Abstract
Infectious uveitis is one of the most common and visually devastating causes of uveitis in the US and worldwide. This review provides a summary of the identification, treatment, and complications associated with certain forms of viral, bacterial, fungal, helminthic, and parasitic uveitis. In particular, this article reviews the literature on identification and treatment of acute retinal necrosis due to herpes simplex virus, varicella virus, and cytomegalovirus. While no agreed-upon treatment has been identified, the characteristics of Ebola virus panuveitis is also reviewed. In addition, forms of parasitic infection such as Toxoplasmosis and Toxocariasis are summarized, as well as spirochetal uveitis. Syphilitic retinitis is reviewed given its increase in prevalence over the last decade. The importance of early identification and treatment of infectious uveitis is emphasized. Early identification can be achieved with a combination of maintaining a high suspicion, recognizing certain clinical features, utilizing multi-modal imaging, and obtaining specimens for molecular diagnostic testing.
Keywords: infectious uveitis, molecular diagnostics, multi-modal ophthalmic imaging
1. Introduction
Uveitis due to infectious etiologies accounts for between 15–20% of all cases of uveitis in certain parts of the United States, and likely accounts for a much more significant proportion of uveitis worldwide. [1,2] At tertiary care referral centers, it represents an even higher proportion of uveitis cases, approximately between 26–35%.[3,4] While infectious uveitis can affect various anatomic locations of the eye ranging from anterior to posterior uveitis, the most devastating cases are represented by those that cause posterior involvement such as acute retinal necrosis due to herpes family viruses, or toxoplasmosis retinochoroiditis. Recognition of an infectious cause of uveitis, and early aggressive treatment with antimicrobial agents are of paramount importance for visual recovery and preservation. Because infectious uveitis can often mimic immune-mediated uveitis, intimate knowledge of the clinical presentation of various types of infectious uveitis and appropriate use of molecular diagnostics to aid in identifying an infectious agent are crucial in the management of these conditions.
2. Viral uveitis
a. Herpes Simplex Virus (HSV) and Varicella Zoster Virus (VZV)
i. Herpetic anterior uveitis
HSV-1/HSV-2 and VZV, in addition to causing a dendritic and pseudodendritic epithelial keratitis, respectively, can cause acute recurrent or chronic anterior uveitis. In the Pacific Ocular Inflammation Study, approximately 8% of uveitis was due to HSV or VZV. [1] Clinical findings associated with herpetic uveitis include the following: elevated intraocular pressure associated with acute iritis, stellate keratic precipitates throughout the corneal endothelium rather than sequestered to the inferior third of the cornea (Arlt’s triangle), large granulomatous keratic precipitates, iris transillumination defects in a sectoral or non-sectoral fashion, atrophy of the iris pigmented epithelium, decreased corneal sensation, and a mydriatic or corectopic pupil in the absence of dilating drops. Elucidating a history of herpetic lesions in the ipsilateral V1 dermatome in VZV or the presence of HSV-type ulcerations on the skin or oral/genital mucosa is an important part of distinguishing viral anterior uveitis from other causes. While usually unilateral, bilateral herpetic anterior uveitis has been shown.[5] Confirmation of VZV or HSV-related anterior uveitis can be obtained by procurement of an aqueous specimen for polymerase chain reaction (PCR) testing, and does not rely on serological testing for antibodies against these viruses given the widespread exposure to HSV and VZV (as well as vaccination against VZV).
In the absence of herpetic keratitis, topical anti-viral agents do not appear to be beneficial in the treatment of HSV or VZV anterior uveitis. Rather, the hallmark of treatment for HSV or VZV anterior uveitis includes the use of systemic antivirals such as oral acyclovir, valacyclovir, or famciclovir (see Table 1 for dosage). The concomitant administration of topical corticosteroids is also quite important, using either topical prednisolone acetate 1% or difluprednate, with the use of a cycloplegic if anterior chamber inflammation exceeds a grade of 1+ cell.
Table 1.
Pharmacologic Treatment of Viral Retinitis and Uveitis
| Drug | Route | Side effects | Viral coverage |
|---|---|---|---|
| Acyclovir |
Intravenous: 1500 mg/m2/day divided Q8h x 14 days followed by Oral: 800 mg five times a day for 6 weeks (also dose for viral anterior uveitis) Prophylactic dose: 400 mg PO BID-TID |
GI symptoms, hypersensitivity reactions, renal or CNS dysfunction (requires renal dosing) | HSV-1, HSV-2, VZV, EBV ≫ CMV |
| Valacyclovir (prodrug) |
Oral: 1 g (viral anterior uveitis)-2 g (viral retinitis) Q8h x 6 weeks Prophylactic dose: 1 g PO BID |
Similar to acyclovir | HSV-1, HSV-2, VZV≫ CMV |
| Ganciclovir |
Intravenous: 500 mg Q12h x 14 days Intravitreal: 2–5 mg/0.1 mL, 3x/week Topical gel: 0.15% Applied 4x/day x 3 months for CMV anterior uveitis Intravitreal surgical implant: lasts 8 months (no longer available) |
Anemia, thrombocytopenia, granulocytopenia | HSV-1, CMV ≫VZV, HSV-2 |
| Valganciclovir |
Oral: 900 mg BID x 3–6 weeks Prophylactic dose: 450 mg PO BID |
HA, GI symptoms, bone marrow suppression, anemia, renal dysfunction | HSV-1, CMV≫VZV, HSV-2 |
| Foscarnet |
Intravenous: 40–60 mg/kg Q8h x 3 weeks Intravitreal: 2.4 mg/0.1 mL every 3–4 days |
HA, GI symptoms, renal or CNS toxicity uncommonly | HSV-1, HSV-2, VZV>CMV |
| Famciclovir (prodrug) | Oral: 500 mg Q8h | HA, GI symptoms, rash | HSV-1>HSV-2>VZV |
GI: gastrointestinal; HA: headache; CNS: central nervous system; PO: oral; BID: twice daily; TID: three times a day; HSV: herpes simplex virus; CMV: cytomegalovirus; EBV: Epstein-Barr virus
With the implementation of the varicella and zoster vaccination programs, the epidemiology of VZV uveitis may change over time since less of the population will be exposed to and develop natural immunity to chickenpox. In fact, reactivation of VZV keratitis has been shown to occur after zoster vaccination, and also has been shown to occur despite varicella vaccination. [6–8]
ii. Acute retinal necrosis
Acute retinal necrosis (ARN) was first described by Urayama as an acute unilateral diffuse necrotizing retinitis with panuveitis progressing to retinal detachment. [9] Young and Bird were subsequently the first describe this syndrome in two cases of bilateral disease and name the disease bilateral ARN or BARN. [10] Patients with ARN present with acute redness, pain, photophobia, and vision loss. The clinical examination reveals granulomatous or non-granulomatous anterior chamber cellular reaction, vitritis, and most typically, a multifocal or confluent full thickness necrotizing retinitis with an accompanying occlusive retinal arteritis and sometimes optic nerve edema (Figure 1). The American Uveitis Society criteria for the diagnosis of ARN are shown in Table 2.[11] The vast majority (over 50%) of ARN is due to VZV, followed by HSV-2 (5.1%), and HSV-1 (3.5%). [9] Interestingly, one study found concomitant Epstein-barr virus (EBV) DNA by PCR with VZV in 3 patients with ARN, although most case series and clinicians have not tested for EBV in ARN to confirm this result.[12] EBV-associated ARN has also been shown histopathologically in one case, although clinically, this case appeared to involve much more extensive hemorrhagic retinitis than in typical ARN. [13] VZV and HSV-1 associated ARN are more commonly found in older and middle-aged patients whereas HSV-2 associated ARN is more common in children and young adults. [14–16]
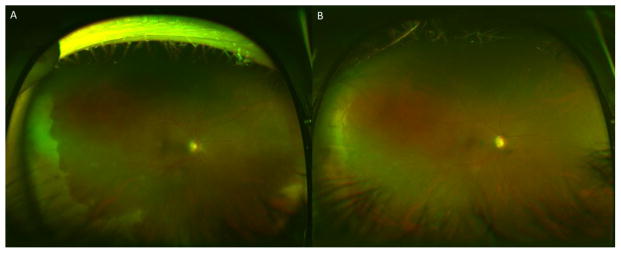
Figure 1.
A 39 year-old pregnant woman at 34 weeks presented with peripheral retinal whitening suggestive of acute retinal necrosis with optic nerve involvement and visual acuity of 20/300 (A). Vitreous paracentesis revealed 800,000,000 copies/mL of VZV DNA by PCR. After 2 weeks of intravitreal foscarnet every 3 days and 2 months of oral valacyclovir 2 g PO TID, the retina remained attached, with resolution of retinitis, and improvement of vision to 20/200, limited by optic atrophy (B).
Table 2.
American Uveitis Society criteria for Acute Retinal Necrosis Syndrome
| Required Clinical Criteria | Supporting Clinical Criteria |
|---|---|
| One or more foci of retinal necrosis with discrete borders, located in peripheral retina | Scleritis |
| Evidence of occlusive vasculopathy and arteriolar involvement | Pain |
| Prominent inflammatory reaction in vitreous and anterior chamber | Optic neuropathy/atrophy |
| Rapid progression of disease without therapy | |
| Circumferential spread of disease |
Without treatment of ARN, there is a much higher rate of poor visual outcomes, but with prompt treatment, resolution of retinitis can be achieved within 4 weeks. Treatment of ARN requires aggressive anti-viral therapy, both with systemic anti-virals to prevent bilateral disease, which can occur in up to one third of patients, and with intravitreal anti-viral agents (Table 1). Second eye involvement usually occurs around 6 weeks after initial eye involvement, but has also been reported to occur years after the first eye was involved. [17] Resolution of active ARN is noted when there is consolidation of the borders of retinitis with subsequent retinal pigment epithelial (RPE) pigmentary changes, retinal atrophy, and often, a scalloped appearance at the junction of affected and unaffected retina. Up to 75% of patients can develop a rhegmatogenous retinal detachment weeks to months after initial presentation. [12,18] Poor visual outcomes are associated with optic nerve involvement, extensive retinitis at the time of presentation, VZV and HSV-2 infection, and inappropriate use of corticosteroids.[9,19] In our experience, optic nerve involvement, which occurs in about 11%,[12] will usually result in poor visual outcomes even if treated early, and even in the absence of retinitis in the macula or retinal detachment. In one large series of ARN, half of all patients had 20/200 or worse vision by 3 months. [20]
There is some, albeit moderate to low level, evidence for prophylactic confluent laser in patients with ARN to prevent retinal detachment and improve outcomes.[21] When applying laser either alone or during retinal detachment surgery in active ARN, it is important to determine where the active border is, and to place 2–3 rows of laser spots posterior to this border given that herpetic infection appears to spread contiguously and infect adjacent retina which may not appear infected early on.[22] As is the case in up to 75% of patients, retinal detachment surgery may be required, in which pars plana vitrectomy, endolaser, and long-acting gas or silicone oil tamponade is the mainstay of surgery to maintain a successful repair. The benefit of early vitrectomy has been proposed, although some find no final visual advantage. [23]
iii. Progressive outer retinal necrosis
Progressive outer retinal necrosis (PORN) is a form of viral retinitis in human immunodeficiency virus/Acquired Immune Deficiency Syndrome (HIV/AIDS) or immunosuppressed patients that is characterized by posterior multifocal outer retinal to full thickness necrotizing retinitis with sparing of the perivascular retina, usually due to VZV. Patients with PORN typically do not have associated vitreous or anterior chamber inflammation. This entity is associated with CD4 cell counts below 50 cells/mL and is treated with a combination of aggressive systemic and local anti-viral therapy (Table 1). In addition to anti-viral therapy, the key to resolution is also immune reconstitution after highly active anti-retroviral therapy (HAART) for HIV patients with PORN. While the prevalence of PORN has decreased in the HAART era, it often resulted in NLP vision in affected eyes previously. [24]
b. Cytomegalovirus (CMV)
i. CMV anterior uveitis
While 80–85% of persons aged ≥ 40 years are seropositive for CMV, few develop infection from CMV unless they are immunosuppressed. In contrast, isolated anterior uveitis due to CMV in immunocompetent patients is an entity that has been more recently been characterized. While clinical findings can be variable, it has been shown to cause a unilateral (occasionally bilateral) anterior chamber inflammation associated with iris sectoral defects, episodes of ocular hypertension, and diffuse fine to medium keratic precipitates, occasionally with focal endotheliitis.[25–27] It can be acute, recurrent, or chronic, and some cases may have been previously characterized as Posner-Schlossman syndrome. This diagnosis can be confirmed with PCR from an aqueous paracentesis. CMV anterior uveitis can be treated successfully with oral valganciclovir (Table 1), intravenous ganciclovir, intravitreal ganciclovir, or topical ganciclovir gel. [25–29] In fact, Chee and colleagues have found that ganciclovir gel 0.15% had moderate response rates for anterior uveitis due to CMV, but may have lower recurrence rates, though this conclusion was drawn from retrospective data.[29]
ii. CMV retinitis
Retinitis due to CMV, unlike isolated anterior uveitis, usually occurs in immunocompromised individuals such as those who have HIV/AIDS, or those who have received chemotherapy for cancer. CMV retinitis usually presents bilaterally, with one of two presentations: a granular necrotizing retinitis or a hemorrhagic necrotizing retinitis, usually associated with an occlusive retinal vasculitis. Typically, none or few vitreous cells are present, and there is almost uniformly an absence of anterior chamber cell. Rarely, CMV retinitis can present as a frosted branch angiitis or an isolated papillitis. While diagnosis is based on the clinical findings and medical history, it can be confirmed by PCR of a vitreous or aqueous paracentesis specimen. Like PORN, CMV retinitis increases when CD4 counts drop below 50/mL. Occasionally, CMV retinitis can occur in patients with CD4 counts above 100/mL, and is thought to be due to an incomplete restoration of immunity against CMV, similar to what occurs in immunosuppressed individuals after bone marrow transplantation or chemotherapy. In the latter scenarios, it is more common for CMV retinitis to present with vitreous or anterior chamber cells (Figure 2).
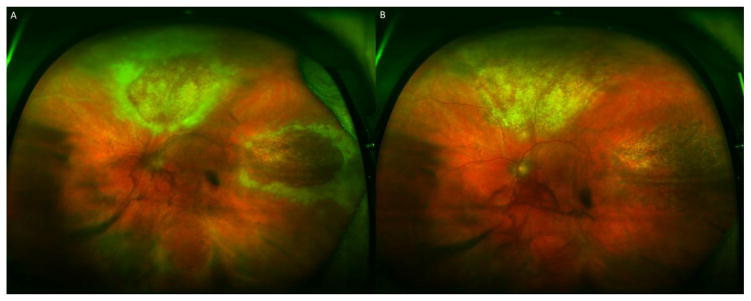
Figure 2.
A 76 year-old man with a history of prostate cancer and follicular lymphoma s/p chemotherapy presented with a panuveitis and granular necrotoizing retinitis OU, left eye shown in (A); visual acuity was 20/50 OS. Vitreous paracentesis revealed 640,000,000 copies/mL of CMV DNA by PCR. After two weeks of intravitreal foscarnet every 3 days and 1 month of oral valganciclovir 900 mg PO BID, retinitis improved, and vision improved to 20/25 OS, without any retinal detachment (B).
Treatment of CMV retinitis requires aggressive systemic and intravitreal antiviral therapies that have increased efficacy against CMV compared to other herpes family viruses. These treatments include ganciclovir, valganciclovir, and foscarnet. Resistance to these common treatments occurs when CMV contains mutations in the UL97 and UL54 genes. UL54 mutations can also cause fosarnet resistance. Intravitreal therapy combined with systemic therapy (foscarnet + ganciclovir/valganciclovir) can, to some extent, mitigate resistant CMV, due to the high concentration of drug being delivered. Complications of CMV retinitis can include rhegmatogenous retinal detachment (28% in one case series) [30], exudative retinal detachment, and immune recovery uveitis (IRU) after resolution of CMV retinitis. IRU is treated with topical or oral corticosteroids.
c. Other viruses
Rubella virus
Rubella virus is thought to be a causal factor in Fuchs’ heterochromic iridocyclitis (FHI), a condition which presents with anterior chamber inflammation, fine stellate keratic precipitates involving the entire corneal endothelium, cataract, glaucoma, and iris heterochromia due to loss of anterior iris pigment. Abnormal bridging vessels in the angle have also been described in FHI, resulting in hyphema during cataract surgery. In one study, de Groot-Mijnes et al demonstrated intraocular antibody production with a Goldmann-Witmer coefficient of > 3, against rubella virus in 13 of 14 cases, while antibodies against HSV, VZV, and Toxoplasmosis were not discovered in these patients. [31] In another study, Birnbaum demonstrated the decreasing prevalence of FHI after the institution of the rubella vaccination program, as well as the higher prevalence of FHI among immigrants in the US who had not been vaccinated. [32] Treatment of FHI includes control of glaucoma and removal of cataract. Topical corticosteroids are often not effective in treating the cellular reaction, but inflammation in FHI seldomly causes sequelae such as cystoid macular edema (CME) or posterior synechiae. Therefore, long-term corticosteroids are not usually indicated although perioperative topical steroids are usually recommended.
Ebola virus
Ebola virus is of the virus family Filoviridae, containing three genera, including Marburgvirus and Cuevavirus. As outlined recently at the keynote session of the Association for Research in Vision and Ophthalmology meeting in May 2015, the current outbreak of Ebola virus disease (EVD) has resulted in over 11,000 deaths among a total of 26,000 cases reported across 9 countries, including Sierra Leone, Liberia, Guinea, Mali, Nigeria, Senegal, the United States, the United Kingdom, and Spain. Ocular complications during convalescence from Ebola virus infection were reported by Kibadi et al in 1999, in which 4 patients who had survived Ebola virus infection developed eye pain, photophobia, and vision loss between 42 and 72 days after diagnosis of EVD. All four of these patients were diagnosed with anterior uveitis, vitritis, or a combination of the two. All patients appeared to respond to topical cycloplegics and topical steroids. One patient in this series had a positive PCR for Ebola virus from a conjunctival swab despite a negative enzyme-linked immunosorbent assay (ELISA) from the blood.
In a very recent report by Varkey and colleagues from the most recent EVD outbreak, a 43 year-old male patient who acquired EVD while treating Ebola patients from Sierra Leone, and who was transferred back to the US for care, developed low back pain, sacroiliitis, bilateral enthesitis of the Achilles’ tendon and paresthesias, as well as photophobia, 10 weeks after the onset of EVD after discharge from the hospital. Sparse pigmented chorioretinal scars with hypopigmented halos were seen on fundus examination. One month later, he presented with acute onset blurry vision, pain, and photophobia of the left eye, with elevated intraocular pressure to 44 mmHg. Clinically, he had non-granulomatous keratic precipitates and 1+ anterior chamber cell which worsened over 48 hours despite frequent topical corticosteroids. Anterior chamber paracentesis was positive for Ebola virus by reverse transcriptase (RT)-PCR and by viral cultures despite negative conjunctival swabs, RT-PCR of tears, and peripheral blood. The patient developed iris heterochromia and increasing vitritis. The patient received experimental systemic treatment for Ebola virus as well as a posterior subtenon triamcinolone injection, and has since resolved to 20/15 visual acuity in the affected eye. In summary, this patient represents the first to have developed documented acute panuveitis due to viable Ebola virus after convalescence, which implies that Ebola virus may persist in sites of immune privilege such as the eye and CNS. 33
West Nile Virus
West Nile virus is a single-stranded RNA mosquito-borne virus that belongs to the Flaviviridae family. Clinical findings in uveitis due to West Nile Virus include elevated intraocular pressure, typically bilateral anterior and intermediate uveitis, and panuveitis, with chorioretinal lesions that have been described as linear, streak-like +/− an occlusive retinal vasculitis. [34] Optic nerve edema has also been described. Chorioretinal lesions have also been described to be nummular with central pigmentation and surrounding haloes of atrophy, similar to the case of Ebola virus described above. [35] Treatment has been supportive, without any known antivirals that are active against West Nile Virus in vivo.
Additional viruses
Other viruses that have also been shown to be associated with uveitis include Dengue virus, another Flavivirus, which causes an often bilateral, retinal vascultiis with retinal hemorrhages, retinal vascular sheathing, yellow subretinal dots, optic neuritis, and an anterior chamber reaction. Chikungunya virus is a single-stranded RNA virus of the Togaviridae family transmitted through Aedes aegypti mosquitoes. Chikungunya causes retinal whitening, occlusive retinal vasculitis, optic neuritis, and can sometimes result in central retinal artery occlusion and exudative retinal detachment. Rift valley fever virus is an arthropod-borne virus of the Bunyaviridae family from sub-Saharan Africa and the Arabian peninsula. The main symptoms include headache, arthralgias, and gastrointestinal symptoms. Ocular findings of Rift valley Fever virus include macular/paramacular retinitis with optic disc edema, retinal vasculitis, and vitritis. These findings can resolve within 2–3 weeks, although with scarring, vision can become limited. Influenza A (H1N1) has also been shown to cause ocular involvement rarely, including retinitis, choroiditis, frosted branch angiitis, and optic disc edema. Severe manifestations can be treated with oral prednisone. [36–38]
3. Spirochetal infections
a. Syphilis
Syphilis is caused by the spirochete Treponema pallidum. A number of factors, including high risk behavior with improvements in pharmacotherapy of HIV and syphilis, have contributed to the increasing prevalence of this disease world-wide over the last 10–15 years compared to the decade before that, especially in men having sex with men, and in patients who are coinfected with HIV. The most common ocular manifestation of syphilis is uveitis, which occurs in up to 5% of patients with tertiary syphilis. [39] Clinical findings range from a granulomatous or non-granulomatous anterior chamber reaction, vitritis, retinal vasculitis, and papillitis, to a retinitis. Retinitis can manifest in one of two main ways: either as a punctate inner retinitis with dew-drop like collections along the surface of the retina,[40] or as a more diffuse yellowish or grayish outer retinitis which can form consolidated lesions termed acute syphilitic posterior placoid chorioretinitis;[41,42] syphilis patients can also present with a combination of the above presentations. An outer retinal wipe-out syndrome due to syphilis which is similar in presentation to acute zonal occult outer retinopathy (or AZOOR) has also been described with an example shown in Figure 3, with recovery to near normal after penicillin treatment. [43] Other ocular findings and manifestations in syphilis include interstitial keratitis, chancre of the conjunctiva, episcleritis, and scleritis, as well as the Argyll Robertson pupil which results in anisocoria with a light-near dissociation. Serous and tractional retinal detachments can also result as a complication of inflammation.
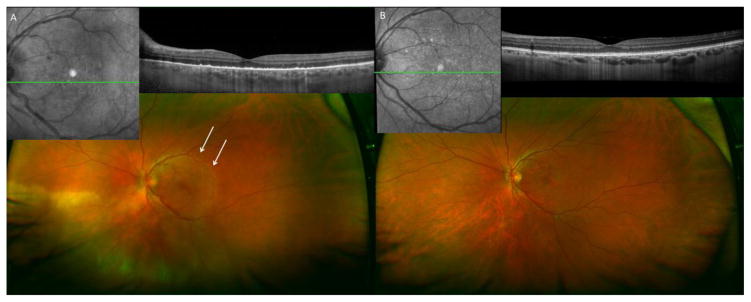
Figure 3.
A 46 year-old previously healthy man presented with counting fingers vision OS, an outer retinitis in the macula and inferonasal retina, and panuveitis (A). Arrows delineate AZOOR-like outer retinopathy in macula. RPR was 1:64 and FTA-ABS was positive. The patient was treated with 21 days of IV penicillin G with resolution of outer retinitis and improvement of visual acuity to 20/30 OS (B).
Diagnosis of syphilis requires maintaining a high suspicion and low threshold for clinical testing, which should include both treponemal tests such as fluorescent treponemal antibody absorption (FTA-ABS) or microhemagglutinin assay for T. pallidum (MHA-TP) tests and non-treponemal tests such as venereal disease research laboratory (VDRL) and rapid plasma reagin (RPR) tests. While the non-treponemal tests can be non-specific, they are useful, once treponemal tests are used to confirm diagnosis, to guide treatment decisions given that the titer can be followed upon treatment. While a positive VDRL test of the CSF is highly specific, it is not sensitive, and a negative test does not rule out neurosyphilis.
Whereas ocular syphilis is always a sign of tertiary syphilis, optic neuritis and retinitis are considered manifestations of neurosyphilis, and should be treated accordingly with a 14 day course of intravenous aqueous penicillin G at 3–4 million units every 4 hours. In the absence of neurologic involvement and just anterior syphilitic uveitis, it is unclear whether or not treatment as tertiary syphilis without neurologic involvement (ie with Benzathine penicillin at 2.3 million units intramuscularly weekly x 3 weeks) is sufficient. [44,45] Involvement of an infectious disease specialist is usually helpful in the treatment of syphilitic uveitis.
b. Lyme disease
Lyme disease is caused by Borrelia burgdorferi, a spirochete transmitted by the Ixodes tick in certain geographical locations in the US, Europe, and Asia. Ocular manifestations of Lyme disease vary, from conjunctivitis in early-stage Lyme disease, to optic neuritis and intermediate uveitis with a retinal vasculitis, usually occurring in stage 2 and 3 disease several years after inoculation. Diagnosis of Lyme disease should utilize a 2 step approach, recommended by the Centers for Disease Control and Prevention, in which an ELISA is first performed to detect IgG and IgM specific for B. burgdoferi, followed by Western blot to determine if there is IgM or IgG against multiple Borrelia antigens.[46] Even with the latter approach, false positives can be present due to the high proportion of patients in the general population who have antibodies reactive against a flagellar antigen of the spirochete. Unless there is a clear history of tick exposure in an endemic location, we do not routinely perform Lyme disease testing in uveitis patients due to this high rate of false positivity.
Treatment for the systemic manifestations of Lyme disease include the use of Doxycycline 100 mg PO BID x 14–21 days, but the treatment of the ocular manifestations is unclear, with some suggesting the use of systemic corticosteroids, which have shown some benefit, while others have reported that they may increase the rate of recurrence. [47]
c. Leptospirosis
Leptospirosis is a zoonotic spirochetal infection transmitted in tropical and subtropical climates through rodents and other domestic livestock. Farmers, veterinarians, miners, sewer workers, and patients exposed through ecotourism, are at higher risk for this infection, which can invade through intact mucosa. A triad of fever, malaise, and uveitis, was first described by Weil in 1886. Ocular manifestations are actually more variable, including conjunctival chemosis, subconjunctival hemorrhage, retinal hemorrhages, retinal vasculitis, and optic disc edema. [48] Diagnosis is by clinical findings in association with elucidation of risk factors, but serological tests and dark field microscopy can also be used, as well as PCR. Treatment of systemic leptospirosis typically utilizes penicillin G or an alternative antibiotic such as doxycycline or azithromycin. Ocular manifestations are concurrently treated with topical corticosteroids.
4. Mycobacterial uveitis
a. Ocular tuberculosis
Mycobacterium tuberculosis (MTB) is a rod-shaped, non-spore-forming obligate intracellular aerobic bacterium usually disseminated by the airborne droplet route. Ocular manifestations of MTB can vary from phlyctenulosis, scleritis, and episcleritis, to multifocal choroiditis, granulomatous papillitis, retinal vasculitis, panuveitis, and serpiginous-like choroiditis mimicking classic serpiginous choroiditis.[49]
Diagnosis of MTB uveitis requires maintaining a high index of suspicion in individuals from endemic areas or who live in high-risk environments such as nursing homes or prisons, but is often presumptive given the difficulty of isolating these bacteria from ocular fluids or tissues. PCR of aqueous or vitreous specimens can be performed to confirm diagnosis, but a negative result may not rule out disease given the slow-growing nature of this organism. A quantiferon test, known commonly as interferon-gamma release assays (IGRAs) measures the ability of the patient’s T cells to release interferon-gamma in response to specific MTB antigens. While it is unclear whether or not this test is any more sensitive or specific for active TB infection than a tuberculin skin test, it does have the advantage of not requiring a return visit from the patient for the result, as well as avoiding a false positive result when there is a history of Bacillus Calmette-Guerin (BCG) vaccination. However, like the skin test, the quantiferon test also cannot distinguish between latent and active MTB infection. Thus, a presumed diagnosis of MTB uveitis can be made with a combination of characteristic clinical findings, a positive quantiferon or positive tuberculin skin test, with concomitant chest X-ray or CT-chest findings. However, it must be noted that even in the absence of pulmonary findings on chest imaging, MTB uveitis can occur. In fact, in one cohort, 57% of histopathologically-proven ocular TB patients had a normal chest X-ray. [50]
Treatment for MTB uveitis includes the use of four-drug therapy (Table 3) for 2 months followed by isoniazid and rifampin for 4 additional months. Coritcosteroids are often administered for enhanced efficacy against inflammatory consequence of MTB ocular infection after antibiotics are initiated. Multiple-drug resistant (MDR) TB (resistant to isoniazid and rifampin), and XDR-TB (resistant to isoniazid, rifampin, any fluoroquinolone, and any one of the injectable second-line drugs) is an emerging problem world-wide, and may require 8–10 drug combinations for 18–24 months. Molecular diagnostics to identify MDR-TB by PCR is now available. [51]
Table 3.
Treatment of Ocular Tuberculosis
| Drug | Dose (oral) | Side effects |
|---|---|---|
| Rifampin*1 | 8–12 mg/kg | Thrombocytopenia, hepatotoxicity |
| Isoniazid*1 | 4–6 mg/kg | Peripheral neuropathy, transient elevation in LFTs |
| Pyrazinamide* | 20–25 mg/kg | Liver toxicity, pruritus |
| Ethambutol* | 15–20 mg/kg | Liver toxicity, possible retrobulbar optic neuritis |
| Fluoroquinolones** | Ofloxacin 15 mg/kg Levofloxacin 15 mg/kg Moxifloxacin 7.5–10 mg/kg |
GI symptoms, CNS side effects, elevated LFTs |
| Injectable drugs# | Capreomycin 15 mg/kg Kanamycin 15 mg/kg Amikacin 15 mg/kg Streptomycin 15 mg/kg |
Nephrotoxicity, ototoxicity, neurotoxicity, hypersensitivity |
First-line therapy for first two months.
First line therapy for subsequent 4 months.
Second line therapy.
One must be used in the treatment of MDR or XDR TB.
GI: gastrointestinal; CNS: central nervous system; LFTs: liver function tests
Eales’ disease is an entity in which there is peripheral occlusive vasculitis with a history of a positive Mantoux test for TB. It is thought to arise from an overactive inflammatory reaction against mycobacterium antigens, and is treated (unlike MTB uveitis) with corticosteroids either locally or systemically, and scatter laser to areas of non-perfused retina. However, recent demonstration of MTB DNA in ocular samples from Eales’ disease patients argues for the treatment of this disease as a primarily infectious rather than inflammatory disease.[52,53]
b. Nontuberculous mycobacterial uveitis
Nontuberculous mycobacterium are organisms found ubiquitously in the environment that can occasionally be introduced via procedures or surgery into the ocular environment causing an indolent infectious uveitis. [54,55] While nontubercular mycobacterium do not respond to typical MTB drugs, they do respond to other antibiotics including amikacin and certain fluoroquinolones.
5. Endogenous Bacterial Endophthalmitis
Endogenous bacterial endophthalmitis can be due to a variety of organisms though most frequently, it is caused by gram positive bacteria such as Staphylcoccus aureus, and streptococcal organisms. Other organisms include group B Streptococcus, Streptococcus pneumonia, Propionibacterium acnes, Listeria monocytogenes, Bacillus spp, Serratia spp, Nocardia spp, Klebsiella pneumonia, Pseudomonas aeruginosa, Escherichia coli, Enterococcus faecalis, Proteus spp, and others. Presenting symptoms can include pain, photophobia, floaters, and vision loss. Clinical findings can include anterior chamber cell, hypopyon with fibrin, vitritis, retinal or choroidal infiltrates, choroidal abscesses, septic emboli, and intraretinal or white-centered hemorrhages. A work up for a systemic source, if not already known, is imperative, given the severe consequences of unrecognized septicemia. Systemic sources can be investigated by blood and urine cultures, cultures of indwelling catheters or feeding tubes, and examination of chest imaging for possible pneumonia.
Management of endogenous bacterial endophthalmitis includes vitreous paracentesis for bacterial culture followed by intravitreal injection of antibiotics and systemic antibiotic treatment directed against the causative organism. In early cases of endogenous bacterial endophthalmitis perhaps sequestered to a small focal area of the choroid or retina, systemic antibiotic treatment alone may be effective. However, if retinal or vitreous involvement is significant, intravitreal treatment is usually added. Early administration of intravitreal antibiotics is likely associated with better visual and anatomical outcomes in endogenous bacterial endophthalmitis. [56]
The role and timing of pars plana vitrectomy in endogenous bacterial endophthalmitis is controversial, although most agree that it is sometimes necessary if there is a sequestered progressive infectious nidus in the eye despite systemic treatment, or if there is a pending or vision-threatening retinal detachment associated with the infectious nidus. In one study from Vietnam, 108 consecutive patients with severe endogenous bacterial endophthalmitis were randomized to either vitrectomy alone with intravitreal antibiotics or vitrectomy with intravitreal antibiotics and silicone oil tamponade. They found that the overall success rate defined as visual acuity ≥ counting fingers at 1 meter, attached retina, without silicone oil at 9 months follow up was higher in the silicone oil group than the vitrectomy alone group. 57
6. Endogenous Fungal Endophthalmitis
Candida albicans is the most common yeast isolated from patients with endogenous fungal endophthalmitis, followed by Aspergillus spp, the most common mold isolate found in fungal endophthalmitis. Other organisms found to cause fungal endophthalmitis include Cryptococcus neoformans, Coccidioides immitis, Histoplasma capsulatum, Sporothrix schenckii, and others. Fungus can be introduced into the blood stream via indwelling catheters, feeding tubes, and/or intravenous drug use. Occasionally, fungal infection can occur without a specific source in immunosuppressed individuals. Diagnosis is determined by vitreous paracentesis for fungal culture, or by inference from positive fungal blood cultures in the setting of certain clinical features. Clinical features can include choroidal or retinal infiltrates, vitritis, and an anterior chamber reaction with keratic precipitates. The characteristic presentation of Candida endophthalmitis is a creamy white or fluffy choroidal or retinal lesion in the posterior pole with overlying vitreous cells or haze in a “string of pearls” configuration over the lesion. Up to 28% of candidemic patients can develop candidal chorioretinitis. [58,59]
Treatment of fungal chorioretinitis includes systemic anti-fungal treatment with agents that have reasonable ocular penetration including voriconazole, fluconazole, flucytosine, and liposomal amphotericin B. Systemic anti-fungals that have poor ocular penetration include the echinocandin class of antifungals and non-liposomal amphotericin B. Intravitreal antifungal therapy should be added if there are sight-threatening lesions, and include amphotericin B 5–10 μg or voriconazole 100 μg. Pars plana vitrectomy is an important adjunct treatment for fungal chorioretinitis, for microbiologic identification, to debulk infectious organism, to prevent or treat retinal detachment, and to facilitate delivery of antifungal drugs to potentially sequestered organism. [60]
7. Toxoplasmosis retinochoroiditis
Toxoplasma gondii is an obligate intracellular protozoan that utilize cats as their primary host. They are inadvertently ingested in the bradyzoite, or tissue cyst, form, and then undergo transformation into the infectious, tachyzoite form. Bradyzoites can remain dormant in the retina for years, and upon rupture, result in release of tachyzoites and surrounding retinitis. While it only represents 25% of posterior uveitis in the US, it represents up to 85% of posterior uveitis in other countries such as Brazil. Clinically, acutely active toxoplasmic uveitis presents with blurry vision, photophobia, and floaters. The most common clinical presentation is a partial or full thickness necrotizing retinitis adjacent to an old hyperpigmented chorioretinal scar (Figure 4). This can be associated with a focal arteritis, overlying vitritis, and anterior chamber cell. Occasionally, it can cause massive vitreous inflammation resulting in the “headlight in fog” appearance. It should be noted that in HIV+ individuals, toxoplasmosis chorioretinitis can appear clinically disparate from that in immunocompetent patients in that in immunocompromised patients, toxoplasmosis retinochoroiditis is much more diffuse, hemorrhagic, and may not be associated with prior chorioretinal scars.
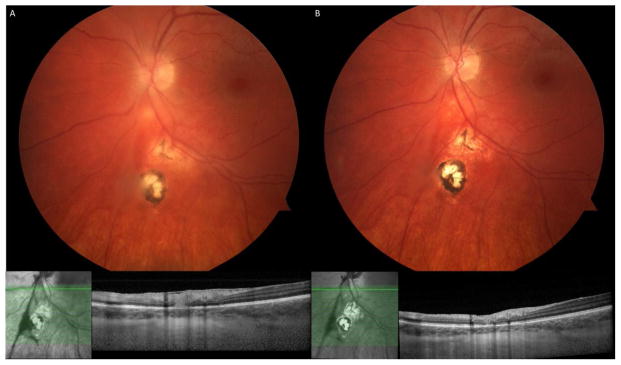
Figure 4.
A 27 year-old healthy woman presented with a focal area of full-thickness retinitis adjacent to the optic nerve and an old pigmented chorioretinal scar, with overlying vitreous cells (A). Initial visual acuity was 20/30. She was treated with Atovaquone 750 mg PO QID for 3 months, as well as a tapering course of oral prednisone starting 3 days after initiating Atovaquone. The retinal lesion consolidated and vitreous cell resolved (B), with return to 20/20 vision OS.
While serologies for IgG and IgM against toxoplasmosis can be corroborative especially if they are negative, high IgG can occur in the absence of active disease with prior exposure, and IgM is often negative in active retinal disease. Toxoplasmosis PCR or a Goldmann Whitmer coefficient from an aqueous or vitreous paracentesis specimen can be very useful in these circumstances.
Treatment of toxoplasmosis retinochoroiditis using the common “classic” therapies (Table 4) does not usually eliminate tissue cysts and therefore does not prevent chronic or recurrent infection. Further, no randomized placebo-controlled trial has demonstrated definite benefit of these treatments over no treatment. Nevertheless, most uveitis specialists agree that vision-threatening or severe disease warrants treatment. However, atovaquone, which is more commonly used for Pneumocystis Jiroveci infection in AIDS, has been shown in animal models to be active against the bradyzoite form of Toxoplasmosis gondii, and additionally, is tolerated well with very few side effects in both healthy and immunocompromised patients, as well as in children. [61] It has been demonstrated to be effective as a single agent. [61] Additionally, trimethoprim/sulfamethoxazole is also usually well-tolerated, and has been tested in a small single-center randomized placebo-controlled double-masked trial, which showed that it can prevent recurrent retinochoroiditis.[62] However, hypersensitivity reactions and bone marrow suppression especially in immunocompromised patients, can sometimes limit its use. Local therapy with intravitreal clindamycin with dexamethasone has also been found to be effective. [63,64] Adjunctive treatment includes topical prednisolone acetate drops for the anterior uveitis component, and oral prednisone after initiation of anti-infective agent(s).
Table 4.
Treatment of Ocular Toxoplasmosis
| Drug | Dose | Side effects |
|---|---|---|
| Pyrimethamine* | 75–100 mg loading over 24 hours, then 25–50 mg daily x 4–6 weeks | Hypersensitivity reaction, photosensitivity, abnormal skin pigmentation, bone marrow suppression |
| Sulfadiazine* | 2–4 g loading dose, then 1 g QID x 4–6 weeks | Hypersensitivity reaction, bone marrow suppression |
| Prednisone* | 40–60 mg daily with taper, starting 3 days after starting antibiotics including Atovaquone | Hyperglycemia, weight gain, mood lability |
| Folinic acid* | 5 mg 2–3 times/week with pyrimethamine | |
| Atovaquone# | 750 mg PO QID x 3 months | Hypersensitivity reaction, |
| Trimethoprim/Sulfamethoxazole | 160 mg/800 mg PO BID x 6 weeks | Hypersensitivity reactions, bone marrow suppression |
| Clindamycin1 | Intravitreal: 1.5 mg/0.1 mL weekly for 4 weeks | Risks of intravitreal injection |
| Dexamethasone1 | Intravitreal: 400 μg/0.1 mL given with intravitreal clindamycin | Elevated intraocular pressure, cataract |
Used in combination as “classic” therapy.
May kill bradyzoite cysts based on animal data.
Used as intravitreal treatment of ocular toxoplasmosis.
8. Other parasites/helminths
a. Ocular Toxocariasis
Toxocariasis is caused by a roundworm, either Toxocara canis or Toxocara catis found in dog or cat feces. It can present a granulomatous fibrous stalk either in the posterior pole or in the retinal periphery. Histopathologically, the fibrous granuloma consists of roundworm remnants surrounded by copious inflammatory cellular infiltrates, many of which are eosinophils. There is usually unilateral involvement, typically found in children, and is therefore on the differential diagnosis for retinoblastoma and Coat’s disease. Chronic endophthalmitis can also result from ocular toxocariasis, especially in younger patients, and can present with a severe intraocular inflammation with hypopyon and granulomatous keratic precipitates. Complications from ocular toxocariasis include tractional retinal detachment, epiretinal membrane formation, rhegmategonous retinal detachment, cyclitic membrane formation, hypotony, and phthisis bulbi. [65] Laboratory diagnosis involves demonstration of positive serum antibody titers against Toxocara canis or catis using an ELISA. While a positive test does not prove causality of Toxocara for ocular involvement, a negative test can help to rule out toxocariasis.
Treatment of ocular toxocariasis involves the use of topical and systemic corticosteroids to reduce the inflammation associated with infection. Because T. canis is not self-replicating, systemic corticosteroids will not promote growth of the infectious agent. Anti-helminthic drugs such as albendazole 400 mg PO BID x 30 days may also be effective in treating ocular toxocariasis but should not be undertaken without concurrent corticosteroid administration due to the severe inflammatory reaction that can occur with dying helminths. Retinal detachment surgery is occasionally required either with vitrectomy or combination with scleral buckle surgery to treat tractional or rhegmatogenous retinal detachments associated with the organizing fibrous membranes over the area of inflammation.
b. Ophthalmomyiasis
Ophthalmomyiasis interna is inflammation secondary to inoculation of the eye with burrowing fly larvae such as the sheep bot fly, Oestris Ovis, which is found throughout much of the world. Dermatobia hominis is another common bot fly found in Central and South America that can cause ophthalmomyiasis. These organisms can invade the anterior chamber, the vitreous, and the subretinal space. When found in the vitreous or anterior chamber, it can induce a severe uveitis. When found in the subretinal space, it can cause subretinal hemorrhage and an outer retinal inflammation that is best noted by criss-crossing tracks throughout the retina. When it is located in the vitreous cavity, it can be removed via vitrectomy with laser to the entry and exit sites if visible, although the importance of keeping the organism whole during extraction should be emphasized to prevent a severe inflammatory reaction. The organism can be identified morphologically by its width and segmented appearance. Subretinal larvae can be removed in toto, by performing a pars plana vitrectomy with retinotomy. [66]Ivermectin 0.2 mg/kg PO once can be used in combination with oral corticosteroids to treat subretinal larvae that cannot be isolated or removed.
c. Diffuse Unilateral Subacute Neuroretinitis (DUSN)
DUSN is caused by a nematode wandering the subretinal space and causing outer retinal atrophy over a number of years. The organisms reported to cause DUSN include Baylisascaris procyonis, Dirofilaria, and Ancylostoma caninum. While Baylisascaris larvae are between 300–2000 μm long and infect raccoons and skunks, A. caninum is a hookworm 650 μm in size, found in dogs. In early DUSN, lesions made by the moving nematode appear as outer retinal gray-white inflammatory lesions. On fluorescein angiography, lesions may be wreath-like hyperfluorescent dots usually with early hypofluorescence. DUSN can also present with perivenous infiltrates similar to sarcoidosis, and later develop multifocal chorioretinal scarring. DUSN can also present with crops of yellowish or whitish outer retinal lesions[67,68] and vitritis, as well as rarely, hypopyon uveitis. [69] End-stage DUSN can appear like unilateral retinitis pigmentosa, with outer retinal atrophy, optic nerve atrophy, attenuated vessels, pigmented bony-spicule formation, and a posterior subcapsular cataract. The nematode itself appears in the subretinal space as a thin S-shaped organism. In addition to identifying the worm by slit lamp microscopy using a 78D or 90D lens, the organism can sometimes be identified by its movement during the acquisition of an optical coherence tomography scan (OCT), as the laser from this imaging modality will sometimes prompt movement.
Treatment of DUSN includes the use of laser to kill or sequester the subretinal nematode. Laser photocoagulation using a 200–500 μm spot size at a duration of 0.2–0.5 seconds, is required to destroy the worm. Oral anti-helminthic agents such as thiabendazole, albendazole, or ivermectin have also been used to treat DUSN with varied success. [70]
d. Cysticercosis
Ocular cysticercosis is usually due to the human tapeworm, Taenia solium, which is acquired via the ingestion of raw or undercooked pork. Anterior chamber cysticercosis can present with a severe non-granulomatous anterior uveitis with keratic precipitates and fibrin, and can be mistaken for an anteriorly dislocated crystalline lens. [71] While a live scolex in the vesicular stage can incite an inflammatory response due to toxin and protein leakage into the eye, a dead scolex can cause an even more severe fibrinous reaction. A live scolex can be distinguished from a dead scolex by movement upon shining a light on the vesicle. Posterior segment cysticercosis is more common than anterior segment involvement and can cause a severe vitritis, pupillary block glaucoma, and rarely, a subretinal cyst. If ocular cysticercosis is suspected, imaging of the CNS should be obtained to rule out neurocysticercosis. Because disruption of the vesicle or killing of the organism incites such a severe inflammatory response, surgical intervention is preferred over anti-helminthic treatment unless there is evidence of systemic disease; in the latter scenario, systemic corticosteroids are required concomitantly with anti-helminthic treatment such as albendazole. Surgical treatment includes viscoelastic-assisted expression of the entire vesicle in total if in the anterior segment. When a cysticercus cyst is present under the retina, pars plana vitrectomy with retinotomy, with engagement of the vesicle using a soft-tipped cannula, and extraction through an enlarged sclerotomy wound, can avoid rupture and the subsequent inflammatory reaction. [72]
9. Molecular diagnostics for infectious uveitis
While microbial culture is the gold-standard for the diagnosis of infectious uveitis, the low volume of ocular specimens and relative inaccessibility of ocular tissues and fluids have limited the reliance on culture results due to low yield and difficulty in culturing indolent or intracellular organisms. Instead, advancements in efficiency and decreases in cost for PCR have resulted in more accurate microbiological classifications of uveitis, such as in the re-identification of many cases of Posner-Schlossman syndrome as CMV anterior uveitis. The sensitivity of PCR to diagnose HSV, VZV, and CMV is upward of 90% with specificities of 95%, with the additional advantage of requiring less time to obtain the results than viral culture. Furthermore, sub-typing of viral strains and molecular identification of resistant strains can also be performed by PCR. 53 In ocular TB, PCR has increased the sensitivity of ocular fluid testing, although this is still low, at only 37%, due to low bacterial load and the thick cell wall of the bacterium. [53] Toxoplasmosis PCR in addition to Goldmann-Witmer coefficient, has increased ocular fluid testing sensitivity and specificity to 80–93% for this disease. [73,74]
10. Summary
A high suspicion for infectious causes of uveitis should always be maintained when managing uveitis patients, given the importance of prompt initiation of antimicrobial therapy to prevent devastating vision loss due to ocular infections from viruses, treponemes, mycobacteria, parasites, or fungi. A full thickness necrotizing retinitis is almost never associated with non-infectious uveitis, and a low threshold to perform molecular diagnostic testing with PCR should be maintained in this setting to guide selection and response to treatment.
Footnotes
Conflict of Interest Statement
Dr. Phoebe Lin has no conflicts of interest to declare
Compliance with Ethics Guidelines
Human and Animal Rights and Informed Consent
This article contains no studies with human or animal subjects performed by the author.
References
Papers of particular interest, published recently, have been highlighted as:
• Of importance
•• Of major importance
- 1.Acharya NR, Tham VM, Esterberg E, et al. Incidence and prevalence of uveitis: results from the Pacific Ocular Inflammation Study. JAMA ophthalmology. 2013 Nov;131(11):1405–1412. doi: 10.1001/jamaophthalmol.2013.4237. [DOI] [PubMed] [Google Scholar]
- 2.Suhler EB, Lloyd MJ, Choi D, Rosenbaum JT, Austin DF. Incidence and prevalence of uveitis in Veterans Affairs Medical Centers of the Pacific Northwest. American journal of ophthalmology. 2008 Dec;146(6):890–896. e898. doi: 10.1016/j.ajo.2008.09.014. [DOI] [PubMed] [Google Scholar]
- 3.McCannel CA, Holland GN, Helm CJ, Cornell PJ, Winston JV, Rimmer TG. Causes of uveitis in the general practice of ophthalmology. UCLA Community-Based Uveitis Study Group. American journal of ophthalmology. 1996 Jan;121(1):35–46. doi: 10.1016/s0002-9394(14)70532-x. [DOI] [PubMed] [Google Scholar]
- 4.Rodriguez A, Calonge M, Pedroza-Seres M, et al. Referral patterns of uveitis in a tertiary eye care center. Archives of ophthalmology. 1996 May;114(5):593–599. doi: 10.1001/archopht.1996.01100130585016. [DOI] [PubMed] [Google Scholar]
- 5.Nalcacioglu-Yuksekkaya P, Ozdal PC, Teke MY, Kara C, Ozturk F. Presumed herpetic anterior uveitis: a study with retrospective analysis of 79 cases. European journal of ophthalmology. 2014 Jan-Feb;24(1):14–20. doi: 10.5301/ejo.5000331. [DOI] [PubMed] [Google Scholar]
- 6.Esmaeli-Gutstein B, Winkelman JZ. Uveitis associated with varicella virus vaccine. American journal of ophthalmology. 1999 Jun;127(6):733–734. doi: 10.1016/s0002-9394(99)00059-8. [DOI] [PubMed] [Google Scholar]
- 7.Lin P, Yoon MK, Chiu CS. Herpes zoster keratouveitis and inflammatory ocular hypertension 8 years after varicella vaccination. Ocular immunology and inflammation. 2009 Jan-Feb;17(1):33–35. doi: 10.1080/09273940802491892. [DOI] [PubMed] [Google Scholar]
- 8.Naseri A, Good WV, Cunningham ET., Jr Herpes zoster virus sclerokeratitis and anterior uveitis in a child following varicella vaccination. American journal of ophthalmology. 2003 Mar;135(3):415–417. doi: 10.1016/s0002-9394(02)01957-8. [DOI] [PubMed] [Google Scholar]
- 9.Wong RW, Jumper JM, McDonald HR, et al. Emerging concepts in the management of acute retinal necrosis. The British journal of ophthalmology. 2013 May;97(5):545–552. doi: 10.1136/bjophthalmol-2012-301983. [DOI] [PubMed] [Google Scholar]
- 10.Young NJ, Bird AC. Bilateral acute retinal necrosis. The British journal of ophthalmology. 1978 Sep;62(9):581–590. doi: 10.1136/bjo.62.9.581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Holland GN. Standard diagnostic criteria for the acute retinal necrosis syndrome. Executive Committee of the American Uveitis Society. American journal of ophthalmology. 1994 May 15;117(5):663–667. doi: 10.1016/s0002-9394(14)70075-3. [DOI] [PubMed] [Google Scholar]
- 12.Lau CH, Missotten T, Salzmann J, Lightman SL. Acute retinal necrosis features, management, and outcomes. Ophthalmology. 2007 Apr;114(4):756–762. doi: 10.1016/j.ophtha.2006.08.037. [DOI] [PubMed] [Google Scholar]
- 13.Schaal S, Kagan A, Wang Y, Chan CC, Kaplan HJ. Acute retinal necrosis associated with Epstein-Barr virus: immunohistopathologic confirmation. JAMA ophthalmology. 2014 Jul;132(7):881–882. doi: 10.1001/jamaophthalmol.2014.266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ganatra JB, Chandler D, Santos C, Kuppermann B, Margolis TP. Viral causes of the acute retinal necrosis syndrome. American journal of ophthalmology. 2000 Feb;129(2):166–172. doi: 10.1016/s0002-9394(99)00316-5. [DOI] [PubMed] [Google Scholar]
- 15.Tran TH, Stanescu D, Caspers-Velu L, et al. Clinical characteristics of acute HSV-2 retinal necrosis. American journal of ophthalmology. 2004 May;137(5):872–879. doi: 10.1016/j.ajo.2003.12.036. [DOI] [PubMed] [Google Scholar]
- 16.Van Gelder RN, Willig JL, Holland GN, Kaplan HJ. Herpes simplex virus type 2 as a cause of acute retinal necrosis syndrome in young patients. Ophthalmology. 2001 May;108(5):869–876. doi: 10.1016/s0161-6420(01)00556-5. [DOI] [PubMed] [Google Scholar]
- 17.Martinez J, Lambert HM, Capone A, et al. Delayed bilateral involvement in the acute retinal necrosis syndrome. American journal of ophthalmology. 1992 Jan 15;113(1):103–104. doi: 10.1016/s0002-9394(14)75765-4. [DOI] [PubMed] [Google Scholar]
- 18.Clarkson JG, Blumenkranz MS, Culbertson WW, Flynn HW, Jr, Lewis ML. Retinal detachment following the acute retinal necrosis syndrome. Ophthalmology. 1984 Dec;91(12):1665–1668. doi: 10.1016/s0161-6420(84)34107-0. [DOI] [PubMed] [Google Scholar]
- 19.Meghpara B, Sulkowski G, Kesen MR, Tessler HH, Goldstein DA. Long-term follow-up of acute retinal necrosis. Retina. 2010 May;30(5):795–800. doi: 10.1097/IAE.0b013e3181c7013c. [DOI] [PubMed] [Google Scholar]
- 20.Tibbetts MD, Shah CP, Young LH, Duker JS, Maguire JI, Morley MG. Treatment of acute retinal necrosis. Ophthalmology. 2010 Apr;117(4):818–824. doi: 10.1016/j.ophtha.2009.09.001. [DOI] [PubMed] [Google Scholar]
- 21.Park JJ, Pavesio C. Prophylactic laser photocoagulation for acute retinal necrosis. Does it raise more questions than answers? The British journal of ophthalmology. 2008 Sep;92(9):1161–1162. doi: 10.1136/bjo.2008.147181. [DOI] [PubMed] [Google Scholar]
- 22.Holland GN, Togni BI, Briones OC, Dawson CR. A microscopic study of herpes simplex virus retinopathy in mice. Investigative ophthalmology & visual science. 1987 Jul;28(7):1181–1190. [PubMed] [Google Scholar]
- 23.Hillenkamp J, Nolle B, Bruns C, Rautenberg P, Fickenscher H, Roider J. Acute retinal necrosis: clinical features, early vitrectomy, and outcomes. Ophthalmology. 2009 Oct;116(10):1971–1975. e1972. doi: 10.1016/j.ophtha.2009.03.029. [DOI] [PubMed] [Google Scholar]
- 24.Engstrom RE, Jr, Holland GN, Margolis TP, et al. The progressive outer retinal necrosis syndrome. A variant of necrotizing herpetic retinopathy in patients with AIDS. Ophthalmology. 1994 Sep;101(9):1488–1502. doi: 10.1016/s0161-6420(94)31142-0. [DOI] [PubMed] [Google Scholar]
- 25.Accorinti M, Gilardi M, Pirraglia MP, et al. Cytomegalovirus anterior uveitis: long-term follow-up of immunocompetent patients. Graefe’s archive for clinical and experimental ophthalmology = Albrecht von Graefes Archiv fur klinische und experimentelle Ophthalmologie. 2014 Nov;252(11):1817–1824. doi: 10.1007/s00417-014-2782-4. [DOI] [PubMed] [Google Scholar]
- 26.de Schryver I, Rozenberg F, Cassoux N, et al. Diagnosis and treatment of cytomegalovirus iridocyclitis without retinal necrosis. The British journal of ophthalmology. 2006 Jul;90(7):852–855. doi: 10.1136/bjo.2005.086546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.van Boxtel LA, van der Lelij A, van der Meer J, Los LI. Cytomegalovirus as a cause of anterior uveitis in immunocompetent patients. Ophthalmology. 2007 Jul;114(7):1358–1362. doi: 10.1016/j.ophtha.2006.09.035. [DOI] [PubMed] [Google Scholar]
- 28.Chee SP, Bacsal K, Jap A, Se-Thoe SY, Cheng CL, Tan BH. Clinical features of cytomegalovirus anterior uveitis in immunocompetent patients. American journal of ophthalmology. 2008 May;145(5):834–840. doi: 10.1016/j.ajo.2007.12.015. [DOI] [PubMed] [Google Scholar]
- 29.Chee SP, Jap A. Cytomegalovirus anterior uveitis: outcome of treatment. The British journal of ophthalmology. 2010 Dec;94(12):1648–1652. doi: 10.1136/bjo.2009.167767. [DOI] [PubMed] [Google Scholar]
- 30.Sandy CJ, Bloom PA, Graham EM, et al. Retinal detachment in AIDS-related cytomegalovirus retinitis. Eye. 1995;9( Pt 3):277–281. doi: 10.1038/eye.1995.54. [DOI] [PubMed] [Google Scholar]
- 31.de Groot-Mijnes JD, de Visser L, Rothova A, Schuller M, van Loon AM, Weersink AJ. Rubella virus is associated with fuchs heterochromic iridocyclitis. American journal of ophthalmology. 2006 Jan;141(1):212–214. doi: 10.1016/j.ajo.2005.07.078. [DOI] [PubMed] [Google Scholar]
- 32.Birnbaum AD, Tessler HH, Schultz KL, et al. Epidemiologic relationship between fuchs heterochromic iridocyclitis and the United States rubella vaccination program. American journal of ophthalmology. 2007 Sep;144(3):424–428. doi: 10.1016/j.ajo.2007.05.026. [DOI] [PubMed] [Google Scholar]
- 33.Varkey JB, Shantha JG, Crozier I, et al. Persistence of Ebola Virus in Ocular Fluid during Convalescence. The New England journal of medicine. 2015 May 7; doi: 10.1056/NEJMoa1500306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Chan CK, Limstrom SA, Tarasewicz DG, Lin SG. Ocular features of west nile virus infection in North America: a study of 14 eyes. Ophthalmology. 2006 Sep;113(9):1539–1546. doi: 10.1016/j.ophtha.2006.04.021. [DOI] [PubMed] [Google Scholar]
- 35.Khairallah M, Ben Yahia S, Ladjimi A, et al. Chorioretinal involvement in patients with West Nile virus infection. Ophthalmology. 2004 Nov;111(11):2065–2070. doi: 10.1016/j.ophtha.2004.03.032. [DOI] [PubMed] [Google Scholar]
- 36.Jo T, Mizota A, Hatano N, Tanaka M. Frosted branch angiitis-like fundus following presumed influenza virus type A infection. Japanese journal of ophthalmology. 2006 Nov-Dec;50(6):563–564. doi: 10.1007/s10384-006-0358-5. [DOI] [PubMed] [Google Scholar]
- 37.Khairallah M, Chee SP, Rathinam SR, Attia S, Nadella V. Novel infectious agents causing uveitis. International ophthalmology. 2010 Oct;30(5):465–483. doi: 10.1007/s10792-009-9319-6. [DOI] [PubMed] [Google Scholar]
- 38.Khairallah M, Kahloun R, Ben Yahia S, Jelliti B, Messaoud R. New infectious etiologies for posterior uveitis. Ophthalmic research. 2013;49(2):66–72. doi: 10.1159/000344009. [DOI] [PubMed] [Google Scholar]
- 39.Aldave AJ, King JA, Cunningham ET., Jr Ocular syphilis. Current opinion in ophthalmology. 2001 Dec;12(6):433–441. doi: 10.1097/00055735-200112000-00008. [DOI] [PubMed] [Google Scholar]
- 40.Wickremasinghe S, Ling C, Stawell R, Yeoh J, Hall A, Zamir E. Syphilitic punctate inner retinitis in immunocompetent gay men. Ophthalmology. 2009 Jun;116(6):1195–1200. doi: 10.1016/j.ophtha.2008.12.055. [DOI] [PubMed] [Google Scholar]
- 41.Gass JD, Braunstein RA, Chenoweth RG. Acute syphilitic posterior placoid chorioretinitis. Ophthalmology. 1990 Oct;97(10):1288–1297. doi: 10.1016/s0161-6420(90)32418-1. [DOI] [PubMed] [Google Scholar]
- 42.Pichi F, Ciardella AP, Cunningham ET, Jr, et al. Spectral domain optical coherence tomography findings in patients with acute syphilitic posterior placoid chorioretinopathy. Retina. 2014 Feb;34(2):373–384. doi: 10.1097/IAE.0b013e3182993f11. [DOI] [PubMed] [Google Scholar]
- 43.Lima BR, Mandelcorn ED, Bakshi N, Nussenblatt RB, Sen HN. Syphilitic outer retinopathy. Ocular immunology and inflammation. 2014 Feb;22(1):4–8. doi: 10.3109/09273948.2013.841960. [DOI] [PubMed] [Google Scholar]
- 44.Chao JR, Khurana RN, Fawzi AA, Reddy HS, Rao NA. Syphilis: reemergence of an old adversary. Ophthalmology. 2006 Nov;113(11):2074–2079. doi: 10.1016/j.ophtha.2006.05.048. [DOI] [PubMed] [Google Scholar]
- 45.Clement ME, Okeke NL, Hicks CB. Treatment of syphilis: a systematic review. Jama. 2014 Nov 12;312(18):1905–1917. doi: 10.1001/jama.2014.13259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Centers for Disease C and Prevention. Recommendations for test performance and interpretation from the Second National Conference on Serologic Diagnosis of Lyme Disease. MMWR. Morbidity and mortality weekly report. 1995 Aug 11;44(31):590–591. [PubMed] [Google Scholar]
- 47.Winterkorn JM. Lyme disease: neurologic and ophthalmic manifestations. Survey of ophthalmology. 1990 Nov-Dec;35(3):191–204. doi: 10.1016/0039-6257(90)90089-e. [DOI] [PubMed] [Google Scholar]
- 48.Rathinam SR. Ocular manifestations of leptospirosis. Journal of postgraduate medicine. 2005 Jul-Sep;51(3):189–194. [PubMed] [Google Scholar]
- 49.De Luigi G, Mantovani A, Papadia M, Herbort CP. Tuberculosis-related choriocapillaritis (multifocal-serpiginous choroiditis): follow-up and precise monitoring of therapy by indocyanine green angiography. International ophthalmology. 2012 Feb;32(1):55–60. doi: 10.1007/s10792-011-9508-y. [DOI] [PubMed] [Google Scholar]
- 50.Wroblewski KJ, Hidayat AA, Neafie RC, Rao NA, Zapor M. Ocular tuberculosis: a clinicopathologic and molecular study. Ophthalmology. 2011 Apr;118(4):772–777. doi: 10.1016/j.ophtha.2010.08.011. [DOI] [PubMed] [Google Scholar]
- 51.Heysell SK, Houpt ER. The future of molecular diagnostics for drug-resistant tuberculosis. Expert review of molecular diagnostics. 2012 May;12(4):395–405. doi: 10.1586/erm.12.25. [DOI] [PubMed] [Google Scholar]
- 52.Singh R, Toor P, Parchand S, Sharma K, Gupta V, Gupta A. Quantitative polymerase chain reaction for Mycobacterium tuberculosis in so-called Eales’ disease. Ocular immunology and inflammation. 2012 Jun;20(3):153–157. doi: 10.3109/09273948.2012.658134. [DOI] [PubMed] [Google Scholar]
- 53.Taravati P, Lam D, Van Gelder RN. Role of molecular diagnostics in ocular microbiology. Current ophthalmology reports. 2013 Dec 1;1(4) doi: 10.1007/s40135-013-0025-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Couto C, Rossetti S, Schlaen A, Hurtado E, D’Alessandro L, Goldstein DA. Chronic postoperative Mycobacterium gordonae endophthalmitis in a patient with phakic intraocular lens. Ocular immunology and inflammation. 2013 Dec;21(6):491–494. doi: 10.3109/09273948.2013.824104. [DOI] [PubMed] [Google Scholar]
- 55.Kuznetcova TI, Sauty A, Herbort CP. Uveitis with occult choroiditis due to Mycobacterium kansasii: limitations of interferon-gamma release assay (IGRA) tests (case report and mini-review on ocular non-tuberculous mycobacteria and IGRA cross-reactivity) International ophthalmology. 2012 Oct;32(5):499–506. doi: 10.1007/s10792-012-9588-3. [DOI] [PubMed] [Google Scholar]
- 56.Nishida T, Ishida K, Niwa Y, Kawakami H, Mochizuki K, Ohkusu K. An eleven-year retrospective study of endogenous bacterial endophthalmitis. Journal of ophthalmology. 2015;2015:261310. doi: 10.1155/2015/261310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Do T, Hon do N, Aung T, Hien ND, Cowan CL., Jr Bacterial endogenous endophthalmitis in Vietnam: a randomized controlled trial comparing vitrectomy with silicone oil versus vitrectomy alone. Clinical ophthalmology. 2014;8:1633–1640. doi: 10.2147/OPTH.S67589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Brooks RG. Prospective study of Candida endophthalmitis in hospitalized patients with candidemia. Archives of internal medicine. 1989 Oct;149(10):2226–2228. [PubMed] [Google Scholar]
- 59.Donahue SP, Greven CM, Zuravleff JJ, et al. Intraocular candidiasis in patients with candidemia. Clinical implications derived from a prospective multicenter study. Ophthalmology. 1994 Jul;101(7):1302–1309. doi: 10.1016/s0161-6420(94)31175-4. [DOI] [PubMed] [Google Scholar]
- 60.Lin P, Wynn P, Stewart JM. Management of a recalcitrant candidal chorioretinal abscess. Retinal cases & brief reports. 2012 Summer;6(3):280–284. doi: 10.1097/ICB.0b013e318228e363. [DOI] [PubMed] [Google Scholar]
- 61.Pearson PA, Piracha AR, Sen HA, Jaffe GJ. Atovaquone for the treatment of toxoplasma retinochoroiditis in immunocompetent patients. Ophthalmology. 1999 Jan;106(1):148–153. doi: 10.1016/S0161-6420(99)90021-0. [DOI] [PubMed] [Google Scholar]
- 62.Felix JP, Lira RP, Zacchia RS, Toribio JM, Nascimento MA, Arieta CE. Trimethoprim-sulfamethoxazole versus placebo to reduce the risk of recurrences of Toxoplasma gondii retinochoroiditis: randomized controlled clinical trial. American journal of ophthalmology. 2014 Apr;157(4):762–766. e761. doi: 10.1016/j.ajo.2013.12.022. [DOI] [PubMed] [Google Scholar]
- 63.Baharivand N, Mahdavifard A, Fouladi RF. Intravitreal clindamycin plus dexamethasone versus classic oral therapy in toxoplasmic retinochoroiditis: a prospective randomized clinical trial. International ophthalmology. 2013 Feb;33(1):39–46. doi: 10.1007/s10792-012-9634-1. [DOI] [PubMed] [Google Scholar]
- 64.Lasave AF, Diaz-Llopis M, Muccioli C, Belfort R, Jr, Arevalo JF. Intravitreal clindamycin and dexamethasone for zone 1 toxoplasmic retinochoroiditis at twenty-four months. Ophthalmology. 2010 Sep;117(9):1831–1838. doi: 10.1016/j.ophtha.2010.01.028. [DOI] [PubMed] [Google Scholar]
- 65.Stewart JM, Cubillan LD, Cunningham ET., Jr Prevalence, clinical features, and causes of vision loss among patients with ocular toxocariasis. Retina. 2005 Dec;25(8):1005–1013. doi: 10.1097/00006982-200512000-00009. [DOI] [PubMed] [Google Scholar]
- 66.Lagace-Wiens PR, Dookeran R, Skinner S, Leicht R, Colwell DD, Galloway TD. Human ophthalmomyiasis interna caused by Hypoderma tarandi, Northern Canada. Emerging infectious diseases. 2008 Jan;14(1):64–66. doi: 10.3201/eid1401.070163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Gass JD, Braunstein RA. Further observations concerning the diffuse unilateral subacute neuroretinitis syndrome. Archives of ophthalmology. 1983 Nov;101(11):1689–1697. doi: 10.1001/archopht.1983.01040020691004. [DOI] [PubMed] [Google Scholar]
- 68.Vezzola D, Kisma N, Robson AG, Holder GE, Pavesio C. Structural and functional retinal changes in eyes with DUSN. Retina. 2014 Aug;34(8):1675–1682. doi: 10.1097/IAE.0000000000000114. [DOI] [PubMed] [Google Scholar]
- 69.Muccioli C, Belfort R., Jr Hypopyon in a patient with presumptive diffuse unilateral subacute neuroretinitis. Ocular immunology and inflammation. 2000 Jun;8(2):119–121. [PubMed] [Google Scholar]
- 70.Casella AM, Farah ME, Belfort R., Jr Antihelminthic drugs in diffuse unilateral subacute neuroretinitis. American journal of ophthalmology. 1998 Jan;125(1):109–111. doi: 10.1016/s0002-9394(99)80248-7. [DOI] [PubMed] [Google Scholar]
- 71.Takkar B, Chandra P, Kumar K, Vanathi M. Toxic granulomatous anterior uveitis in live intracameral cysticercosis masquerading as leukocoria. Canadian journal of ophthalmology. Journal canadien d’ophtalmologie. 2014 Dec;49(6):e140–141. doi: 10.1016/j.jcjo.2014.08.011. [DOI] [PubMed] [Google Scholar]
- 72.Wani VB, Kumar N, Uboweja AK, Kazem MA. A case of submacular cysticercosis treated by pars plana vitrectomy in Kuwait. Oman journal of ophthalmology. 2014 Sep;7(3):144–146. doi: 10.4103/0974-620X.142599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Fekkar A, Bodaghi B, Touafek F, Le Hoang P, Mazier D, Paris L. Comparison of immunoblotting, calculation of the Goldmann-Witmer coefficient, and real-time PCR using aqueous humor samples for diagnosis of ocular toxoplasmosis. Journal of clinical microbiology. 2008 Jun;46(6):1965–1967. doi: 10.1128/JCM.01900-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Talabani H, Asseraf M, Yera H, et al. Contributions of immunoblotting, real-time PCR, and the Goldmann-Witmer coefficient to diagnosis of atypical toxoplasmic retinochoroiditis. Journal of clinical microbiology. 2009 Jul;47(7):2131–2135. doi: 10.1128/JCM.00128-09. [DOI] [PMC free article] [PubMed] [Google Scholar]