Abstract
Background
The apolipoprotein-E (APOE) ε4 allele is a risk factor for vascular dementia and Alzheimer’s disease. Recent studies are equivocal with regards to whether or not the ε4 allele confers increased risk for the development of human immunodeficiency virus (HIV)-associated neurocognitive disorder (HAND), but suggest that age and/or disease severity may be modulating factors. The aim of this study was to assess the interactions and contributions of APOE genotype, age, and HIV disease severity as risk factors for HAND in HIV-infected adults.
Methods
Participants were 259 HIV-positive individuals who underwent APOE genotyping, a standardized neurological evaluation, a comprehensive neuropsychological evaluation, and laboratory testing.
Results
Older ε4 carriers showed a higher frequency of HAND compared with age-matched non-ε4 carriers. Analysis by discrete neurocognitive domain revealed that advanced age modulated the effect of the ε4 allele, such that older ε4 allele carriers showed reduced executive functioning and information processing speed. Exploratory analyses assessing the relationship between ε4 and disease severity in the overall sample revealed that disease severity modulated the effect of the ε4 allele on cognition. Lower absolute CD4+ cell count among ε4 allele carriers was associated with poorer working memory ability.
Conclusion
Advancing age and degree of immunosuppression may influence the association between APOE ε4 allele status and HAND. These two factors need to be taken into account in future research.
Keywords: apolipoprotein-E, human immunodeficiency virus, aging, neurocognition
Background
The introduction of combined antiretroviral therapy has resulted in a significant decrease in human immunodeficiency virus-1 (HIV)-related morbidity and mortality,1 giving rise to a growing population of older individuals living with HIV. The Centers for Disease Control estimates that approximately 50% of the HIV population will be over the age of 50 years in the next few years.2 A persistence of HIV-associated neurocognitive disorder (HAND) has been observed despite a decline in the overall incidence of new cases of HIV-associated dementia, which is the most severe form of HAND.3–5 The prevalence of HAND has been particularly pronounced in older HIV-positive adults (defined as age over 50 years). For example, HIV-positive adults over the age of 50 years are almost three times more likely to develop HIV-associated dementia than younger HIV-positive individuals.6 In light of these statistics, further study of the factors that place older HIV-positive adults at disproportionate risk for neurocognitive decline is clearly warranted.
One factor that has started to receive attention is the apolipoprotein-E (APOE) genotype. The APOE ε4 allele is a risk factor for the development of vascular dementia,7,8 cognitive impairment after traumatic brain injury9,10 and, most noteworthy, for Alzheimer’s disease.11 APOE genotype has also been associated with cardiovascular health, longevity, and cancer.12–16 More recently, ε4 has been examined for its relationship with other neuromuscular17 and infectious diseases,18–21 the latter possibly through direct effects on inflammatory processes.22 An association between the ε4 allele and cognition in an HIV sample was first reported over a decade ago in the pre-combined antiretroviral therapy era by researchers who found a higher rate of HIV-associated dementia and peripheral neuropathy among HIV-positive ε4 carriers as compared with HIV-positive ε4 noncarriers.23 However, subsequent studies have yielded inconsistent findings.18,24–31 Although a variety of methodological differences exist between these studies that may account for some of these discrepancies, only two studies, to our knowledge,24,25 have directly assessed the interaction between HIV (using an HIV seronegative control group) and APOE genotype on objective measures of neurocognitive performance. Both studies found an interaction between HIV and APOE genotype, such that HIV-positive APOE ε4 carriers demonstrated poorer cognition compared with seronegative controls. This suggests that the combination of HIV and the APOE ε4 allele puts individuals at increased risk for neurocognitive decline. However, the majority of the above-mentioned studies have assessed the effects of APOE genotype on cognition within HIV samples (ie, without seronegative controls), and the variability within these studies suggests that other factors modulate the relationship between APOE genotype and cognition in HIV. Ascertaining factors that place subgroups of individuals at risk for HAND is of considerable clinical import.
Among the studies assessing differences in APOE genotype within an HIV sample, one potential explanation put forth to explain the discrepant findings was that age modulated the relationship between ε4 and cognition in HIV.31 This is consistent with the age-dependent effects of APOE found in other illnesses.32,33 Valcour et al31 found no relationship between ε4 and HIV-associated dementia in a sample of HIV-positive individuals. When age groups were analyzed separately, ε4 conferred a significant risk for older individuals (≥50 years) but not younger individuals. Two studies to our knowledge have directly assessed the relationship between advanced age and APOE in the development of HAND and failed to replicate those findings. However, small sample sizes may have precluded the ability to ascertain an age by ε4 effect.25,29
There is also evidence to suggest that the APOE ε4 allele has a deleterious impact on the course of HIV disease in the pre-combined antiretroviral therapy era.18,34 In a large sample of HIV-positive individuals, Burt et al18 found that individuals with the APOE ε4/ε4 genotype showed accelerated disease progression and a shorter time until death compared with those with the APOE ε3/ε3 genotype. The interplay between the APOE ε4 allele, disease severity, and HAND warrants further attention. Given that studies using HIV seronegative control samples have found a deleterious interaction between APOE genotype and HIV on cognition, it is logical to opine that disease severity may be another marker that modulates the impact of APOE genotype on cognition in HIV-positive samples. Corder et al23 not only demonstrated a deleterious relationship between ε4 and cognition, independent of disease severity, but found an interaction between the ε4 allele and cluster of differentiation 4-positive (CD4+) lymphocyte counts in plasma, such that HIV-positive ε4+ individuals who presented with lower CD4+ levels had higher rates of dementia across a 5-year study. This study was conducted in the pre-combined antiretroviral therapy era and it is unclear whether the ε4 allele would interact with CD4+ in the same manner in the context of combined antiretroviral therapy. To our knowledge, this has been the only study to date assessing the interplay between the APOE ε4 allele, disease severity, and cognition in HIV.
The objective of the current study was to further delineate the relationship between APOE genotype, age, and HIV disease severity in a large, well-characterized sample of HIV-positive adults.
Materials and methods
Participants
The current study received institutional ethics approval from each of the participating sites’ institutional review boards. All participants were recruited from the National NeuroAIDS Tissue Consortium (NNTC) and provided their written informed consent to participate in the study. The NNTC sample and research methods have been described in detail elsewhere.35 Briefly, the NNTC consists of four sites within the United States: The National NeuroAIDS Bank in Los Angeles, CA; the Texas NeuroAIDS Research Center, Galveston, TX; the Manhattan HIV Brain Bank, New York, NY; and the California NeuroAIDS Tissue Network, San Diego, CA. HIV-positive participants were recruited for the purpose of brain banking and selected based on having a high risk for imminent death. At study entry, neuromedical, neuropsychological, psychiatric, psychosocial, substance abuse, laboratory (including viral load and absolute CD4+ cell count), cerebrospinal fluid (when available), and neuroimaging (when available) assessments were made.
When this analysis was conducted, the NNTC sample consisted of a total of 1642 HIV-positive individuals with neurocognitive data. Of these, 467 individuals were selected for genetic testing based on whether they were neurologically normal, or had subsyndromic impairment, mild cognitive/motor disorder, or HIV-associated dementia, using established criteria at study entry.36 This selection process has been detailed elsewhere.37 From the data available, participants were then excluded based on whether they presented with a history of non-HIV-related neurological illness (eg, stroke or traumatic brain injury with loss of consciousness longer than 30 minutes), opportunistic infection affecting the brain (eg, toxoplasmosis, cryptococcal meningitis, progressive multifocal leukoencephalopathy, or cytomegalovirus encephalitis), neurosyphilis, or brain tumor (eg, primary central nervous system lymphoma) and for missing key demographic or disease severity (CD4+ T cell count) data, or if their APOE genotyping data were not reliable. A total of 259 participants remained in the study. Compared with the larger NNTC sample, the current sample was similar in regard to mean age (43.52 ± 7.82 versus NNTC sample 43.52 ± 8.92, P > 0.05) and had a higher mean level of education (13.35 ± 2.57 versus NNTC sample 12.15 ± 3.13, P < 0.05). Ethnicity varied between samples, with more Caucasians and fewer Latinos in the current sample (African American 28.6%, Latino 12%, Caucasian 55.2%, other 4.2%; NNTC sample, African American, 31.9%, Latino, 27.8%, Caucasian, 37%, other 3.3%, P < 0.05). There was also a trend for a higher proportion of males in the current sample (84.6% versus NNTC sample 79.2%, P = 0.05).
Participants with substance use diagnoses, psychiatric diagnoses, and hepatitis C virus (HCV) coinfection were not excluded because of the high base rate of these diagnoses in this population, (excluding such participants would not yield a representative sample of the HIV-positive population). Rather, we chose to include these participants in the current study and conduct follow-up analyses using these conditions as covariates.
Neurocognitive diagnosis
Neurocognitive diagnosis was determined via consensus agreement between the examining study neurologist and a board-certified neuropsychologist, with consideration of laboratory results (eg, viral load and CD4+ T cell count), neuroimaging (when available), and results of comprehensive neuropsychological testing. For the purposes of this study, individuals were diagnosed either as neurologically normal, or as having HAND, which included mild cognitive/motor disorder or HIV-associated dementia per established criteria,36 or subsyndromic HIV-related neurocognitive impairment (equivalent to asymptomatic neurocognitive impairment as per 2007 Frascati criteria).38 NNTC diagnostic work sheets with an algorithmic approach were used to maximize reliability.
Neuropsychological functioning
As part of our study, we also examined the interactive effects of APOE genotype, age, and disease severity upon specific domains of neurocognitive functioning. All NNTC participants underwent a comprehensive neuropsychological evaluation (see Table 1) at study entry by trained psychometrists under the supervision of study neuropsychologists. For this study, we focused on the domains of executive functions, information processing speed, working memory, learning, and memory, because these are most affected by age and HIV disease severity. Individual test scores were converted to z-scores based on the mean and standard deviation of the larger overall NNTC sample (n = 1642). By using the NNTC as the normative sample, we were better able to detect deviations from “normalcy” due to genotype, disease severity, and age. Characteristics of the overall NNTC sample and this normative approach have been published elsewhere.37 Given that the analyses were addressing the impact of age, normative data correcting for age were not used in order to avoid “double correcting” for this variable. Rather, the effects of demographics (age, education/premorbid IQ, gender, and ethnicity) were controlled for within the analyses. Further, this method offers the same sample from which to derive normative data across domains. Each of the tests was grouped by domain, and domain z-scores were calculated as the average of test scores within each domain.
Table 1.
Neuropsychological tests
| Domain/test |
|---|
| Executive Functioning |
| Trail Making Test B58 |
| Wisconsin Card Sorting Test59,60 |
| Information Processing Speed |
| Digit Symbol61 |
| Symbol Search61 |
| Trail Making Test-Form A58 |
| Working Memory |
| PASAT Trial 162 |
| WAIS-III Letter-Number Sequencing61 |
| Learning |
| HVLT–Revised Learning Trials total63 |
| BVMT–Revised Learning Trials total64 |
| Memory |
| HVLT–Revised Free Recall63 |
| BVMT–Revised Free Recall64 |
Abbreviations: PASAT, Paced Auditory Serial Addition Task; WAIS, Wechsler Adult Intelligence Scale; HVLT, Hopkins Verbal Learning Test; BVMT, Brief Visuospatial Memory Test.
Cognitive reserve
Participants were administered the reading subtest of the Wide Range Achievement Test, 3rd Edition39 as an estimate of premorbid intellectual functioning. The averaged sum of premorbid intellectual functioning (standard score) and standardized years of education was used as the cognitive reserve composite score.40
Systemic disease severity
Systemic disease severity was determined using absolute CD4+ at study entry. A cut-point of 200 cells/mm3 (severe < 200 cells/mm3; not severe ≥ 200 cells/mm3) was used in analyses focusing on disease severity. Nadir CD4+ data were not available for the majority of participants. Length of known HIV infection was calculated based on the difference between their self-reported year of infection and the year that the evaluation was conducted. Exact dates of seroconversion were typically not available.
Hepatitis C virus
HCV was measured using self-report. A small sample (n = 48) was formally tested for HCV. Self-reports were combined with formal testing. Participants were characterized as having HCV if they formally tested positive for viral RNA or if they self-reported having the virus.
Psychiatric and substance use diagnoses
Participants were administered the affective and substance use disorder sections of the Psychiatric Research Interview for Substance or Mental Disorders,41 a structured diagnostic interview that yields Diagnostic and Statistical Manual of Mental Disorders Fourth Edition diagnoses.42 They were classified as being currently depressed or not depressed if they met the diagnostic criteria for current major depressive disorder. They were classified as being substance users if they met diagnostic criteria for current cocaine, methamphetamine, heroin, or alcohol abuse and/or dependence or if their urine toxicology at study entry was positive for nonmedically prescribed opiates, cocaine, or amphetamines. These data were available for a smaller subsample (though still the majority) of participants and used as covariates in follow-up analyses.
APOE genotype
Peripheral blood mononuclear cells and/or frozen tissue samples were shipped to the UCLA Biological Samples Processing Core from the four NNTC sites for DNA extraction. The Autopure LSTM nucleic acid purification instrument was used for extracting DNA. Extracted DNA was then sent to the UCLA Genotyping Core for genotyping. Genotype was evaluated according to a number of quality parameters. Participants were characterized as ε4 carriers if they had at least one ε4 allele.
Statistical analyses
Three sets of analyses were conducted. First, in order to assess the effects of the ε4 allele on neurocognitive diagnosis, Chi-squared statistics were conducted for each of the age groups (<50 years versus ≥50 years) separately. Given the potential of ethnic admixture confounding these analyses,43,44 we also reran these analyses with the Caucasian sample alone. We did not rerun these analyses with the African American sample because of reduced sample size. Second, in order to assess the independent effects of the ε4 allele and the interactive effects of the ε4 allele and age on discrete neu-rocognitive domains, we used multiple linear regression analyses. For each of the cognitive domains, pertinent demographic information was placed in the first block (ethnicity, gender, and cognitive reserve). Next, ε4 allele status and age (continuous) were placed in the second block. The interaction between age and ε4 allele status was placed in the third and final block. Domain z-scores were used as the outcome variables. In order to be consistent with the above analyses, we centered age at 50 years, a cut-point that has been used frequently in studies assessing the effects of age in HIV infection.45–47 In addition, 50 years is the age at which longitudinal studies suggest that the apolipoprotein ε4 allele begins to exert deleterious effects on cognition.48,49 These main analyses were followed by controlling for the effects of systemic disease severity (CD4+ using a cut-point of 200 cells/mm3 and length of infection), psychiatric status (depression and substance use/abuse), and HCV serostatus simultaneously. Next, to assess the relationship between ε4 status, disease severity, and neurocognitive diagnosis, a binary logistic regression was used. Disease severity (based on the above-described cut-point of 200 cells/mm3) was entered in the first block. APOE genotype and the interaction between disease severity and APOE genotype was placed in the second step. Next, we assessed the interactive effects of disease severity and APOE genotype on each of the cognitive domains with multiple regressions. Pertinent demographic information (including age) was placed in the first block. APOE ε4 carrier status and CD4+ status were placed in the second block. The interaction between ε4 allele carrier status and CD4+ status was placed in the final block. Two-tailed tests were used. Descriptive statistics were examined to ensure that statistical assumptions were met. Robust standard errors were provided for analyses that exhibited mild heteroskedasticity (ie, executive functions). To correct for multiple comparisons conducted when neurocognitive domains were assessed separately, the false discovery rate was used.50 This approach controls for the expected proportion of false positives based on the total number of hypotheses by calculating different q values for each of the analyses. Each of the significant analyses was checked to ensure that it was below the expected q value.
Results
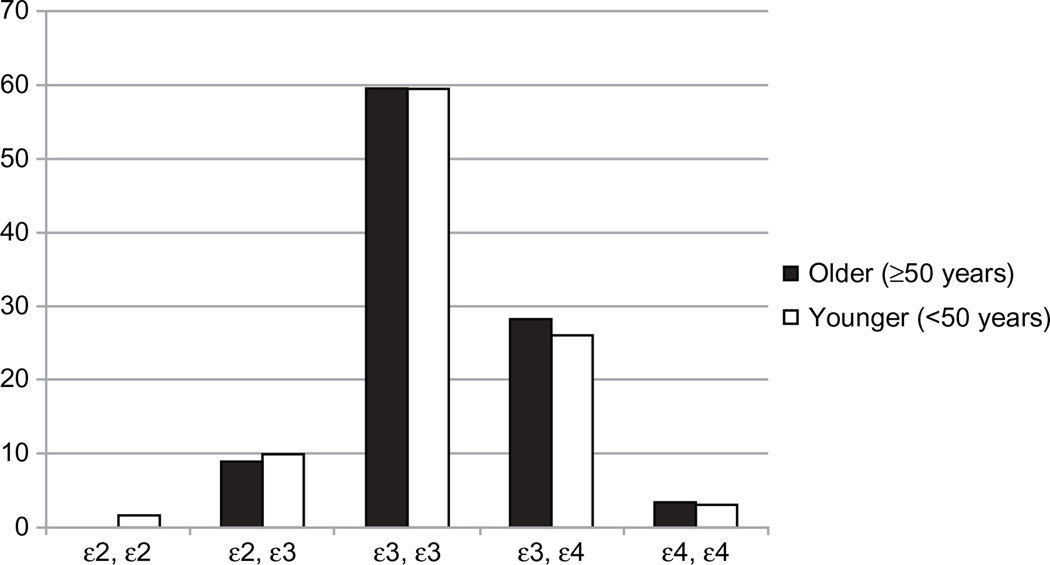
See Table 2 for key demographic and clinical data. Figure 1 shows the frequency of APOE variants between younger and older adults. There were a total of 77 participants who were carriers of at least one ε4 allele, the proportion of which was comparable between age groups [χ2 (1, N = 259) = 0.12, P = 0.73]. A total of eight participants were homozygous and 69 were heterozygous for ε4, both equally distributed between the older and younger groups. The ε4 groups (carriers versus noncar-riers) were comparable in regards to ethnic distribution [χ2 (3, N = 259) = 3.02, P = 0.39], ethnic minority status [χ2 (1, N = 259) = 0.92, P = 0.34], and gender [χ2 (1, N = 259) = 0.51, P = 0.48]. APOE ε4 allele carriers in the current sample had higher levels of cognitive reserve than ε4 noncarriers [F(1, 257) = 4.90, P = 0.03]. Age groups (older versus younger) were comparable in regards to ethnic minority status [χ2 (1, N = 259) = 1.87, P = 0.17] and ethnic distribution [χ2 (3, N = 259) = 3.89, P = 0.27]. The older group had a higher percentage of male participants than did the younger participants [χ2 (1, N = 259) = 3.97, P = 0.05] and evidenced higher cognitive reserve [F (1, 257) = 7.89, P = 0.01].
Table 2.
Demographic and clinical characteristics
| ε4+ (n = 77) Mean (SD) |
ε4− (n = 182) Mean (SD) |
|
|---|---|---|
| Age (years) | 43.91 (7.72) | 43.34 (7.88) |
| Age over 50 years (%) | 23.4 | 21.4 |
| Education (years)a | 14.00 (2.50) | 13.07 (2.55) |
| WRAT-III (SS) | 98.62 (14.83) | 96.52 (14.14) |
| Cognitive reservea | 101.21 (13.08) | 97.45 (12.26) |
| Sex (% male) | 87.0 | 83.5 |
| Ethnicity (%) | ||
| African American | 35.1 | 25.8 |
| Latino | 9.1 | 13.2 |
| Caucasian | 50.6 | 57.1 |
| Other | 5.2 | 3.8 |
| CD4+ (T cell count) | 219.40 (243.93) | 219.23 (243.03) |
| Length of infection (years)b | 11.12 (5.12) | 11.61 (5.54) |
| HAART (% prescribed) | 84.4 | 84.6 |
| Hepatitis C (% positive) | 11.7 | 11.0 |
| Substance use/abuse (%)c | 27.3 | 25.9 |
| Major depression (%)d | 19.7 | 24.7 |
Notes:
P < 0.05;
n = 252;
n = 194;
n = 216.
Abbreviations: HAART, highly active antiretroviral therapy; SD, standard deviation; WRAT-III, Wide Range Achievement Test, 3rd edition.
Figure 1.
Percentage of apolipoprotein-E allele combinations by age group.
ε4, age, and neurocognitive diagnosis
In the first set of analyses, the relationship between ε4 allele status and neurocognitive diagnosis was assessed. In the overall sample, there was no significant relationship between ε4 allele status and HAND, [χ2 (1, N = 259) = 0.74, P = 0.39, odds ratio = 1.31]. However, when the age groups (younger versus older) were analyzed separately, there was a higher frequency of HAND diagnoses among older ε4 carriers compared with their ε4 noncarrier age-matched peers. In fact, all but one participant with the ε4 allele carried a diagnosis of HAND [χ2 (1, N = 57) = 8.25, P = 0.004, odds ratio = 13.14; Fisher’s exact test, P = 0.005 (Table 3)]. Among the younger sample, the frequency of HAND diagnoses was comparable between ε4 carriers and ε4 noncarriers, [χ2 (1, N = 202) = 0.41, P = 0.52, odds ratio = 0.80].
Table 3.
Proportion of HAND by APOE ε4 allele status and age
| APOE ε4 allele status |
Age group |
|||
|---|---|---|---|---|
| Younger* |
Older** |
|||
| HAND n (%) |
No HAND n (%) |
HAND n (%) |
No HAND n (%) |
|
| Carriers | 42 (71.2) | 17 (28.8) | 17 (94.4) | 1 (5.6) |
| Noncarriers | 108 (75.5) | 35 (24.5) | 22 (56.4) | 17 (43.6) |
Notes:
χ2 (1, N = 202) = 0.41, P = 0.52, odds ratio = 0.80;
χ2 (1, N = 57) = 8.25, P = 0.004, odds ratio = 13.14; Fisher’s exact test, P = 0.005.
Abbreviations: APOE, apolipoprotein-E; HAND, human immunodeficiency virus-associated neurocognitive disorder.
When running the analyses separately for the Caucasian sample, the findings remained the same. There was no association between APOE genotype and HAND in the overall sample [χ2 (1, N = 143) = 0.15, P = 0.70], an association between the ε4 allele and HAND among the older participants [χ2 (1, N = 36) = 6.09, P = 0.01, Fisher’s exact test P = 0.02], and no association between the ε4 allele and HAND among the younger participants [χ2 (1, N = 107) = 1.11, P = 0.29].
Interaction between age and ε4 carrier status on cognition by domain
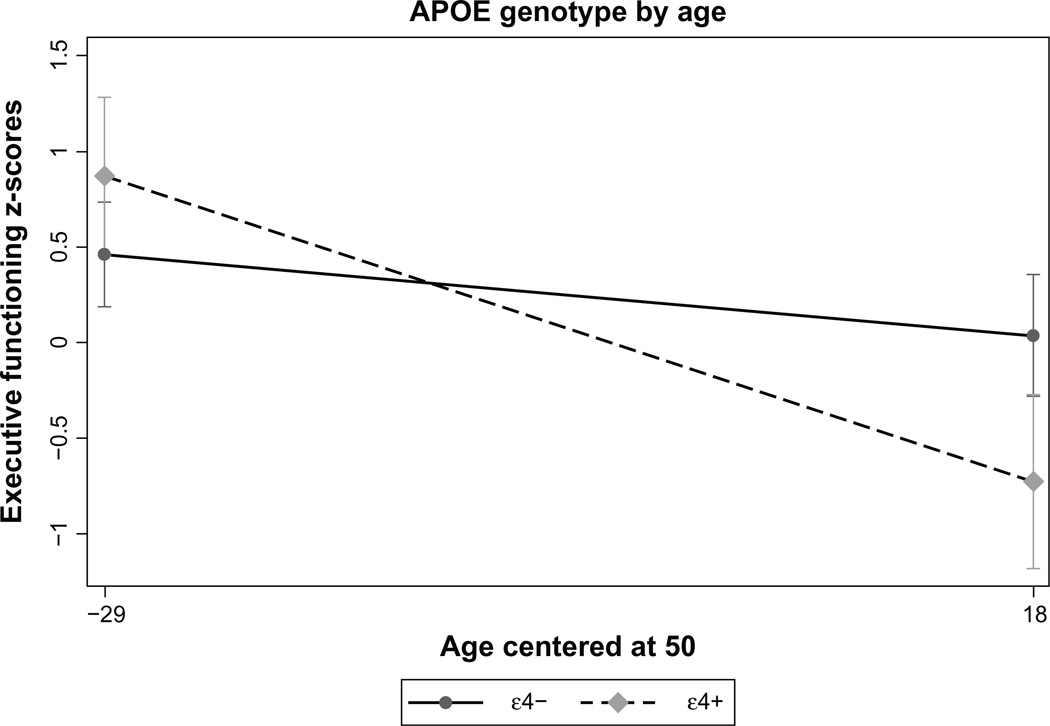
Next, we examined the effect of age and ε4 allele status on five discrete neurocognitive domains. We focused on the domains of executive functions, information processing speed, working memory, learning, and memory, because these are most affected by age and HIV disease. In the domain of executive functioning, demographics were significantly related to executive function in the first step of the model [F(3,252) = 11.05, P , 0.001]. In the second step, there was no main effect of the ε4 allele on cognition (b = −0.16, P = 0.11), although there was a significant effect of age (b = −0.02, P = 0.002). In the third step, there was a significant ε4 allele by age interaction (b = −0.02, P = 0.02, Table 4 and Figure 2), indicating that the combination of advanced age and the ε4 allele contribute to decline. The model was improved in each of the steps. Of note, there were two outliers in the dataset. When these were removed and the analyses were rerun, there was still no effect of the ε4 allele (P = 0.36) and the results of the interaction remained unchanged (P = 0.02). Results of the interaction also remained unchanged (P < 0.05) after simultaneously controlling for the effects of systemic disease severity (CD4+), length of infection, psychiatric diagnoses (depression and substance use), and HCV serostatus. There were no other significant main ε4 effects or age by ε4 allele interactions for any of the other domains (see Table 5). However, after controlling for the effects of systemic disease severity, length of infection, psychiatric diagnoses, and HCV serostatus simultaneously, there was a significant ε4 allele by age interaction in the domain of information processing (b = −0.03, P = 0.03), such that the impact of the ε4 allele contributed to decline as individuals advanced in age.
Table 4.
Interactive effects of ε4 and age on executive functioning
| B | SE B (robust) |
t | P | 95% CI |
Partial η2 | ||
|---|---|---|---|---|---|---|---|
| Lower | Upper | ||||||
| Step 1a | |||||||
| Ethnicityb | 0.20 | 0.09 | 2.09 | 0.04 | 0.01 | 0.38 | 0.02 |
| Sexc | 0.23 | 0.09 | 2.49 | 0.01 | 0.05 | 0.41 | 0.02 |
| Cognitive reserve | 0.01 | 0.003 | 4.02 | <0.001 | 0.01 | 0.02 | 0.08 |
| Step 2 | |||||||
| Aged | −0.02 | 0.01 | −3.18 | 0.002 | −0.03 | −0.01 | 0.04 |
| ε4 allelee | −0.16 | 0.10 | −1.61 | 0.11 | −0.36 | 0.04 | 0.01 |
| Step 3 | |||||||
| ε4 allele × age | −0.02 | 0.01 | −2.37 | 0.02 | −0.05 | −0.004 | 0.02 |
Notes:
ΔR2 = 0.10 for step 1, ΔR2 = 0.044 for step 2 (P = 0.002), ΔR2 = 0.016 for step 3 (P = 0.03);
reference group is ethnic minority;
reference group is male;
centered at 50 years;
reference group is ε4-.
Abbreviations: CI, confidence interval; SE, standard error.
Figure 2.
Interaction between apolipoprotein-E genotype on executive functions.
Table 5.
The interaction between APOE ε4 and age on cognition by other domains (Step 3)
| B | SE B | t | P | 95% Confidence Interval |
||
|---|---|---|---|---|---|---|
| Lower | Upper | |||||
| Working memory | ||||||
| Agea | −0.02 | 0.01 | −2.31 | 0.02 | −0.03 | −0.003 |
| Sexb | 0.04 | 0.15 | 0.26 | 0.80 | −0.25 | 0.33 |
| Ethnicityc | 0.34 | 0.11 | 3.14 | <0.01 | 0.13 | 0.55 |
| Cognitive reserve | 0.03 | 0.004 | 5.69 | <0.001 | 0.02 | 0.03 |
| ε4 alleled | −0.09 | 0.14 | −0.64 | 0.53 | −0.37 | 0.19 |
| ε4 allele x age | −0.02 | 0.01 | −1.17 | 0.24 | −0.05 | 0.01 |
| Information Processing Speed | ||||||
| Agea | −.02 | 0.01 | −3.18 | 0.002 | −0.04 | −.01 |
| Sexb | 0.11 | 0.13 | 0.90 | 0.37 | −0.13 | 0.36 |
| Ethnicityc | 0.12 | 0.09 | 1.33 | 0.19 | −0.06 | 0.31 |
| Cognitive reserve | 0.02 | 0.004 | 4.28 | <0.001 | 0.01 | 0.02 |
| ε4 alleled | −0.21 | 0.12 | −1.68 | 0.09 | −0.45 | 0.04 |
| ε4 allele x age | −0.02 | 0.01 | −1.54 | 0.13 | −0.04 | 0.01 |
| Learning | ||||||
| Agea | −0.01 | 0.01 | −1.50 | 0.14 | −0.03 | 0.004 |
| Sexb | 0.09 | 0.14 | 0.65 | 0.52 | −0.18 | 0.36 |
| Ethnicityc | 0.23 | 0.10 | 02.19 | 0.03 | 0.02 | 0.43 |
| Cognitive reserve | 0.02 | 0.004 | 04.63 | <0.001 | 0.01 | 0.03 |
| ε4 alleled | −0.20 | 0.14 | −1.50 | 0.14 | −0.47 | 0.06 |
| ε4 allele x age | −0.02 | 0.01 | −1.20 | 0.23 | −0.04 | 0.01 |
| Memory | ||||||
| Agea | −0.01 | 0.01 | −1.41 | 0.16 | −0.03 | 0.004 |
| Sexb | 0.08 | 0.14 | 0.56 | 0.58 | −0.20 | 0.36 |
| Ethnicityc | 0.21 | 0.10 | 1.99 | 0.05 | 0.002 | 0.41 |
| Cognitive reserve | 0.02 | 0.004 | 4.55 | <0.001 | 0.01 | 0.03 |
| ε4 alleled | −0.13 | 0.14 | −0.94 | 0.35 | −0.40 | 0.14 |
| ε4 allele x age | −0.02 | 0.01 | −0.09 | 0.27 | −0.04 | 0.01 |
Note:
Centered at 50 years;
Reference group is male;
Reference group is ethnic minority;
Reference group is ε4–.
Abbreviation: SE, standard error.
ε4 allele and disease severity
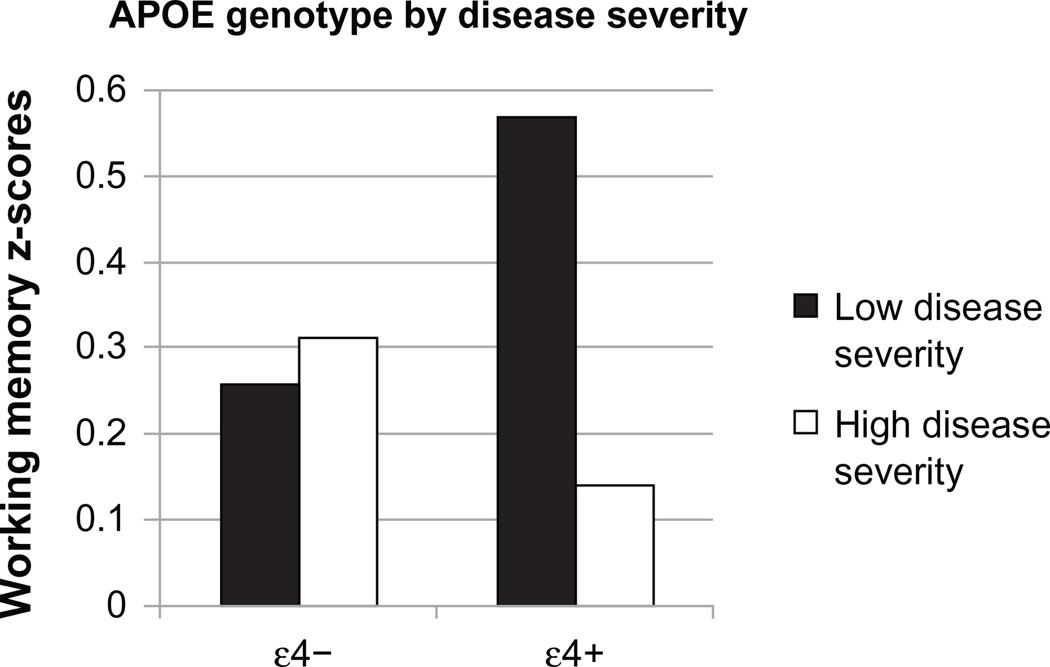
The relationship between ε4, systemic HIV disease severity, and cognition was further explored using the entire sample. In the first analysis, the relationship between disease severity and APOE genotype on HAND diagnosis was explored. There was no significant relationship between disease severity and HAND [b = −0.21, Wald χ2 (1) = 0.54, P = 0.46] or interaction between disease severity and APOE genotype on risk for HAND [b = −0.04, Wald χ2 (1) = 0.003, P = 0.96]. The results remained unchanged when the Caucasians were assessed separately. In the next set of analyses, the interactive effects of APOE genotype and disease severity on each of the cognitive domains was explored. Demographics (including age) were entered in the first step, ε4 carrier status and CD4+ (split at 200 cells/mm3) were entered in the second step, and the interaction between ε4 and CD4+ were entered in the third step. Analyses by five discrete neurocognitive domains revealed a significant interaction between ε4 and CD4+ in the domain of working memory (b = −0.48, P = 0.03, see Table 6 and Figure 3). The interaction improved the model. Among ε4 carriers, individuals with higher disease severity performed significantly worse. There were no other significant ε4 allele by disease severity interactions (see Table 7).
Table 6.
Interaction between apolipoprotein-E ε4 and disease severity on working memory
| B | SE B | t | P | 95% CI |
Partial η2 | ||
|---|---|---|---|---|---|---|---|
| Lower | Upper | ||||||
| Step 1a | |||||||
| Age | −0.02 | 0.01 | −3.49 | 0.001 | −0.04 | −0.01 | 0.05 |
| Ethnicityb | 0.34 | 0.11 | 3.14 | 0.002 | 0.13 | 0.55 | 0.04 |
| Cognitive reserve | 0.03 | 0.004 | 5.79 | <0.001 | 0.02 | 0.03 | 0.12 |
| Sexc | 0.02 | 0.15 | 0.11 | 0.91 | −0.27 | 0.30 | 0.00 |
| Step 2 | 0.00 | ||||||
| Disease severityd | −0.09 | 0.11 | −0.86 | 0.39 | −0.30 | 0.12 | 0.00 |
| ε4 allelee | 0.01 | 0.11 | 0.12 | 0.91 | −0.21 | 0.23 | 0.00 |
| Step 3 | |||||||
| ε4 allele × disease severity | −0.48 | 0.22 | −2.16 | 0.03 | −0.93 | −0.04 | 0.02 |
Notes:
R2 = 0.21 for step 1, ΔR2 = 0.002 for step 2 (P = 0.69), ΔR2 = 0.01 for step 3 (P = 0.03);
reference group is ethnic minority;
reference group is male;
reference group is CD4+ ≥ 200;
reference group is ε4–.
Abbreviations: CI, confidence interval; SE, standard error.
Figure 3.
Interaction between apolipoprotein-E genotype and disease severity on working memory.
Table 7.
Interaction between APOE ε4 and disease severity by other cognitive domains (Step 3)
| B | SE B | t | P | 95% CI |
||
|---|---|---|---|---|---|---|
| Lower | Upper | |||||
| Executive functioning | ||||||
| Age | −0.02 | 0.01 | −3.07 | 0.002 | −0.03 | −0.01 |
| Ethnicitya | 0.17 | 0.09 | 1.93 | 0.06 | −0.003 | 0.34 |
| Cognitive reserve | 0.02 | 0.004 | 4.51 | <0.001 | 0.01 | 0.02 |
| Sexb | 0.18 | 0.12 | 1.54 | 0.13 | −0.05 | 0.42 |
| Disease severityc | −0.04 | 0.10 | −0.43 | 0.67 | −0.24 | 0.15 |
| ε4 alleled | −0.22 | 0.14 | −1.52 | 0.13 | −0.50 | 0.06 |
| ε4 allele × disease severity | 0.09 | 0.18 | 0.50 | 0.62 | −0.27 | 0.45 |
| Information processing speed | ||||||
| Age | −0.03 | 0.01 | −4.39 | <0.001 | −0.04 | −0.01 |
| Ethnicitya | 0.13 | 0.09 | 1.38 | 0.17 | −0.06 | 0.31 |
| Cognitive reserve | 0.02 | 0.004 | 4.16 | <0.001 | 0.01 | 0.02 |
| Sexb | 0.11 | 0.13 | 0.90 | 0.37 | −0.13 | 0.36 |
| Disease severityc | 0.14 | 0.11 | 1.32 | 0.19 | −0.07 | 0.35 |
| ε4 alleled | −0.05 | 0.15 | −0.32 | 0.75 | −0.35 | 0.25 |
| ε4 allele × disease severity | −0.07 | 0.20 | −0.33 | 0.74 | −0.45 | 0.32 |
| Learning | ||||||
| Age | −0.02 | 0.01 | −2.39 | 0.02 | −0.03 | −0.003 |
| Ethnicitya | 0.23 | 0.10 | 2.18 | 0.03 | 0.02 | 0.43 |
| Cognitive Reserve | 0.02 | 0.004 | 4.58 | <0.001 | 0.01 | 0.03 |
| Sexb | 0.08 | 0.14 | 0.57 | 0.57 | −0.20 | 0.36 |
| Disease Severityc | 0.03 | 0.12 | 0.28 | 0.78 | −0.20 | 0.27 |
| ε4 alleled | −0.12 | 0.17 | −0.70 | 0.48 | −0.46 | 0.22 |
| ε4 allele × disease severity | 0.03 | 0.22 | 0.13 | 0.90 | −0.40 | 0.46 |
| Memory | ||||||
| Age | −0.01 | 0.01 | −2.18 | 0.03 | −0.03 | −0.001 |
| Ethnicitya | 0.21 | 0.11 | 2.02 | 0.04 | 0.01 | 0.42 |
| Cognitive reserve | 0.02 | 0.004 | 4.44 | <0.001 | 0.01 | 0.03 |
| Sexb | 0.07 | 0.17 | 0.50 | 0.62 | −0.21 | 0.35 |
| Disease severityc | 0.06 | 0.12 | 0.10 | 0.49 | −0.18 | 0.29 |
| ε4 alleled | −0.08 | 0.17 | −0.46 | 0.65 | −0.18 | 0.29 |
| ε4 allele × disease severity | 0.07 | 0.22 | 0.33 | 0.74 | −0.36 | 0.50 |
Notes:
Reference group is ethnic minority;
reference group is male;
reference group is CD4+ ≥ 200;
comparison group is ε4-.
Abbreviations: CI, confidence interval; SE, standard error.
Discussion
The aim of the current study was to assess potential interactions between APOE genotype, aging, and disease severity on neurocognitive functioning in a sample of HIV-positive adults. The results indicate that age augments the relationship between the ε4 allele and neurocognitive dysfunction in HIV-positive adults. Although we found no impact of ε4 on neurocognitive functioning in the overall sample, when age groups were analyzed separately, older ε4 carriers were at a disproportionate risk for developing HAND compared with age-matched ε4 noncarriers. When diagnosis was used as the outcome variable, 94% of older ε4 carriers in the current sample carried a diagnosis of HAND. Examination by discrete individual cognitive domains revealed age by APOE genotype interactions in the domains of executive functions and information processing speed such that the combination of the ε4 allele and advanced age resulted in reduced performance. These findings were independent of disease severity (CD4+ cell count and duration of HIV infection).
Interestingly, although the combined effects of the ε4 allele and advanced age were evident at a sub/syndromic level (as seen in HAND) and on a domain level, there seemed to be a discrepancy between the diagnostic findings and the domain level analyses. Whereas almost all of the older ε4 allele carriers carried a diagnosis of HAND, interactions between advanced age and the ε4 allele were evident only in the domains of executive functioning and information processing speed. This distinction warrants attention because it may explain some of the variability across studies assessing the relationship between APOE genotype and HAND. There are a number of additional factors that are considered when arriving at a diagnosis (eg, psychiatric and substance abuse history, neurological history, and functional ability) that are not considered in deriving neuropsychometric scores. This may contribute to the discrepancy.
The current results are consistent with the findings of Valcour et al,31 who found no association between HIV-associated dementia and the presence of one or more ε4 alleles among their entire cohort or when examining only younger (age < 40 years) participants, but did find a significant association between ε4 and HIV-associated dementia among older (age ≥ 50 years) participants. Conversely, the current findings are inconsistent with two previous studies directly assessing the interplay between age, ε4, and cognition in HIV.25,29 Both Chang et al and Spector et al found a negative effect of the ε4 allele on neurocognition in HIV-positive individuals in their overall sample (older and younger combined); however, when the age groups were analyzed separately, there was no increased risk for older participants. Methodological differences between the current study and these studies in regards to sample size, clinical features, and demographic characteristics of the participants may account for some of these differences.
Chang et al25 studied 70 HIV-seronegative and 69 HIV-positive individuals with neuropsychological tests and quantitative neuroimaging. Among their seronegative controls, they found evidence suggesting that the ε4 allele had beneficial effects in younger but not older adults (suggesting antagonistic pleiotropic effects of the ε4 allele on the brain). However, the opposite was found in the HIV sample, with younger participants demonstrating smaller brain volumes and poorer cognitive performance. There was no evidence of worsening with advanced age. One explanation put forward was that younger HIV-positive individuals might have a more robust neuroinflammatory response. A small sample size may have precluded them from finding any additional worsening with age. In their study, HIV-positive ε4 carriers also had lower CD4+ than noncarriers, although they were comparable in regards to nadir CD4+. The extent to which disease severity between age groups within their ε4 allele groups differed is unclear. Spector et al29 studied a predominantly male, HCV-positive Chinese sample of HIV-positive individuals and found a deleterious effect of ε4 on cognition at baseline in the overall (older and younger combined) sample; however, when age groups were analyzed separately, there was no increased risk for older participants with the ε4 allele. The authors opined that this might have been influenced by the small number of subjects aged over 50 years. The extent to which HCV also had an adverse impact on cognition and may have contributed to their findings is unclear.
Demographic differences between samples in the studies are also noteworthy. The current study was comprised of 28.6% African Americans, 12% Latinos, 55.2% Caucasians, and 4.2% “others”, and differs from both Chang et al25 and Spector et al.29 There is evidence to suggest that the risk for conferring the ε4 allele differs between ethnic distributions, as does the strength of the relationship between the ε4 allele and dementia.43,44 Compared to Caucasians, the association between APOE genotype and dementia has been found to be weaker among African American and Hispanic samples. Conversely, a stronger association has been seen in Japanese participants. Ethnic differences between study samples may contribute to the variable findings.
Compared with the previous studies, ours is the only study, to our knowledge, to control for the effects of cognitive reserve. Cognitive reserve, often measured with indices of crystallized intelligence and years of education, refers to the degree to which an individual can compensate for insults to the brain. Among HIV-positive individuals, individuals with higher cognitive reserve capacity may be able to shoulder greater insult to the brain before they show overt signs of cognitive dysfunction.40,51–53 In the current study, older participants presented with higher levels of cognitive reserve, as did ε4 allele carriers. Failure to control for the effects of cognitive reserve when assessing the interactive effects of ε4 and age (should this be the case for older HIV-positive adults in other samples) on cognition may mask findings. Interestingly, there is now emerging evidence that supports the hypothesis of the antagonistic pleiotropic effects of the ε4 allele (ie, the ε4 allele having a differential effect across the lifespan, exhibiting beneficial effects when individuals are younger and deleterious effects when they are older; for review, see Tuminello and Han54). Among the research supportive of this hypothesis, there is some evidence to suggest that young ε4 carriers have higher IQ than young ε4 noncarriers.55 In another study, infant ε4 carriers were found to have higher scores on a mental development scale compared with infant ε4 noncarriers.56 It may not be until older adulthood that a threshold is reached after which the protective influence of cognitive reserve against the adverse impact of neuropathology is reduced.
Additional exploratory assessment of the interplay between disease severity, ε4, and cognition suggested that there was no consistently significant relationship between APOE genotype, disease severity, and HAND. This contrasts with previous findings in the pre-combined antiretroviral therapy era, when the combination of the ε4 allele and advanced disease severity had synergistic deleterious effects on cognition.23 It may be that the effect of the ε4 allele on disease severity is no longer significant in the post-combined antiretroviral therapy era or that the combined effects are no longer significant on cognition in the post-combined antiretroviral therapy era. However, the current sample used to study this interplay between APOE genotype and disease severity may also have precluded us from ascertaining this relationship. The NNTC was initially established for the purpose of brain banking. As such, individuals who were at risk for imminent death were specifically recruited. This may have truncated the range of CD4+ and limited the potential for ascertaining differences in CD4+ between ε4 carriers and ε4 noncarriers. This is a limitation to our assessment of disease severity and APOE genotype. Nevertheless, we found an effect on one domain of cognition (working memory). Although the impact of ε4 allele on neurocognitive functioning was not initially evident, differences emerged when the groups were characterized by disease severity. Among ε4 carriers, individuals who presented with advanced disease had poorer working memory.
The current findings should be considered with the following caveats. First, given the previously established relationship between ε4 and disease progression,18 the possibility that older individuals with ε4 may not be accurately represented in the current sample, and in other studies assessing the relationship between ε4 and cognition in HIV, should be considered. Valcour et al57 noted a lower ε4 allele frequency among older participants compared with younger ones. More recently, in a young adult South African sample, Joska et al26 found that the ε4 allele was less common among individuals with HIV-associated dementia. These individuals were just entering HIV care. The extent to which survival rates may have influenced these findings and the extent to which this contributed to the overall lack of findings among younger participants or more significant findings in older participants is unknown and would best be assessed in a longitudinal design. However, it should be noted that a Hardy–Weinberg equilibrium analysis in that study indicated no differences in the proportion of South African infant ε4 allele carriers or the proportion of ε4 allele carriers in their study sample. Second, the current study employed a cross-sectional design. Although we have documented associations between ε4, age, disease severity, and cognition, a longitudinal design is necessary to confirm these findings and determine whether ε4 alone or in combination with disease severity leads to a progressive decline in cognition or whether the effects are static.
Acknowledgments
This study was funded through the California HIV/AIDS Research Program grant ID06-LA-187, of which Dr Levine was the principal investigator. This study was also made possible through grants U01-MH08021 and R24-NS38841 (National Neurological AIDS Bank), awarded to Dr Singer, U01-MH083507 and R24-NS45491 (Texas NeuroAIDS Research Center), U01-MH083501 and R24-MH59724 (Manhattan HIV Brain Bank), U01-MH083506 and R24-MH59745 (California NeuroAIDS Tissue Network). Drs Panos and Patel are funded through the NIMH T-32 MH19535 (PI: Dr Hinkin). Additional funding was provided to Dr Panos through the NIH Loan Repayment Program. Dr Thames is supported by the NIMH K23MH095661 (PI: A Thames). We would like to thank Drs John Ringman, Seth Sherman, and Alyssa Arentoft, who provided insightful suggestions and technical assistance along the way. Analyses and manuscript preparation was completed at the University of California, Los Angeles. Parts of this manuscript have been presented at the American Academy of Clinical Neuropsychology (annual meeting) and the International Neuropsychological Society (annual meeting).
Footnotes
Disclosure
The authors report no conflicts of interest in this work.
References
- 1.Detels R, Munoz A, McFarlane G, et al. Effectiveness of potent anti-retroviral therapy on time to AIDS and death in men with known HIV infection duration. Multicenter AIDS Cohort Study Investigators. JAMA. 1998;280(17):1497–1503. doi: 10.1001/jama.280.17.1497. [DOI] [PubMed] [Google Scholar]
- 2.Centers for Disease Control. HIV/AIDS Surveillance Report, 2005. Atlanta, GA: US Department of Health and Human Services; 2007. [Accessed January 13, 2013]. Available from: http://www.cdc.gov/hiv/topics/surveillance/resources/reports/2005report/pdf/2005surveillancereport.pdf. [Google Scholar]
- 3.Dilley JW, Schwarcz S, Loeb L, Hsu L, Nelson K, Scheer S. The decline of incident cases of HIV-associated neurological disorders in San Francisco, 1991–2003. AIDS. 2005;19(6):634–635. doi: 10.1097/01.aids.0000163944.39306.7c. [DOI] [PubMed] [Google Scholar]
- 4.Heaton RK, Clifford DB, Franklin DR, Jr, et al. HIV-associated neurocognitive disorders persist in the era of potent antiretroviral therapy: CHARTER study. Neurology. 2010;75(23):2087–2096. doi: 10.1212/WNL.0b013e318200d727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sacktor N. The epidemiology of human immunodeficiency virus-associated neurological disease in the era of highly active antiretroviral therapy. J Neurovirol. 2002;(8 Suppl 2):115–121. doi: 10.1080/13550280290101094. [DOI] [PubMed] [Google Scholar]
- 6.Valcour VG, Shikuma C, Shiramizu B, et al. Higher frequency of dementia in older HIV-1 individuals: the Hawaii Aging with HIV-1 Cohort. Neurology. 2004;63(5):822–827. doi: 10.1212/01.wnl.0000134665.58343.8d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chuang YF, Hayden KM, Norton MC, et al. Association between APOE epsilon4 allele and vascular dementia: the Cache County study. Dement Geriatr Cogn Disord. 2010;29(3):248–253. doi: 10.1159/000285166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Liu X, Li L, Liu F, et al. ApoE gene polymorphism and vascular dementia in Chinese population: a meta-analysis. J Neural Transm. 2012;119(3):387–394. doi: 10.1007/s00702-011-0714-6. [DOI] [PubMed] [Google Scholar]
- 9.Teasdale GM, Murray GD, Nicoll JA. The association between APOE epsilon4, age and outcome after head injury: a prospective cohort study. Brain. 2005;128(Pt 11):2556–2561. doi: 10.1093/brain/awh595. [DOI] [PubMed] [Google Scholar]
- 10.Teasdale GM, Nicoll JA, Murray G, Fiddes M. Association of apolipoprotein-E polymorphism with outcome after head injury. Lancet. 1997;350(9084):1069–1071. doi: 10.1016/S0140-6736(97)04318-3. [DOI] [PubMed] [Google Scholar]
- 11.Jarvik L, LaRue A, Blacker D, et al. Children of persons with Alzheimer disease: what does the future hold? Alzheimer Dis Assoc Disord. 2008;22(1):6–20. doi: 10.1097/WAD.0b013e31816653ac. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Drenos F, Kirkwood TB. Selection on alleles affecting human longevity and late-life disease: the example of apolipoprotein-E. PLoS One. 2010;5(4):e10022. doi: 10.1371/journal.pone.0010022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Jofre-Monseny L, Minihane AM, Rimbach G. Impact of apoE genotype on oxidative stress, inflammation and disease risk. Mol Nutr Food Res. 2008;52(1):131–145. doi: 10.1002/mnfr.200700322. [DOI] [PubMed] [Google Scholar]
- 14.Kulminski AM, Culminskaya I, Ukraintseva SV, et al. Trade-off in the effects of the apolipoprotein-E polymorphism on the ages at onset of CVD and cancer influences human lifespan. Aging Cell. 2011;10(3):533–541. doi: 10.1111/j.1474-9726.2011.00689.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Moore RJ, Chamberlain RM, Khuri FR. Apolipoprotein E and the risk of breast cancer in African-American and non-Hispanic white women. A review. Oncology. 2004;66(2):79–93. doi: 10.1159/000077433. [DOI] [PubMed] [Google Scholar]
- 16.Smith JD. Apolipoproteins and aging: emerging mechanisms. Ageing Res Rev. 2002;1(3):345–365. doi: 10.1016/s1568-1637(02)00005-3. [DOI] [PubMed] [Google Scholar]
- 17.Bedlack RS, Strittmatter MD, Morgenlander JC. Apolipoprotein E and neuromuscular disease: a critical review of the literature. Arch Neurol. 2000;57:1561–1565. doi: 10.1001/archneur.57.11.1561. [DOI] [PubMed] [Google Scholar]
- 18.Burt TD, Agan BK, Marconi VC, et al. Apolipoprotein (apo) E4 enhances HIV-1 cell entry in vitro, and the APOE epsilon4/ epsilon4 genotype accelerates HIV disease progression. Proc Natl Acad Sci U S A. 2008;105(25):8718–8723. doi: 10.1073/pnas.0803526105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hill JM, Bhattacharjee PS, Neumann DM. Apolipoprotein E alleles can contribute to the pathogenesis of numerous clinical conditions including HSV-1 corneal disease. Exp Eye Res. 2007;84(5):801–811. doi: 10.1016/j.exer.2006.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Jayasuriya AN, Itzhaki RF, Wozniak MA, et al. Apolipoprotein E-epsilon 4 and recurrent genital herpes in individuals co-infected with herpes simplex virus type 2 and HIV. Sex Transm Infect. 2008;84(7):516–517. doi: 10.1136/sti.2008.032367. [DOI] [PubMed] [Google Scholar]
- 21.Kuhlmann I, Minihane AM, Huebbe P, Nebel A, Rimbach G. Apolipoprotein E genotype and hepatitis C, HIV and herpes simplex disease risk: a literature review. Lipids Health Dis. 2010;9:8. doi: 10.1186/1476-511X-9-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Vitek MP, Brown CM, Colton CA. APOE genotype-specific differences in the innate immune response. Neurobiol Aging. 2009;30(9):1350–1360. doi: 10.1016/j.neurobiolaging.2007.11.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Corder EH, Robertson KR, Lannfelt L, et al. HIV-infected subjects with E4 allele for APOE have excess dementia and peripheral neuropathy. Nat Med. 1998;4(10):1182–1184. doi: 10.1038/2677. [DOI] [PubMed] [Google Scholar]
- 24.Andres MA, Feger U, Nath A, Munsaka S, Jiang CS, Chang L. APOE epsilon 4 allele and CSF APOE on cognition in HIV-infected subjects. J Neuroimmune Pharmacol. 2011;6(3):389–398. doi: 10.1007/s11481-010-9254-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Chang L, Andres M, Sadino J, et al. Impact of apolipoprotein-E epsilon4 and HIV on cognition and brain atrophy: antagonistic pleiotropy and premature brain aging. Neuroimage. 2011;58(4):1017–1027. doi: 10.1016/j.neuroimage.2011.07.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Joska JA, Combrinck M, Valcour VG, et al. Association between apolipoprotein-E4 genotype and human immunodeficiency virus-associated dementia in younger adults starting antiretroviral therapy in South Africa. J Neurovirol. 2010;16(5):377–383. doi: 10.3109/13550284.2010.513365. [DOI] [PubMed] [Google Scholar]
- 27.Pemberton LA, Stone E, Price P, van Bockxmeer F, Brew BJ. The relationship between ApoE, TNFA, IL1a, IL1b and IL12b genes and HIV-1-associated dementia. HIV Med. 2008;9(8):677–680. doi: 10.1111/j.1468-1293.2008.00614.x. [DOI] [PubMed] [Google Scholar]
- 28.Pomara N, Belzer KD, Silva R, Cooper TB, Sidtis JJ. The apolipoprotein-E epsilon4 allele and memory performance in HIV-1 seropositive subjects: differences at baseline but not after acute oral lorazepam challenge. Psychopharmacolog y (Berl) 2008;201(1):125–135. doi: 10.1007/s00213-008-1253-1. [DOI] [PubMed] [Google Scholar]
- 29.Spector SA, Singh KK, Gupta S, et al. APOE e4 and MBL-2 O/O genotypes are associated with neurocognitive impairment in HIV-infected plasma donors. AIDS. 2010;24(10):1471–1479. doi: 10.1097/QAD.0b013e328339e25c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sun B, Abadjian L, Rempel H, Calosing C, Rothlind J, Pulliam L. Peripheral biomarkers do not correlate with cognitive impairment in highly active antiretroviral therapy-treated subjects with human immunodefciency virus type 1 infection. J Neurovirol. 2010;16(2):115–124. doi: 10.3109/13550280903559789. [DOI] [PubMed] [Google Scholar]
- 31.Valcour VG, Shikuma C, Shiramizu B, et al. Age, apolipoprotein-E4, and the risk of HIV dementia: the Hawaii Aging with HIV Cohort. J Neuroimmunol. 2004;157(1–2):197–202. doi: 10.1016/j.jneuroim.2004.08.029. [DOI] [PubMed] [Google Scholar]
- 32.Jarvik GP, Austin MA, Fabsitz RR, et al. Genetic influences on age-related change in total cholesterol, low density lipoprotein-cholesterol, and triglyceride levels: longitudinal apolipoprotein-E genotype effects. Genet Epidemiol. 1994;11(4):375–384. doi: 10.1002/gepi.1370110407. [DOI] [PubMed] [Google Scholar]
- 33.Kulminski A, Culminskaya I, Arbeev K, Ukraintseva S, Arbeeva L, Yashin AI. Trade-off in the effect of the APOE gene on the ages at onset of CVD and cancer across ages, gender, and human generations. Rejuvenation Res. 2012 Oct 25; doi: 10.1089/rej.2012.1362. [Epub ahead of print.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Corder EH, Galeazzi L, Franceschi C, et al. Differential course of HIV-1 infection and apolipoprotein-E polymorphism. Proc Natl Acad Sci U S A. 2008;105(46):E87. doi: 10.1073/pnas.0808164105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Morgello S, Gelman BB, Kozlowski PB, et al. The National NeuroAIDS Tissue Consortium: a new paradigm in brain banking with an emphasis on infectious disease. Neuropathol Appl Neurobiol. 2001;27(4):326–335. doi: 10.1046/j.0305-1846.2001.00334.x. [DOI] [PubMed] [Google Scholar]
- 36.American Academy of Neurology. Nomenclature and research case definitions for neurologic manifestations of human immunodefciency virus-type 1 (HIV-1) infection. Report of a Working Group of the American Academy of Neurology AIDS Task Force. Neurology. 1991;41(6):778–785. doi: 10.1212/wnl.41.6.778. [DOI] [PubMed] [Google Scholar]
- 37.Levine AJ, Sinsheimer JS, Bilder R, Shapshak P, Singer EJ. Functional polymorphisms in dopamine-related genes: effect on neurocognitive functioning in HIV+ adults. J Clin Exp Neuropsychol. 2012;34(1):78–91. doi: 10.1080/13803395.2011.623118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Antinori A, Arendt G, Becker JT, et al. Updated research nosology for HIV-associated neurocognitive disorders. Neurology. 2007;69(18):1789–1799. doi: 10.1212/01.WNL.0000287431.88658.8b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Wilkinson GS. The Wide Range Achievement Test: Manual. 3rd ed. Wilmington, DE: Wide Range; 1993. [Google Scholar]
- 40.Thames AD, Foley JM, Panos SE, Singer EJ, El-Saden S, Hinkin CH. Cognitve reserve masks neurobehavioral expression of immuno-defciency virus-associated neurological disorder in older patients. Neurobehav HIV Med. 2011;3:87–93. [Google Scholar]
- 41.Hasin DS, Trautman KD, Miele GM, Samet S, Smith M, Endicott J. Psychiatric Research Interview for Substance and Mental Disorders (PRISM): reliability for substance abusers. Am J Psychiatry. 1996;153(9):1195–1201. doi: 10.1176/ajp.153.9.1195. [DOI] [PubMed] [Google Scholar]
- 42.American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders. 4th ed. Washington, DC: American Psychiatric Association; 1994. [Google Scholar]
- 43.Farrer LA, Cupples LA, Haines JL, et al. Effects of age, sex, and ethnicity on the association between apolipoprotein-E genotype and Alzheimer disease. A meta-analysis. APOE and Alzheimer Disease Meta Analysis Consortium. JAMA. 1997;278(16):1349–1356. [PubMed] [Google Scholar]
- 44.Teruel BM, Rodriguez JJ, McKeigue P, et al. Interactions between genetic admixture, ethnic identity, APOE genotype and dementia prevalence in an admixed Cuban sample; a cross-sectional population survey and nested case-control study. BMC Med Genet. 2011;12:43. doi: 10.1186/1471-2350-12-43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Ettenhofer ML, Hinkin CH, Castellon SA, et al. Aging, neurocognition, and medication adherence in HIV infection. Am J Geriatr Psychiatry. 2009;17(4):281–290. doi: 10.1097/JGP.0b013e31819431bd. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Hinkin CH, Castellon SA, Durvasula RS, et al. Medication adherence among HIV+ adults: effects of cognitive dysfunction and regimen complexity. Neurology. 2002;59(12):1944–1950. doi: 10.1212/01.wnl.0000038347.48137.67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Valcour V, Shikuma C, Shiramizu B, et al. Age, apolipoprotein-E4, and the risk of HIV dementia: the Hawaii Aging with HIV Cohort. J Neuroimmunol. 2004;157(1–2):197–202. doi: 10.1016/j.jneuroim.2004.08.029. [DOI] [PubMed] [Google Scholar]
- 48.Caselli RJ, Dueck AC, Osborne D, et al. Longitudinal modeling of age-related memory decline and the APOE epsilon4 effect. N Engl J Med. 2009;361(3):255–263. doi: 10.1056/NEJMoa0809437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Liu F, Pardo LM, Schuur M, et al. The apolipoprotein-E gene and its age-specific effects on cognitive function. Neurobiol Aging. 2010;31(10):1831–1833. doi: 10.1016/j.neurobiolaging.2008.09.015. [DOI] [PubMed] [Google Scholar]
- 50.Benjamini Y, Hochberg Y. The adaptive control of the false discovery rate in multiple comparison problems. J Educ Behav Stat. 2000;25(1):60–83. [Google Scholar]
- 51.Satz P. Brain reserve capacity on symptom onset after brain injury. A formulation and review of evidence for threshold theory. Neuropsychology. 1993;7(3):273–295. [Google Scholar]
- 52.Satz P, Morgenstern H, Miller EN, et al. Low education as a possible risk factor for cognitive abnormalities in HIV-1: fndings from the Multicenter AIDS cohort study (MACS) J Acquir Immune Defc Syndr. 1993;6(5):503–511. [PubMed] [Google Scholar]
- 53.Stern Y. Cognitive reserve and Alzheimer’s disease. Alzheimer Dis Assoc Disord. 2006;20:S69–S74. doi: 10.1097/00002093-200607001-00010. [DOI] [PubMed] [Google Scholar]
- 54.Tuminello ER, Han SD. The apolipoprotein e antagonistic pleiotropy hypothesis: review and recommendations. Int J Alzheimers Dis. 2011;2011:726197. doi: 10.4061/2011/726197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Yu YW, Lin CH, Chen SP, Hong CJ, Tsai SJ. Intelligence and event-related potentials for young female human volunteer apolipoprotein-E epsilon4 and non-epsilon4 carriers. Neurosci Lett. 2000;294(3):179–181. doi: 10.1016/s0304-3940(00)01569-x. [DOI] [PubMed] [Google Scholar]
- 56.Wright RO, Hu H, Silverman EK, et al. Apolipoprotein E genotype predicts 24-month bayley scales infant development score. Pediatr Res. 2003;54(6):819–825. doi: 10.1203/01.PDR.0000090927.53818.DE. [DOI] [PubMed] [Google Scholar]
- 57.Valcour VG, Shiramizu B, Shikuma C. Frequency of apolipoprotein-E4 among older compared with younger HIV patients: support for detrimental effect of E4 on survival. Proc Natl Acad Sci U S A. 2008;105(41):E66. doi: 10.1073/pnas.0806919105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Battery AIT. Manual of Directions and Scoring. Washington, DC: War Department, Adjutant General’s Offce; 1944. [Google Scholar]
- 59.Grant EA, Berg DA. A behavioral analysis of degree of reinforcement and ease of shifting to new responses in a Weigl-type card sorting problem. J Exp Psychol. 1948;38(4):404–411. doi: 10.1037/h0059831. [DOI] [PubMed] [Google Scholar]
- 60.Heaton RK. A Manual for the Wisconsin Card Sorting Test. Odessa, FL: Psychological Assessment Resources; 1981. [Google Scholar]
- 61.Wechsler D. Wechsler Adult Intelligence Scale-III. San Antonio, TX: The Psychological Corporation; 1997. [Google Scholar]
- 62.Wiens AN, Fuller KH, Crossen JR. Paced Auditory Serial Addition Test: adult norms and moderator variables. J Clin Exp Neuropsychol. 1997;19(4):473–483. doi: 10.1080/01688639708403737. [DOI] [PubMed] [Google Scholar]
- 63.Shapiro AM, Benedict RH, Schretlen D, Brandt J. Construct and concurrent validity of the Hopkins Verbal Learning Test-Revised. Clin Neuropsychol. 1999;13(3):348–358. doi: 10.1076/clin.13.3.348.1749. [DOI] [PubMed] [Google Scholar]
- 64.Benedict RHB, Schretlen D, Groninger L, Dobraski M. Revision of the Brief Visuospatial Memory Test: Studies of normal performance, reliability, and validity. Psychol Assess. 1996;8(2):145–153. [Google Scholar]