lung function in patients at risk of ARDS (NCT01783821). This trial has been designed to test the previous observational study results and provide essential data for a future multi-center phase-III trial.
Heparin/Thrombolytics
The interplay between local alveolar inflammation and dysregulated coagulation with fibrin deposition/accumulation is integral to the pathogenesis of ARDS. This makes agents targeting the coagulation cascade attractive potential therapeutic options. Although systemic administration of anticoagulant medications would possess the same proposed effect, it carries significant risk of bleeding complications, leading to the investigation into use of inhaled anticoagulant medications.
Heparin is unique in its ability to bind not only antithrombin III, but dozens of other so-called ‘heparin binding proteins’ such as complement proteins, interferons, fibroblast growth factor and other cytokines (Young 2008). These biologic actions likely mediate the previously documented but not completely understood anti-inflammatory effects of heparinoids. Miller et al. recently published a review of available human and animal data relating to the use of inhaled heparin and heparin related compounds in smoke inhalation injury. Collectively these data show decreased morbidity and may suggest potential mortality benefit for the use of inhaled heparin in smoke inhalation injury without increasing markers of coagulopathy or increasing the risk of bleeding complications (Miller, Elamin et al. 2014). Whether these data will have generalizable applications to the at-risk ARDS population remains to be seen, and further investigation into the utility of routine use of inhaled heparin in smoke inhalation injury is merited and already registered in the clinicaltrial.gov database (#NCT014548690).
In a murine model of hemorrhagic shock, it has been shown that microthrombus formation precedes the subsequent accumulation of leukocytes and is likely due to sluggish blood-flow in the low-pressure pulmonary circulation (Conhaim, Mangino et al. 2010). As such, inhaled thrombolytic therapy has been of interest for prevention of ARDS and has been studied in rat models showing mitigation of microthrombus formation and subsequent leukocyte accumulation (Conhaim, Watson et al. 2014).
Hypertonic saline
Hypertonic saline has been established as a safe and effective tool in the management of cystic fibrosis (Elkins, Robinson et al. 2006), and non-CF bronchiectasis (Kellett and Robert 2011). It is postulated that in addition to its effects on sputum viscosity, the efficacy of nebulized hypertonic saline is due at least in part to its ability to inhibit neutrophil and macrophage mediated cytokine production (Cuschieri, Gourlay et al. 2002), (Zallen, Moore et al. 2000), (Deitch, Shi et al. 2003). In a rat model of traumatic shock, Wohlauer et al. demonstrated a convincing protective effect of inhaled hypertonic saline against pulmonary vascular permeability, neutrophil chemotaxis, matrix metalloprotease activity, IL-8 activity and histologic changes consistent with ARDS (Wohlauer, Moore et al. 2012). A recent preclinical study demonstrated the cytoprotective effect of extracellular hypertonicity induced by aerosolized hypertonic saline on alveolar resident cells, resulting in their resistance to injury. (Brian, Rolf et al.). Although routine use of intravenous hypertonic saline for resuscitation of hypovolemic shock in trauma was shown not to improve mortality (Bulger, May et al. 2011) owing to disturbance of coagulation cascade, inhaled hypertonic saline is unlikely to have this systemic effect. A prospective study is being planned by the LIPS investigators.
N-Acetyl Cysteine
N-Acetyl Cysteine (NAC) is known for its ability to reduce the burden of oxidative stress by maintaining intracellular stores of reduced glutathione (Lauterburg, Corcoran et al. 1983). There is considerable evidence that oxidative stress stemming from depleted glutathione stores contributes to the pathogenesis of ARDS (Soltan-Sharifi, Mojtahedzadeh et al. 2007). In one small prospective clinical trial enrolling patients with smoke inhalation injury, the combination of inhaled NAC, heparin and albuterol were superior to albuterol and placebo with respect to mortality and lung injury scores (Miller, Rivero et al. 2009). Two additional clinical trials enrolling patients with active ARDS or undergoing esophagectomy (high risk surgery) have shown promise for intravenous NAC with regards to surrogate markers of pulmonary morbidity (Zingg, Hofer et al. 2007), (Soltan-Sharifi, Mojtahedzadeh et al. 2007). Owing to its well-established safety profile and promising preliminary clinical data, NAC should be of particular interest moving forward.
On The Horizon
Myriad additional agents are at various stages of preclinical and/or clinical investigation with regard to their potential use in the prevention of ARDS. Focus of our attention to these agents is not all inclusive as this area of research is rapidly evolving.
In a recently published, industry-sponsored phase 1/2 trial, Interferon-β-1a (FP-1201) showed promising, significant results with regard to improving oxygenation and 28 day mortality (OR 0.19, 95% CI 0.03 – 0.72) in 37 patients receiving treatment vs 59 matched controls with established ARDS. The proposed mechanism is via decreased vascular leakage through upregulation of pulmonary capillary CD73 (Bellingan, Maksimow et al. 2014).
Mesenchymal stem cell (MSC) therapy has been shown in animal models of sepsis to reduce markers of inflammation and incidence/severity of ARDS (Mei, Haitsma et al. 2010), and decrease histopathologic evidence of ARDS in ex-vivo and in-vitro studies using human lungs and lung tissue (Lee, Krasnodembskaya et al. 2013). An additional recently published study using human MSC in a rat ARDS model improved lung compliance, oxygenation, alveolar edema and promoted return to normal lung architecture (Hayes, Masterson et al. 2014). MSC have been shown to lead to more efficient tissue repair, and their use in ARDS is an exciting clinical prospect. One challenge of translating this promising bench research to the clinical setting is the optimal route of delivery of stem cell therapy; which in animal models has shown promise via the intravenous and intratracheal routes. Two clinical trials are ongoing (NCT01775774, NCT01902082) to assess the optimal doses of IV MSC therapy, and an additional phase 1 trial (NCT01632475) is underway assessing the safety of intratracheal administration of MSC in neonates with severe bronchopulmonary dysplasia (Lee, Rocco et al. 2014).
It has been postulated that at least part of the protective effects of exogenous MSC administration is mediated via their ability to secrete Keratinocyte Growth Factor (KGF) (Zhu, Feng et al. 2014). As such, KGF has been under investigation as a therapeutic agent in and of itself. Pretreatment with KGF prior to pulmonary acid injury in mice decreased markers of inflammation (Yano, Deterding et al. 1996). The mechanism by which KGF protects pulmonary epithelial cells from apoptosis and promotes survival and epithelial integrity has been elucidated in exquisite detail (Bao, Wang et al. 2005). KGF therefore may prove to have direct applications in the prevention of ARDS.
Curcumin, a natural phenol found in the spice turmeric has gained attention for its potential ability to modulate mammalian inflammation. In a murine model of virally induced ARDS, pretreatment with curcumin significantly reduced a number of surrogate severity markers of ARDS (Avasarala, Zhang et al. 2013). There has been interest among LIPS investigators to further study prospectively curcumin for ARDS prevention (personal communication, Ruxana Sadikot, MD).
Adrenomedullin, a vasoactive peptide hormone initially isolated from pheochromocytoma tissue has been gaining interest as a potential biomarker for ARDS and may have therapeutic potential. Rat models of ARDS have shown exogenous adrenomedullin to be a potent inhibitor of lung injury in gut ischemia/reperfusion (Dwivedi, Wu et al. 2007) as well as endotoxinemia (Itoh, Obata et al. 2007). The potential role of this agent for humans remains to be seen and to our knowledge the only ongoing research using human subjects involves its use as a biomarker in various conditions.
Conclusion
Years of research on pharmacologic therapy for established ARDS have not yielded a single effective medication. However, this might not have been in vain. Many of the previously studied pharmacologic compounds, as well as new ones, when used early in the disease evolution may prove effective in the prevention of ARDS. We especially favor medications that have been proven to be safe, inexpensive and widely available. As such, these medications should be first studied in smaller, phase 2 trials that are much more feasible.
The completed LIPS-A trial and ongoing LIPS-B trial have already demonstrated the feasibility of early administration of systemic and inhaled medications to patients deemed at risk for ARDS development, frequently started while the patient is still in the emergency room. We postulate that overall morbidity and mortality rates from ARDS in the future will be contingent upon decreasing the overall incidence of ARDS through effective identification of those at risk and early application of proven supportive care and pharmacologic interventions.
Investigators, however, need to change the way of conducting randomized clinical trials to discern effective interventions. Some of the proposed strategies should include the following: smaller phase II trials of relatively homogenous patient populations, assessment of outcome variables other than mortality, focus on patient-centered outcomes including longer-term outcomes, pragmatic and adaptive clinical trials, as well as the “platform” trials, which could simultaneously evaluate multiple treatments efficiently.
Supplementary Material
Acknowledgments
Funding: This study was done in part with the support of NIH 5KL2 TR000136 and UL1 TR000135 grants and Mayo Clinic Foundation.
Footnotes
Conflict of Interest: Neither author has any potential conflicts of interest related to the manuscript.
References
- Acute Respiratory Distress Syndrome Network. Ventilation with lower tidal volumes as compared with traditional tidal volumes for acute lung injury and the acute respiratory distress syndrome. The New England journal of medicine. 2000;342(18):1301–1308. doi: 10.1056/NEJM200005043421801. [DOI] [PubMed] [Google Scholar]
- Arnaud C, Veillard NR, et al. Cholesterol-independent effects of statins in inflammation, immunomodulation and atherosclerosis. Current drug targets. Cardiovascular & haematological disorders. 2005;5(2):127–134. doi: 10.2174/1568006043586198. [DOI] [PubMed] [Google Scholar]
- Avasarala S, Zhang F, et al. Curcumin modulates the inflammatory response and inhibits subsequent fibrosis in a mouse model of viral-induced acute respiratory distress syndrome. PloS one. 2013;8(2):e57285. doi: 10.1371/journal.pone.0057285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bajwa EK, Malhotra CK, et al. Statin therapy as prevention against development of acute respiratory distress syndrome: an observational study. Critical care medicine. 2012;40(5):1470–1477. doi: 10.1097/CCM.0b013e3182416d7a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bao S, Wang Y, et al. Keratinocyte growth factor induces Akt kinase activity and inhibits Fas-mediated apoptosis in A549 lung epithelial cells. American journal of physiology. Lung cellular and molecular physiology. 2005;288(1):L36–L42. doi: 10.1152/ajplung.00309.2003. [DOI] [PubMed] [Google Scholar]
- Beitler JR, Schoenfeld DA, et al. Preventing ARDS: progress, promise, and pitfalls. Chest. 2014;146(4):1102–1113. doi: 10.1378/chest.14-0555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bellingan G, Maksimow M, et al. The effect of intravenous interferon-beta-1a (FP-1201) on lung CD73 expression and on acute respiratory distress syndrome mortality: an open-label study. The Lancet. Respiratory medicine. 2014;2(2):98–107. doi: 10.1016/S2213-2600(13)70259-5. [DOI] [PubMed] [Google Scholar]
- Belvisi MG, Hele DJ. Peroxisome proliferator-activated receptors as novel targets in lung disease. Chest. 2008;134(1):152–157. doi: 10.1378/chest.08-0019. [DOI] [PubMed] [Google Scholar]
- Bosma KJ, Taneja R, et al. Pharmacotherapy for prevention and treatment of acute respiratory distress syndrome: current and experimental approaches. Drugs. 2010;70(10):1255–1282. doi: 10.2165/10898570-000000000-00000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brian W, Rolf H, et al. The Use Of Hypertonic Saline Aerosols As Cytoprotective Adjunct In Murine Hydrochloric Aspiration And Ventilation Induced Injury. D24. THE VILLAIN BEHIND VENTILATOR-INDUCED LUNG INJURY. American Thoracic Society. :A5446–A5446. [Google Scholar]
- Brown JD, Plutzky J. Peroxisome proliferator-activated receptors as transcriptional nodal points and therapeutic targets. Circulation. 2007;115(4):518–533. doi: 10.1161/CIRCULATIONAHA.104.475673. [DOI] [PubMed] [Google Scholar]
- Bulger EM, May S, et al. Out-of-hospital hypertonic resuscitation after traumatic hypovolemic shock: a randomized, placebo controlled trial. Annals of surgery. 2011;253(3):431–441. doi: 10.1097/SLA.0b013e3181fcdb22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cepkova M, Matthay MA. Pharmacotherapy of acute lung injury and the acute respiratory distress syndrome. Journal of intensive care medicine. 2006;21(3):119–143. doi: 10.1177/0885066606287045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen W, Janz DR, et al. Prehospital Aspirin Use Is Associated With Reduced Risk of Acute Respiratory Distress Syndrome in Critically Ill Patients: A Propensity-Adjusted Analysis. Critical care medicine. 2014 doi: 10.1097/CCM.0000000000000789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conhaim RL, Mangino MJ, et al. Microthrombus formation may trigger lung injury after acute blood loss. Shock. 2010;34(6):601–607. doi: 10.1097/SHK.0b013e3181e46e2a. [DOI] [PubMed] [Google Scholar]
- Conhaim RL, Watson KE, et al. Inhaled thrombolytics reduce lung microclot and leukocyte infiltration after acute blood loss. Shock. 2014;41(6):528–536. doi: 10.1097/SHK.0000000000000149. [DOI] [PubMed] [Google Scholar]
- Cuschieri J, Gourlay D, et al. Hypertonic preconditioning inhibits macrophage responsiveness to endotoxin. Journal of immunology. 2002;168(3):1389–1396. doi: 10.4049/jimmunol.168.3.1389. [DOI] [PubMed] [Google Scholar]
- Deitch EA, Shi HP, et al. Hypertonic saline resuscitation limits neutrophil activation after trauma-hemorrhagic shock. Shock. 2003;19(4):328–333. doi: 10.1097/00024382-200304000-00006. [DOI] [PubMed] [Google Scholar]
- Dwivedi AJ, Wu R, et al. Adrenomedullin and adrenomedullin binding protein-1 prevent acute lung injury after gut ischemia-reperfusion. Journal of the American College of Surgeons. 2007;205(2):284–293. doi: 10.1016/j.jamcollsurg.2007.03.012. [DOI] [PubMed] [Google Scholar]
- Elkins MR, Robinson M, et al. A controlled trial of long-term inhaled hypertonic saline in patients with cystic fibrosis. The New England journal of medicine. 2006;354(3):229–240. doi: 10.1056/NEJMoa043900. [DOI] [PubMed] [Google Scholar]
- Erlich JM, Talmor DS, et al. Prehospitalization antiplatelet therapy is associated with a reduced incidence of acute lung injury: a population-based cohort study. Chest. 2011;139(2):289–295. doi: 10.1378/chest.10-0891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Estenssoro E, Dubin A, et al. Incidence, clinical course, and outcome in 217 patients with acute respiratory distress syndrome. Critical care medicine. 2002;30(11):2450–2456. doi: 10.1097/00003246-200211000-00008. [DOI] [PubMed] [Google Scholar]
- Falagas ME, Makris GC, et al. Statins for infection and sepsis: a systematic review of the clinical evidence. The Journal of antimicrobial chemotherapy. 2008;61(4):774–785. doi: 10.1093/jac/dkn019. [DOI] [PubMed] [Google Scholar]
- Festic E, Bansal V, et al. SpO2/FiO2 Ratio on Hospital Admission Is an Indicator of Early Acute Respiratory Distress Syndrome Development Among Patients at Risk. Journal of intensive care medicine. 2013 doi: 10.1177/0885066613516411. [DOI] [PubMed] [Google Scholar]
- Festic E, Ortiz-Diaz E, et al. Prehospital use of inhaled steroids and incidence of acute lung injury among patients at risk. Journal of Critical Care. 28(6):985–991. doi: 10.1016/j.jcrc.2013.08.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forsgren PE, Modig JA, et al. Prophylactic treatment with an aerosolized corticosteroid liposome in a porcine model of early ARDS induced by endotoxaemia. Acta Chirurgica Scandinavica. 1990;156(6–7):423–431. [PubMed] [Google Scholar]
- Frieri M. Corticosteroid effects on cytokines and chemokines. Allergy and asthma proceedings : the official journal of regional and state allergy societies. 1999;20(3):147–159. doi: 10.2500/108854199778553082. [DOI] [PubMed] [Google Scholar]
- Gajic O, Dabbagh O, et al. Early identification of patients at risk of acute lung injury: evaluation of lung injury prediction score in a multicenter cohort study. American Journal of Respiratory and Critical Care Medicine. 2011;183(4):462–470. doi: 10.1164/rccm.201004-0549OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gajic O, Rana R, et al. Transfusion-related acute lung injury in the critically ill: prospective nested case-control study. American Journal of Respiratory and Critical Care Medicine. 2007;176(9):886–891. doi: 10.1164/rccm.200702-271OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greenwood J, Mason JC. Statins and the vascular endothelial inflammatory response. Trends in immunology. 2007;28(2):88–98. doi: 10.1016/j.it.2006.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayes M, Masterson C, et al. Therapeutic Efficacy of Human Mesenchymal Stromal Cells in the Repair of Established Ventilator-induced Lung Injury in the Rat. Anesthesiology. 2014 doi: 10.1097/ALN.0000000000000545. [DOI] [PubMed] [Google Scholar]
- Honiden S, Gong MN. Diabetes, insulin, and development of acute lung injury. Critical care medicine. 2009;37(8):2455–2464. doi: 10.1097/CCM.0b013e3181a0fea5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Idell S, Kueppers F, et al. Angiotensin converting enzyme in bronchoalveolar lavage in ARDS. Chest. 1987;91(1):52–56. doi: 10.1378/chest.91.1.52. [DOI] [PubMed] [Google Scholar]
- Itoh T, Obata H, et al. Adrenomedullin ameliorates lipopolysaccharide-induced acute lung injury in rats. American journal of physiology. Lung cellular and molecular physiology. 2007;293(2):L446–L452. doi: 10.1152/ajplung.00412.2005. [DOI] [PubMed] [Google Scholar]
- Jacobson JR, Barnard JW, et al. Simvastatin attenuates vascular leak and inflammation in murine inflammatory lung injury. American journal of physiology. Lung cellular and molecular physiology. 2005;288(6):L1026–L1032. doi: 10.1152/ajplung.00354.2004. [DOI] [PubMed] [Google Scholar]
- Jansson AH, Eriksson C, et al. Effects of budesonide and N-acetylcysteine on acute lung hyperinflation, inflammation and injury in rats. Vascul Pharmacol. 2005;43(2):101–111. doi: 10.1016/j.vph.2005.03.006. [DOI] [PubMed] [Google Scholar]
- Kellett F, Robert NM. Nebulised 7% hypertonic saline improves lung function and quality of life in bronchiectasis. Respiratory medicine. 2011;105(12):1831–1835. doi: 10.1016/j.rmed.2011.07.019. [DOI] [PubMed] [Google Scholar]
- Khan H, Belsher J, et al. Fresh-frozen plasma and platelet transfusions are associated with development of acute lung injury in critically ill medical patients. Chest. 2007;131(5):1308–1314. doi: 10.1378/chest.06-3048. [DOI] [PubMed] [Google Scholar]
- Kor DJ, Erlich J, et al. Association of prehospitalization aspirin therapy and acute lung injury: results of a multicenter international observational study of at-risk patients. Critical care medicine. 2011;39(11):2393–2400. doi: 10.1097/CCM.0b013e318225757f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kor DJ, Lingineni RK, et al. Predicting risk of postoperative lung injury in high-risk surgical patients: a multicenter cohort study. Anesthesiology. 2014;120(5):1168–1181. doi: 10.1097/ALN.0000000000000216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kor DJ, Talmor DS, et al. Lung Injury Prevention with Aspirin (LIPS-A): a protocol for a multicentre randomised clinical trial in medical patients at high risk of acute lung injury. BMJ open. 2012;2(5) doi: 10.1136/bmjopen-2012-001606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kor DJ, Warner DO, et al. Derivation and diagnostic accuracy of the surgical lung injury prediction model. Anesthesiology. 2011;115(1):117–128. doi: 10.1097/ALN.0b013e31821b5839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kovalovsky D, Refojo D, et al. Molecular mechanisms and Th1/Th2 pathways in corticosteroid regulation of cytokine production. Journal of neuroimmunology. 2000;109(1):23–29. doi: 10.1016/s0165-5728(00)00298-8. [DOI] [PubMed] [Google Scholar]
- Kruger P, Fitzsimmons K, et al. Statin therapy is associated with fewer deaths in patients with bacteraemia. Intensive care medicine. 2006;32(1):75–79. doi: 10.1007/s00134-005-2859-y. [DOI] [PubMed] [Google Scholar]
- Lauterburg BH, Corcoran GB, et al. Mechanism of action of N-acetylcysteine in the protection against the hepatotoxicity of acetaminophen in rats in vivo. The Journal of clinical investigation. 1983;71(4):980–991. doi: 10.1172/JCI110853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee JW, Krasnodembskaya A, et al. Therapeutic effects of human mesenchymal stem cells in ex vivo human lungs injured with live bacteria. American Journal of Respiratory and Critical Care Medicine. 2013;187(7):751–760. doi: 10.1164/rccm.201206-0990OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee JW, Rocco PR, et al. Mesenchymal Stem Cell Therapy for Acute Respiratory Distress Syndrome: A Light at the End of the Tunnel? Anesthesiology. 2014 doi: 10.1097/ALN.0000000000000546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levitt JE, Bedi H, et al. Identification of early acute lung injury at initial evaluation in an acute care setting prior to the onset of respiratory failure. Chest. 2009;135(4):936–943. doi: 10.1378/chest.08-2346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li G, Malinchoc M, et al. Eight-year trend of acute respiratory distress syndrome: a population-based study in Olmsted County, Minnesota. American Journal of Respiratory and Critical Care Medicine. 2011;183(1):59–66. doi: 10.1164/rccm.201003-0436OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liao JK. Isoprenoids as mediators of the biological effects of statins. The Journal of clinical investigation. 2002;110(3):285–288. doi: 10.1172/JCI16421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu H, Zhao J. An experimental study of therapeutic effect of ACEI on chemical-induced ARDS in rats. Zhonghua yu fang yi xue za zhi [Chinese journal of preventive medicine] 2002;36(2):93–96. [PubMed] [Google Scholar]
- Looney MR, Nguyen JX, et al. Platelet depletion and aspirin treatment protect mice in a two-event model of transfusion-related acute lung injury. The Journal of clinical investigation. 2009;119(11):3450–3461. doi: 10.1172/JCI38432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marshall RP, Gohlke P, et al. Angiotensin II and the fibroproliferative response to acute lung injury. American journal of physiology. Lung cellular and molecular physiology. 2004;286(1):L156–L164. doi: 10.1152/ajplung.00313.2002. [DOI] [PubMed] [Google Scholar]
- Marshall RP, Webb S, et al. Angiotensin converting enzyme insertion/deletion polymorphism is associated with susceptibility and outcome in acute respiratory distress syndrome. American Journal of Respiratory and Critical Care Medicine. 2002;166(5):646–650. doi: 10.1164/rccm.2108086. [DOI] [PubMed] [Google Scholar]
- Matthay MA, Folkesson HG, et al. Lung epithelial fluid transport and the resolution of pulmonary edema. Physiological Reviews. 82(3):569–600. doi: 10.1152/physrev.00003.2002. [DOI] [PubMed] [Google Scholar]
- Matthay MA, Ware LB, et al. The acute respiratory distress syndrome. The Journal of clinical investigation. 2012;122(8):2731–2740. doi: 10.1172/JCI60331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mei SH, Haitsma JJ, et al. Mesenchymal stem cells reduce inflammation while enhancing bacterial clearance and improving survival in sepsis. American Journal of Respiratory and Critical Care Medicine. 2010;182(8):1047–1057. doi: 10.1164/rccm.201001-0010OC. [DOI] [PubMed] [Google Scholar]
- Miller AC, Elamin EM, et al. Inhaled anticoagulation regimens for the treatment of smoke inhalation-associated acute lung injury: a systematic review. Critical care medicine. 2014;42(2):413–419. doi: 10.1097/CCM.0b013e3182a645e5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller AC, Rivero A, et al. Influence of nebulized unfractionated heparin and N-acetylcysteine in acute lung injury after smoke inhalation injury. Journal of burn care & research : official publication of the American Burn Association. 2009;30(2):249–256. doi: 10.1097/BCR.0b013e318198a268. [DOI] [PubMed] [Google Scholar]
- National Heart, L., N. Blood Institute Acute Respiratory Distress Syndrome Clinical Trials et al. Comparison of two fluid-management strategies in acute lung injury. New England Journal of Medicine. 2006;354(24):2564–2575. doi: 10.1056/NEJMoa062200. [DOI] [PubMed] [Google Scholar]
- O'Neal HR, Jr, Koyama T, et al. Prehospital statin and aspirin use and the prevalence of severe sepsis and acute lung injury/acute respiratory distress syndrome. Critical care medicine. 2011;39(6):1343–1350. doi: 10.1097/CCM.0b013e3182120992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohkubo T, Chapman N, et al. Effects of an angiotensin-converting enzyme inhibitor-based regimen on pneumonia risk. American Journal of Respiratory and Critical Care Medicine. 2004;169(9):1041–1045. doi: 10.1164/rccm.200309-1219OC. [DOI] [PubMed] [Google Scholar]
- Ortiz-Diaz E, Festic E, et al. Emerging pharmacological therapies for prevention and early treatment of acute lung injury. Seminars in respiratory and critical care medicine. 2013;34(4):448–458. doi: 10.1055/s-0033-1351118. [DOI] [PubMed] [Google Scholar]
- Phua J, Badia JR, et al. Has mortality from acute respiratory distress syndrome decreased over time?: A systematic review. American Journal of Respiratory and Critical Care Medicine. 2009;179(3):220–227. doi: 10.1164/rccm.200805-722OC. [DOI] [PubMed] [Google Scholar]
- Ranieri VM, Rubenfeld GD, et al. Acute respiratory distress syndrome: the Berlin Definition. JAMA. 2012;307(23):2526–2533. doi: 10.1001/jama.2012.5669. [DOI] [PubMed] [Google Scholar]
- Rivers E, Nguyen B, et al. Early goal-directed therapy in the treatment of severe sepsis and septic shock. The New England journal of medicine. 2001;345(19):1368–1377. doi: 10.1056/NEJMoa010307. [DOI] [PubMed] [Google Scholar]
- Rosenthal VD, Rodrigues C, et al. Effectiveness of a multidimensional approach for prevention of ventilator-associated pneumonia in adult intensive care units from 14 developing countries of four continents: findings of the International Nosocomial Infection Control Consortium. Critical care medicine. 2012;40(12):3121–3128. doi: 10.1097/CCM.0b013e3182657916. [DOI] [PubMed] [Google Scholar]
- Schein RM, Bergman R, et al. Complement activation and corticosteroid therapy in the development of the adult respiratory distress syndrome. Chest. 1987;91(6):850–854. doi: 10.1378/chest.91.6.850. [DOI] [PubMed] [Google Scholar]
- Seam N, Meduri GU, et al. Effects of methylprednisolone infusion on markers of inflammation, coagulation, and angiogenesis in early acute respiratory distress syndrome. Critical care medicine. 2012;40(2):495–501. doi: 10.1097/CCM.0b013e318232da5e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soltan-Sharifi MS, Mojtahedzadeh M, et al. Improvement by N-acetylcysteine of acute respiratory distress syndrome through increasing intracellular glutathione, and extracellular thiol molecules and anti-oxidant power: evidence for underlying toxicological mechanisms. Human & experimental toxicology. 2007;26(9):697–703. doi: 10.1177/0960327107083452. [DOI] [PubMed] [Google Scholar]
- Song C, Suzuki S, et al. Effects of antiplatelet agents on pulmonary haemodynamic response to fMLP in endotoxin primed rats. Thorax. 2004;59(1):39–44. doi: 10.1136/thx.2003.002022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson BT. Glucocorticoids and acute lung injury. Critical care medicine. 2003;31(4 Suppl):S253–S257. doi: 10.1097/01.CCM.0000057900.19201.55. [DOI] [PubMed] [Google Scholar]
- Thompson BT. The Berlin Definition of ARDS versus Pathological Evidence of Diffuse Alveolar Damage. American Journal of Respiratory and Critical Care Medicine. 2014;187:675–677. doi: 10.1164/rccm.201302-0385ed. [DOI] [PubMed] [Google Scholar]
- Toy P, Gajic O, et al. Transfusion-related acute lung injury: incidence and risk factors. Blood. 2012;119(7):1757–1767. doi: 10.1182/blood-2011-08-370932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trillo-Alvarez CA, et al. American Thoracic Society. San Diego: American Journal of Respiratory and Critical Care Medicine; 2009. Chronic Use of Angiotensin Pathway Inhibitors Is Associated with a Decreased Risk of Acute Respiratory Distress Syndrome. [Google Scholar]
- Truwit JD, Bernard GR, et al. Rosuvastatin for sepsis-associated acute respiratory distress syndrome. The New England journal of medicine. 2014;370(23):2191–2200. doi: 10.1056/NEJMoa1401520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walther S, Jansson I, et al. Pulmonary granulocyte accumulation is reduced by nebulized corticosteroid in septic pigs. Acta Anaesthesiol Scand. 1992;36(7):651–655. doi: 10.1111/j.1399-6576.1992.tb03537.x. [DOI] [PubMed] [Google Scholar]
- Walther S, Jansson I, et al. Corticosteroid by aerosol in septic pigs--effects on pulmonary function and oxygen transport. Intensive Care Med. 1993;19(3):155–160. doi: 10.1007/BF01720531. [DOI] [PubMed] [Google Scholar]
- Wang J, Zhang L, et al. Inhaled budesonide in experimental chlorine gas lung injury: influence of time interval between injury and treatment. Intensive Care Medicine. 2002;28(3):352–357. doi: 10.1007/s00134-001-1175-4. [DOI] [PubMed] [Google Scholar]
- Wang J, Zhang L, et al. Administration of aerosolized terbutaline and budesonide reduces chlorine gas-induced acute lung injury. Journal of Trauma-Injury Infection & Critical Care. 2004;56(4):850–862. doi: 10.1097/01.ta.0000078689.45384.8b. [DOI] [PubMed] [Google Scholar]
- Watkins TR, et al. American Thoracic Society. Denver, CO: American Journal of Respiratory and Critical Care Medicine; 2011. Use of Angiotensin Converting Enzyme Inhibitors or Angiotensin Receptor Blockers and Clinical Outcomes Among Patients At-Risk for Acute Lung Injury. [Google Scholar]
- Wohlauer M, Moore EE, et al. Nebulized hypertonic saline attenuates acute lung injury following trauma and hemorrhagic shock via inhibition of matrix metalloproteinase-13. Critical care medicine. 2012;40(9):2647–2653. doi: 10.1097/CCM.0b013e3182592006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wosten-van Asperen RM, Lutter R, et al. ACE mediates ventilator-induced lung injury in rats via angiotensin II but not bradykinin. The European respiratory journal. 2008;31(2):363–371. doi: 10.1183/09031936.00060207. [DOI] [PubMed] [Google Scholar]
- Yano T, Deterding RR, et al. Keratinocyte growth factor reduces lung damage due to acid instillation in rats. American journal of respiratory cell and molecular biology. 1996;15(4):433–442. doi: 10.1165/ajrcmb.15.4.8879176. [DOI] [PubMed] [Google Scholar]
- Yao S, Feng D, et al. Losartan attenuates ventilator-induced lung injury. The Journal of surgical research. 2008;145(1):25–32. doi: 10.1016/j.jss.2007.03.075. [DOI] [PubMed] [Google Scholar]
- Yealy DM, Kellum JA, et al. A randomized trial of protocol-based care for early septic shock. The New England journal of medicine. 2014;370(18):1683–1693. doi: 10.1056/NEJMoa1401602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yiming MT, Lederer DJ, et al. Platelets enhance endothelial adhesiveness in high tidal volume ventilation. American journal of respiratory cell and molecular biology. 2008;39(5):569–575. doi: 10.1165/rcmb.2007-0332OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young E. The anti-inflammatory effects of heparin and related compounds. Thrombosis Research. 2008;122(6):743–752. doi: 10.1016/j.thromres.2006.10.026. [DOI] [PubMed] [Google Scholar]
- Yu S, Christiani DC, et al. Role of diabetes in the development of acute respiratory distress syndrome. Critical care medicine. 2013;41(12):2720–2732. doi: 10.1097/CCM.0b013e318298a2eb. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu Z, Ouyang J-P, et al. Dexamethasone attenuated endotoxin-induced acute lung injury through inhibiting expression of inducible nitric oxide synthase. Clinical Hemorheology & Microcirculation. 2009;41(2):117–125. doi: 10.3233/CH-2009-1162. [DOI] [PubMed] [Google Scholar]
- Zallen G, Moore EE, et al. Hypertonic saline resuscitation abrogates neutrophil priming by mesenteric lymph. The Journal of trauma. 2000;48(1):45–48. doi: 10.1097/00005373-200001000-00008. [DOI] [PubMed] [Google Scholar]
- Zarbock A, Ley K. The role of platelets in acute lung injury (ALI) Frontiers in bioscience. 2009;14:150–158. doi: 10.2741/3236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zarbock A, Singbartl K, et al. Complete reversal of acid-induced acute lung injury by blocking of platelet-neutrophil aggregation. The Journal of clinical investigation. 2006;116(12):3211–3219. doi: 10.1172/JCI29499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu YG, Feng XM, et al. Human mesenchymal stem cell microvesicles for treatment of Escherichia coli endotoxin-induced acute lung injury in mice. Stem cells. 2014;32(1):116–125. doi: 10.1002/stem.1504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zingg U, Hofer CK, et al. High dose N-acetylcysteine to prevent pulmonary complications in partial or total transthoracic esophagectomy: results of a prospective observational study. Diseases of the esophagus : official journal of the International Society for Diseases of the Esophagus / I.S.D.E. 2007;20(5):399–405. doi: 10.1111/j.1442-2050.2007.00690.x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.