Abstract
To respond to infection, resting or naïve T cells must undergo activation, clonal expansion, and differentiation into specialized functional subsets of effector T cells. However, to prevent excessive or self-destructive immune responses, regulatory T cells (Tregs) are instrumental in suppressing the activation and function of effector cells, including effector T cells. The transcription factor Forkhead box P3 (Foxp3) regulates the expression of genes involved in the development and function of Tregs. Foxp3 interacts with other transcription factors and with epigenetic elements such as histone deacetylases (HDACs) and histone acetyltransferases. Treg suppressive function can be increased by exposure to HDAC inhibitors. The individual contributions of different HDAC family members to Treg function and their respective mechanisms of action, however, remain unclear. A study showed that HDAC6, HDAC9, and Sirtuin-1 had distinct effects on Foxp3 expression and function, suggesting that selectively targeting HDACs individually or in combination may enhance Treg stability and suppressive function. Another study showed that the receptor programmed death 1 (PD-1), a well-known inhibitor of T cell activation, halted cell cycle progression in effector T cells by inhibiting the transcription of the gene encoding the substrate-recognition component (Skp2) of the ubiquitin ligase SCFSkp2. Together, these findings reveal new signaling targets for enhancing Treg or effector T cell function that may be helpful in designing future therapies, either to increase Treg suppressive function in transplantation and autoimmune diseases or to block PD-1 function, thus increasing the magnitude of antiviral or antitumor immune responses of effector T cells.
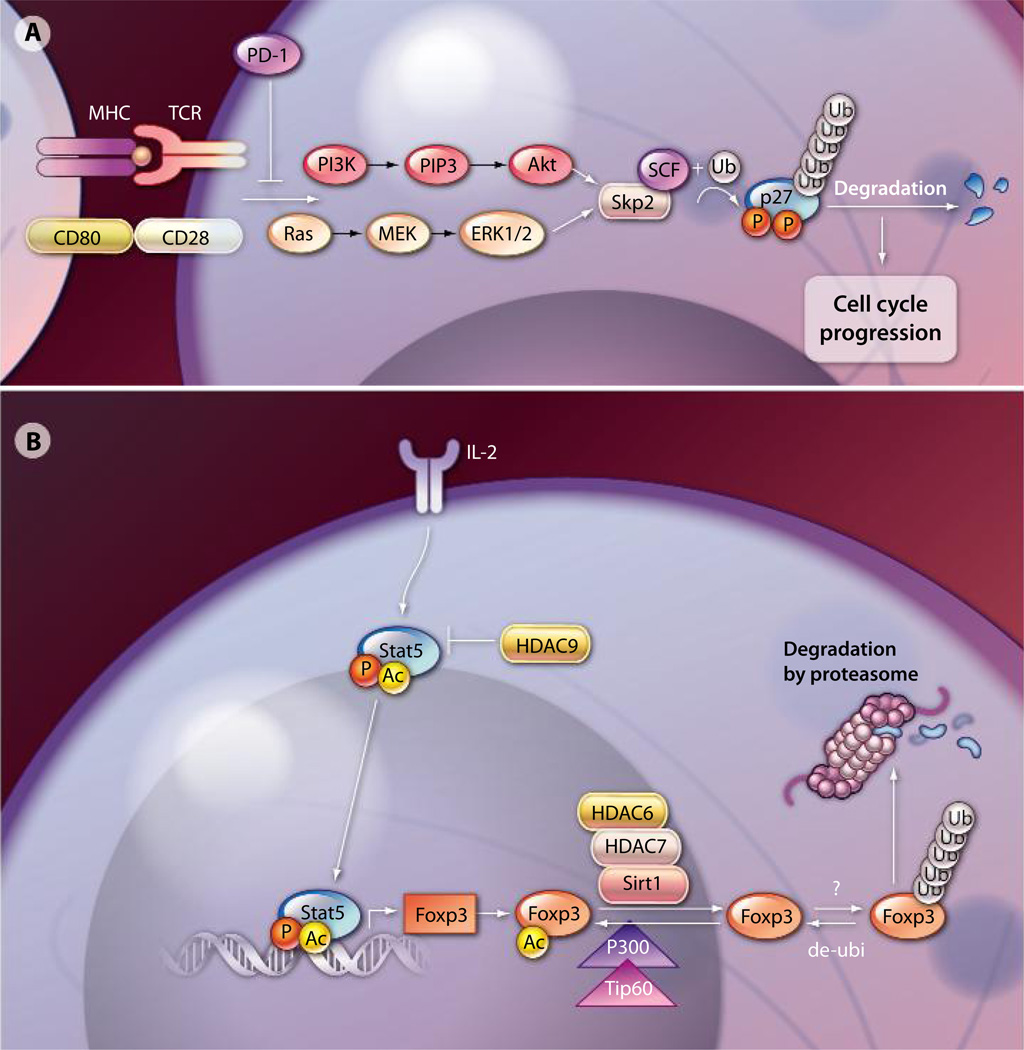
T cell activation requires two signals. Signal 1 arises from T cell receptor (TCR) engagement by the major histocompatability complex (MHC) and its cognate antigen. The critical element of signal 2 is provided by CD28 costimulation by the antigen-presenting cell (APC), which activates phosphatidylinositol 3-kinase (PI3K) and its downstream target Akt (also known as protein kinase B), leading to increased abundance of glucose transporters on the plasma membrane and an increase in glycolytic enzyme activity. Blocking PI3K activation would effectively prevent T cell activation. Programmed cell death protein 1 (PD-1), a cell-surface molecule serving as an inhibitory receptor, inhibits the CD28-mediated activation of PI3K upon engagement of PD-1 with its ligand (1–3). However, the molecular mechanism by which PD-1 affects cell cycle progression and T cell proliferation remains largely unknown. Patsoukis et al. demonstrated that PD-1 activation halted the cell cycle in the G1 phase by inhibiting both PI3K and the Rasmitogen–activated and extracellular signal– regulated kinase kinase (MEK) pathway, as well as extracellular signal–regulated kinase (ERK) signaling pathways (4). This important signaling network is required for expression of the gene encoding interleukin 2 (IL-2) and T cell proliferation. Patsoukis et al. showed that a key aspect of PD-1–mediated suppression of T cell activation through the cell cycle machinery was the negative effect of PD-1 signaling on the expression of Skp2, the substrate-recognition component of ubiquitin ligase SCFSkp2. By interrupting the SCFSkp2-mediated activation of cyclin-dependent kinases (CDKs), PD-1–mediated suppressive signals prevented T cell proliferation by locking T cells in the G1 phase (4) (Fig. 1A). This mechanistic insight may provide potential therapeutic opportunities to increase effector T cell immune responses against cancer and viral infection by targeting PD-1 signaling.
Fig. 1.
(A) PD-1 signaling in effector T cells. PD-1 inhibits the PI3K and Ras pathways resulting in decreased activity of the ubiquitin ligase SCFSkp2. This ligase promotes the activation of CDKs and is involved in cell cycle progression. (B) Transcriptional and posttranslational regulation of Foxp3. HATs and HDACs have opposing roles in the modification of Foxp3 acetylation status. Acetylation of Foxp3 relies on the accessibility of HATs (Tip60 and p300) or HDACs (HDAC6, HDAC7, HDAC9, and Sirt1), as well as the activity of the enzymes. Deacetylated Foxp3 can be further ubiquitinated by E3 ligases and is subject to the proteasome-dependent degradation pathway. AC inducates acetylation.
The engagement of PD-1 signaling leads to the inhibition of effector T cell activation. Regulatory T cells (Tregs), on the other hand, are dedicated suppressor cells, playing a pivotal role in the control of immunological self-tolerance and immune responses to pathogens and tumor antigens (5–13). The Forkhead family transcription factor Foxp3 is the master transcription factor for Treg development and function (8–13). Scurfy mice, which lack Foxp3, are deficient in Tregs and develop severe lymphoproliferative autoimmune disease. Furthermore, mutations in the Foxp3 gene in humans give rise to immune dysregulation and polyendocrinopathy enteropathy X-linked syndrome (IPEX), which is a life-threatening severe autoimmune disorder (8–13). Tregs can reverse or even cure established autoimmune diseases, and Treg therapies can be efficacious in controlling autoimmune responses in organ and cell transplantation in animal models (5–13). The application of Tregs or the enhancement of their suppressive function to cure autoimmune diseases and prevent organ transplant rejection and graft-versus-host disease (GVHD) after bone marrow transplantation in humans is an active area of research (8–14). Recent phase I clinical trials have shown that Tregs are safe and well tolerated in patients and have potential efficacy in treating GVHD (15–18). However, one major problem associated with Treg therapy is that the phenotype of the administered Tregs is unstable, and there is potential loss of suppressive activity over time in vivo (18). A better understanding of the factors governing Treg function and stability will be crucial for the advancement of Treg therapy to the clinic.
The methylation states of distinct regions of DNA in Foxp3 contribute to the stability or relative instability of the Treg phenotype. Repeated in vitro activation of human Tregs results in CpG island methylation within a conserved region of the Foxp3 gene, which precipitates a loss of its expression and leads to proinflammatory cytokine production by the conversion of Tregs into effector T cells (19). In contrast, pharmacological inhibition of DNA methylation in vivo increases the number of Tregs and enhances the suppression of diabetes in mice (20).
Adding another layer of complexity, another mechanism for the regulation of Foxp3 at the protein level integrates physiological cues from the microenvironment, such as hypoxia (21, 22). This ubiquitination-dependent pathway is likely to play a role in the newly appreciated potential for metabolic control of Treg and effector T cell balance. Because ubiquitination-mediated removal of Foxp3 protein inhibits Treg function, it is reasonable to argue that inhibiting Foxp3 degradation should have potential therapeutic implications.
Acetylation is another important posttranslational modification of Foxp3 that affects its stability and activity. Acetylation of Foxp3 is regulated by components of a Foxp3-associated supermolecular complex containing multiple histone acetyltransferases (HATs), histone deacetylases (HDACs), and other transcriptional co-regulators (23). HATs and HDACs play defining roles in the regulation of Foxp3 activity (Fig. 1B); thus, it is reasonable to expect that modulating their activity will correspondingly affect Treg suppressive activity. For example, acetylation of Foxp3 by the HAT p300 can be reversed by the histone deacetylase Sirtuin-1 (Sirt1) (24). HDAC9, on the other hand, co-localizes with Foxp3 in resting Tregs. HDAC9 interaction with Foxp3 can be disrupted by TCR engagement because of the translocation of HDAC9 from the nucleus to the cytosol, which can be reversed by the pretreatment of Tregs with an HDAC inhibitor such as trichostatin A (25). HDAC9 interaction with and deacetylation of Foxp3 destabilizes Treg-specific transcriptional programs. Hancock and colleagues previously demonstrated that either exposure to HDAC inhibitors (26) or genetic deletion of HDAC family members (27) increases the suppressive capacity of both murine and human Foxp3+ Tregs by enhancing Foxp3 acetylation.
Beier et al. (28) further dissected the molecular mechanism by which isotype-specific inhibition or deletion of HDACs affects Treg function. The authors demonstrated that Treg function was significantly enhanced by inhibition of Sirt1. They also provided evidence to suggest that HDAC6 deacetylated Foxp3 in the nucleus. The loss of HDAC6 promoted the longevity of acetylated Foxp3, with a consequent increase in the resistance of Foxp3 to proteasomal degradation. In addition, they showed that HDAC6 was involved in promoting the activities of other transcriptional factors required for Treg function, such as cyclic adenosine monophosphate response element–binding protein. They further showed that the loss of HDAC9, but not other HDACs, was associated with stabilization of the acetylated form of IL-2–mediated signal transducer and activator of transcription 5 (STAT5) and promoted its transcriptional activity in Tregs (28).
Given that HDAC6, HDAC9, and Sirt1 possess distinct mechanisms of enhancing Treg suppressive activity, Beier et al. also explored the efficacy of combinatorial targeting of HDAC6, HDAC9, and Sirt1. The combined loss of HDAC6, HDAC9, and Sirt1 augmented Treg function, and pharmacological inhibition of Sirt1 and HDAC6 was very effective in enhancing Treg function in vivo (28). These studies provide a framework for further testing other small-molecule inhibitors of HDACs in relevant mouse models, as well as in clinical trials. A combined therapy using HDAC inhibitors in conjunction with small molecules targeting posttranslational pathways such as E3 ligase–mediated protein degradation warrants further investigation.
In summary, the dissection of the mechanisms underlying key processes of immune regulation—namely, PD-1 signaling in effector T cells and the activity of the transcription factor Foxp3 in Tregs—revealed important roles for these signaling molecules in effector T cell and Treg function. Furthermore, these studies provide new avenues to explore the potential benefits of modulating the activities of these molecules and their signaling pathways. Further investigation of these targets is likely to yield promising immunotherapies against autoimmune diseases or viral infection and cancer by enhancing Treg or effector T cell function, respectively.
Acknowledgments
Work in the Pan laboratory is supported by an NIH grant. F.P. is a recipient of a Stewart Trust Scholar award and a young investigator award from the Melanoma Research Alliance.
References and Notes
- 1.Parry RV, Chemnitz JM, Frauwirth KA, Lanfranco AR, Braunstein I, Kobayashi SV, Linsley PS, Thompson CB, Riley JL. CTLA-4 and PD-1 receptors inhibit T-cell activation by distinct mechanisms. Mol. Cell. Biol. 2005;25:9543–9553. doi: 10.1128/MCB.25.21.9543-9553.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Francisco LM, Salinas VH, Brown KE, Vanguri VK, Freeman GJ, Kuchroo VK, Sharpe AH. PD-L1 regulates the development, maintenance, and function of induced regulatory T cells. J. Exp. Med. 2009;206:3015–3029. doi: 10.1084/jem.20090847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Francisco LM, Sage PT, Sharpe AH. The PD-1 pathway in tolerance and autoimmunity. Immunol. Rev. 2010;236:219–242. doi: 10.1111/j.1600-065X.2010.00923.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Patsoukis N, Brown J, Petkova V, Liu F, Li L, Boussiotis VA. Selective effects of PD-1 on Akt and Ras pathways regulate molecular components of the cell cycle and inhibit T cell proliferation. Sci. Signal. 2012;5:ra46. doi: 10.1126/scisignal.2002796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sakaguchi S, Sakaguchi N, Asano M, Itoh M, Toda M. Immunologic self-tolerance maintained by activated T cells expressing IL-2 receptor alpha-chains (CD25). Breakdown of a single mechanism of self-tolerance causes various autoimmune diseases. J. Immunol. 1995;155:1151–1164. [PubMed] [Google Scholar]
- 6.Asano M, Toda M, Sakaguchi N, Sakaguchi S. Autoimmune disease as a consequence of developmental abnormality of a T cell subpopulation. J. Exp. Med. 1996;184:387–396. doi: 10.1084/jem.184.2.387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Thornton AM, Shevach EM. CD4+CD25+ immunoregulatory T cells suppress polyclonal T cell activation in vitro by inhibiting interleukin 2 production. J. Exp. Med. 1998;188:287–296. doi: 10.1084/jem.188.2.287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wing K, Sakaguchi S. Regulatory T cells exert checks and balances on self tolerance and autoimmunity. Nat. Immunol. 2010;11:7–13. doi: 10.1038/ni.1818. [DOI] [PubMed] [Google Scholar]
- 9.Littman DR, Rudensky AY. Th17 and regulatory T cells in mediating and restraining inflammation. Cell. 2010;140:845–858. doi: 10.1016/j.cell.2010.02.021. [DOI] [PubMed] [Google Scholar]
- 10.Campbell DJ, Koch MA. Phenotypical and functional specialization of FOXP3+ regulatory T cells. Nat. Rev. Immunol. 2011;11:119–130. doi: 10.1038/nri2916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Feuerer M, Hill JA, Mathis D, Benoist C. Foxp3+ regulatory T cells: Differentiation, specification, subphenotypes. Nat. Immunol. 2009;10:689–695. doi: 10.1038/ni.1760. [DOI] [PubMed] [Google Scholar]
- 12.Sakaguchi S, Miyara M, Costantino CM, Hafler DA. FOXP3+ regulatory T cells in the human immune system. Nat. Rev. Immunol. 2010;10:490–500. doi: 10.1038/nri2785. [DOI] [PubMed] [Google Scholar]
- 13.O’Shea JJ, Paul WE. Mechanisms underlying lineage commitment and plasticity of helper CD4+ T cells. Science. 2010;327:1098–1102. doi: 10.1126/science.1178334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Fan H, Cao P, Game DS, Dazzi F, Liu Z, Jiang S. Regulatory T cell therapy for the induction of clinical organ transplantation tolerance. Semin. Immunol. 2011;23:453–461. doi: 10.1016/j.smim.2011.08.012. [DOI] [PubMed] [Google Scholar]
- 15.Trzonkowski P, Bieniaszewska M, Juścińska J, Dobyszuk A, Krzystyniak A, Marek N, Myśliwska J, Hellmann A. First-in-man clinical results of the treatment of patients with graft versus host disease with human ex vivo expanded CD4+CD25+CD127− T regulatory cells. Clin. Immunol. 2009;133:22–26. doi: 10.1016/j.clim.2009.06.001. [DOI] [PubMed] [Google Scholar]
- 16.Brunstein CG, Miller JS, Cao Q, McKenna DH, Hippen KL, Curtsinger J, Defor T, Levine BL, June CH, Rubinstein P, McGlave PB, Blazar BR, Wagner JE. Infusion of ex vivo expanded T regulatory cells in adults transplanted with umbilical cord blood: Safety profile and detection kinetics. Blood. 2011;117:1061–1070. doi: 10.1182/blood-2010-07-293795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Di Ianni M, Falzetti F, Carotti A, Terenzi A, Castellino F, Bonifacio E, Del Papa B, Zei T, Ostini RI, Cecchini D, Aloisi T, Perruccio K, Ruggeri L, Balucani C, Pierini A, Sportoletti P, Aristei C, Falini B, Reisner Y, Velardi A, Aversa F, Martelli MF. Tregs prevent GVHD and promote immune reconstitution in HLA-haploidentical transplantation. Blood. 2011;117:3921–3928. doi: 10.1182/blood-2010-10-311894. [DOI] [PubMed] [Google Scholar]
- 18.Wang X, Lu L, Jiang S. Regulatory T cells: Customizing for the clinic. Sci. Transl. Med. 2011;3:83ps19. doi: 10.1126/scitranslmed.3001819. [DOI] [PubMed] [Google Scholar]
- 19.Hoffmann P, Boeld TJ, Eder R, Huehn J, Floess S, Wieczorek G, Olek S, Dietmaier W, Andreesen R, Edinger M. Loss of FOXP3 expression in natural human CD4+CD25+ regulatory T cells upon repetitive in vitro stimulation. Eur. J. Immunol. 2009;39:1088–1097. doi: 10.1002/eji.200838904. [DOI] [PubMed] [Google Scholar]
- 20.Zheng Q, Xu Y, Liu Y, Zhang B, Li X, Guo F, Zhao Y. Induction of Foxp3 demethylation increases regulatory CD4+CD25+ T cells and prevents the occurrence of diabetes in mice. J. Mol. Med. (Berlin) 2009;87:1191–1205. doi: 10.1007/s00109-009-0530-8. [DOI] [PubMed] [Google Scholar]
- 21.Dang EV, Barbi J, Yang HY, Jinasena D, Yu H, Zheng Y, Bordman Z, Fu J, Kim Y, Yen HR, Luo W, Zeller K, Shimoda L, Topalian SL, Semenza GL, Dang CV, Pardoll DM, Pan F. Control of TH17/Treg balance by hypoxia-inducible factor 1. Cell. 2011;146:772–784. doi: 10.1016/j.cell.2011.07.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Shi LZ, Wang R, Huang G, Vogel P, Neale G, Green DR, Chi H. HIF1alpha-dependent glycolytic pathway orchestrates a metabolic checkpoint for the differentiation of TH17 and Treg cells. J. Exp. Med. 2011;208:1367–1376. doi: 10.1084/jem.20110278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Li B, Samanta A, Song X, Iacono KT, Bembas K, Tao R, Basu S, Riley JL, Hancock WW, Shen Y, Saouaf SJ, Greene MI. FOXP3 interactions with histone acetyltransferase and class II histone deacetylases are required for repression. Proc. Natl. Acad. Sci. U.S.A. 2007;104:4571–4576. doi: 10.1073/pnas.0700298104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.van Loosdregt J, Vercoulen Y, Guichelaar T, Gent YY, Beekman JM, van Beekum O, Brenkman AB, Hijnen DJ, Mutis T, Kalkhoven E, Prakken BJ, Coffer PJ. Regulation of Treg functionality by acetylation-mediated Foxp3 protein stabilization. Blood. 2010;115:965–974. doi: 10.1182/blood-2009-02-207118. [DOI] [PubMed] [Google Scholar]
- 25.Akimova T, Beier UH, Liu Y, Wang L, Hancock WW. Histone/protein deacetylases and T-cell immune responses. Blood. 2012;119:2443–2451. doi: 10.1182/blood-2011-10-292003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Akimova T, Ge G, Golovina T, Mikheeva T, Wang L, Riley JL, Hancock WW. Histone/protein deacetylase inhibitors increase suppressive functions of human FOXP3+ Tregs. Clin. Immunol. 2010;136:348–363. doi: 10.1016/j.clim.2010.04.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Tao R, de Zoeten EF, Ozkaynak E, Chen C, Wang L, Porrett PM, Li B, Turka LA, Olson EN, Greene MI, Wells AD, Hancock WW. Deacetylase inhibition promotes the generation and function of regulatory T cells. Nat. Med. 2007;13:1299–1307. doi: 10.1038/nm1652. [DOI] [PubMed] [Google Scholar]
- 28.Beier UH, Wang L, Han R, Akimova T, Liu Y, Hancock WW. Histone deacetylases 6 and 9 and sirtuin-1 control foxp3+ regulatory T cell function through shared and isoform-specific mechanisms. Sci. Signal. 2012;5:ra45. doi: 10.1126/scisignal.2002873. [DOI] [PMC free article] [PubMed] [Google Scholar]