Abstract
Venous thromboembolism (VTE) is a common complication in patients with high-grade gliomas. The purpose of this prospective multicenter study was to determine the hazard rate of first symptomatic VTE in newly-diagnosed glioma patients and identify clinical and laboratory risk factors. On enrollment, demographic and clinical information were recorded and a comprehensive coagulation evaluation was performed. Patients were followed until death. The study end point was objectively-documented symptomatic VTE. One hundred seven patients were enrolled with a median age of 57 years (range 29–85) between June 2005 and April 2008. Ninety-one (85 %) had glioblastoma multiforme (GBM). During an average survival of 17.7 months, 26 patients (24 %) (95 % CI 17–34 %) developed VTE (hazard rate 0.15 per person-year) and 94 patients (88 %) died. Median time to VTE was 14.2 weeks post-operation (range 3–126).
Patients with an initial tumor biopsy were 3.0 fold more likely to suffer VTE (p = 0.02). Patients with an elevated factor VIII activity (>147 %) were 2.1-fold more likely to develop VTE. ABO blood group, D dimer and thrombin generation were not associated with VTE. No fatal VTE occurred. VTE is a common complication in patients with newly-diagnosed high grade gliomas, particularly in the first six months after diagnosis. Patients with an initial tumor biopsy and elevated factor VIII levels are at increased risk. However, VTE was not judged to be pri-marily responsible for any patient deaths. Therefore, out-patient primary VTE prophylaxis remains investigational until more effective primary prophylaxis strategies and therapies for glioma are identified.
Keywords: Venous thromboembolism, Cancer, Risk factors, Glioma, Factor VIII, Glioblastoma
Introduction
Venous thromboembolism (VTE) is a common complication of cancer and its treatment. Cancer patients have a 4–20 fold increased risk of VTE that is further accentuated by chemotherapy [1, 2]. Patients with high grade gliomas have been noted to be at particularly high risk for VTE [3]. The cumulative incidence of symptomatic VTE among glioma patients has been estimated to be as high as 36 % during their course of therapy [4–8]. A recent population-based observational study estimated the incidence of VTE among glioma patients to be 16.1 events per 100 patient years during the first six months after diagnosis [9].
Prevention is important as thrombosis and its treatment are associated with significant morbidity, mortality and reduced quality of life [10]. VTE has been identified as the second leading cause of death among ambulatory cancer patients receiving chemotherapy [11]. Furthermore, cancer patients with VTE have a twofold higher risk of death than patients without VTE [12]. Consequently, VTE prevention has received increasing attention in cancer patients. Despite the high incidence of VTE in glioma patients, concerns about bleeding complications have limited the use of primary anticoagulant prophylaxis [13]. The PRODIGE study, a randomized controlled trial of dalteparin thromboprophylaxis in glioma patients, found a non-significant reduction in VTE but a trend toward more hemorrhagic complications [14]. A phase 1 study of tinzaparin reported similar results [15], while Robins et al. did not note any VTE or bleeding events among 42 patients receiving dalteparin [16].
Identification of VTE risk factors in glioma patients would facilitate targeting prophylaxis to the patients at greatest risk. Previous retrospective studies have identified patient age [8, 9, 17], tumor size [18], tumor grade [4, 9, 19], long operative times (more than 4 h) [20], chemotherapy [6], and the presence of leg paresis [6–8] as clinical risk factors for VTE. Laboratory VTE risk factors include an increased prothrombin time, reduced plas-minogen activator activity, ABO blood type, low platelet counts (<25th percentile), elevated WBC counts, P-selectin, D dimer and tissue factor-bearing microparticles (TFMP) [21–26]. Only age, tumor grade and leg paresis have been noted in more than one study.
In a retrospective cohort study, we demonstrated that ABO blood group was a previously unrecognized risk factor for symptomatic VTE in glioma patients. Blood group AB was associated with a hazard ratio (HR) of 9.4 (95 % confidence interval [CI] 2.7–32) compared to those with blood group O (p <0.0001). Blood group A was also associated with greater risk (HR = 2.7, 95 % CI 1.0–7.0, p = 0.045) [23]. ABO blood group has a significant influence on factor VIII levels with blood group AB being associated with the highest levels [27]. Since elevated levels of factor VIII are associated with a 4–6 fold increased risk of VTE [28, 29], our finding that ABO group was associated with an increased risk of VTE in glioma patients was biologically plausible.
The purpose of the current study was three-fold: (1) to prospectively determine the hazard rate of VTE in a population of patients with newly-diagnosed grade 3 or 4 glioma (2) to confirm the association of ABO blood type on the hazard rate of VTE and (3) to identify novel clinical and laboratory risk factors for VTE.
Materials and methods
This prospective multicenter study of patients with newly-diagnosed, pathologically-confirmed, grade III and IV glioma was conducted in member institutions of the National Cancer Institute-funded “New Approaches to Brain Tumor Therapy” (NABTT) and the Adult Brain Tumor Consortium. (Grant 2UM1 CA137443) The study protocol was reviewed and approved by the National Cancer Institute’s Cancer Therapy Evaluation Program (CTEP) and the Institutional Review Board of each participating institution. The study was initiated in October 2004.
The study eligibility criteria were that patients had to be adults (age 18 years or older) with histologically-confirmed, newly-diagnosed, supratentorial grade III or IV astrocytoma. Potential subjects were approached for study participation once their diagnosis of grade III or IV glioma was pathologically confirmed. All patients underwent complete tumor resections unless they had serious underlying medical issues precluding surgery, extensive or bilateral disease or disease in eloquent areas where surgery would have resulted in a high likelihood of severe postoperative disabilities. Patients could not have received prior radiation therapy, chemotherapy, immunotherapy or therapy with biologic agents or hormonal therapy. Glucocorticoid therapy was allowed. Patients had to have a Karnofsky performance status (KPS) of at least 60 %. Patients had to identify a caregiver to provide information if the patient became unable to remember or communicate during their course of therapy. Patients also had to be able to provide written informed consent.
Patients were ineligible if they had a history of prior cranial irradiation, previous thromboembolism or an indication for chronic anticoagulation. Patients were allowed to use chronic anti-platelet therapies including aspirin, clopidogrel or non-steroidal anti-inflammatory drugs. VTE prophylaxis was permitted during hospitalizations in accordance with routine clinical practice but outpatient prophylaxis was not permitted.
At study enrollment, demographic and clinical information were recorded including age, gender, ethnicity, tumor histology, size and location, treatments, KPS, and any limb paresis. Blood was drawn for ABO blood type, fibrinogen, factor VIII activity, D dimer, and endogenous thrombin potential. Patients were followed at monthly intervals in clinic or by telephone. Symptomatic VTE had to be objectively confirmed with standard radiological diagnostic methods (duplex ultrasound, computer tomography angiography, etc). Routine radiographic VTE surveillance was not permitted. VTE treatment was left to the discretion of the treating physician. Patients with VTE were followed every 2 months until death or withdrawal of consent.
Blood samples were collected at the time of study enrollment into blue-top vacutainer tubes containing 3.2 % sodium citrate. After collection, samples were centrifuged twice at 15,000 rpm for 3 min. Plasma was separated into 1 milliliter aliquots for storage in Nalgene sample vials at −80 °C for later analysis. All laboratory samples were shipped to the Johns Hopkins Special Coagulation laboratory for testing. Factor VIII activity was determined with a single-stage factor VIII activity assay using the Siemens Factor VIII assay on the BCS® coagulation analyzer. (Normal range, 50–150 %) (Siemens AG, Marburg Germany) Fibrinogen levels were determined using the Clauss technique.(Normal range, 150–400 mg/dL) Quantitative D dimer levels were determined using the quantitative Advanced D dimer assay. (Normal range, 0.17–0.88 mg/L Fibrinogen Equivalent Units) (Siemens AG, Marburg, Germany) Endogenous thrombin potential (ETP) was measured using Innovin® and a synthetic chromogenic thrombin substrate. (Siemens AG, Marburg Germany)
Statistical analysis
The purpose of the study was to prospectively determine the hazard rate of first symptomatic VTE in a population of patients with newly-diagnosed grade 3 or 4 glioma, assess the association of ABO blood type with VTE and to identify novel VTE risk factors. The trial design was based upon the results of Brandes et al. and Dhami et al., which estimated the hazard rate of venous thromboembolism to be 0.18 and 0.3 per person-year follow-up, respectively [4, 6]. Using these data, we estimated that we would need at least 107 participants to obtain 32 events assuming a 30 % cumulative incidence of VTE. The overall incidence rate was calculated as the total number of events divided by the total number of study participants.
The overall hazard rate was expressed as the hazard of symptomatic VTE per person-year of follow-up. Differences in baseline patient and disease characteristics were compared using the Student t test and Chi square test for continuous data and categorical data, respectively. In comparisons, the levels of each laboratory variable were considered elevated if they were above the median value of the entire patient population. The probability of a first episode of symptomatic VTE and overall survival were calculated using the non-parametric method of Kaplan–Meier. Subgroup comparisons were performed using the Log-rank test. The Logistic regression model was used for the univariate and multivariate analyses to estimate the risk ratio of symptomatic VTE associated with the clinical and laboratory measurements. All P values are reported as 2-sided and all analyses were conducted using SAS software (version 9.2, SAS Institute).
Results
A total of 107 patients (55 women, 52 men) were enrolled in the study a median of 23 days (range, 0–81 days) after pathologic diagnosis of a grade III or IV glioma. The median age was 57 years (range, 28–85 years). The histologic diagnosis was glioblastoma multiforme in 91 patients (85 %), anaplastic astrocytoma in 12 (11 %), anaplastic oligodendroglioma and unclassified malignant glioma in 2 patients each (2 %). The demographic and clinical characteristics are shown in Table 1.
Table 1.
Baseline clinical and laboratory characteristics of the study population
| Characteristic | |
|---|---|
| No. of patients | N = 107 |
| Median age (range) | 57 (28–85) |
| Gender | |
| Male | 52 (49 %) |
| Female | 55 (51 %) |
| Histologic diagnosis | |
| Glioblastoma multiforme | 91 (85 %) |
| Anaplastic astrocytoma | 12 (11 %) |
| Anaplastic oligodendroglioma | 2 (2 %) |
| Malignant glioma | 2 (2 %) |
| Median initial KPS (range) | 90 (60–100) |
| Surgical procedure | |
| Biopsy | 23 (21 %) |
| Resection | 84 (79 %) |
| ABO blood type | |
| O | 55 (52 %) |
| A | 31 (29 %) |
| B | 13 (12 %) |
| AB | 7 (6 %) |
| Rh+ | 91 (86 %) |
| D dimer μg/L (mean ± Std) | 2 ± 2 |
| Factor VIII activity (%) (mean ± Std) | 154 ± 55 |
| Fibrinogen mg/dL (mean ± Std) | 385 ± 154 |
| ETP % | 114 ± 53 |
| ETP maximal amplitude (mean ± Std) | 440 ± 78 |
| Tmax | 56 ± 47 |
| Cmax | 194 ± 168 |
| AUC | 451 ± 89 |
ETP endogenous thrombin potential, Tmax time required to reach maximal thrombin concentration, Cmax maximal thrombin concentration, AUC area under the thrombin concentration curve
During an average survival of 17.7 months, 26 patients (24 %; 95 % CI 17–34 %) developed symptomatic VTE. In patients with glioblastoma multiforme (GBM), the incidence was 26 % (95 % CI 18–37 %). GBM patients constituted 92 % of all patients with symptomatic VTE. The median time from initial surgery to VTE diagnosis was 14.2 weeks (range, 3–126 weeks). The hazard rate of first symptomatic VTE was 0.15 (95 % CI 0.1–0.22) per-person year of follow-up among all patients and 0.2 (95 % CI 0.13–0.3) for patients with newly-diagnosed GBM. Among patients with symptomatic VTE, 25 had a lower extremity deep venous thrombosis (DVT) and one had a pulmonary embolism (PE). No fatal VTE occurred. All patients were treated with anticoagulation.
Baseline clinical and laboratory characteristics were similar between patients who did and did not develop symptomatic VTE during their clinical course (Table 2). Notably, there was no difference in the frequency of symptomatic VTE by ABO blood type (Table 2). Patients who subsequently developed symptomatic VTE were more likely to have had an initial tumor biopsy only rather than a complete resection (p = 0.02) (Table 2). However, there was no difference in KPS between patients who underwent tumor biopsy only or complete resection. Among the 23 patients who underwent biopsy only, 15 (65 %) had a KPS of 90–100 and 8 (35 %) had a KPS of 60–80. In comparison, among the 84 patients who underwent resection, 68 (81 %) had a KPS of 90–100 and 16 (19 %) had a KPS at 60–80. (p = 0.1). Male gender was associated with a greater risk of symptomatic VTE but only among GBM patients. (p = 0.02) A total of 39 (37 %) patients had a second surgery. There was no difference in the rate of symptomatic VTE between patients who had a second surgery compared to those who did not (7/39, 17.9 % vs. 19/67, 28.4 % respectively, p = 0.2). In fact, among patients who had a second surgery and a symptomatic VTE, all VTE occurred prior to the second surgery except for one patient whose initial surgery was a tumor biopsy.
Table 2.
Comparison of baseline clinical and laboratory characteristic in patients with and without VTE
| Characteristic | No VTE | VTE | p value |
|---|---|---|---|
| No. of patients (107) | N = 81 | N = 26 | |
| Median age (range) | 57(28–85) | 57 (41–77) | 0.2 |
| Median KPS (range) | 90 (70–100) | 90 (60–100) | 0.09 |
| Gender | 0.1 | ||
| Male | 36 (44 %) | 16 (62 %) | |
| Female | 45 (56 %) | 10 (38 %) | |
| Histologic diagnosis | 0.2 | ||
| Glioblastoma multiforme | 67 (83 %) | 24 (92 %) | |
| Other | 14 (17 %) | 2 (8 %) | |
| Surgical procedure | 0.02 | ||
| Biopsy | 13 (16 %) | 10 (38 %) | |
| Resection | 68 (84 %) | 16 (62 %) | |
| ABO blood type | 0.9 | ||
| O | 41 (51 %) | 14 (54 %) | |
| A | 24 (30 %) | 7 (27 %) | |
| B | 9 (11 %) | 4 (15 %) | |
| AB | 6 (8 %) | 1 (4 %) | |
| Rh+ | 68 (85 %) | 23 (88 %) | 0.7 |
| Mean D dimer lg/L (± Std) | 2 (±2) | 2 (±1) | 0.5 |
| Mean % factor VIII (± Std) | 147 (±51) | 175(±60) | 0.02 |
| Mean fibrinogen mg/dL (± Std) | 388 (±166) | 379 (±116) | 0.8 |
| Mean ETP % | 110 (±43) | 125 (±75) | 0.4 |
| Mean ETP maximal amplitude (±Std) | 437(±74) | 450 (±90) | 0.5 |
| Mean Tmax (±Std) | 50 (±12) | 73 (±94) | 0.3 |
| Mean Cmax (±Std) | 166 (±32) | 284 (±330) | 0.1 |
| Mean AUC (±Std) | 440 (±64) | 487 (±139) | 0.1 |
Statistically significant values are represented in bold
ETP endogenous thrombin potential, Tmax time required to reach maximal thrombin concentration, Cmax maximal thrombin concentration, AUC area under the thrombin concentration curve
The median factor VIII activity in the study population was 147 %. The mean baseline factor VIII activity was significantly higher among patients who subsequently developed symptomatic VTE compared with those who did not among the entire study population (factor VIII activity, 175 ± 60 vs. 147 ± 51, p = 0.02). This difference was also noted in the subpopulation of patients with GBM (180 ± 60 vs. 146 ± 52, p = 0.01). Thirty-two percent of patients (95 % CI 20–47 %) with elevated factor VIII at baseline developed VTE within 24 months compared to 18 % (95 % CI 9–31 %) among those without elevated factor VIII activity (Fig. 1). A similar trend was observed among GBM patients [36 % (95 % CI 22–51 %) vs. 17 % (95 % CI 7–32 %)]. The estimated risk ratio (RR) of developing VTE was 2.1 (95 % CI 0.8–5.5; p = 0.11) among the entire population with elevated factor VIII and 2.7 (95 % CI 0.97–7.41; p = 0.05) among GBM patients. The risk ratio was 2.5 (95 % CI 0.9–7.1; p = 0.09) among GBM patients after adjusting for baseline surgical procedure and gender. Patients who had a biopsy as an initial surgical procedure were 3.0 fold more likely (95 % CI 1.2–8.8; p = 0.02) to develop symptomatic VTE than patients who underwent resection. Interestingly, D dimer and thrombin generation were similar in patients who did and did not have symptomatic VTE (Table 2).
Fig. 1.
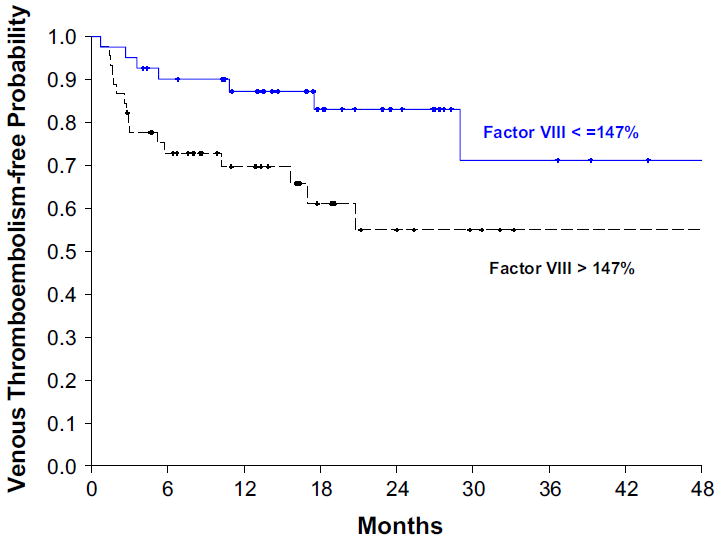
Elevated factor VIII activity increases the risk of VTE in glioma patients
At the time of data analyses, 94 (88 %) patients had died. The average overall survival (OS) was 17.7 months (95 % CI 15.4–20.5 months). Average OS was 15.7 months (95 % CI 14.1–18.7) for patients with VTE and 18.3 months (95 % CI 16.1–23.5) for those without VTE (p = 0.09 Log-rank test).
Discussion
Venous thromboembolism is a common complication in patients with malignant glioma. However, previous studies of primary thromboprophylaxis in unselected glioma patients have not been associated with improved outcomes. Targeting high risk patients could improve the risk:benefit balance of prophylaxis. In this large prospective multi-center study of newly-diagnosed patients with high-grade glioma, we noted symptomatic VTE was common in the first six months after tissue diagnosis. Patients with an initial tumor biopsy, elevated factor VIII activity and men with GBM were at high risk for VTE. However, progressive cancer not VTE was responsible for all the patient deaths during follow up.
Brandes and colleagues noted 26 % of their 77 glioma patients developed VTE, an incidence of 0.15 VTE per patient month [4]. In a retrospective analysis of the California discharge database, Semrad et al. noted an incidence of 16.1 VTE per 100 patient years during the first six months after diagnosis of a malignant glioma [9]. Sub-total resection has been associated with an increased risk of VTE in two previous studies [14, 19]. In contrast, elevated factor VIII activity has not been previously identified as a risk factor for VTE in glioma patients, although factor VIII activity has been associated with VTE in other patient populations [28, 30]. Likewise, male gender, a VTE risk factor in GBM patients in our study, is a novel finding in glioma patients but has been identified as VTE risk factor in the general population [31, 32].
In contrast to our earlier study, we did not identify ABO blood type as a risk factor for VTE which underscores the importance of validating risk factors in prospective studies. Nevertheless, it is worthwhile noting that only seven patients had AB blood group. Therefore it is possible that a larger study might be necessary to confirm the influence of ABO blood group on VTE risk in glioma patients. We were also surprised that D dimer and thrombin generation were not associated with VTE. Prell et al. and Thaler and colleagues have identified D dimer as a risk factor for VTE [25, 24]. We suspect that differences in study populations and the timing of study procedures are responsible for these conflicting results.
The most surprising result of our study was the absence of any VTE-related mortality among our study population. All but one of the thrombotic events during our study was a DVT and no patient deaths were attributable to VTE. In the PRODIGE study, 74 % of thrombotic events were DVT alone and no fatal PE was identified [14]. In contrast, Thaler et al. noted 11 PE (46 % of all VTE) including two fatal events [24]. Although we cannot be certain as to the exact reason for the low VTE-related mortality in our study, one possible reason may be that all our co-investigators are experienced neuro-oncologists who have a high index of suspicion for VTE and order objective testing at the first signs or symptoms of DVT. It is also possible larger studies focused on high-risk patient populations may be required to identify survival differences associated with VTE and its prevention.
Our study has several strengths and weaknesses. Our study is the first prospective multicenter study of risk factors for symptomatic VTE in patients with newly-diagnosed stage III/IV malignant glioma. All participants were recruited after tissue diagnosis and before initiation of chemotherapy or radiotherapy. Therefore, there was no impact of these procedures on the results of laboratory testing. In addition, initial therapy consisting of temo-zolomide-based combination chemo-radiotherapy was uniform in all but two patients. All VTE were symptomatic and objectively confirmed with standardized radiologic procedures so the likelihood of misclassification is low. Plasma samples were collected prior to the initiation of therapy and were available on 93 % of participants, so we are confident our results are representative of the study population. All laboratory studies were performed using the same assay and coagulation analyzer in a single reference laboratory eliminating the possibility of inter-laboratory differences influencing results. Since we recruited patients from multiple centers, we believe our results are generalizable to the overall population of glioma patients treated in the United States.
Our study also has some limitations. Laboratory samples were obtained after diagnosis but the timing of study enrollment varied between subjects depending upon the timing of referral for neuro-oncology consultation. This could have influenced laboratory results. Furthermore, we did not prohibit or standardize the use of corticosteroids which are routinely prescribed in glioma patients. Corticosteroids have been shown to influence coagulation factor levels and thus could have influenced assay results [33]. However, we felt that prohibition of corticosteroids prior to blood sampling would have adversely affected patient care and study enrollment, so we did not exclude patients on this basis.
How can we incorporate these findings into clinical care and the research agenda for patients with high-risk glioma? VTE is a common complication among glioma patients, particularly in the first six months after diagnosis. Therefore, clinicians should maintain a high index of suspicion for DVT and PE when patients report symptoms (e.g., leg swelling or cramping, dyspnea, fatigue) that could indicate VTE. Risk factors identified by this study and others can help clinicians identify patients who may be at greater risk. However, our study also emphasizes that clinically significant morbidity and mortality posed by VTE is limited compared to that associated with the underlying cancer. Therefore, investigative efforts should continue to focus on improving therapy for glioma. Once therapeutic outcomes are better, we suspect the absolute clinical impact of VTE and other complications will be greater as will the rewards of effective prevention. To this end, Thaler et al. have recently proposed the first VTE risk assessment tool for glioma patients [24]. Once more effective therapy is available, we envision that studies of targeted thrombo-prophylaxis (perhaps employing new non-vitamin K antagonist oral anticoagulants) may provide an effective strategy to further improve the outcomes for patients with glioma.
Acknowledgments
The authors would like to acknowledge the efforts of all the investigators and staff at the member institutions of the National Cancer Institute-funded “New Approaches to Brain Tumor Therapy” (NABTT) and Adult Brain Tumor Consortium. (Grant 2UM1 CA137443).
Funding This study was conducted by the Adult Brain Tumor Consortium (ABTC) which is funded by the National Cancer Institute (Grant #2UM1 CA137443).
Potential conflict of interest Michael Streiff has received research funding from Bristol Myers Squibb, Portola and the Patient Centered Outcomes Research Institute, honoraria for CME lectures from Sanofi-Aventis and consulted for Sanofi-Aventis, Eisai, Daiichi-Sankyo, Boehringer-Ingelheim, Pfizer and Janssen HealthCare and has given expert witness testimony in various medical malpractice cases.
Thomas Kickler receives patent fees for inventions licensed to Siemens that are administered thru Johns Hopkins University. He recieves no cost research reagents for performance of the ETP assay.
Footnotes
Conflict of interest Xiaobu Ye, Serena Desideri, Jayesh Jani, Joy Fisher, Stuart Grossman has no conflicts of interest to report.
References
- 1.Heit JA, Silverstein MD, Mohr DN, Petterson TM, O’Fallon WM, Melton LJ. Risk factors for deep vein thrombosis and pulmonary embolism: a population-based case-control study. Arch Intern Med. 2000;160:809–815. doi: 10.1001/archinte.160.6.809. [DOI] [PubMed] [Google Scholar]
- 2.Blom JW, Doggen CJ, Osanto S, Rosendaal FR. Malignancies, prothrombotic mutations, and the risk of venous thrombosis. JAMA. 2005;293:715–722. doi: 10.1001/jama.293.6.715. [DOI] [PubMed] [Google Scholar]
- 3.Wun T, White RH. Venous thromboembolism (VTE) in patients with cancer: epidemiology and risk factors. Cancer Investig. 2009;27(Suppl 1):63–74. doi: 10.1080/07357900802656681. [DOI] [PubMed] [Google Scholar]
- 4.Brandes AA, Scelzi E, Salmistraro G, Ermani M, Carollo C, Berti F, Zampieri P, Baiocchi C, Fiorentino MV. Incidence of risk of thromboembolism during treatment high-grade gliomas: a prospective study. Eur J Cancer. 1997;33:1592–1596. doi: 10.1016/s0959-8049(97)00167-6. [DOI] [PubMed] [Google Scholar]
- 5.Cheruku R, Tapazoglou E, Ensley J, Kish JA, Cummings GD, Al-Sarraf M. The incidence and significance of thromboembolic complications in patients with high-grade gliomas. Cancer. 1991;68:2621–2624. doi: 10.1002/1097-0142(19911215)68:12<2621::aid-cncr2820681218>3.0.co;2-7. [DOI] [PubMed] [Google Scholar]
- 6.Dhami MS, Bona RD, Calogero JA, Hellman RM. Venous thromboembolism and high grade gliomas. Thromb Haemost. 1993;70:393–396. [PubMed] [Google Scholar]
- 7.Quevedo JF, Buckner JC, Schmidt JL, Dinapoli RP, O’Fallon JR. Thromboembolism in patients with high-grade glioma. Mayo Clin Proc. 1994;69:329–332. doi: 10.1016/s0025-6196(12)62216-2. [DOI] [PubMed] [Google Scholar]
- 8.Ruff RL, Posner JB. Incidence and treatment of peripheral venous thrombosis in patients with glioma. Ann Neurol. 1983;13:334–336. doi: 10.1002/ana.410130320. [DOI] [PubMed] [Google Scholar]
- 9.Semrad TJ, O’Donnell R, Wun T, Chew H, Harvey D, Zhou H, White RH. Epidemiology of venous thromboembolism in 9489 patients with malignant glioma. J Neurosurg. 2007;106:601–608. doi: 10.3171/jns.2007.106.4.601. [DOI] [PubMed] [Google Scholar]
- 10.Prandoni P, Lensing AW, Piccioli A, Bernardi E, Simioni P, Girolami B, Marchiori A, Sabbion P, Prins MH, Noventa F, et al. Recurrent venous thromboembolism and bleeding complications during anticoagulant treatment in patients with cancer and venous thrombosis. Blood. 2002;100:3484–3488. doi: 10.1182/blood-2002-01-0108. [DOI] [PubMed] [Google Scholar]
- 11.Khorana AA, Francis CW, Culakova E, Kuderer NM, Lyman GH. Thromboembolism is a leading cause of death in cancer patients receiving outpatient chemotherapy. J Thromb Haemost. 2007;5:632–634. doi: 10.1111/j.1538-7836.2007.02374.x. [DOI] [PubMed] [Google Scholar]
- 12.Khorana AA, Francis CW, Culakova E, Fisher RI, Kuderer NM, Lyman GH. Thromboembolism in hospitalized neu-tropenic cancer patients. J Clin Oncol. 2006;24:484–490. doi: 10.1200/JCO.2005.03.8877. [DOI] [PubMed] [Google Scholar]
- 13.Trujillo-Santos J, Herrera S, Page MA, Soto MJ, Raventos A, Sanchez R, Monreal M RIETE Investigators. Predicting adverse outcome in outpatients with acute deep vein thrombosis. Findings from the RIETE Registry. J Vasc Surg. 2006;44:789–793. doi: 10.1016/j.jvs.2006.06.032. [DOI] [PubMed] [Google Scholar]
- 14.Perry JR, Julian JA, Laperriere NJ, Geerts W, Agnelli G, Rogers LR, Malkin MG, Sawaya R, Baker R, Falanga A, et al. PRODIGE: a randomized placebo-controlled trial of dalteparin low-molecular-weight heparin thromboprophylaxis in patients with newly diagnosed malignant glioma. J Thromb Haemost. 2010;8:1959–1965. doi: 10.1111/j.1538-7836.2010.03973.x. [DOI] [PubMed] [Google Scholar]
- 15.Perry SL, Bohlin C, Reardon DA, Desjardins A, Friedman AH, Friedman HS, Vredenburgh JJ. Tinzaparin prophylaxis against venous thromboembolic complications in brain tumor patients. J Neurooncol. 2009;95:129–134. doi: 10.1007/s11060-009-99117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Robins HI, O’Neill A, Gilbert M, Olsen M, Sapiente R, Berkey B, Mehta M. Effect of dalteparin and radiation on survival and thromboembolic events in glioblastoma multiforme: a phase II ECOG trial. Cancer Chemother Pharmacol. 2008;62:227–233. doi: 10.1007/s00280-007-0596-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kayser-Gatchalian MC, Kayser K. Thrombosis and intracranial tumors. J Neurol. 1975;209:217–224. doi: 10.1007/BF00312543. [DOI] [PubMed] [Google Scholar]
- 18.Sawaya R, Zuccarello M, Elkalliny M, Nishiyama H. Postoperative venous thromboembolism and brain tumors: part I. Clinical profile. J Neurooncol. 1992;14:119–125. doi: 10.1007/BF00177615. [DOI] [PubMed] [Google Scholar]
- 19.Simanek R, Vormittag R, Hassler M, Roessler K, Schwarz M, Zielinski C, Pabinger I, Marosi C. Venous thromboem-bolism and survival in patients with high-grade glioma. Neuro Oncol. 2007;9:89–95. doi: 10.1215/15228517-2006-035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Valladares JB, Hankinson J. Incidence of lower extremity deep vein thrombosis in neurosurgical patients. Neurosurgery. 1980;6:138–141. doi: 10.1227/00006123-198002000-00004. [DOI] [PubMed] [Google Scholar]
- 21.Sawaya R, Glas-Greenwalt P. Postoperative venous thromboembolism and brain tumors: part II. Hemostatic profile. J Neurooncol. 1992;14:127–134. doi: 10.1007/BF00177616. [DOI] [PubMed] [Google Scholar]
- 22.Sawaya R, Highsmith RF. Postoperative venous throm-boembolism and brain tumors: Part III. Biochemical profile. J Neurooncol. 1992;14:113–118. doi: 10.1007/BF00177614. [DOI] [PubMed] [Google Scholar]
- 23.Streiff MB, Segal J, Grossman SA, Kickler TS, Weir EG. ABO blood group is a potent risk factor for venous thromboembolism in patients with malignant gliomas. Cancer. 2004;100:1717–1723. doi: 10.1002/cncr.20150. [DOI] [PubMed] [Google Scholar]
- 24.Thaler J, Ay C, Kaider A, Reitter EM, Haselbock J, Mannhalter C, Zielinski C, Marosi C, Pabinger I. Biomarkers predictive of venous thromboembolism in patients with newly diagnosed high-grade gliomas. Neuro Oncol. 2014;16:1645–1651. doi: 10.1093/neuonc/nou106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Prell J, Rachinger J, Smaczny R, Taute BM, Rampp S, Illert J, Koman G, Marquart C, Rachinger A, Simmermacher S, et al. D-dimer plasma level: a reliable marker for venous thromboembolism after elective craniotomy. J Neurosurg. 2013;119:1340–1346. doi: 10.3171/2013.5.JNS13151. [DOI] [PubMed] [Google Scholar]
- 26.Sartori M, Favaretto E, Cini M, Legnani C, Palareti G, Cosmi B. D-dimer, FVIII and thrombotic burden in the acute phase of deep vein thrombosis in relation to the risk of post-thrombotic syndrome. Thromb Res. 2014;134:320–325. doi: 10.1016/j.thromres.2014.05.043. [DOI] [PubMed] [Google Scholar]
- 27.Bertina RM. Elevated clotting factor levels and venous thrombosis. Pathophysiol Haemost Thromb. 2003;33:395–400. doi: 10.1159/000083835. [DOI] [PubMed] [Google Scholar]
- 28.Koster T, Blann AD, Briet E, Vandenbroucke JP, Rosendaal FR. Role of clotting factor VIII in effect of von Willebrand factor on occurrence of deep-vein thrombosis. Lancet. 1995;345:152–155. doi: 10.1016/s0140-6736(95)90166-3. [DOI] [PubMed] [Google Scholar]
- 29.Kyrle PA, Minar E, Hirschl M, Bialonczyk C, Stain M, Schneider B, Weltermann A, Speiser W, Lechner K, Eichinger S. High plasma levels of factor VIII and the risk of recurrent venous thromboembolism. N Engl J Med. 2000;343:457–462. doi: 10.1056/NEJM200008173430702. [DOI] [PubMed] [Google Scholar]
- 30.Vormittag R, Simanek R, Ay C, Dunkler D, Quehenberger P, Marosi C, Zielinski C, Pabinger I. High factor VIII levels independently predict venous thromboembolism in cancer patients: the cancer and thrombosis study. Arterioscler Thromb Vasc Biol. 2009;29:2176–2181. doi: 10.1161/ATVBAHA.109.190827. [DOI] [PubMed] [Google Scholar]
- 31.Douketis J, Tosetto A, Marcucci M, Baglin T, Cosmi B, Cushman M, Kyrle P, Poli D, Tait RC, Iorio A. Risk of recurrence after venous thromboembolism in men and women: patient level meta-analysis. BMJ. 2011;342:d813. doi: 10.1136/bmj.d813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Roach RE, Lijfering WM, Rosendaal FR, Cannegieter SC, le Cessie S. Sex difference in risk of second but not of first venous thrombosis: paradox explained. Circulation. 2014;129:51–56. doi: 10.1161/CIRCULATIONAHA.113.004768. [DOI] [PubMed] [Google Scholar]
- 33.Brotman DJ, Girod JP, Posch A, Jani JT, Patel JV, Gupta M, Lip GY, Reddy S, Kickler TS. Effects of short-term gluco-corticoids on hemostatic factors in healthy volunteers. Thromb Res. 2006;118:247–252. doi: 10.1016/j.thromres.2005.06.006. [DOI] [PubMed] [Google Scholar]


