The severity of atopic dermatitis (AD) and intragenic filaggrin (FLG; OMIM 135940) copy number variant (CNV) genotypes were assessed in African American pediatric patients, a health disparities group that is disproportionately affected with AD.1
METHODS
The study was approved by Washington University School of Medicine’s institutional review board. Eligibility criteria for recruited pediatric patients were (1) age 3 months – 18 years, (2) United Kingdom Working Party’s Diagnostic Criteria for Atopic Dermatitis,2 (3) African American ethnicity (self-reported), (4) moderate to severe AD (SCORAD index, >25),3 and (5) written informated assent or consent. Common European FLG R501X and 2282del4 mutations and intragenic FLG CNV (3 alleles of either 10, 11, or 12 FLG monomer repeats), upon high-quality DNA assessment, were genotyped4 and correlated with AD severity.
RESULTS
Thirty-nine pediatric African American AD patients were recruited with a mean (range) age of 6.7 (0.4–15) years (Table 1). Thirty-five patients reported a first-degree family member with atopy and 30 patients reported AD onset before age 2 years. Of the 31 patients who were 4 years or older at the time of visit, a history of asthma and allergic rhinitis and/or hay fever were reported in 24 (77%) and 16 (52%), respectively. Food allergies were reported as well (51% [n=20]), most commonly peanut (n=10) and fish and/or shellfish (n=10) that were not coincident. All but 1 were either being treated with or had been prescribed topical triamcinolone ointment, 0.1%.
Table 1.
Characteristics of 39 African American Pediatric Patients with Atopic Dermatitis (AD)
| Variable | Value |
|---|---|
|
| |
| Sex, No. (%) | |
| Female | 18 (46) |
| Male | 21 (54) |
|
| |
| Age, mean (range), y | 6.7 (0.4–15) |
|
| |
| Atopy in immediate family, No. (%) | 35 (90) |
|
| |
| Onset age, No. (%), y | |
|
| |
| <2 | 30 (77) |
|
| |
| ≥2 | 9 (23) |
|
| |
| Asthma at age ≥4 y, No. (%) | 24 (77) |
|
| |
| Hay Fever at age ≥4 y, No. (%) | 16 (52) |
|
| |
| Allergy | |
| Food | 20 (51) |
| Peanut | 10 (26) |
| Fish or Shellfish | 10 (26) |
|
| |
| Quality of life disruption, mean (range)a | 7.9 (1–10) |
|
| |
| Triamcinolone ointment, 0.1%, treatment, No. (%) | 38 (97) |
|
| |
| SCORAD Index, mean (range)b | 58.5 (28.0–94.1) |
|
| |
| Moderate AD (SCORAD ≥ 25 – 49), No. (%) | 15 (38) |
|
| |
| Severe AD (SCORAD ≥ 50), No. (%) | 24 (62) |
|
| |
| FLG CNV Allele, R501X excluded, No. (%) | |
|
| |
| 10 | 47 (64) |
|
| |
| 11 | 13 (18) |
|
| |
| 12 | 14 (19) |
Abbrevation: CNV, copy number variant.
Scale of 0 to 10.
Scale of 0 to 103.
The mean (range) SCORAD of the participants was 58.5 (28.0–94.1) (Table 1) with severe pruritus (mean, 7.8) and moderate sleep loss (mean 5.7, both scales 0–10). Twenty-four patients (62%) exhibited severe AD. The mean lesional body extent was 44% and mean lesional intensity was 10 (scale, 0–15; 15 = worst). Lichenification and dryness (both means, 2.2; scale 0–3, 3=worst) contributed the most to the lesional intensity (Figure, A).
Figure.
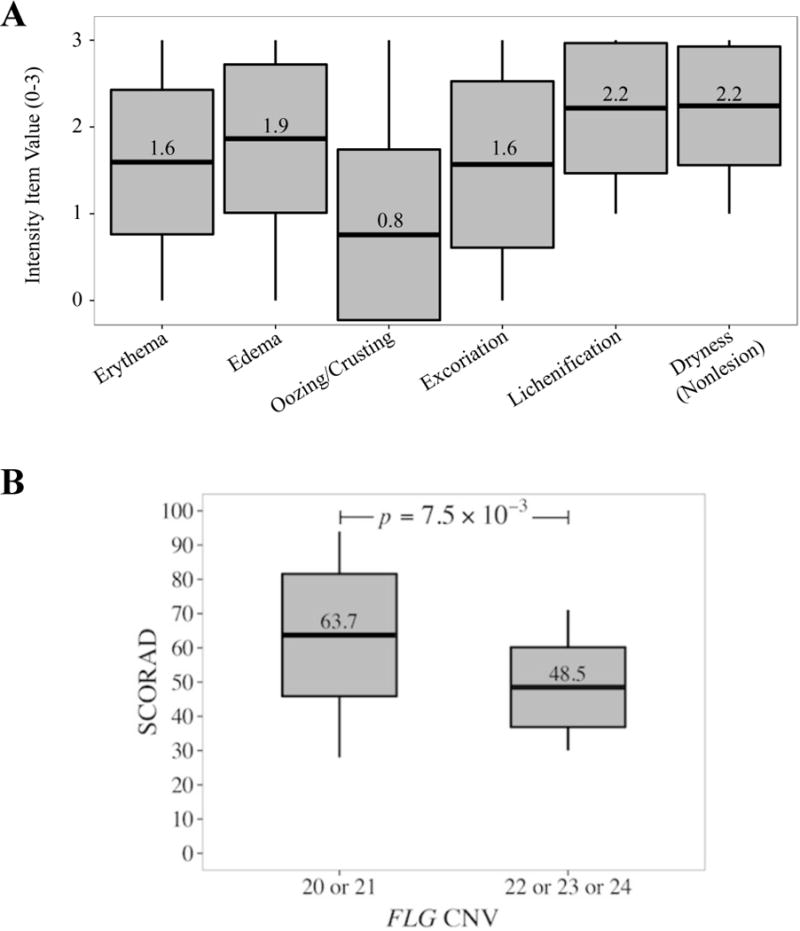
A, Intensity item values that contribute to the collective intensity measure of a representative lesion. B, Distribution of total FLG repeats with respect to SCORAD, The horizontal line in the middle of each box indicates the mean, while the top and bottom borders of the box mark 1 standard deviation above and below the mean. The whiskers above and below the box mark the maximum and minimum values, respectively. Statistics, 2-sided Wilcoxon rank-sum test.
We sought to explain the associated severe nonlesional skin dryness in our participants by genotyping a previously described European dose-dependent risk factor for AD, intragenic FLG repeats, or CNV.4 Excluding 2 FLG R501X heterozygous patients (no FLG 2282del4 mutations identified), 16 cases (43%) were homozygous for the FLG CNV 10 allele, thus totaling 20 filaggrin monomers (Table 2). Of the total 74 FLG alleles in our cases (n = 37), the FLG CNV 10 allele made up 64% (Table 1). Patients with a total of either 20 or 21 FLG CNV exhibited higher SCORAD (mean, 63.7) and hence severe AD compared with those participants with 22, 23, and 24 FLG CNV (mean SCORAD, 48.5) (P=0.015) (Figure, B). Moreover, we found that individuals with 20 total filaggrin monomers are 1.9 times more likely to have severe AD (Table 2). However, this was not statistically significant (P=0.33).
Table 2.
Total FLG Copy Number Variants (CNVs) and Unadjusted Odds Ratios (ORs)for participants with Severe Atopic Dermatitis (AD) (SCORAD Index >50), R501X excluded
| Total FLG CNV | Patients, No. (%) | Severe AD (95% CI) | P value |
|---|---|---|---|
| 20 | 16 (43) | 1.91 (0.51–7.12) | 0.33 |
| 21 | 8 (22) | 1.12 (0.24–5.15) | 0.88 |
| 22 | 8 (22) | 1.12 (0.24–5.15) | 0.88 |
| 23 | 3 (8) | 0.38 (0.04–3.19) | 0.35 |
| 24 | 2 (5) | Unable to estimate |
DISCUSSION
Despite epidemiological data supporting a marked increase in AD in African American children,1 to our knowledge, a quantitative measure of AD severity and an investigation of FLG CNV in this health disparities group have not been reported until now. We identify a significant difference between low FLG CNV (20 or 21) with severe AD versus FLG CNV (22, 23, or 24) with moderate AD (P=.01). Each FLG repeat encodes 1 posttranslationally modified active filaggrin monomer that is further degraded to metabolites such as urocanic acid that comprises part of the skin’s natural moisturizing factor.4 Addition of each FLG monomer decreases the odds ratio of disease risk of AD by 0.88.4 A reduction of NMF metabolites was also observed in skin in South African patients with AD.5 Although NMF metabolites were not assessed in this study, the parallels between our study and that of Brown et al.4 with respect to low FLG CNV and AD suggest a reduction in filaggrin metabolites contributing to our patients’ skin dryness. Observed low if not absent frequencies of FLG and/or FLG-2 stop-gain mutations in African Americans6–8 and Africans5 suggest decreased likelihoods for these mutations in AD risk specific to this ancestry. Future case-control studies specific to this health disparities group are warranted to more fully understand the genetics of AD.
Acknowledgments
Funding/Support: This study was supported in parts by Dean’s Faculty Diversity Scholar Award (Washington University School of Medicine, Dr de Guzman Strong) and from the National Institutes of Health grants T32 HG000045 (Mr Goodwin) and R00AR055948 (Dr de Guzman Strong). The Dean’s Faculty Diversity Funds provided the materials for the study.
Footnotes
Author Contributions: Drs Bayliss and de Guzman Strong had full access to all of the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis.
Study concept and design: Marfatia, de Guzman Strong.
Acquisition, analysis, and interpretation of data: All authors.
Drafting of the manuscript: Quiggle, Bayliss, de Guzman Strong.
Critical revision of the manuscript for important intellectual content: Quiggle, Goodwin, Marfatia, Kumar, Ciliberto, Bayliss, de Guzman Strong.
Statistical analysis: Goodwin, de Guzman Strong.
Obtained funding: de Guzman Strong.
Administrative, technical, or material support: All authors.
Study supervision: Bayliss, de Guzman Strong.
Role of the Sponsor: The sponsor had no role in the design and conduct of the study; collection, management, analysis, and interpretation of data, preparation, review, or approval of the manuscript, and decision to submit the manuscript for publication.
Conflict of Interest Disclosures: None reported.
Additional Contributions: Emily Beck, M.D., Emily Gurnee, M.D, Kara J. Gulewicz, M.D., and Colleen Cotton, M.D. assisted with patient recruitment and were not compensated.
Previous Presentations: Presented in part at the 73rd Annual Meeting of the Society of Investigative Dermatology; May 8–9, 2014; Albuquerque, NM.
References
- 1.Shaw TE, Currie GP, Koudelka CW, Simpson EL. Eczema prevalence in the United States: data from the 2003 National Survey of Children’s Health. J Invest Dermatol. 2011;131(1):67–73. doi: 10.1038/jid.2010.251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Williams HC, Burney PG, Hay RJ, et al. The UK Working Party’s Diagnostic Criteria for Atopic Dermatitis. 1. derivation of a minimum set of discriminators for atopic dermatitis. Br J Dermatol. 1994;131(3):383–396. doi: 10.1111/j.1365-2133.1994.tb08530.x. [DOI] [PubMed] [Google Scholar]
- 3.Severity scoring of atopic dermatitis: the SCORAD index; Consensus Report of the European Task Force on Atopic Dermatitis. Dermatology. 1993;186(1):23–31. doi: 10.1159/000247298. [DOI] [PubMed] [Google Scholar]
- 4.Brown SJ, Kroboth K, Sandilands A, et al. Intragenic copy number variation within filaggrin contributes to the risk of atopic dermatitis with a dose-dependent effect. J Invest Dermatol. 2012;132(1):98–104. doi: 10.1038/jid.2011.342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Thawer-Esmail F, Jakasa I, Todd G, et al. South African amaXhosa patients with atopic dermatitis have decreased levels of filaggrin breakdown products but no loss-of-function mutations in filaggrin. J Allergy Clin Immunol. 2014;133(1):280–282. doi: 10.1016/j.jaci.2013.09.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Margolis DJ, Gupta J, Apter AJ, et al. Exome Sequencing of Filaggrin and Related Genes in African-American Children with Atopic Dermatitis. J Invest Dermatol. 2014;134(1):2272–2274. doi: 10.1038/jid.2014.126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Polcari I, Becker L, Stein SL, et al. Filaggrin gene mutations in African Americans with both ichthyosis vulgaris and atopic dermatitis. Pediatr Dermatol. 2014;31(4):489–492. doi: 10.1111/pde.12355. [DOI] [PubMed] [Google Scholar]
- 8.Margolis DJ, Gupta J, Apter AJ, et al. Filaggrin-2 variation is associated with more persistent atopic dermatitis in African American subjects. J Allergy Clin Immunol. 2014;133(3):784–789. doi: 10.1016/j.jaci.2013.09.015. [DOI] [PMC free article] [PubMed] [Google Scholar]


