Abstract
Atherosclerosis is a systemic disease characterized by the development of multifocal plaque lesions within vessel walls and extending into the vascular lumen. The disease takes decades to develop symptomatic lesions, affording opportunities for accurate detection of plaque progression, analysis of risk factors responsible for clinical events, and planning personalized treatment. Of the available molecular imaging modalities, radionuclide-based imaging strategies have been favored due to their sensitivity, quantitative detection and pathways for translational research. This review summarizes recent advances of radiolabeled small molecules, peptides, antibodies and nanoparticles for atherosclerotic plaque imaging during disease progression.
Keywords: Atherosclerosis, molecular imaging, PET, SPECT, radionuclide, peptide, nanoparticle
Introduction
1.1 Biology of atherosclerosis
Atherosclerosis, the leading cause of morbidity and mortality in Westernized societies, is a progressive disease characterized by the development of lipid-rich plaque lesions within vessel walls and extending into the vascular lumen. It is the underlying basis of cardiovascular diseases including myocardial infarction, stroke, and peripheral arterial disease [1]. Several factors, including hypertension, lifestyle choices (smoking, diet, sedentary habits), and genetic predisposition increase the risk of developing atherosclerosis. The disease begins with the accumulation of low-density lipoproteins (LDL) on the arterial wall which triggers an inflammatory process through modifications including oxidation, glycation, acetylation and carbamylation [2]. Tissue undergoing the initiation of atheroma becomes more permeable to leukocytes which are recruited by molecules in the vascular endothelium. Adhesion molecules, containing vascular cell adhesion molecule-1 (VCAM-1) and intercellular adhesion molecule (ICAM-1), and selectins, containing P-selectin and E-selectin, are overexpressed by endothelial cells [3]. The activated endothelium produces monocyte chemoattractant protein-1 (MCP-1), a chemokine for leukocyte recruitment, in addition to growth factors such as macrophage colony-stimulating factor (M-CSF) and granulocyte-macrophage colony-stimulating factor (GM-CSF) [4]. Reactive oxygen species (ROS), which are produced by activated macrophages, endothelial cells and smooth muscle cells (SMCs), generate the most important modification in LDL - oxidation [5]. Cells of the innate immune system express pattern-recognition receptors (PRRs) such as scavenger receptors, especially CD36 and SRA (scavenger receptor A, CD204) [6]. These are known as endocytic type receptors for recognizing and capturing oxidized LDL (oxLDL), the main process responsible for the formation of foam cells. Signaling receptors are also PRRs playing key roles in the activation of proinflammatory factors [7].
In early atherosclerotic lesions, foam cells containing large amounts of LDL undergo apoptosis and are removed from the lesion. With the progression of the plaque the apoptotic process slows down. Accumulating foam cell deposits in the lesion lead to the formation of a necrotic core [8]. Moreover, neoangiogenic processes in the plaque provide supplemental oxygen while facilitating the mobilization of leukocytes for plaque development. In advanced plaque, these fragile microvessels are prone to break, resulting in thrombus formation which can collaborate with the inflammatory process and inhibit macrophage migration. Interestingly, with the formation of necrotic core and plaque progression, the plaque tends to be hypoxic which correlates with inflammation, angiogenesis and apoptosis [9], and has been demonstrated in a recent study showing that the reversal of hypoxia could decrease necrotic core formation and prevent apoptotic cell accumulation in atherosclerotic plaques in LDLR-/- mice [10].
Adaptive immune response participates in the progression of atherosclerosis through interactions between antigen-presenting cells and T-cells. CD4+ T-cell is a subtype found in the atherosclerotic lesion that presents epitopes bound to the major hiscompatibility complex class II (MHC II) [11]. These T-cells can also differentiate into proinflammatory T-helper 1 (Th-1) cells which secretes interferon gamma (IFN-γ) to decreases collagen synthesis, narrow the thickness of fibrotic cap, and perpetuate the Th-1 inflammatory response [12]. The antigens exposed by antigen-presenting cells are presented to adaptive immunity cells, activating T-cells and inducing antibody production by B-cells. The association between autoantigens produced during atherogenesis and their respective autoantibodies has been studied as a target for atherosclerosis imaging [13-15].
The stability of atherosclerotic lesions depends on the balance between inflammatory response and those mechanisms that stimulate extracellular matrix synthesis. When the fibrotic cap thins and tensile strength decreases the chance of rupture increases. The mechanism that elicits this clinical consequence involves the process of thrombosis resulting from the extravasation of the necrotic lipid core and subsequent contact with proteins of the coagulation cascade [16]. Important clinical outcomes of thrombosis and disruptions of the plaque include acute myocardial infarction and unstable angina.
1.2 Challenges in clinical diagnosis
Clinical challenges in CVD include predicting when a plaque may rupture and managing treatment to avoid associated fatal consequences. The need for early diagnosis is critical since atherosclerosis is a silent disease that progresses slowly over years. Factors influencing plaque vulnerability include plaque thickness, composition and degree of inflammation. The amount of cholesterol in the plaque is also important as cholesterol esters may be softer, thus increasing the pressure on the fibrous cap. Additionally, the intense activity of inflammatory cells may increase the temperature of the plaque, thus reducing the rigidity of the lipid core [17].
Currently, imaging techniques for the diagnosis of atherosclerosis include both noninvasive methods [computed tomography (CT), magnetic resonance imaging (MRI), single-photon emission computed tomography (SPECT), positron emission tomography (PET) and fluorescence molecular tomography (FMT)] and invasive methods [intravascular ultrasound (IVUS), optical coherence tomography (OCT)], as summarized in Table 1.
Table 1. Comparison of imaging modalities for atherosclerosis [55, 56].
| Modality | Advantages | Disadvantages |
|---|---|---|
| SPECT | High sensitivity (10-14 – fmol) Signal quantification |
Low spatial resolution (1-2mm) Radiation exposure |
| PET | High sensitivity (10-15 – fmol) Signal quantification Use of short half-life radionuclides |
Low spatial resolution (1-2mm) Radiation exposure |
| CT | High spatial resolution (50-200μm) | Low sensitivity (10-6 - μmol) Radiation exposure |
| MRI | High spatial resolution (10-100μm) | Low sensitivity (10-9 – nmol) Use of contrast agents |
| IVUS | Differentiation between layers in the arterial wall High axial resolution (∼100μm) Details about plaque composition |
Invasive method by catheter |
| OCT | Higher resolution than IVUS (∼10μm) | Invasive method by catheter Requires saline flushing of the lumen Limited depth of view |
| FMT | Versatility of use | Limited depth of view for infrared probes |
Invasive techniques provide valuable morphological information while affording high-resolution images of the atherosclerotic plaque. Noninvasive imaging platforms such as CT and MRI show high-resolution images but may require the administration of contrast agents due to their low sensitivity. In order to improve MRI images, contrast agents in the form of iron oxide nanoparticles have been developed for directing the localization of activated macrophages, since these cells are able to phagocytose the nanoparticles. Although this approach correlates with inflammatory activity of an atherosclerotic plaque, it does not present sufficient specificity to accurately identify specific biomarkers expressed on the plaque [18]. Nuclear imaging with PET and SPECT can characterize atherosclerotic plaques with the highest sensitivity allowing quantification of the biologic process and tracking of disease progression during treatment. PET and SPECT represent areas of molecular imaging in which relevant biological targets are identified by specific radiotracers. The co-registration with MRI or CT can combine high sensitivity with increased resolution to better determine the plaque activity [19, 20]. Some of these dual-modality imaging techniques are already being used in translational research [21], providing great anatomical detail coupled with information at the cellular and molecular level. Of the above imaging modalities, CT is able to report on the degree of plaque stenosis, and those with high-grade stenosis are certainly at increased risk of interruption of blood flow. However, there is a positive remodeling that occurs in the beginning of plaque formation towards the adventitia rather than the lumen, suggesting that when the stenosis begins, there is already a large and complex atherosclerotic plaque [3]. Studies with human arteries found early stenosis occurs only when the growth of an atherosclerotic plaque reaches 40% of the cross sectional area of the artery and then the growth begins in opposite direction [22]. This data reinforces the need for advances in early diagnosis.
Due to the high sensitivity and specificity, radionuclide imaging techniques are valuable tools in imaging the biological processes during the plaque development, providing information about plaque composition and status of the disease. We discuss here the recent advances in radionuclide-based molecular imaging for PET and SPECT in preclinical studies of atherosclerosis, covering discoveries of potential targets for distinct biological processes and their imaging by radiolabeled compounds such as peptides, nanoparticles, small molecules and antibodies.
2. Radionuclides
Mode of decay and half-life typically direct the choice of radionuclide for molecular imaging. In general, SPECT probes are labeled with γ-emitting radionuclides as the SPECT camera is coupled to lead collimators which detect γ-rays in different energy levels, whereas PET probes utilize positron emitting radionuclides with detection of γ photons resulting from positron-electron annihilation [21].
Molecular imaging probes need to follow certain criteria in order to achieve the best contrast against normal tissue surrounding the pathological disease. In this context, key characteristics of the imaging agent include high affinity for the molecular target, the ability to overcome biological barriers, low toxicity, efficient clearance in vivo, straightforward production [23, 24], and high specific activity for radioactive probes which is defined as radioactivity per mass unit of radionuclide or radiolabeled compound [25]. The number of targets and the affinity of the imaging agent to these targets play an important role in its effectiveness because the presence of non-radioactive components may compete with the radiolabeled compound by binding to the target and decreasing signal uptake in the tissue [26]. Some radiolabeled compounds are already being used in clinical practice, however, most are dedicated to cancer imaging. In contrast, in the field of cardiac research few molecular imaging probes can be considered effective in accurate diagnosis.
2.1. PET
In contrast to other imaging modalities, PET has the advantages of high sensitivity and quantification at the region of interest. Basically, upon decay of a PET radionuclide, a positron migrates a small distance until meeting a surrounding electron, suffering an annihilation event in which both are destroyed. Their masses are converted into two photons of γ-radiation that move in opposite directions forming an angle of 180° between their paths, which is then detected by pairs of oppositely disposed detectors in the PET scanner. The sensitivity of detection in concentration ranges from nanomolar (10-9) to picomolar (10-12) and permits quantitative imaging of the signal [27]. Typical PET radionuclides used in imaging applications are listed in Table 2.
Table 2. Characteristics of Selected PET Radionuclides and Applications [57-60].
| Nuclide | Half-life | Decay (%) | Maximum β+ energy | γ Energy (keV) | Nuclear reaction | Application |
|---|---|---|---|---|---|---|
| 11C | 20.4 min | β+ (99.8) EC (0.2) | 960 keV | 511 | 14N (p, α) 11C | [61, 62] |
| 18F | 109.8 min | β+ (97) EC (3) | 635 keV | 511 | 18O (p, n) 18F | [32, 63-66] |
| 64Cu | 12.7 h | β+ (18) β- (37) EC (45) | 653 keV | 511 | 64Ni (p, n) 64Cu | [38, 50, 67, 68] |
| 68Ga | 67.7 min | β+ (90) EC (10) | 1.9 MeV | 511 | 68Ge /68Ga Generator | [69-71] |
| 89Zr | 78.5 h | β+(22.7) EC (77) | 2.8 MeV | 511 909 |
89Y(p, n) 89Zr | [51, 72-75] |
| 124I | 4.2 d | β+ (23) EC (77) | 2.2 MeV | 511 603 723 |
124Te (p, n) 124I 124Te (d, 2n) 124I |
[76-78] |
2.2. SPECT
SPECT is able to detect γ photons by utilizing a gamma camera and a lead or tungsten collimator that physically selects photons by defining the incidence angle of γ-rays. The acceptable range of energy for SPECT cameras is between 90 and 300 keV but the ideal γ-ray energy is 150 keV for which value the SPECT instrumentation has been optimized. Unlike PET imaging which is unable to distinguish between radionuclides, SPECT can identify the different energy emissions in one image, thus allowing the tracking of multiple biological pathways simultaneously [28]. Furthermore, the relatively long half-lives of some SPECT radionuclides permit more elaborate syntheses of radiotracers and allow delivery to distant imaging sites. 99mTc eluted from 99Mo/99mTc generator is the most widely used SPECT radionuclides in clinical applications [29, 30]. Commonly used SPECT radionuclides are summarized in Table 3.
Table 3. Characteristics of Selected SPECT Radionuclides and Applications [57, 79].
3. Biological processes
3.1. Radiotracers imaging early stage atherosclerotic lesions
As discussed earlier, some of the first molecules expressed by endothelial cells in atherosclerotic lesion formation are adhesion molecules such as VCAM-1 [31, 32]. Of the agents developed for VCAM-1 imaging, nanobodies are a new class of probe which carry the smallest functional unit of a whole antibody for antigen recognition. They are highly versatile, combining the specificity of an antibody with rapid clearance, making them ideal imaging agents. In a recent study, up to 10 anti-VCAM-1 nanobodies were screened for VCAM-1 imaging and the candidate agent was radiolabeled with 99mTc to detect the (99mTc-cAbVCAM1-5) atherosclerotic lesions [33]. SPECT imaging showed sensitive and specific detection of VCAM-1 with high lesion-to-blood (4.32 ± 0.48) and lesion-to-heart (8.30 ± 1.11) ratios (fig.1A). The ex vivo autoradiography also confirmed the localization of tracer in the aorta and the up-regulation of VCAM-1 was verified by immunohistochemistry.
Figure 1.
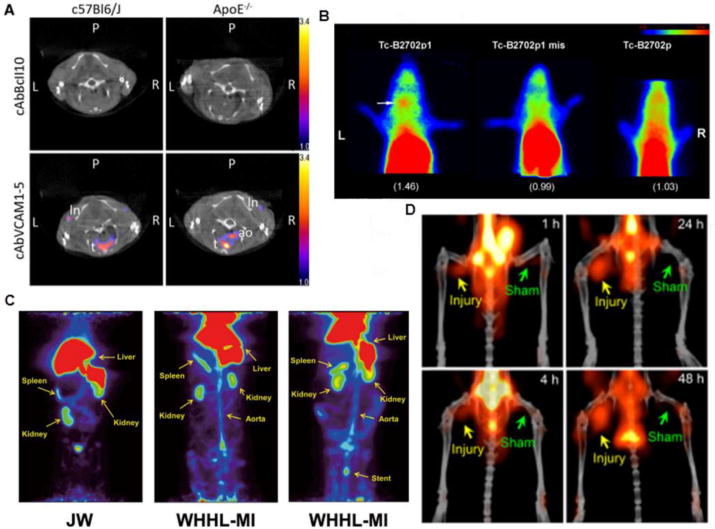
Representative images detecting early stage atherosclerosis. (A) SPECT/CT imaging showed uptake of 99mTc-cAbVCAM1-5 in the aortic arch of C57Bl/6J and apoE-/- mice in coronal views, demonstrating higher signal as compared to non-specific control nanobody 99mTc-cAbBcll10. (B) Planar images after injection of 99mTc-B2702p1, 99mTc-B2702p1 mismatch and 99mTc-B2702p with higher left carotid uptake indicated by white arrow. (C) 68Ga-DOTA-FAMP peptide uptake in PET imaging of Japanese white (JW) control rabbit and WHHL-MI, without and with stent implanted presenting high uptake by atherosclerosis model which was more significant with the presence of the stent. (D) PET/CT images of 64Cu-DOTA-DAPTA-comb detecting plaque at right femoral arteries of apoE-/- mice 1, 4, 24 and 48 hours post injection, demonstrating increasing uptake over time. Images modified with permission [33, 36, 38, 85].
Owing to the straightforward preparation and fast in vivo clearance, peptides have been extensively explored in atherosclerosis molecular imaging, especially in cases of cell surface receptor recognition. During plaque development, major histocompatibility complex 1 (MHC-I) expressed on leukocytes is actively involved in immune response [34, 35] Thus, out of 20 peptides (B2702p1 to B2702p20) derived from MHC-1, B2702p1 showed the best binding affinity and specificity in detecting plaque. In the carotid region of ApoE-/- mice, 99mTc radiolabeled B2702p showed specific accumulation in the left carotid artery and 3.4 fold increase in contrast to the blood pool retention, indicating targeting specificity (fig. 1B). During the plaque progression, HDL (high-density lipoprotein), especially apolipoprotein A-I (apo A-I), plays an important function in the reverse transport of cholesterol from cholesterol-rich cells to the liver, making it an interesting target for atherosclerosis imaging. Thus, a FAMP peptide (Fukuoka University apo A-I Mimetic Peptide) was radiolabeled with 68Ga for in vivo atherosclerosis imaging in a myocardial infarction-prone Watanabe heritable hyperlipidemic rabbit model (WHHL-MI) and demonstrated favorable pharmacokinetics and specific accumulation in the plaque (fig. 1C) [36]. Further study should focus on biological assays to confirm the expression levels of HDL during disease progression and its correlation to PET signals.
Another interesting target is the folate receptor present in activated macrophages [37]. Compared to uptake in the aortic arch of ApoE-/- mice fed with normal chow, the accumulation of 99mTc-EC20 in ApoE-/- mice fed a high fat diet showed 70% higher signal as detected by γ-scintigraphy imaging, which was also confirmed by γ-counting of the dissected aorta. Flow cytometry analysis showed 33% of macrophages were positive for folate receptors in mice fed a high fat diet in contrast to the 11% positivity in mice fed with normal chow.
Chemokine receptors such as CCR5 hold potential as biomarkers to determine plaque progression and activity. In a vascular injury accelerated ApoE-/- mouse atherosclerosis model, a CCR5 binding peptide D-Ala1-peptide T-amide (DAPTA) labeled with 64Cu showed specific plaque accumulation. With the conjugation of DAPTA peptide onto a multivalent comb-like nanoparticle (64Cu-DOTA-DAPTA-comb) with well-controlled structure to extend blood circulation, the imaging specificity and targeting efficiency were significantly improved compared to DAPTA peptide tracer alone. At the injured artery, targeted 64Cu-DOTA-DAPTA-comb demonstrated specific accumulation and binding to the CCR5 receptor. The quantification of PET images showed the targeted 64Cu-DOTA-DAPTA-comb had more than 3 times higher uptake in the plaque relative to the non-targeted 64Cu-DOTA-comb, indicating the potential of this targeted nanoprobe for plaque detection (fig. 1D) [38].
Of the targets evaluated for atherosclerosis imaging, the selectin family (P-selectin and E-selectin) has been widely studied due to their role in promoting arterial inflammation. Through radiolabeling with 64Cu, the aortic uptake of 64Cu-anti-P-selectin mAb in LDLR-/- mice fed with high-cholesterol diet for 12 weeks was 6 fold higher than the uptake obtained in chow-diet mice. PET/CT imaging clearly showed the specific accumulation of this tracer at the aortic arch (fig. 2A) [39]. The role of oxLDL in the formation of foam cells is well established and oxidation-specific epitopes have been explored as biomarkers of disease risk and for therapeutic treatments. Malondialdehyde 2 monoclonal antibody (MDA2 mAb) radiolabeled with 125I targeting MDA-LDL, LDL oxidized in vitro by malondialdehyde (MDA), was studied by gamma camera imaging in a rabbit atherosclerosis model. In contrast to control rabbits, the uptake of 125I-MDA2 mAb in the aorta of WHHL rabbits was 17.4 fold higher, indicating the potential of this agent for plaque detection [40]. In another study, lectin-like oxidized LDL receptor 1 (LOX-1), which is overexpressed by activated endothelial cells and macrophages in the early stage of atherosclerosis development, was imaged with 99mTc-LOX-1-mAb. Compared to control rabbit, 99mTc-LOX-1-mAb planar images showed specific and higher accumulation in the abdominal aorta of WHHL-MI rabbit, which was also more than the data acquired with 99mTc-IgG2 control radiotracer (fig. 2B) [41].
Figure 2.
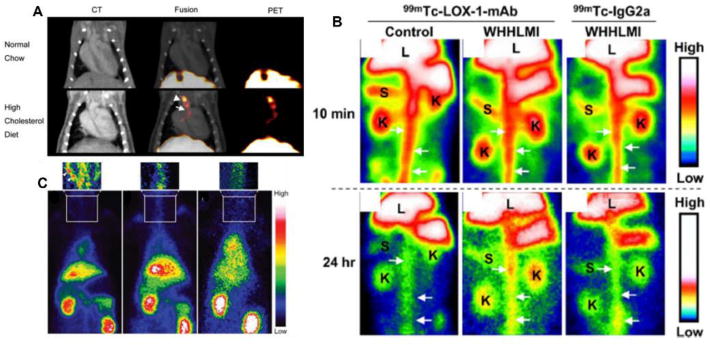
Representative images using antibodies as radiotracers for atherosclerosis imaging. (A) In vivo PET/CT imaging in LDLR-/- mice injected with 64Cu-DOTA-anti-P-selectin mAb, demonstrating signal uptake in the brachiocephalic artery and aortic arch, as indicated by arrowhead and arrow, respectively. (B) Planar images after injection 99mTc-LOX-1-mAb compared to 99mTc-IgG2a in WHHL-MI and control rabbits. White arrows indicate aorta, K is kidney, L is liver, S is spleen. (C) Noninvasive planar images with injection of 99mTc-chP3R99 mAb by atherosclerotic (left image) and healthy (center image) rabbits, demonstrating the accumulation in the carotid indicated by arrowheads, compared to the injection of control radiotracer 99mTc-chT3 mAb (right image). Modified with permission [39, 41, 42].
As atherosclerosis develops, chondroitin sulfate on proteoglycans is produced and interacts with LDL, leading to the retention and accumulation of LDL in the arterial wall. Thus, an antibody-based tracer of 99mTc-chP3R99 mAb targeting chondroitin sulfate was developed for atherosclerosis imaging in a lipofundin-induced New Zealand White (NZW) rabbit atherosclerotic model using immunoscintigram planar imaging (fig. 2C) [42]. Compared to non-atherosclerotic rabbits, the uptake of 99mTc-chP3R99 mAb at the atherosclerotic lesion was 3.9 fold higher. However, the decay half-life of 99mTc does not match the long blood retention of chP3R99 mAb. Further studies may need to focus on the use of antibody fragments or peptides.
3.2. Radiotracers imaging the progression of atherosclerosis
Metalloproteinases (MMPs) are enzymes that degrade protein constituents of the extracellular matrix. Their activity is strongly induced by inflammatory cells during atherogenesis. More specifically, RP782 is a small molecule able to identify MMP activation epitopes. When radiolabeled with 111In (fig. 3A), evaluation of the effects of diet on ApoE-/- mice was possible, correlating macrophage density found on atherosclerotic plaques with uptake signal [43]. Most macrophage cells were found in the atherosclerotic plaques of animals on a prolonged high fat diet (> 3 months). Moreover, ApoE-/- mice fed a high fat diet for 2 to 3 months followed by a chow diet for 1 month presented reduced relative plaque area by 30%.
Figure 3.
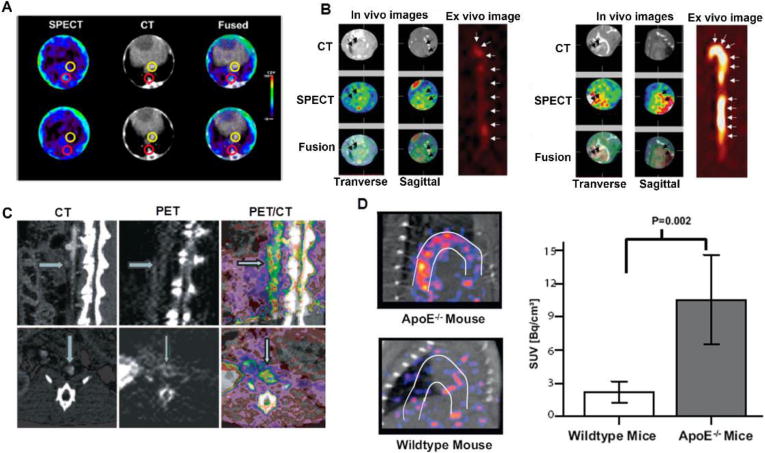
Representative images determining the progression of atherosclerosis. (A) Transversal SPECT/CT imaging using 111In-RP782 radiotracer in apoE-/- mice with aorta uptake indicated by red circles and the inferior vena cava circled in yellow. (B) In vivo SPECT/CT and ex vivo autoradiography images of the aorta after injection of 99mTc-annexin A5 in the control (left image) and LDLR-/- (right image) mice with the highest uptake evident in the atherosclerotic model. (C) PET/CT imaging in an atherosclerotic rabbit injected with 18F-AppCHFppA by sagittal sections in the upper row and transversal sections in the lower row, with arrows indicating the lumen by CT and arterial wall by PET imaging. (E) Higher uptake in aortic arch of apoE-/- compared to wild type mice that received 64Cu-CD68-Fc-NOTA by PET/CT images with the SUV data quantification from the images. Modified with permission [43-46].
When a cell becomes apoptotic, phosphatidylserine (PS) present in the cell membrane is exposed. Because a massive presence of apoptotic macrophages could cause plaque rupture, Annexin V, a molecule with an affinity to PS in nanomole concentrations, was studied as a 99mTc-Annexin V tracer to monitor different stages of atherosclerosis in LDLR-/- and ApoE-/- mice on high cholesterol and chow diet [44]. SPECT/CT imaging and aorta autoradiography showed highest tracer uptake in cholesterol-diet ApoE-/- mice (0.88 ± 0.27%ID/g), followed by chow-diet apoE-/- mice (0.60 ± 0.16%ID/g) and cholesterol-diet LDLR-/- mice (0.59 ± 0.14%ID/g) (fig. 3B). Immunohistochemistry demonstrated that apoptosis was correlated to macrophage cell number and Annexin V uptake. These results suggested that radiotracer uptake may detect different stages of disease, presenting an option for monitoring therapeutic intervention.
Macrophage-induced lesion inflammation has been studied as a means to follow disease progression. The molecule diadenosine-5′,5‴-P1,P4-tetraphosphate (Ap4A) and its analogue P2,P3-monochloromethylene diadenosine-5′,5‴-P1, P4-tetraphosphate (AppCHClppA) are competitive inhibitors of platelet aggregation. 18F-AppCHFppA was investigated for imaging inflamed atherosclerotic plaques in male NZW rabbits (fig. 3D) [45]. Uptake correlated to the presence of macrophages by histological examinations (r= 0.87, P= 0.0001). 18F-AppCHFppA shows potential for use in in vivo quantification of atherosclerosis progression.
A strategy based on CD68-Fc soluble protein which targets oxLDL foam cells was investigated [46]. ApoE-/- mice fed a high-fat diet for 12 weeks were analyzed for detection of atherosclerotic lesion by 64Cu-CD68-Fc-NOTA [46]. Standardized uptake value (SUV) quantification by PET/CT showed higher values in the aortic arch of apoE-/- mice (10.5 ± 1.5 Bq/cm3) than wild type (WT) mice (2.1 ± 0.3 Bq/cm3) (fig. 3D). In addition, higher R1 relaxation rates by MRI imaging using gadolinium-based elastin-specific contrast agent in apoE-/- mice (1.47 ± 0.06 s-1) compared to WT mice (0.92 ± 0.05 s-1) were observed, demonstrating increased vessel wall remodeling and strong correlation data (r=0.946; P=0.042), which suggests the potential of 64Cu-CD68-Fc-NOTA as a useful tool for cardiovascular risk stratification and for monitoring treatment effects from statin therapy.
3.3. Radiotracers imaging the complications of atherosclerosis
The efficacy of treatment and healing following myocardial infarction in apoE-/- mice was evaluated by using 18F-FXIII (coagulation factor) and myeloperoxidase coupled with gadolinium (MPO-Gd) (fig. 4A) [47]. PET/MRI imaging could monitor inflammation resolution which was useful in evaluating the nanoparticle-guided iRNA silencing of CCR2, wherein monocyte migration to inflamed sites was inhibited.
Figure 4.
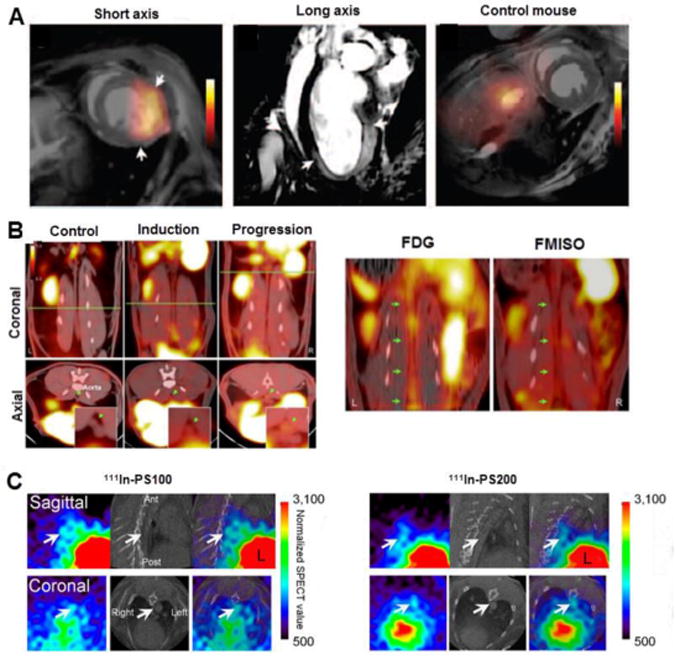
Radiotracers imaging atherosclerotic plaque complication. (A) PET/MRI imaging after injection of 18F-FXIII/myeloperoxidase-MPO-Gd in the myocardial infarction model WT mice, with the arrows indicating border regions of infarct, with increased signal by MRI image (center) and no uptake of 18F-FXIII/MPO-Gd observed in control mice. (B) Uptake of 18F-FMISO for hypoxia imaging in atherosclerotic rabbits with 8 months (induction) and 16 months of atherogenic diet (progression) by PET/CT imaging, demonstrating higher signal compared to control rabbits. The green line is related to axial views captured in bottom of the image and green arrows are showing abdominal aorta. Comparisons between FDG and FMISO uptake specificity by PET/CT imaging were made, indicated by green arrows. (C) In vivo SPECT/CT imaging in the aorta (arrows) of WHHL rabbits injected with 111In-PS100 and 111In-PS200. Images modified with permission [47-49].
Hypoxia imaging was reported in an advanced atherosclerosis rabbit model since this pathogenic process triggers inflammation and angiogenesis, two critical factors for the disease [48]. This study made comparisons between PET imaging of atherosclerosis using 18F-FMISO and 18F-FDG by evaluating radiotracer uptake during disease progression until advanced lesions were obtained (fig. 4B). 18F-FMISO showed higher signal (SUV mean of 0.20 ± 0.03 vs 0.10 ± 0.01 in healthy animals) with uptake increasing over the time of atherogenic diet. 18F-FMISO and 18F-FDG signals in the abdominal aorta were poorly correlated, suggesting the uptake of these two radiotracers does not share the same biological process.
Apoptotic cells present in plaques are recognized and phagocytized by macrophages through the surface presentation of phosphatidylserine (PS). 111In radiolabeled liposome nanoparticles ranging from 100-200 nm were used as carriers of PS in a study designed to image the presence of vulnerable plaques [49]. Uptake of 111In-PS200 was greater than that of 111In-PS100 in vivo (fig. 4C), however, both particles showed high liver accumulation. The authors suggested that PEGylation of the liposomes may increase blood retention and decrease uptake by the liver.
The expression of MMPs has also been studied to evaluate rupture risk because their intense activity can destabilize atherosclerotic plaques. Membrane type 1 matrix metalloproteinase (MT1-MMP) was evaluated as a target for advanced stages of atherosclerosis by nuclear imaging using anti-MT1-MMP monoclonal antibody radiolabeled with 99mTc [15]. 99mTc-MT1-MMP mAb had 3.9-6.6 fold higher uptake in WHHL-MI atherosclerotic rabbits as compared to control models. MT1-MMP expression levels correlated closely with tracer uptake. Also, according to atherosclerotic lesion stage classification by histological analysis, 99mTc-MT1-MMP showed higher uptake in atheromatous lesion (stage IV), which presents vulnerable lesion characteristics such as a large lipid necrotic core and many macrophage-derived foam cells.
A wide range of possible targets and imaging agents were studied but in preclinical settings the challenge remains finding appropriate animal models of plaque rupture. This is still a matter of much discussion among researchers in the field, considering the general animal utilized in experiments does not have propensity to events like myocardial infarction found in humans.
4. Multimodality probes for atherosclerosis imaging
Multimodality imaging is a fast-growing area because these imaging platforms can provide both functional and anatomic information to better characterize the progression and activity of plaque. Of the various molecules developed for multimodality imaging of plaque, nanoparticles have directed the advancement in this field, thus boosting the development of new hybrid systems for clinical use. Nanoparticles targeting monocytes and macrophages can characterize the degree of plaque inflammation. Trireporter nanoparticle (TNP) derivatized to DTPA for 64Cu radiolabeling and to a dextran-coated VT680 infrared fluorochrome for macrophage targeting was evaluated [50]. Biodistribution indicated higher uptake in aortas (260%) and carotid arteries (392%) in apoE-/- mice compared to wild type animals. Uptake of 64Cu-TNP was observed by PET/CT and MRI images (fig. 5A) and colocalization was demonstrated by autoradiography and Oil Red-O staining of excised aortas. Tissues from the aortic arch, root and carotid arteries were strongly positive by ex vivo fluorescence imaging. In another application, 13 nm dextran nanoparticles (DNP) were labeled with 89Zr and Vivotag-680 (VT680) fluorochrome to investigate atherosclerotic apoE-/- mice using PET imaging and flow cytometry, respectively [51]. In these mice, higher PET signal was found in the aortic arch which correlated to excised aortas in autoradiography (fig. 5B). Additionally, the radiotracer was able to monitor treatment with siRNA-CCR2 which was characterized by decreased PET signal and inflammatory gene expression.
Figure 5.
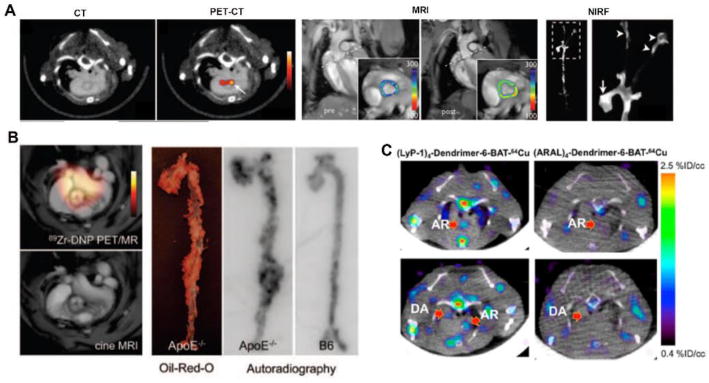
Studies involving multimodal imaging agents and new hybrid systems. (A) PET/CT imaging with 64Cu-TNP with uptake in aortic arch demonstrated by arrow, MRI images showing signal in the aortic root pre and post-injection, respectively and versatility of the tracer showed by ex vivo near-infrared fluorescence reflectance imaging (NIRF) of the aorta with signal observed in the root (arrow) and carotid bifurcation (arrowhead). (B) Uptake of 89Zr-DNP in atherosclerotic plaque of aortic root from apoE-/- mice. Hybrid PET/MRI (top) and MRI (bottom) images demonstrating the signal from radiotracer and Oil-Red O staining and autoradiography of aorta correlating uptake in PET/MRI imaging. (C) PET/CT images in axial views demonstrating uptake of (LyP-1)4-dendrimer-6-BAT-64Cu and (ARAL)4-dendrimer-6-BAT-64Cu in the aortic root (AR) and descending aorta (DA). Modified with permission [50-52].
LyP-1, a cyclic peptide that binds to overexpressed p32 protein on the surface of macrophages, was radiolabeled with 64Cu to evaluate its potential for atherosclerotic molecular imaging and labeled with fluorescent probes (FAM or 6-BAT) for optical imaging [52]. This study compared the uptake of (LyP-1)4-dendrimer-64Cu with control (ARAL)4-dendrimer. The dynamics of the tracer demonstrated higher uptake and accumulation in the aortic arch of apoE-/- mice by PET/CT (fig. 5C), biodistribution analysis and fluorescence imaging of the aortic atherosclerotic plaques with (LyP-1)4-dendrimer-FAM.
Nevertheless, concerns about nanoparticle clearance properties are great because of their potential toxicity. Efforts to improve this characteristic, such as using renal clearable nanostructures, have been undertaken [53]. Currently, PET/CT and SPECT/CT configurations have been commonly used for preclinical setting and new hybrid systems are currently in evaluation for assembly. Furthermore, effort has been devoted to study the data from PET, SPECT, fluorescence and MRI images by utilizing landmarks in the aorta regions in order to find correlations between these modalities, taking in to consideration the benefits offered by each [54].
5. Conclusions and prospective
Despite the immense volume of papers published in the area of atherosclerosis imaging, there are still many challenges and issues to be clarified due to the complexity of the disease through formation, evolution and rupture of the plaque. Of the various imaging platforms, small molecules and peptides have proven to be valuable tools for atherosclerosis detection due to their specificity and high binding affinity. However, fast in vivo pharmacokinetics and clearance may limit their capability to deliver high target-to-background contrast ratio. Despite the high binding specificity to targets expressed on the plaque, antibody-based tracers also face the challenge of generating high contrast ratio for accurate and sensitive detection of atherosclerotic lesions due to their extended blood circulation. Interestingly, engineered antibody fragments may have the potential for improved imaging owing to their optimized pharmacokinetics. Nanoparticles, due to their improved binding affinity and tunable pharmacokinetics, have shown great potential for plaque detection. However, in vivo clearance and toxicity need further confirmation.
Owing to the longitudinal nature of the inflammation process numerous atherosclerosis biomarkers have been identified and imaged. However, the connection between the expression of these targets and progression or severity of disease warrants further investigation, which will significantly affect the development of prognostic imaging agents to detect plaque activity and prone-to-rupture risk.
Taken together, radionuclide-based imaging agents have been widely used for pre-clinical research and show promise. However, there is a long way to go to develop prognostic agents to sensitively and specifically detect atherosclerotic plaque, track the progression and predict the rupture.
Acknowledgments
This work is supported by 1R01HL125655-01 from NHLBI. We would like to thank FAPESP (Fundacao de Amparo a Pesquisa do Estado de Sao Paulo) for the scholarship support of SMK. The manuscript is published on Current Pharmaceutical Design and the published manuscript is available at EurekaSelect via http://www.eurekaselect.com/openurl/content.php?genre=article&doi=[DOI: 10.2174/1381612821666150915104529].
References
- 1.Alwan A. Global status report on noncommunicable diseases 2010. World Health Organization; Geneva: 2010. p. 176. [Google Scholar]
- 2.Steinbrecher UP, Fisher M, Witztum JL, Curtiss LK. Immunogenicity of homologous low density lipoprotein after methylation, ethylation, acetylation, or carbamylation: generation of antibodies specific for derivatized lysine. J Lipid Res. 1984;25:1109–16. [PubMed] [Google Scholar]
- 3.Libby P. The Vascular Biology of Atherosclerosis. In: Mann DL, Zipes DP, Libby P, Bonow RO, Braunwald E, editors. Braunwald's heart disease : a textbook of cardiovascular medicine. Saunders; Philadelphia: 2012. pp. 995–1009. [Google Scholar]
- 4.Bentzon JF, Otsuka F, Virmani R, Falk E. Mechanisms of plaque formation and rupture. Circ Res. 2014;114:1852–66. doi: 10.1161/CIRCRESAHA.114.302721. [DOI] [PubMed] [Google Scholar]
- 5.Libby P, DiCarli M, Weissleder R. The vascular biology of atherosclerosis and imaging targets. J Nucl Med. 2010;51(Suppl 1):33S–37S. doi: 10.2967/jnumed.109.069633. [DOI] [PubMed] [Google Scholar]
- 6.Libby P, Lichtman AH, Hansson GK. Immune effector mechanisms implicated in atherosclerosis: from mice to humans. Immunity. 2013;38:1092–104. doi: 10.1016/j.immuni.2013.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lundberg AM, Hansson GK. Innate immune signals in atherosclerosis. Clin Immunol. 2010;134:5–24. doi: 10.1016/j.clim.2009.07.016. [DOI] [PubMed] [Google Scholar]
- 8.Poon IK, Lucas CD, Rossi AG, Ravichandran KS. Apoptotic cell clearance: basic biology and therapeutic potential. Nat Rev Immunol. 2014;14:166–80. doi: 10.1038/nri3607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Marsch E, Sluimer JC, Daemen MJ. Hypoxia in atherosclerosis and inflammation. Curr Opin Lipidol. 2013;24:393–400. doi: 10.1097/MOL.0b013e32836484a4. [DOI] [PubMed] [Google Scholar]
- 10.Marsch E, Theelen TL, Demandt JA, Jeurissen M, van Gink M, Verjans R, Janssen A, Cleutjens JP, Meex SJ, Donners MM, Haenen GR, Schalkwijk CG, Dubois LJ, Lambin P, Mallat Z, Gijbels MJ, Heemskerk JW, Fisher EA, Biessen EA, Janssen BJ, Daemen MJ, Sluimer JC. Reversal of hypoxia in murine atherosclerosis prevents necrotic core expansion by enhancing efferocytosis. Arterioscler Thromb Vasc Biol. 2014;34:2545–53. doi: 10.1161/ATVBAHA.114.304023. [DOI] [PubMed] [Google Scholar]
- 11.Andersson J, Libby P, Hansson GK. Adaptive immunity and atherosclerosis. Clin Immunol. 2010;134:33–46. doi: 10.1016/j.clim.2009.07.002. [DOI] [PubMed] [Google Scholar]
- 12.Tousoulis D, Psarros C, Demosthenous M, Patel R, Antoniades C, Stefanadis C. Innate and adaptive inflammation as a therapeutic target in vascular disease: the emerging role of statins. J Am Coll Cardiol. 2014;63:2491–502. doi: 10.1016/j.jacc.2014.01.054. [DOI] [PubMed] [Google Scholar]
- 13.Li D, Patel AR, Klibanov AL, Kramer CM, Ruiz M, Kang BY, Mehta JL, Beller GA, Glover DK, Meyer CH. Molecular imaging of atherosclerotic plaques targeted to oxidized LDL receptor LOX-1 by SPECT/CT and magnetic resonance. Circ Cardiovasc Imaging. 2010;3:464–72. doi: 10.1161/CIRCIMAGING.109.896654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Tsimikas S, Shortal BP, Witztum JL, Palinski W. In vivo uptake of radiolabeled MDA2, an oxidation-specific monoclonal antibody, provides an accurate measure of atherosclerotic lesions rich in oxidized LDL and is highly sensitive to their regression. Arterioscler Thromb Vasc Biol. 2000;20:689–97. doi: 10.1161/01.atv.20.3.689. [DOI] [PubMed] [Google Scholar]
- 15.Kuge Y, Takai N, Ogawa Y, Temma T, Zhao Y, Nishigori K, Ishino S, Kamihashi J, Kiyono Y, Shiomi M, Saji H. Imaging with radiolabelled anti-membrane type 1 matrix metalloproteinase (MT1-MMP) antibody: potentials for characterizing atherosclerotic plaques. Eur J Nucl Med Mol Imaging. 2010;37:2093–104. doi: 10.1007/s00259-010-1521-2. [DOI] [PubMed] [Google Scholar]
- 16.Quillard T, Libby P. Molecular imaging of atherosclerosis for improving diagnostic and therapeutic development. Circ Res. 2012;111:231–44. doi: 10.1161/CIRCRESAHA.112.268144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Stefanadis C, Vavuranakis M, Toutouzas P. Vulnerable plaque: the challenge to identify and treat it. J Interv Cardiol. 2003;16:273–80. doi: 10.1034/j.1600-0854.2003.8043.x. [DOI] [PubMed] [Google Scholar]
- 18.Wildgruber M, Swirski FK, Zernecke A. Molecular imaging of inflammation in atherosclerosis. Theranostics. 2013;3:865–84. doi: 10.7150/thno.5771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Rosa GM, Bauckneht M, Masoero G, Mach F, Quercioli A, Seitun S, Balbi M, Brunelli C, Parodi A, Nencioni A, Vuilleumier N, Montecucco F. The vulnerable coronary plaque: update on imaging technologies. Thromb Haemost. 2013;110:706–22. doi: 10.1160/TH13-02-0121. [DOI] [PubMed] [Google Scholar]
- 20.Bala G, Cosyns B. Recent advances in visualizing vulnerable plaque: focus on noninvasive molecular imaging. Curr Cardiol Rep. 2014;16:520. doi: 10.1007/s11886-014-0520-5. [DOI] [PubMed] [Google Scholar]
- 21.Chen IY, Wu JC. Cardiovascular molecular imaging: focus on clinical translation. Circulation. 2011;123:425–43. doi: 10.1161/CIRCULATIONAHA.109.916338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Glagov S, Weisenberg E, Zarins CK, Stankunavicius R, Kolettis GJ. Compensatory Enlargement of Human Atherosclerotic Coronary Arteries. New Engl J Med. 1987;316:1371–1375. doi: 10.1056/NEJM198705283162204. [DOI] [PubMed] [Google Scholar]
- 23.Wickline SA, Lanza GM. Molecular imaging, targeted therapeutics, and nanoscience. J Cell Biochem Suppl. 2002;39:90–7. doi: 10.1002/jcb.10422. [DOI] [PubMed] [Google Scholar]
- 24.Liu Y, Welch MJ. Molecular imaging of small animals : instrumentation and applications. Spring New York; New York: 2014. Advances in Radiotracer Development for Molecular Imaging; pp. 275–318. [Google Scholar]
- 25.Saha GB. Fundamentals of nuclear pharmacy. Springer; New York: 2010. [Google Scholar]
- 26.Velikyan I, Beyer GJ, Bergstrom-Pettermann E, Johansen P, Bergstrom M, Langstrom B. The importance of high specific radioactivity in the performance of 68Ga-labeled peptide. Nucl Med Biol. 2008;35:529–36. doi: 10.1016/j.nucmedbio.2008.03.002. [DOI] [PubMed] [Google Scholar]
- 27.Rowland DJ, Lewis JS, Welch MJ. Molecular imaging: the application of small animal positron emission tomography. J Cell Biochem Suppl. 2002;39:110–5. doi: 10.1002/jcb.10417. [DOI] [PubMed] [Google Scholar]
- 28.Uhl P, Fricker G, Haberkorn U, Mier W. Radionuclides in drug development. Drug Discov Today. 2014;20:198–208. doi: 10.1016/j.drudis.2014.09.027. [DOI] [PubMed] [Google Scholar]
- 29.Pimlott SL, Sutherland A. Molecular tracers for the PET and SPECT imaging of disease. Chem Soc Rev. 2011;40:149–62. doi: 10.1039/b922628c. [DOI] [PubMed] [Google Scholar]
- 30.Koba W, Jelicks LA, Fine EJ. MicroPET/SPECT/CT imaging of small animal models of disease. Am J Pathol. 2013;182:319–24. doi: 10.1016/j.ajpath.2012.09.025. [DOI] [PubMed] [Google Scholar]
- 31.Broisat A, Riou LM, Ardisson V, Boturyn D, Dumy P, Fagret D, Ghezzi C. Molecular imaging of vascular cell adhesion molecule-1 expression in experimental atherosclerotic plaques with radiolabelled B2702-p. Eur J Nucl Med Mol Imaging. 2007;34:830–40. doi: 10.1007/s00259-006-0310-4. [DOI] [PubMed] [Google Scholar]
- 32.Nahrendorf M, Keliher E, Panizzi P, Zhang H, Hembrador S, Figueiredo JL, Aikawa E, Kelly K, Libby P, Weissleder R. 18F-4V for PET-CT imaging of VCAM-1 expression in atherosclerosis. JACC Cardiovasc Imaging. 2009;2:1213–22. doi: 10.1016/j.jcmg.2009.04.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Broisat A, Hernot S, Toczek J, De Vos J, Riou LM, Martin S, Ahmadi M, Thielens N, Wernery U, Caveliers V, Muyldermans S, Lahoutte T, Fagret D, Ghezzi C, Devoogdt N. Nanobodies targeting mouse/human VCAM1 for the nuclear imaging of atherosclerotic lesions. Circ Res. 2012;110:927–37. doi: 10.1161/CIRCRESAHA.112.265140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hansson GK. Immune mechanisms in atherosclerosis. Arterioscler Thromb Vasc Biol. 2001;21:1876–90. doi: 10.1161/hq1201.100220. [DOI] [PubMed] [Google Scholar]
- 35.Rodriguez JM, Wolfrum S, Robblee M, Chen KY, Gilbert ZN, Choi JH, Teupser D, Breslow JL. Altered expression of Raet1e, a major histocompatibility complex class 1-like molecule, underlies the atherosclerosis modifier locus Ath11 10b. Circ Res. 2013;113:1054–64. doi: 10.1161/CIRCRESAHA.113.302052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kawachi E, Uehara Y, Hasegawa K, Yahiro E, Ando S, Wada Y, Yano T, Nishikawa H, Shiomi M, Miura S, Watanabe Y, Saku K. Novel molecular imaging of atherosclerosis with gallium-68-labeled apolipoprotein A-I mimetic peptide and positron emission tomography. Circ J. 2013;77:1482–9. doi: 10.1253/circj.cj-12-0736. [DOI] [PubMed] [Google Scholar]
- 37.Ayala-Lopez W, Xia W, Varghese B, Low PS. Imaging of atherosclerosis in apoliprotein e knockout mice: targeting of a folate-conjugated radiopharmaceutical to activated macrophages. J Nucl Med. 2010;51:768–74. doi: 10.2967/jnumed.109.071324. [DOI] [PubMed] [Google Scholar]
- 38.Luehmann HP, Pressly ED, Detering L, Wang C, Pierce R, Woodard PK, Gropler RJ, Hawker CJ, Liu Y. PET/CT imaging of chemokine receptor CCR5 in vascular injury model using targeted nanoparticle. J Nucl Med. 2014;55:629–34. doi: 10.2967/jnumed.113.132001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Nakamura I, Hasegawa K, Wada Y, Hirase T, Node K, Watanabe Y. Detection of early stage atherosclerotic plaques using PET and CT fusion imaging targeting P-selectin in low density lipoprotein receptor-deficient mice. Biochem Biophys Res Commun. 2013;433:47–51. doi: 10.1016/j.bbrc.2013.02.069. [DOI] [PubMed] [Google Scholar]
- 40.Tsimikas S, Palinski W, Halpern SE, Yeung DW, Curtiss LK, Witztum JL. Radiolabeled MDA2, an oxidation-specific, monoclonal antibody, identifies native atherosclerotic lesions in vivo. J Nucl Cardiol. 1999;6:41–53. doi: 10.1016/s1071-3581(99)90064-8. [DOI] [PubMed] [Google Scholar]
- 41.Ishino S, Mukai T, Kuge Y, Kume N, Ogawa M, Takai N, Kamihashi J, Shiomi M, Minami M, Kita T, Saji H. Targeting of lectinlike oxidized low-density lipoprotein receptor 1 (LOX-1) with 99mTc-labeled anti-LOX-1 antibody: potential agent for imaging of vulnerable plaque. J Nucl Med. 2008;49:1677–85. doi: 10.2967/jnumed.107.049536. [DOI] [PubMed] [Google Scholar]
- 42.Soto Y, Mesa N, Alfonso Y, Perez A, Batlle F, Grinan T, Pino A, Viera J, Frometa M, Brito V, Olivera A, Zayas F, Vazquez AM. Targeting arterial wall sulfated glycosaminoglycans in rabbit atherosclerosis with a mouse/human chimeric antibody. MAbs. 2014;6:1340–1346. doi: 10.4161/mabs.29970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Razavian M, Tavakoli S, Zhang J, Nie L, Dobrucki LW, Sinusas AJ, Azure M, Robinson S, Sadeghi MM. Atherosclerosis plaque heterogeneity and response to therapy detected by in vivo molecular imaging of matrix metalloproteinase activation. J Nucl Med. 2011;52:1795–802. doi: 10.2967/jnumed.111.092379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Isobe S, Tsimikas S, Zhou J, Fujimoto S, Sarai M, Branks MJ, Fujimoto A, Hofstra L, Reutelingsperger CP, Murohara T, Virmani R, Kolodgie FD, Narula N, Petrov A, Narula J. Noninvasive imaging of atherosclerotic lesions in apolipoprotein E-deficient and low-density-lipoprotein receptor-deficient mice with annexin A5. J Nucl Med. 2006;47:1497–505. [PubMed] [Google Scholar]
- 45.Elmaleh DR, Fischman AJ, Tawakol A, Zhu A, Shoup TM, Hoffmann U, Brownell AL, Zamecnik PC. Detection of inflamed atherosclerotic lesions with diadenosine-5′,5‴-P1,P4-tetraphosphate (Ap4A) and positron-emission tomography. Proc Natl Acad Sci U S A. 2006;103:15992–6. doi: 10.1073/pnas.0607246103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Bigalke B, Phinikaridou A, Andia ME, Cooper MS, Schuster A, Wurster T, Onthank D, Munch G, Blower P, Gawaz M, Nagel E, Botnar RM. PET/CT and MR imaging biomarker of lipid-rich plaques using [(64)Cu]-labeled scavenger receptor (CD68-Fc) Int J Cardiol. 2014;177:287–91. doi: 10.1016/j.ijcard.2014.09.017. [DOI] [PubMed] [Google Scholar]
- 47.Majmudar MD, Keliher EJ, Heidt T, Leuschner F, Truelove J, Sena BF, Gorbatov R, Iwamoto Y, Dutta P, Wojtkiewicz G, Courties G, Sebas M, Borodovsky A, Fitzgerald K, Nolte MW, Dickneite G, Chen JW, Anderson DG, Swirski FK, Weissleder R, Nahrendorf M. Monocyte-directed RNAi targeting CCR2 improves infarct healing in atherosclerosis-prone mice. Circulation. 2013;127:2038–46. doi: 10.1161/CIRCULATIONAHA.112.000116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Mateo J, Izquierdo-Garcia D, Badimon JJ, Fayad ZA, Fuster V. Noninvasive assessment of hypoxia in rabbit advanced atherosclerosis using (1)(8)F-fluoromisonidazole positron emission tomographic imaging. Circ Cardiovasc Imaging. 2014;7:312–20. doi: 10.1161/CIRCIMAGING.113.001084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Ogawa M, Umeda IO, Kosugi M, Kawai A, Hamaya Y, Takashima M, Yin H, Kudoh T, Seno M, Magata Y. Development of 111In-labeled liposomes for vulnerable atherosclerotic plaque imaging. J Nucl Med. 2014;55:115–20. doi: 10.2967/jnumed.113.123158. [DOI] [PubMed] [Google Scholar]
- 50.Nahrendorf M, Zhang H, Hembrador S, Panizzi P, Sosnovik DE, Aikawa E, Libby P, Swirski FK, Weissleder R. Nanoparticle PET-CT imaging of macrophages in inflammatory atherosclerosis. Circulation. 2008;117:379–87. doi: 10.1161/CIRCULATIONAHA.107.741181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Majmudar MD, Yoo J, Keliher EJ, Truelove JJ, Iwamoto Y, Sena B, Dutta P, Borodovsky A, Fitzgerald K, Di Carli MF, Libby P, Anderson DG, Swirski FK, Weissleder R, Nahrendorf M. Polymeric nanoparticle PET/MR imaging allows macrophage detection in atherosclerotic plaques. Circ Res. 2013;112:755–61. doi: 10.1161/CIRCRESAHA.111.300576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Seo JW, Baek H, Mahakian LM, Kusunose J, Hamzah J, Ruoslahti E, Ferrara KW. (64)Cu-labeled LyP-1-dendrimer for PET-CT imaging of atherosclerotic plaque. Bioconjug Chem. 2014;25:231–9. doi: 10.1021/bc400347s. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Zhao Y, Sultan D, Detering L, Luehmann H, Liu Y. Facile synthesis, pharmacokinetic and systemic clearance evaluation, and positron emission tomography cancer imaging of Cu-Au alloy nanoclusters. Nanoscale. 2014;6:13501–9. doi: 10.1039/c4nr04569f. [DOI] [PubMed] [Google Scholar]
- 54.Nahrendorf M, Sosnovik DE, French BA, Swirski FK, Bengel F, Sadeghi MM, Lindner JR, Wu JC, Kraitchman DL, Fayad ZA, Sinusas AJ. Multimodality cardiovascular molecular imaging, Part II. Circ Cardiovasc Imaging. 2009;2:56–70. doi: 10.1161/CIRCIMAGING.108.839092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Teresa Albelda M, Garcia-Espana E, Frias JC. Visualizing the atherosclerotic plaque: a chemical perspective. Chem Soc Rev. 2014;43:2858–76. doi: 10.1039/c3cs60410a. [DOI] [PubMed] [Google Scholar]
- 56.Tardif JC, Lesage F, Harel F, Romeo P, Pressacco J. Imaging biomarkers in atherosclerosis trials. Circ Cardiovasc Imaging. 2011;4:319–33. doi: 10.1161/CIRCIMAGING.110.962001. [DOI] [PubMed] [Google Scholar]
- 57.Qaim SM. Nuclear data relevant to the production and application of diagnostic radionuclide. Radiochimica Acta. 2001;89:223–32. [Google Scholar]
- 58.Rob TL, Simon M. PET Chemistry: An Introduction Basic sciences of nuclear medicine. Springer; Heidelberg: 2011. pp. 65–101. [Google Scholar]
- 59.Correia JD, Paulo A, Raposinho PD, Santos I. Radiometallated peptides for molecular imaging and targeted therapy. Dalton Trans. 2011;40:6144–67. doi: 10.1039/c0dt01599g. [DOI] [PubMed] [Google Scholar]
- 60.Cherry SR, Sorenson JA, Phelps ME. Physics in Nuclear Medicine. Elsevier/Saunders; Philadelphia: 2012. [Google Scholar]
- 61.Laitinen I, Marjamaki P, Nagren K, Laine VJ, Wilson I, Leppanen P, Yla-Herttuala S, Roivainen A, Knuuti J. Uptake of inflammatory cell marker [11C]PK11195 into mouse atherosclerotic plaques. Eur J Nucl Med Mol Imaging. 2009;36:73–80. doi: 10.1007/s00259-008-0919-6. [DOI] [PubMed] [Google Scholar]
- 62.Laitinen IE, Luoto P, Nagren K, Marjamaki PM, Silvola JM, Hellberg S, Laine VJ, Yla-Herttuala S, Knuuti J, Roivainen A. Uptake of 11C-choline in mouse atherosclerotic plaques. J Nucl Med. 2010;51:798–802. doi: 10.2967/jnumed.109.071704. [DOI] [PubMed] [Google Scholar]
- 63.Ogawa M, Ishino S, Mukai T, Asano D, Teramoto N, Watabe H, Kudomi N, Shiomi M, Magata Y, Iida H, Saji H. (18)F-FDG accumulation in atherosclerotic plaques: immunohistochemical and PET imaging study. J Nucl Med. 2004;45:1245–50. [PubMed] [Google Scholar]
- 64.Saraste A, Laitinen I, Weidl E, Wildgruber M, Weber AW, Nekolla SG, Holzlwimmer G, Esposito I, Walch A, Leppanen P, Lisinen I, Luppa PB, Yla-Herttuala S, Wester HJ, Knuuti J, Schwaiger M. Diet intervention reduces uptake of alphavbeta3 integrin-targeted PET tracer 18F-galacto-RGD in mouse atherosclerotic plaques. J Nucl Cardiol. 2012;19:775–84. doi: 10.1007/s12350-012-9554-5. [DOI] [PubMed] [Google Scholar]
- 65.Silvola JM, Saraste A, Forsback S, Laine VJ, Saukko P, Heinonen SE, Yla-Herttuala S, Roivainen A, Knuuti J. Detection of hypoxia by [18F]EF5 in atherosclerotic plaques in mice. Arterioscler Thromb Vasc Biol. 2011;31:1011–5. doi: 10.1161/ATVBAHA.110.221440. [DOI] [PubMed] [Google Scholar]
- 66.Laitinen I, Saraste A, Weidl E, Poethko T, Weber AW, Nekolla SG, Leppanen P, Yla-Herttuala S, Holzlwimmer G, Walch A, Esposito I, Wester HJ, Knuuti J, Schwaiger M. Evaluation of alphavbeta3 integrin-targeted positron emission tomography tracer 18F-galacto-RGD for imaging of vascular inflammation in atherosclerotic mice. Circ Cardiovasc Imaging. 2009;2:331–8. doi: 10.1161/CIRCIMAGING.108.846865. [DOI] [PubMed] [Google Scholar]
- 67.Liu Y, Abendschein D, Woodard GE, Rossin R, McCommis K, Zheng J, Welch MJ, Woodard PK. Molecular imaging of atherosclerotic plaque with (64)Cu-labeled natriuretic peptide and PET. J Nucl Med. 2010;51:85–91. doi: 10.2967/jnumed.109.066977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Liu Y, Pierce R, Luehmann HP, Sharp TL, Welch MJ. PET imaging of chemokine receptors in vascular injury-accelerated atherosclerosis. J Nucl Med. 2013;54:1135–41. doi: 10.2967/jnumed.112.114777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Johanna H, Iina L, Pauliina L, Peter I, Ian W, Hege K, Alan C, Jukka L, Pia L, Yla-Herttula S, Knuuti J, Roivainen A. 68Ga-DOTA-RGD peptide: biodistribution and binding into atherosclerotic plaques in mice. Eur J Nucl Med Mol Imaging. 2009;36:2058–67. doi: 10.1007/s00259-009-1220-z. [DOI] [PubMed] [Google Scholar]
- 70.Silvola JM, Laitinen I, Sipila HJ, Laine VJ, Leppanen P, Yla-Herttuala S, Knuuti J, Roivainen A. Uptake of 68gallium in atherosclerotic plaques in LDLR-/-ApoB100/100 mice. EJNMMI Res. 2011;1:14. doi: 10.1186/2191-219X-1-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Paeng JC, Lee YS, Lee JS, Jeong JM, Kim KB, Chung JK, Lee DS. Feasibility and kinetic characteristics of (68)Ga-NOTA-RGD PET for in vivo atherosclerosis imaging. Ann Nucl Med. 2013;27:847–54. doi: 10.1007/s12149-013-0757-x. [DOI] [PubMed] [Google Scholar]
- 72.Doran MG, Watson PA, Cheal SM, Spratt DE, Wongvipat J, Steckler JM, Carrasquillo JA, Evans MJ, Lewis JS. Annotating STEAP1 regulation in prostate cancer with 89Zr immuno-PET. J Nucl Med. 2014;55:2045–9. doi: 10.2967/jnumed.114.145185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Marquez BV, Zheleznyak A, Lapi SE. Glypican-3-targeted 89Zr PET imaging of hepatocellular carcinoma: where antibody imaging dares to tread. J Nucl Med. 2014;55:708–9. doi: 10.2967/jnumed.113.136234. [DOI] [PubMed] [Google Scholar]
- 74.Sham JG, Kievit FM, Grierson JR, Chiarelli PA, Miyaoka RS, Zhang M, Yeung RS, Minoshima S, Park JO. Glypican-3-targeting F(ab')2 for 89Zr PET of hepatocellular carcinoma. J Nucl Med. 2014;55:2032–7. doi: 10.2967/jnumed.114.145102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Sugyo A, Tsuji AB, Sudo H, Nagatsu K, Koizumi M, Ukai Y, Kurosawa G, Zhang MR, Kurosawa Y, Saga T. Preclinical evaluation of (8)(9)Zr-labeled human antitransferrin receptor monoclonal antibody as a PET probe using a pancreatic cancer mouse model. Nucl Med Commun. 2015;36:286–94. doi: 10.1097/MNM.0000000000000245. [DOI] [PubMed] [Google Scholar]
- 76.Knowles SM, Tavare R, Zettlitz KA, Rochefort MM, Salazar FB, Jiang ZK, Reiter RE, Wu AM. Applications of immunoPET: using 124I-anti-PSCA A11 minibody for imaging disease progression and response to therapy in mouse xenograft models of prostate cancer. Clin Cancer Res. 2014;20:6367–78. doi: 10.1158/1078-0432.CCR-14-1452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Chacko AM, Li C, Nayak M, Mikitsh JL, Hu J, Hou C, Grasso L, Nicolaides NC, Muzykantov VR, Divgi CR, Coukos G. Development of 124I immuno-PET targeting tumor vascular TEM1/endosialin. J Nucl Med. 2014;55:500–7. doi: 10.2967/jnumed.113.121905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Su YC, Cheng TC, Leu YL, Roffler SR, Wang JY, Chuang CH, Kao CH, Chen KC, Wang HE, Cheng TL. PET imaging of beta-glucuronidase activity by an activity-based 124I-trapping probe for the personalized glucuronide prodrug targeted therapy. Mol Cancer Ther. 2014;13:2852–63. doi: 10.1158/1535-7163.MCT-14-0212. [DOI] [PubMed] [Google Scholar]
- 79.Holzwarth U. Radiopharmaceutical Production. In: Cantone MC, Hoeschen C, editors. Radiation physics for nuclear medicine. Springer; Berlin; London: 2011. p. 285. [Google Scholar]
- 80.Schafers M, Riemann B, Kopka K, Breyholz HJ, Wagner S, Schafers KP, Law MP, Schober O, Levkau B. Scintigraphic imaging of matrix metalloproteinase activity in the arterial wall in vivo. Circulation. 2004;109:2554–9. doi: 10.1161/01.CIR.0000129088.49276.83. [DOI] [PubMed] [Google Scholar]
- 81.Wu MC, Ho HI, Lee TW, Wu HL, Lo JM. In vivo examination of 111In-bis-5HT-DTPA to target myeloperoxidase in atherosclerotic ApoE knockout mice. J Drug Target. 2012;20:605–14. doi: 10.3109/1061186X.2012.702768. [DOI] [PubMed] [Google Scholar]
- 82.Starmans LW, van Duijnhoven SM, Rossin R, Berben M, Aime S, Daemen MJ, Nicolay K, Grull H. Evaluation of 111In-labeled EPep and FibPep as tracers for fibrin SPECT imaging. Mol Pharm. 2013;10:4309–21. doi: 10.1021/mp400406x. [DOI] [PubMed] [Google Scholar]
- 83.Zhang J, Nie L, Razavian M, Ahmed M, Dobrucki LW, Asadi A, Edwards DS, Azure M, Sinusas AJ, Sadeghi MM. Molecular imaging of activated matrix metalloproteinases in vascular remodeling. Circulation. 2008;118:1953–60. doi: 10.1161/CIRCULATIONAHA.108.789743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Kolodgie FD, Petrov A, Virmani R, Narula N, Verjans JW, Weber DK, Hartung D, Steinmetz N, Vanderheyden JL, Vannan MA, Gold HK, Reutelingsperger CP, Hofstra L, Narula J. Targeting of apoptotic macrophages and experimental atheroma with radiolabeled annexin V: a technique with potential for noninvasive imaging of vulnerable plaque. Circulation. 2003;108:3134–9. doi: 10.1161/01.CIR.0000105761.00573.50. [DOI] [PubMed] [Google Scholar]
- 85.Dimastromatteo J, Broisat A, Perret P, Ahmadi M, Boturyn D, Dumy P, Fagret D, Riou LM, Ghezzi C. In vivo molecular imaging of atherosclerotic lesions in ApoE-/- mice using VCAM-1-specific, 99mTc-labeled peptidic sequences. J Nucl Med. 2013;54:1442–9. doi: 10.2967/jnumed.112.115675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Broisat A, Toczek J, Dumas LS, Ahmadi M, Bacot S, Perret P, Slimani L, Barone-Rochette G, Soubies A, Devoogdt N, Lahoutte T, Fagret D, Riou LM, Ghezzi C. 99mTc-cAbVCAM1-5 Imaging Is a Sensitive and Reproducible Tool for the Detection of Inflamed Atherosclerotic Lesions in Mice. J Nucl Med. 2014:1678–1684. doi: 10.2967/jnumed.114.143792. [DOI] [PubMed] [Google Scholar]
- 87.Ohshima S, Petrov A, Fujimoto S, Zhou J, Azure M, Edwards DS, Murohara T, Narula N, Tsimikas S, Narula J. Molecular imaging of matrix metalloproteinase expression in atherosclerotic plaques of mice deficient in apolipoprotein e or low-density-lipoprotein receptor. J Nucl Med. 2009;50:612–7. doi: 10.2967/jnumed.108.055889. [DOI] [PubMed] [Google Scholar]


