Abstract
Immunology offers an unprecedented opportunity for the science-driven development of therapeutics. The successes of antibodies to the immunomodulatory receptor CTLA-4 and blockade of the immunoinhibitory receptor PD-1 in cancer immunotherapy, from gene discovery to patient benefit, have created a paradigm for driving such endeavors.
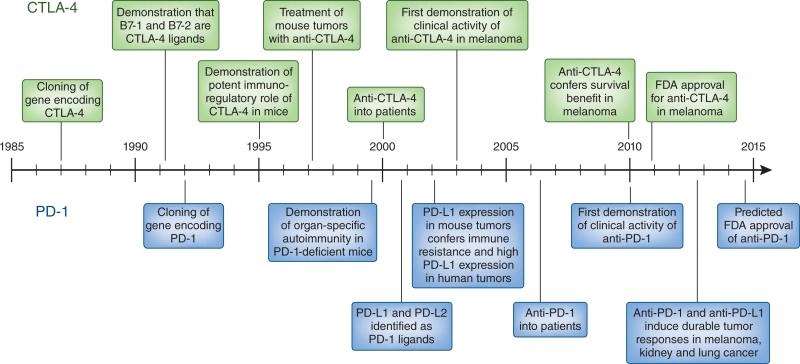
One of the most important turning points in US and Canadian medical education came with the 1906 Flexner report1, which proposed that medical schools should incorporate the study of the natural sciences into the teaching and practice of medicine, according to the paradigm of the then recently founded Johns Hopkins School of Medicine. Over time, this educational transformation, linking physiology and clinical medicine, became the forerunner of what is now called ‘translational medicine’, whereby advances come not only from clinical observation and empiric clinical testing but also directly from scientific discoveries in the laboratory. Two immunotherapies, one based on blockade of the immunomodulatory receptor CTLA-4 and the other based on blockade of the immunoinhibitory receptor PD-1 and its ligand PD-L1, have created a revolution in clinical oncology and are being embraced by clinicians who may have forgotten the difference between a CD4+ T cell and a CD8+ T cell2–5. This Historical Commentary recounts the journey for each of these agents, from gene discovery to the establishment of substantial clinical benefit in subsets of patients with cancer. Both the milestones of advance and the timelines—roughly 25 years from gene discovery to definitive establishment of clinical benefit—are remarkably similar for both, offset by only about 5 years (Fig. 1). Once the respective genes encoding these molecules were discovered, the clinical translation of each agent required six key sequential milestones: discovery of the ligands and linkage between ligand(s) and receptor; definition of biological function through the use of antibodies and mouse genetics; key preclinical experiments in mouse models demonstrating the effects of antibodies to the target receptor or ligand; development of clinical antibodies; initial clinical evidence of efficacy; and, finally, definitive demonstration of clinical efficacy. In the movies, all these milestones are achieved, often in a period of days, by one or two heroes (for example, the characters played by Cuba Gooding and Dustin Hoffman in the movie Outbreak). In real life, key contributions are made by many people, both sung and unsung, which makes these translational successes a triumph to be ‘owned’ by the entire field.
Figure 1.
Parallel timelines and milestones of the development of therapies involving anti-CTLA-4 and anti-PD-1, from gene discovery through clinical development. The timelines for almost all major scientific and clinical advances are strikingly similar, offset only by about 5 years.
Blockade of CTLA-4 in melanoma
In 2010, an antibody to CTLA-4 (anti-CTLA-4) became the first agent in history shown to provide a survival benefit for patients with melanoma in a randomized phase 3 trial4, which led to approval by the US Food and Drug Administration (FDA) in 2011. The gene encoding CTLA-4 was first cloned 23 years earlier from a cDNA library derived from a cytotoxic T lymphocyte (CTL) clone (its name refers to ‘CTL antigen number 4’)6. As is true for many such designations, its name does not reflect its function; to this day, no role for CTLA-4 in CTL function has been demonstrated (although it has been reported to modulate the priming of CD8+ T cells). CTLA-4 soon became interesting to immunologists because of its homology to CD28, then considered the main costimulatory receptor on T cells. Peter Linsley and Jeffrey Ledbetter and their colleagues, who had discovered that B7-1 is a major ligand of CD28 (ref. 7), showed that this same molecule is the ligand for CTLA-4 and in fact has a much higher affinity for CTLA-4 than for CD28 (ref. 8).
Linsley's finding that anti-CTLA-4 enhances the costimulation of T cells when used together with agonist anti-CD28 was initially interpreted as evidence that CTLA-4 is a second costimulatory receptor on T cells9. Conversely, on the basis of analyses of the effects of Fab fragments of anti-CTLA-4 and crosslinking of CTLA-4 in the presence or absence of B7 ligands, Jeff Bluestone and colleagues and Jim Allison and colleagues suggested that CTLA-4 might be an inhibitory receptor10,11. It is difficult to make definitive conclusions about the function of a receptor from in vitro experiments with antibodies, as an antagonist antibody to an inhibitory receptor can enhance the activation of T cells similarly to an agonist antibody to a costimulatory receptor. When neither the receptor's function nor the agonist-versus-antagonist nature of an antibody is known, a ‘catch-22’ predicament ensues. Ultimately, the controversy was settled by genetics. In 1995, Arlene Sharpe and colleagues and Tak Mak and colleagues reported that CTLA-4-deficient mice develop a massive generalized lymphoproliferative syndrome characterized by lymphadenopathy and lymphocytic infiltration of all organs12,13. These studies definitively established CTLA-4 as an inhibitory receptor on T cells and brought into focus the concept of balancing the binding of costimulatory and coinhibitory receptors to the same ligand(s)—in this case, B7-1 and B7-2.
Translational research requires a leap that links basic science to a medically useful therapeutic composition such as an antibody and demonstration of utility. In the case of CTLA-4, this was accomplished by Allison and colleagues, who demonstrated in a series of reports, beginning in 1997, that anti-CTLA-4 induces the rejection of established tumors in mice14,15. Anti-CTLA-4 used alone treated only immunogenic mouse tumors, but combinations of anti-CTLA-4 with vaccines and regulatory T cell–depleting antibodies induced the rejection of aggressive, poorly immunogenic tumors15. Notably, antibody treatment did not cause overt systemic hyperimmunity similar to that found in CTLA-4-deficient mice, which suggested that ‘partial’ blockade of CTLA-4 could create a therapeutic window for efficacy versus toxicity.
Although the seminal studies noted above provided the scientific basis for a new therapeutic strategy for the treatment of cancer, the creation of a real drug remained—a process that was not straightforward. Alan Korman, then at the small biotechnology company Nexstar Pharmaceuticals, began a collaboration with Allison to develop a biological agent that would block human CTLA-4 and have therapeutic activity in the clinic, analogous to the mouse studies. In 1998, Korman teamed up with Nils Lonberg at Medarex, another small biotechnology company. Lonberg and his colleagues at Medarex had developed a platform for creating fully human monoclonal antibodies in mice through replacement of the mouse immunoglobulin locus with partial human loci encoding the immunoglobulin heavy chains and light chains. In 2000, Korman and Lonberg made the unprecedented strategic leap of developing anticancer therapeutic antibodies that did not target tumors (as other biotechnology and pharmacological companies were doing) but instead targeted cells of the immune system to modulate endogenous antitumor immunity. In 2000, ipilumumab, an antibody to CTLA-4, was their first antibody to reach the clinic.
The pathway from the creation of a human antibody to FDA approval in 2011 took many twists and turns. Steve Hodi and Glenn Dranoff first published the finding that tumors in a handful of patients treated with ipilimumab became inflamed with lymphocytic infiltration and, in some cases, destruction of blood vessels with intratumoral hemorrhage16. Although that clinical finding intrigued cancer immunologists, oncologists paid little attention, because formal tumor regressions were not reported. Shortly thereafter, Steve Rosenberg and colleagues reported that in a larger cohort of patients with advanced melanoma, anti-CTLA-4 produced a 12% objective response rate according to standard oncology criteria (Response Evaluation Criteria in Solid Tumors)17. Although systemic interleukin 2 also induces tumor regressions in melanoma, many of the patients who responded to ipilumumab were those whose tumors had not responded to treatment with interleukin 2. Although the clinical responses grabbed the attention of the oncology community, so did the toxicities. As might have been predicted from the phenotype of the CTLA-4 deficient mice, anti-CTLA-4 produced substantial immune system–related toxicity in 25–30% of patients, which commonly involved the skin, liver or colon. Nevertheless, under the clinical direction of Michael Yellen and Israel Lowy, Medarex launched a large randomized clinical trial of patients with advanced melanoma, eventually partnering with Bristol-Myers Squibb for further development under the direction of Axel Hoos and Rachel Humphrey. In any medical field other than oncology, the toxicity of ipilimumab would have prevented its development beyond phase 1 trials. However, the risk/benefit ratio for patients with advanced cancer is different from that of patients with almost any other common disease, and oncologists are thus accustomed to learning how to manage and mitigate toxicities before discarding any drug with clinical antitumor activity. The immunological mechanism of action of ipilimumab was so different from that of any previous oncology drug that there was no precedent for the mitigation and management of toxicity. This in part sunk a competing antibody to CTLA-4 tested prematurely in a phase 3 trial by Pfizer. However, careful development of paradigms to manage toxicity and also to define new measures of patient benefit for an immunotherapy versus a cytotoxic chemotherapy, led by Jedd Wolchok, Steve Hodi and members of the Medarex–Bristol-Myers Squibb clinical teams, carried the day and resulted in two successful phase 3 trials4,18.
Many interesting clinical insights came from the 12 years of clinical experience with ipilimumab. First, the kinetics of clinical responses to anti-CTLA-4 (that is, tumor shrinkage) tend to be much slower than those of chemotherapy or tyrosine-kinase inhibitors, and in some patients, tumor regression is preceded by apparent progression, as assessed by computerized tomography or magnetic resonance imaging. Such findings suggest a mechanism of action compatible with the original report by Hodi and Dranoff showing that ultimate tumor regression is preceded by activation of the immune system and infiltration of the tumor by activated lymphocytes. Additionally, although formal regressions are induced in a relatively small proportion of patients and complete responses, as assessed by computerized tomography or magnetic resonance imaging, are rare (<5%), roughly 20% of patients treated with only four doses of ipilimumab remained alive over 4 years after therapy (in contrast to ~5% of control patients). Wolchok, Hoos and colleagues recognized that these response qualities were different from those of conventional cancer therapies and developed the concept of ‘immune-response criteria’ to help clinicians evaluate the efficacy of this new class of immunotherapy in their patients19. The durable responses in advanced melanoma suggest that the dream of cancer immunotherapy—an appropriately activated endogenous antitumor immune response that can have memory and keep cancers at bay long after the cessation of therapy—may not be so fanciful. With the approval for the use of ipilimumab to treat melanoma in hand, analysis of the use of ipilimumab to treat many other cancers is being aggressively pursued.
Blockade of PD-1: similar but different
Antibodies that block the PD-1 pathway have not yet been approved by the FDA; however, given the dramatic clinical results that have been reported2,3,5, one or more will undoubtedly be approved by the FDA by 2015. The gene encoding PD-1 was discovered in 1992 by Tsuku Honjo's group as encoding a T cell molecule potentially involved in programmed cell death20; thus, the time span from the discovery of this gene to the (predicted) FDA approval of a PD-1-blocking antibody is almost identical to that of CTLA-4. As with CTLA-4, the name PD-1 (‘programmed death 1’) does not reflect its main function, which is to inhibit the activation and effector function of T cells dependent on T cell antigen receptors (TCRs) through the recruitment of phosphatases to the immunological synapse and subsequent dephosphorylation of many proximal TCR-signaling molecules21. In striking contrast to the phenotype of CTLA-4-deficient mice, the alteration in the immunological phenotype of unimmunized PD-1-deficient mice is mild and does not manifest itself at all until 6–9 months after birth. To a student spending 2 or more years to make and characterize a knockout mouse, the absence of an overtly distinct phenotype is always a huge disappointment but, as it turns out, this relatively mildly deleterious phenotype reflects an advantage for the PD-1 pathway as a target for cancer immunotherapy. Fortunately for Hiroyuki Nishimura in the Honjo group, some PD-1-deficient mice were left to age a bit and eventually developed strain-specific and organ-specific autoimmune syndromes: PD-1-deficient mice on the C57BL/6 background developed a lupus-like syndrome involving the joints and kidneys, whereas PD-1-deficient mice on the BALB/c background developed myocarditis22,23. As with the CTLA-4-deficient mice, these findings established PD-1 as an immunoregulatory receptor, but a more subtle receptor with very different function. Together with subsequent studies by Sharpe and colleagues demonstrating more rapid development of organ-specific immunity in autoimmune-prone mice deficient in PD-1 ligands24, a picture began to emerge of a pathway that selectively regulates immune responses in tissues rather than globally regulating the activation of T cells, as does CTLA-4. The ligands for PD-1—B7-H1 (now called PD-L1) and B7-DC (now called PD-L2)—were discovered as a homolog of B7-1 by Lieping Chen and colleagues and as a dendritic cell–specific B7 family member by Drew Pardoll and colleagues25,26. Shortly thereafter, Gordon Freeman and colleagues discovered that these molecules are indeed the ligands for PD-1 and thus have major roles in the inhibition of T cells27,28. Even more so than PD-1-deficient mice, unimmunized mice deficient in PD-L1 or PD-L2 have extremely mildly deleterious phenotypes when not on an autoimmune-prone background or challenged by infection. However, blockade or deletion of these molecules enhances responses to immunization and can partially reverse chronic virus-induced exhaustion of T cells29.
Just as Allison published the key anti-CTLA-4 tumor-therapy experiments exactly 10 years after the gene encoding CTLA-4 was discovered, the critical connection between the PD-1 pathway and cancer immunotherapy came 10 years after discovery of the gene encoding PD-1. In 2002, Chen's group showed that mouse tumors with forced expression of PD-L1 become resistant to elimination by the immune system and, furthermore, that expression of the gene encoding PD-L1 is selectively upregulated in many human cancers30. Those dual findings motivated acceleration of the clinical development of human anti-PD-1 and anti-PD-L1 by Medarex through collaborations with investigators from Johns Hopkins and other academic cancer-research centers, and a phase 1 trial of anti-PD-1 led by Julie Brahmer, Charles Drake and Suzanne Topalian at Johns Hopkins University and Israel Lowy at Medarex was initiated in 2006. Despite the fact that most of the patients on this trial had end-stage disease, many dramatic clinical responses were observed in multiple cancer types, with clinically significant toxicity in only one patient2. That led immediately to larger clinical trials of both anti-PD-1 and anti-PD-L1 led by those same investigators, who were joined by Jon Wigginton, Ashok Gupta, David Feltquate and Fouad Namouni at Bristol-Myers Squibb (which purchased Medarex in 2010); this culminated in the demonstration of considerable clinical efficacy (in terms of objective response and disease stabilization of over 6 months) for anti-PD-1 not only in the advanced chemotherapy of refractory melanoma (~35%) but also in renal cancer (~50%) and lung cancer (~25%)3,5. The durability of these responses, even after cessation of treatment, far exceeds that of chemotherapy or tyrosine-kinase inhibitors. Such findings reinforce the notion that a ‘reeducated’ immune system not only has memory but also can adapt to maneuvers by the tumor to develop resistance. Just as predicted from mutations of the mouse genome, toxicity from therapy with anti-PD-1 or anti-PD-L1 is lower than that from therapy with anti-CTLA-4 and is also of a different distribution than that of anti-CTLA-4 (with colitis being most common with anti-CTLA-4 and pneumonitis being most common with anti-PD-1). Consistent with the important role of the PD-1 pathway in tissue protection under physiological conditions and in the resistance of tumors to the immune response in cancer, the expression of PD-L1 on tumor cells correlates with clinical response to anti-PD-1, which suggests that the assessment of PDL-1 expression on tumor biopsies could be a ‘biomarker’ potentially applicable to patient selection5. At least six different companies are now developing antibodies or recombinant molecules that target the PD-1 pathway.
Translation: beyond CTLA-4 and PD-1
The experience with anti-CTLA-4 and anti-PD-1 has demonstrated, for the first time, the efficacy of a ‘generic’ (that is, non-individualized) immunotherapy that targets a patient's endogenous immune system. However, when the total burden of morbidity and mortality from all cancers is considered, these antibodies as monotherapy only scratch the surface of the overall therapeutic potential. The potential to target multiple immunoregulatory molecules is great. There are ten to twenty additional confirmed coinhibitory ligands and receptors, as well as a similar number of costimulatory ligand-receptor pairs. These are not totally redundant, as genetic studies have demonstrated, and thus it makes sense to develop combination therapies that hit multiple immunological targets simultaneously. Furthermore, early studies of the tumor microenvironment have demonstrated considerable heterogeneity among human cancers in their expression of distinct immunoregulatory ligands and receptors, which suggests the proposal of biomarker-directed therapy determined by the analysis of individual tumor biopsies. Moreover, the advent of immunoregulatory antibodies will not make cancer vaccines obsolete; in contrast, this will probably rejuvenate the use of vaccines in the form of combinatorial strategies. Already combinations of cancer vaccines and inhibitors of regulatory pathways have shown tremendous synergy in preclinical models.
Now that a paradigm for immunotherapy translation has been outlined, how can the process be accelerated such that the period from gene discovery to broad clinical use is less than the 25 years it took for the blockade of CTLA-4 and PD-1? There have been many commentaries on the importance of changing corporate culture and FDA policies, but little has been written about how the scientific community can promote translation. That also needs a culture shift—one that could be facilitated by intelligent incentives from governmental funding agencies such as the US National Institutes of Health. Fortunately, private foundations such as the Melanoma Research Alliance, the Prostate Cancer Foundation and Stand Up To Cancer are bridging the ‘translational canyon’. First and foremost, researchers in basic immunology should not be reluctant to ‘get in the game’ of translation. This means not simply throwing in a contrived therapy experiment on the ‘hot’ mouse model du jour to publish a high-profile paper but really challenging and improving on a therapeutic idea in an iterative way. Additionally, researchers in basic immunology should not be reluctant to assertively engage with clinicians and companies, an endeavor that goes far beyond simply ‘out-licensing’ a patent and then retreating to the sidelines. Jim Allison, who has no medical degree, was the paragon of this model: as the anti-CTLA-4 ‘promoter in chief’, he engaged, badgered, charmed and cajoled whomever was in a position to move the therapy forward. No one understands the wonders that the immune system can perform better than immunologists, so translation now represents not only an opportunity but also a mandate for immunologists.
Footnotes
COMPETING FINANCIAL INTERESTS
The author declares no competing financial interests.
References
- 1.Flexner A. Bull. World Health Organ. 2002;80:594–602. [PMC free article] [PubMed] [Google Scholar]
- 2.Brahmer JR, et al. J. Clin. Oncol. 2010;28:3167–3175. doi: 10.1200/JCO.2009.26.7609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Brahmer JR, et al. N. Engl. J. Med. 2012;366:2455–2465. doi: 10.1056/NEJMoa1200694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hodi FS, et al. N. Engl. J. Med. 2010;363:711–723. doi: 10.1056/NEJMoa1003466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Topalian SL, et al. N. Engl. J. Med. 2012;366:2443–2454. doi: 10.1056/NEJMoa1200690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Brunet JF, et al. Nature. 1987;328:267–270. doi: 10.1038/328267a0. [DOI] [PubMed] [Google Scholar]
- 7.Linsley PS, et al. J. Exp. Med. 1991;173:721–730. doi: 10.1084/jem.173.3.721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Linsley PS, et al. J. Exp. Med. 1991;174:561–569. doi: 10.1084/jem.174.3.561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Linsley PS, et al. J. Exp. Med. 1992;176:1595–1604. doi: 10.1084/jem.176.6.1595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Krummel MF, Allison JP. J. Exp. Med. 1995;182:459–465. doi: 10.1084/jem.182.2.459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Walunas TL, et al. Immunity. 1994;1:405–413. [Google Scholar]
- 12.Tivol EA, et al. Immunity. 1995;3:541–547. doi: 10.1016/1074-7613(95)90125-6. [DOI] [PubMed] [Google Scholar]
- 13.Waterhouse P, et al. Science. 1995;270:985–988. doi: 10.1126/science.270.5238.985. [DOI] [PubMed] [Google Scholar]
- 14.Leach DR, Krummel MF, Allison JP. Science. 1996;271:1734–1736. doi: 10.1126/science.271.5256.1734. [DOI] [PubMed] [Google Scholar]
- 15.Sutmuller RP, et al. J. Exp. Med. 2001;194:823–832. doi: 10.1084/jem.194.6.823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hodi FS, et al. Proc. Natl. Acad. Sci. USA. 2003;100:4712–4717. doi: 10.1073/pnas.0830997100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Phan GQ, et al. Proc. Natl. Acad. Sci. USA. 2003;100:8372–8377. doi: 10.1073/pnas.1533209100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Robert C, et al. N. Engl. J. Med. 2011;364:2517–2526. doi: 10.1056/NEJMoa1104621. [DOI] [PubMed] [Google Scholar]
- 19.Hoos A, et al. J. Natl. Cancer Inst. 2010;102:1388–1397. doi: 10.1093/jnci/djq310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ishida Y, Agata Y, Shibahara K, Honjo T. EMBO J. 1992;11:3887–3895. doi: 10.1002/j.1460-2075.1992.tb05481.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Yokosuka T, et al. J. Exp. Med. 2012;209:1201–1217. doi: 10.1084/jem.20112741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Nishimura H, Nose M, Hiai H, Minato N, Honjo T. Immunity. 1999;11:141–151. doi: 10.1016/s1074-7613(00)80089-8. [DOI] [PubMed] [Google Scholar]
- 23.Nishimura H, et al. Science. 2001;291:319–322. doi: 10.1126/science.291.5502.319. [DOI] [PubMed] [Google Scholar]
- 24.Sharpe AH, Wherry EJ, Ahmed R, Freeman GJ. Nat. Immunol. 2007;8:239–245. doi: 10.1038/ni1443. [DOI] [PubMed] [Google Scholar]
- 25.Dong H, Zhu G, Tamada K, Chen L. Nat. Med. 1999;5:1365–1369. doi: 10.1038/70932. [DOI] [PubMed] [Google Scholar]
- 26.Tseng SY, et al. J. Exp. Med. 2001;193:839–846. doi: 10.1084/jem.193.7.839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Freeman GJ, et al. J. Exp. Med. 2000;192:1027–1034. doi: 10.1084/jem.192.7.1027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Latchman Y, et al. Nat. Immunol. 2001;2:261–268. doi: 10.1038/85330. [DOI] [PubMed] [Google Scholar]
- 29.Barber DL, et al. Nature. 2006;439:682–687. doi: 10.1038/nature04444. [DOI] [PubMed] [Google Scholar]
- 30.Dong H, et al. Nat. Med. 2002;8:793–800. doi: 10.1038/nm730. [DOI] [PubMed] [Google Scholar]