Abstract
OBJECTIVE
To evaluate the association between serum prostate-specific antigen (PSA) concentration from a screening test and prostate cancer mortality in an Asian population.
METHODS
We included 118,665 men in the Korean Heart Study, a large prospective cohort study of participants who voluntarily underwent private health examinations that included PSA-based prostate cancer screening. The baseline visit occurred between January 1994 and December 2004, and follow-up was through December 2011. Deaths from prostate cancer were ascertained from the underlying cause of death from a computerized search of death certificate data from the National Statistical Office in Korea. We used the Cox proportional hazards regression to estimate the association between serum PSA and risk of prostate cancer death adjusting the baseline age, cigarette smoking status, and body mass index.
RESULTS
During 1,381,901 person-years of follow-up, 6036 men died of any cause, and of these, 56 men died of prostate cancer. The multivariate-adjusted hazard ratio for prostate cancer death statistically significantly increased across PSA concentrations (P trend <.0001). The hazard ratio increased 7% per 1-ng/mL increase in PSA. The association between PSA concentration and death from prostate cancer was stronger in younger than in older men and in heavier than leaner men.
CONCLUSION
In conclusion, an increased screening PSA level is associated with an increased risk of prostate cancer death in Korean men. Our findings may have implications for the development of targeted PSA cutpoints for biopsy recommendation.
Prostate cancer is the second most diagnosed cancer worldwide and is a leading cause of cancer mortality in Western and other developed countries.1 The number of men diagnosed with prostate cancer is rapidly increasing around the world; the annual percentage increase in Korea was 12.6% from 1999 to 2010.2
The measurement of serum prostate-specific antigen (PSA) concentration has been used extensively in the United States since the early 1990s to screen for prostate cancer. The US Preventive Services Task Force does not currently recommend PSA-based prostate cancer screening after an assessment of the risks vs the benefits,3 whereas the American Urological Association continues to recommend its use in the urologic setting for screening of men aged 55-69 years old.4 Nevertheless, use of PSA to screen for prostate cancer has been partially credited with the observed decline in prostate cancer mortality in the United States.5
Prostate cancer mortality rates have steadily decreased over the past 10 years, including in the United States6 and Europe,7 but are increasing in some Asian countries including Korea2 and Eastern European countries.8 Aside from the United States, PSA-based prostate cancer screening has not been routinely performed globally, although uptake has occurred to some extent.8 Thus, it is unknown whether the decline in the prostate cancer mortality rate observed in some countries is due to PSA screening or improved treatment of prostate cancer,5 or other cause.
Prospective studies using banked blood specimens have reported that the higher the serum PSA concentration the greater the incidence of prostate cancer years to decades later.9 However, only a few prospective observational studies of men without a diagnosis of prostate cancer at baseline have reported on the association between serum PSA concentration in banked specimens and prostate cancer mortality.10,11 No prospective studies have been conducted to address this association in Asian countries, where the prostate cancer mortality rate is lower than that in North America and Europe,8 which, in part, might be attributable to differences in access to PSA testing.12
Thus, the objective of this study was to evaluate the association between serum PSA concentration from a screening test and prostate cancer mortality in an Asian population. We analyzed data from the Korean Heart Study, a large prospective cohort study of participants who voluntarily underwent private health examinations that included PSA-based prostate cancer screening.
METHODS
Study Population
The Korean Heart Study is a prospective cohort study formed from data from men and women who have voluntarily undergone private health examinations in 18 health promotion centers located in 1 capital and 6 provinces in South Korea from 1996 to 2004.13 We conducted this analysis using data from 15 of the 18 centers that provided approval for research on cause-specific mortality. South Korean citizens are assigned a personal identification number at birth. This number was used to link the participants’ health examination records with death registers. The linkage resulted in 521,585 individuals aged 20 years older at their health examination that occurred at the earliest between January 1994 and December 2004. That health examination was considered to be their baseline.
Of this population, 296,771 men were the source population for this prostate cancer study. From these men, we excluded 101,001 men with missing information on PSA level; information was missing because 2 of the centers did not perform PSA-based prostate cancer screening, and in several of the centers, the men had to opt-into an optional examination that included a screening PSA test. Men excluded from the analysis for missing PSA information had a similar age-standardized prostate cancer mortality rate as the men included in the analysis, although their all-cause mortality rate was higher (Supplementary Table 1). We also excluded 612 men who already had a cancer diagnosis of any site by their baseline health assessment and 76,483 participants with missing baseline smoking status, body mass index (BMI), systolic or diastolic blood pressure, total cholesterol, and fasting glucose information. The final analytical study sample included 118,665 men.
Under the January 2005 Bioethics and Safety Act (no. 7159) of the Ministry of Health and Welfare, the Korean Heart study was conducted as a retrospective study was performed using the data collected from individuals who underwent medical examinations before December 2004 (waiver of informed consent). The Institutional Review Board of Human Research of Yonsei University and all the individual health promotion centers participating in the Korean Heart Study approved the investigation.
Data Collection
In this study, we used data, including on PSA concentration that were collected during the voluntary private health examinations. We obtained the following data from the health examination records: smoking status (never, past, or current smoker), history of cancer, cardiovascular disease, diabetes or hypertension, history of use of medications for diabetes or hypertension, weight and height, which were measured during health examination while the examinees were wearing light clothing, and blood pressure, which was measured either by registered nurses or technicians.
We obtained from the health examination record serum total PSA concentration, which was measured immunochemically using the ADVIA Centaur XP Immunoassay (Siemens Diagnostics, Deerfield, IL), which is standardized to the World Health Organization international reference standard for PSA (90:10) 96/670 and has an assay range of 0.01-100 ng/mL. Per the health promotion center protocol, samples with a PSA concentration >100 ng/mL were diluted and remeasured. We also obtained from the health examination record serum total cholesterol, triglycerides, high-density lipoprotein cholesterol, and fasting glucose concentrations, and among other clinical chemistry tests, which were measured at each health promotion center using auto analyzers. Per the health promotion center protocol, each measurement laboratory had internal and external quality control procedures as required by the Korean Association of Laboratory Quality Control.13
Follow-up for Mortality
The men were followed up from their baseline health examination occurring between 1994 and 2004 to death or December 30, 2011, whichever was earlier. The primary outcome for this analysis was death from prostate cancer (International Classification of Diseases-10 code C61). Deaths were ascertained from the underlying cause of death on death certificates. Computerized searches of death certificate data were provided by the National Statistical Office in Korea.
Statistical Analysis
In the analysis, we used the PSA test performed at the baseline health examination. For the main analysis, we used the same cutpoints (<1.0, 1.0-1.9, 2.0-2.9, 3.0-3.0, 4.0-9.9, and ≥10.0 ng/mL) as were used in previous studies,11 rather than distributional cutpoints. The PSA 3.0-3.9 ng/mL category had few deaths, and thus, we combined PSA 2.0-2.9 and 3.0- 3.9 ng/mL categories. Therefore, we divided the men into 5 PSA concentration categories (<1.0, 1.0-1.9, 2.0-3.9, 4.0-9.9, and ≥10.0 ng/mL). In an alternative analysis, we divided PSA concentration into quintiles. We calculated age-specific and overall mortality rates for all causes and prostate cancer. To evaluate the association between PSA concentration and risk of prostate cancer death, we used the Cox proportional hazards regression and adjusted for risk factors for death from prostate cancer—age, cigarette smoking status,14 and BMI15 at the baseline. To avoid the possible confounding effects of health status on mortality, in a subanalysis, we excluded the person-time at risk and deaths from prostate cancer during the first 2 years of follow-up. We stratified the multivariate-adjusted analysis by the baseline age (cutpoint at the median of 60 years) and BMI (cutpoint at 25 kg/m2). Statistical interaction between PSA concentration and age or BMI was assessed by likelihood ratio tests. We repeated the main analysis for prostate cancer mortality using a competing risks model.16 Analyses were performed using Stata, version 12. All statistical tests were 2 sided, and P <.05 was considered to be statistically significant.
RESULTS
At the baseline, the average age of the men included in the analysis was 47.1 years, and mean follow-up was 11.6 years. Men who had higher PSA concentrations were older and after adjusting for age were slightly leaner, had lower fasting glucose concentrations, and were less likely to currently smoke (Table 1). The distribution of selected characteristics of the men by age category is shown in Supplementary Table 2. Of the 118,665 men and during 1,381,901 person-years of follow-up, 6036 men died of any cause, and of these, 56 men died of prostate cancer (Table 2). The prostate cancer mortality rates (per 100,000 person-years) by age category were as follows: 0.2 for 40-49 years, 4.6 for 50-59 years, 11.5 for 60-69 years, and 99.3 for ≥70 years (Table 2). The all-cause mortality rates (per 100,000 person-years) were as follows: 186.9 for 40-49 years, 524.5 for 50-59 years, 1359.2 for 60-69 years, and 3916.9 for ≥70 years (Table 2).
Table 1.
Baseline characteristics by serum PSA concentration at baseline, Korean Heart Study, 1994-2011*
| Serum PSA Concentration, ng/mL | P Trend | |||||
|---|---|---|---|---|---|---|
| <1.0 | 1.0-1.9 | 2.0-3.9 | 4.0-9.9 | ≥10.0 | ||
| N | 71,524 | 36,809 | 8313 | 1747 | 272 | |
| Mean (SE) age, y | 45.6 (0.0) | 48.1 (0.1) | 52.6 (0.1) | 56.4 (0.2) | 60.3 (0.6) | <.0001 |
| Mean (SE) body mass index, kg/m2 | 23.9 (0.0) | 23.8 (0.0) | 23.7 (0.0) | 23.8 (0.1) | 23.6 (0.2) | <.0001 |
| Mean (SE) systolic blood pressure, mm Hg | 124.0 (0.1) | 123.8 (0.1) | 124.6 (0.2) | 125.1 (0.4) | 123.8 (1.0) | .033 |
| Mean (SE) diastolic blood pressure, mm Hg | 77.9 (0.0) | 78.1 (0.1) | 78.2 (0.1) | 77.3 (0.3) | 75.0 (0.7) | .65 |
| Mean (SE) total cholesterol, mg/dL | 195.1 (0.1) | 195.6 (0.2) | 195.1 (0.4) | 194.2 (0.8) | 193.1 (2.1) | .65 |
| Mean (SE) fasting glucose, mg/dL | 101.2 (0.1) | 99.1 (0.1) | 98.6 (0.3) | 97.1 (0.6) | 96.0 (1.5) | <.0001 |
| Mean (SE) PSA, ng/mL | 0.6 (0.0) | 1.3 (0.0) | 2.5 (0.0) | 5.5 (0.0) | 19.3 (0.0) | <.0001 |
| Smoking status (%) | ||||||
| Never | 22.2 | 21.8 | 23.3 | 25.3 | 26.5 | <.0001 |
| Past | 23.8 | 27.2 | 30.9 | 33.0 | 34.9 | |
| Current | 54.1 | 51.0 | 45.8 | 41.7 | 38.6 | |
PSA, prostate-specific antigen; SE, standard error of the mean.
Adjusted for age.
Table 2.
Number of deaths and age-specific mortality rates for deaths from all causes and prostate cancer, Korean Heart Study, 1994-2011
| Age (y) | Number of Men (%) | Person-years at Risk | Number of Deaths (Mortality Rate Per 100,000 Person-years)
|
|
|---|---|---|---|---|
| All Causes | Prostate Cancer | |||
| 20-29 | 3637 (3.1) | 40,765 | 31 (76.1) | 0 (0.0) |
| 30-39 | 25,697 (21.7) | 296,668 | 291 (98.1) | 0 (0.0) |
| 40-49 | 43,018 (36.3) | 507,842 | 949 (186.9) | 1 (0.2) |
| 50-59 | 30,816 (26.0) | 367,753 | 1929 (524.5) | 17 (4.6) |
| 60-69 | 13,330 (11.2) | 147,734 | 2008 (1359.2) | 17 (11.5) |
| ≥70 | 2167 (1.8) | 21,139 | 828 (3916.9) | 21 (99.3) |
| Total | 118,665 (100.0) | 1,381,901 | 6036 (436.8*) | 56 (4.1*) |
The rate in the study population; not standardized to an external age distribution.
The age-adjusted and multivariate-adjusted hazard ratios (HRs) for prostate cancer death statistically significantly increased across PSA concentration (P trend <.0001; Table 3). Even men with a PSA concentration between 2.5 and <4 ng/mL, which is below the usual cutpoint for biopsy recommendation, appeared to have more than twice the risk of death from prostate cancer compared with men with a concentration <1 ng/mL. After excluding the first 2 years of follow-up, the association between PSA concentration and prostate cancer death was similar to overall (Table 3). When we considered competing risk analyses, the overall results were not changed (Supplementary Table 3). When we modeled PSA concentration in quintiles, the trends for the age-adjusted analysis, multivariate adjusted analysis, and the analysis excluding the first 2 years of follow-up remained statistically significant (data not shown).
Table 3.
Hazard ratio and 95% confidence interval of prostate cancer death by serum PSA concentration at baseline, Korean Heart Study, 1994-2011
| Serum PSA Concentration at Baseline, ng/mL | Deaths | Person-years | HR (95% CI)
|
||
|---|---|---|---|---|---|
| Age Adjusted | Adjusted for Age, BMI, and Smoking Status | Excluding the First 2 years of Follow-up* | |||
| <1.0 | 11 | 818,989.3 | 1.00 | 1.00 | 1.00 |
| 1-<2.0 | 14 | 441,292.5 | 1.57 (0.71-3.47) | 1.57 (0.71-3.47) | 1.88 (0.81-4.38) |
| 2-<4.0 | 9 | 98,878.0 | 2.40 (0.97-5.93) | 2.41 (0.97-5.95) | 2.85 (1.10-7.36) |
| 4-<10.0 | 5 | 19,707.7 | 4.31 (1.44-12.91) | 4.32 (1.44-13.0) | 4.09 (1.21-13.8) |
| ≥10.0 | 17 | 3033.4 | 64.4 (27.9-148.6) | 65.0 (28.2-150.0) | 72.4 (29.7-176.8) |
| Per 1-ng/mL increase in PSA | 1.07 (1.06-1.09) | 1.07 (1.06-1.09) | 1.08 (1.07-1.09) | ||
| P trend | <.0001 | <.0001 | <.0001 | ||
BMI, body mass index; CI, con3dence interval; HR, hazard ratio; other abbreviation as in Table 1.
Excluded 4 prostate cancer deaths and 236,698.3 person-years.
Unlike for prostate cancer death, the age-adjusted and multivariate-adjusted HRs for all-cause mortality did not linearly increase across increasing PSA concentration, although when modeling PSA as a continuous variable, a weak positive association of borderline statistical significance was observed (Supplementary Table 4).
For each 1-ng/mL increase in PSA concentration, the multivariate-adjusted HR of prostate cancer death increased by 7% (Table 3; Fig. 1). The association between PSA concentration and death from prostate cancer was stronger in men who were younger than in those who were older at the baseline (P interaction <.0001; Fig. 1) but was similar in men who were heavier and leaner at the baseline (P interaction = 0.12; Fig. 1).
Figure 1.
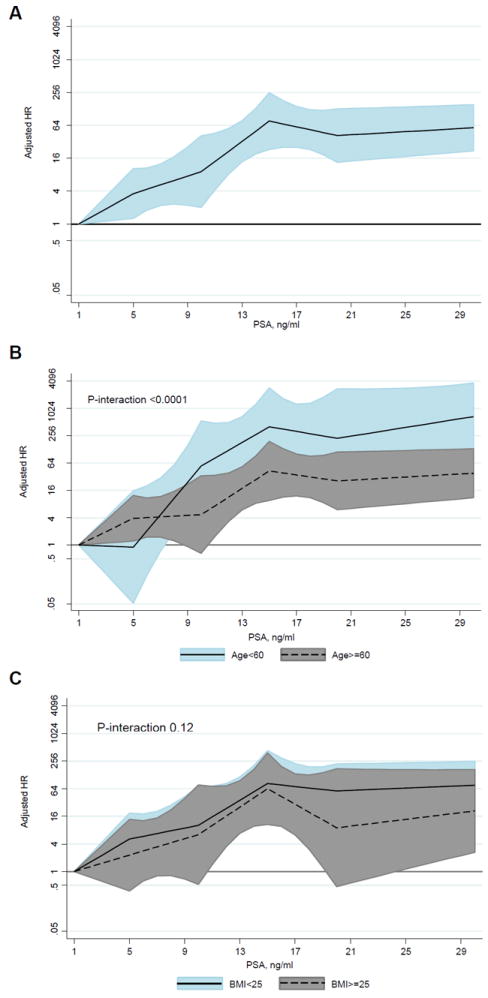
Multivariate-adjusted hazard ratio of prostate cancer mortality for screening prostate-specific antigen concentration overall (A), by baseline age (B), and body mass index (C). BMI, body mass index; HR, hazard ratio; PSA, prostate-specific antigen.
COMMENT
In this large prospective Korean cohort study, we found that the risk of death from prostate cancer increased with increasing serum PSA concentration in men screened for the disease, including when considering competing risks of death. The common threshold for biopsy is >4.0 ng/mL,17 but our results support that any PSA level when compared with ≤1 ng/mL is associated with an increased risk of death from prostate cancer in this Korean study population.
Our results on prostate cancer mortality support the results from previous studies examining PSA levels and risk of prostate cancer mortality.10,11,18 Unlike our study, which used PSA levels from prostate cancer screening in a prospective cohort study, most previous studies on prostate cancer mortality were prospective, but measured PSA in banked specimens for research purposes, or were case-control studies.
The association between baseline PSA levels and incidence of prostate cancer is well known.9 A previous study reported that even low levels of PSA (≤0.70 ng/mL) reflect a higher risk of prostate cancer.19 The most important goal of PSA screening is to reduce the risk of death from prostate cancer.20 Two major randomized trials on PSA screening and prostate cancer mortality have been published. In the US trial, prostate cancer mortality did not differ between the screen and control groups,21 but PSA screening reduced prostate cancer mortality in the European trial.22 Reasons for differences in the findings of the 2 trials have been extensively discussed. 23 The detection of prostate cancer is increased by PSA screening, but such screening also increases the rate of detection of cases that may never manifest clinically and may not have the potential to cause death.21,22 Early detection through PSA screening may lead to unnecessary biopsies, whereas postponing biopsy may lead to a missed opportunity for early treatment of men with prostate cancer.11 Therefore, it is important to detect and treat the appropriate men at the appropriate time in the natural history of this cancer.
We found that PSA concentration was more strongly associated with prostate cancer mortality in younger men (aged <60 years); the risk of prostate cancer death increased by 20% per 1 ng/mL in younger men and 7% per 1 ng/mL in older men. As men age, their PSA concentration increases because of increasing prostate volume24 and increasing prevalence of benign prostatic hyperplasia,25 and they experience competing causes of death (eg, from cardiovascular disease).26 Therefore, higher reference serum PSA ranges for use in screening for prostate cancer may be prudent in older men as Oesterling et al have suggested previously.24
Asian populations tend to have higher proportion of men diagnosed at an advanced stage of prostate cancer compared with Western population,12 despite a lower prostate cancer mortality rate.8 When comparing across countries, however, prostate cancer death rates and HRs by PSA concentration categories are similar (Supplementary Table 5). When compared across studies, age-specific PSA concentrations in Asian countries, the United States, and Europe are generally similar.12,27,28 A previous review suggested that differences in uptake of PSA screening and access to urology clinics could explain the discrepancy in the diagnosis of advanced stage disease.12
In our data, risk of all-cause mortality did not linearly increase across increasing PSA concentration, although when modeling PSA as a continuous variable, a weak positive association was observed. Unlike our study, 1 previous study reporting a significant relationship between continuous PSA concentration and all-cause mortality did not support our study.29 When we analyzed finer causes of death, risk of cause-specific death other than prostate cancer was lower in men with a PSA level of 1-<4.0 ng/mL compared with men with a PSA level of <1 ng/mL.
Most men in the Korean Heart Study had a PSA level measured automatically as a component of a panel of tests performed during their health examination. Thus, in these men, the likelihood of differential uptake of PSA screening based on the patient’s characteristics, which may themselves be risk factors for prostate cancer death, is reduced. However, at some centers, PSA-based prostate cancer screening was part of an opt-in examination. At 2 centers, screening PSA testing was not performed. To assess whether differential uptake of PSA testing may have occurred in the source population, we compared men with (eligible to be included in the analysis) and without (not eligible to be included in the analysis) information on PSA. The 2 groups of men had similar self-rated health status and income status, suggesting lack of major selection bias.
Our study has a number of strengths. This cohort includes a large sample size and a wide age range. Although the number of prostate cancer deaths was not large, the number observed was higher than what was expected (data not shown) based on national data.30 Also, we investigated the association between PSA level and death from prostate cancer in an Asian population. However, our study has several limitations. First, we did not have information on previous PSA screening tests, prostate biopsies, digital rectal examination, incidence of prostate cancer, stage at diagnosis, and treatment for prostate cancer. As a consequence, we could not determine the association between PSA level and prostate cancer incidence in the cohort, or prostate cancer death in men in the cohort who were diagnosed with prostate cancer. Furthermore, we cannot determine whether the increased risk of prostate cancer death in men with higher PSA was due to greater inherent disease aggressiveness (ie, irrespective of early detection and appropriate treatment, the cancer would still have caused death) or poorer ability to treat disease diagnosed at a later phase in its natural history. Second, we used only the PSA level measured at the baseline. Finally, because all men included in the analysis were screened, we cannot comment on the effectiveness of prostate cancer screening vs no screening for the reduction in prostate cancer mortality.
CONCLUSION
In conclusion, an increased screening of the PSA level is associated with an increased risk of prostate cancer death in Korean men. Our findings may have implications for the development of targeted PSA cutpoints for biopsy recommendation.
Supplementary Material
Acknowledgments
Financial Disclosure: Elizabeth A. Platz is supported, in part, by National Cancer Institute Cancer Center support grant P30 CA006973 (Principal Investigator: William G. Nelson).
Funding Support: This work was supported by the National Research Foundation of Korea grant funded by the Korean government (Ministry of Education Science Technology; 2011-0029348).
Korean Heart Study (mortality study) participating institutes and principal collaborators: Hyon-Suk Kim (Shinchon Severance Hospital), Duk Chul Lee (Gangnam Severance Hospital), Moon Jong Kim (CHA Bundang Medical Center), Gyu Jang Lee (Korea Medical Institute), Jidong Sung (Samsung Medical Center), BeLong Cho (Seoul National University), Eung Soo Kim (Daejeon Sun General Hospital), Byung-Yeon Yu (Konyang University Hospital), Tae-Yong Lee (Chungnam National University), Jong Sung Kim (Chungnam National University), Sung Hi Kim (Daegu Catholic University Hospital), Jong-Ku Park (Yonsei University Wonju College of Medicine), Sang Baek Koh (Yonsei University Wonju College of Medicine), Sat Byul Park (Ajou University School of Medicine), Soon Young Lee (Ajou University School of Medicine), Cheol-In Yoo (University of Ulsan College of Medicine), Moon Chan Kim (University of Ulsan College of Medicine), and Joo-sung Park (Dong-A University Medical Center).
Footnotes
APPENDIX
Supplementary Data
Supplementary data associated with this article can be found, in the online version, at http://dx.doi.org/10.1016/j.urology.2015.02.014.
The remaining authors declare that they have no relevant financial interests.
References
- 1.Jemal A, Siegel R, Xu J, et al. Cancer statistics, 2010. CA Cancer J Clin. 2010;60:277–300. doi: 10.3322/caac.20073. [DOI] [PubMed] [Google Scholar]
- 2.Jung KW, Won YJ, Kong HJ, et al. Cancer statistics in Korea: incidence, mortality, survival and prevalence in 2010. Cancer Res Treat. 2013;45:1–14. doi: 10.4143/crt.2013.45.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Screening for Prostate Cancer. Vol. 2014. U.S.: U.S. Preventive Services Task Force; 2012. [Google Scholar]
- 4.Carter HB, Albertsen PC, Barry MJ, et al. Early detection of prostate cancer: AUA guideline. J Urol. 2013;190:419–426. doi: 10.1016/j.juro.2013.04.119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Etzioni R, Tsodikov A, Mariotto A, et al. Quantifying the role of PSA screening in the US prostate cancer mortality decline. Cancer Causes Control. 2008;19:175–181. doi: 10.1007/s10552-007-9083-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Edwards BK, Noone AM, Mariotto AB, et al. Annual Report to the Nation on the status of cancer, 1975-2010, featuring prevalence of comorbidity and impact on survival among persons with lung, colorectal, breast, or prostate cancer. Cancer. 2014;120:1290–1314. doi: 10.1002/cncr.28509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bray F, Lortet-Tieulent J, Ferlay J, et al. Prostate cancer incidence and mortality trends in 37 European countries: an overview. Eur J Cancer. 2010;46:3040–3052. doi: 10.1016/j.ejca.2010.09.013. [DOI] [PubMed] [Google Scholar]
- 8.Jemal A, Center MM, DeSantis C, et al. Global patterns of cancer incidence and mortality rates and trends. Cancer Epidemiol Biomarkers Prev. 2010;19:1893–1907. doi: 10.1158/1055-9965.EPI-10-0437. [DOI] [PubMed] [Google Scholar]
- 9.Gann PH, Hennekens CH, Stampfer MJ. A prospective evaluation of plasma prostate-specific antigen for detection of prostatic cancer. JAMA. 1995;273:289–294. [PubMed] [Google Scholar]
- 10.Orsted DD, Nordestgaard BG, Jensen GB, et al. Prostate-specific antigen and long-term prediction of prostate cancer incidence and mortality in the general population. Eur Urol. 2012;61:865–874. doi: 10.1016/j.eururo.2011.11.007. [DOI] [PubMed] [Google Scholar]
- 11.Connolly D, Black A, Gavin A, et al. Baseline prostate-specific antigen level and risk of prostate cancer and prostate-specific mortality: diagnosis is dependent on the intensity of investigation. Cancer Epidemiol Biomarkers Prev. 2008;17:271–278. doi: 10.1158/1055-9965.EPI-07-0515. [DOI] [PubMed] [Google Scholar]
- 12.Ito K. Prostate cancer in Asian men. Nat Rev Urol. 2014;11:197–212. doi: 10.1038/nrurol.2014.42. [DOI] [PubMed] [Google Scholar]
- 13.Jee SH, Batty GD, Jang Y, et al. The Korean Heart Study: rationale, objectives, protocol, and preliminary results for a new prospective cohort study of 430,920 men and women. Eur J Prev Cardiol. 2013;21:1484–1492. doi: 10.1177/2047487313497602. [DOI] [PubMed] [Google Scholar]
- 14.The Health Consequences of Smoking—50 Years of Progress: A Report of the Surgeon General, 2014. Vol. 2014. U.S.: Surgeon General; 2014. [Google Scholar]
- 15.Cao Y, Ma J. Body mass index, prostate cancer-specific mortality, and biochemical recurrence: a systematic review and meta-analysis. Cancer Prev Res. 2011;4:486–501. doi: 10.1158/1940-6207.CAPR-10-0229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Fine J, Gray R. A proportional hazards model for the subdistribution of a competing risk. J Amer Stat Assoc. 1999;94:496–509. [Google Scholar]
- 17.Catalona WJ, Richie JP, deKernion JB, et al. Comparison of prostate specific antigen concentration versus prostate specific antigen density in the early detection of prostate cancer: receiver operating characteristic curves. J Urol. 1994;152:2031–2036. doi: 10.1016/s0022-5347(17)32299-1. [DOI] [PubMed] [Google Scholar]
- 18.Wallner LP, Jacobsen SJ. Prostate-specific antigen and prostate cancer mortality: a systematic review. Am J Prev Med. 2013;45:318–326. doi: 10.1016/j.amepre.2013.04.015. [DOI] [PubMed] [Google Scholar]
- 19.Fang J, Metter EJ, Landis P, et al. Low levels of prostate-specific antigen predict long-term risk of prostate cancer: results from the Baltimore Longitudinal Study of Aging. Urology. 2001;58:411–416. doi: 10.1016/s0090-4295(01)01304-8. [DOI] [PubMed] [Google Scholar]
- 20.Brawley OW, Ankerst DP, Thompson IM. Screening for prostate cancer. CA Cancer J Clin. 2009;59:264–273. doi: 10.3322/caac.20026. [DOI] [PubMed] [Google Scholar]
- 21.Andriole GL, Crawford ED, Grubb RL, 3rd, et al. Mortality results from a randomized prostate-cancer screening trial. N Engl J Med. 2009;360:1310–1319. doi: 10.1056/NEJMoa0810696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Schroder FH, Hugosson J, Roobol MJ, et al. Screening and prostate-cancer mortality in a randomized European study. N Engl J Med. 2009;360:1320–1328. doi: 10.1056/NEJMoa0810084. [DOI] [PubMed] [Google Scholar]
- 23.Hayes JH, Barry MJ. Screening for prostate cancer with the prostate-specific antigen test: a review of current evidence. JAMA. 2014;311:1143–1149. doi: 10.1001/jama.2014.2085. [DOI] [PubMed] [Google Scholar]
- 24.Oesterling JE, Jacobsen SJ, Chute CG, et al. Serum prostate-specific antigen in a community-based population of healthy men. Establishment of age-specific reference ranges. JAMA. 1993;270:860–864. [PubMed] [Google Scholar]
- 25.Ellis WJ, Brawer MK. PSA in benign prostatic hyperplasia and prostatic intraepithelial neoplasia. Urol Clin North Am. 1993;20:621–625. [PubMed] [Google Scholar]
- 26.Albertsen PC, Moore DF, Shih W, et al. Impact of comorbidity on survival among men with localized prostate cancer. J Clin Oncol. 2011;29:1335–1341. doi: 10.1200/JCO.2010.31.2330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Saraiya M, Kottiri BJ, Leadbetter S, et al. Total and percent free prostate-specific antigen levels among U.S. men, 2001-2002. Cancer Epidemiol Biomarkers Prev. 2005;14:2178–2182. doi: 10.1158/1055-9965.EPI-05-0206. [DOI] [PubMed] [Google Scholar]
- 28.Lilja H, Ulmert D, Vickers AJ. Prostate-specific antigen and prostate cancer: prediction, detection and monitoring. Nat Rev Cancer. 2008;8:268–278. doi: 10.1038/nrc2351. [DOI] [PubMed] [Google Scholar]
- 29.Carter HB, Metter EJ, Wright J, et al. Prostate-specific antigen and all-cause mortality: results from the Baltimore Longitudinal Study on Aging. J Natl Cancer Inst. 2004;96:557–558. doi: 10.1093/jnci/djh111. [DOI] [PubMed] [Google Scholar]
- 30.GLOBOCAN 2012: Estimated Cancer Incidence, Mortality and Prevalence Worldwide in 2012.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


