Abstract
Chagas disease, caused by the parasite Trypanosoma cruzi, is an emerging infectious disease in the United States. In our study, 24 out of 39 triatomines, from the specie Triatoma rubida, were infected with Trypanosoma cruzi. Additionally, only the genotype TcI was characterized among the parasite specimens. Improved knowledge of local epidemiology is needed to prevent transmission of Chagas disease.
Keywords: Chagas disease, Trypanosoma cruzi, triatomine, Triatoma rubida, Indio Mountains Reseach Station
1. Introduction
Chagas disease or American Trypanosomiasisis caused by the protozoan parasite Trypanosoma cruzi. It is estimated that 12 million people are infected worldwide, and approximately 30% of the Chagas patients develop cardiomyopathy, a life-threatening symptom (1). Insect-born transmission is the primarily route for T. cruzi infection, but it can also be transmitted through blood transfusion, heart transplant, and congenitally (2). In the United States, seven autochthonous human infections have been reported (3–6), and a considerable number of seropositive blood donors have been identified(7). In effect, Chagas disease is becoming an emerging infectious disease in the United States. Therefore, epidemiological and ecological studies are needed to allow the implementation of effective preventive resources and raise awareness of Chagas disease (8).
Based on the genetic diversity of the protozoan parasite, T. cruzi has been genotyped into discrete typing units (DTUs), and recently the nomenclature was revised to be from TcI to TcVI (9). In the United States, only 2 genotypes had been characterized, TcI and TcIV (10), TcI being the most common in human infections. In a recent study conducted in Arizona, 41.5 % of the 164 collected triatomine insects were infected with T. cruzi (11), but the genotype DTUs was not reported. Since the clinical manifestations of Chagas disease are related to the genetic diversity of the parasite (12), it is important that the current epidemiological and ecological studies report the genotypes from the field isolates in order to develop awareness and prevent vector-borne infections. In this study, we used a set of molecular techniques (13–16) to identify the incidence of T. cruzi infection in the region and characterize the parasite's genotype from a number of triatomines collected at the University of Texas El Paso's Indio Mountains Research Station.
2. Materials and Methods
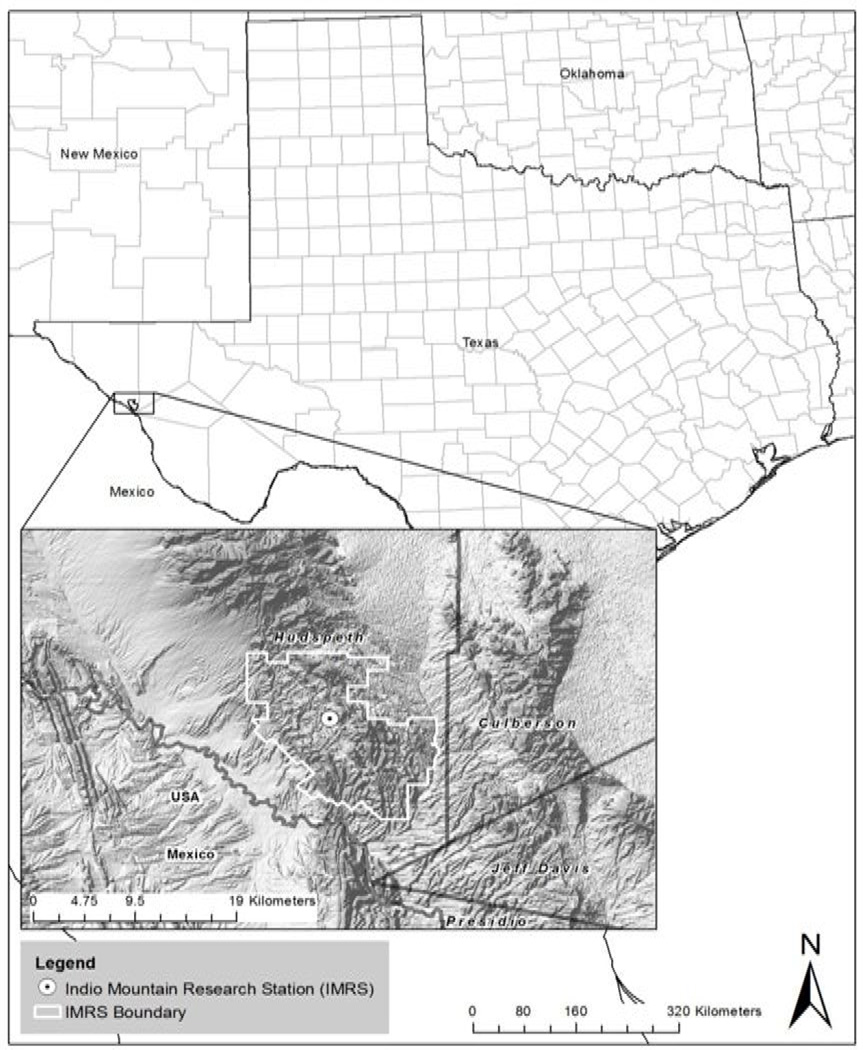
The study samples were collected at Indio Mountains Research Station located southeastern Hudspeth County, about 26 miles southwest of Van Horn, Texas (Figure 1) . Light traps were placed during the month of June 2013 before rainy season, and triatomine bugs were selected out of the insects trapped. A total of 39 triatomine vector bugs were collected. They were individually placed in 95% ethanol immediately after collection and preserved at room temperature until analysis. The intestinal tract of each insect was dissected and isolated in a phosphate-buffered saline suspension. DNA extraction was performed according to the Wizard® Genomic DNA Purification Kit (Promega A7933;Promega Corporation, USA).The presence of T. cruzi from the isolated genetic material was assessed by PCR amplification of a kinetoplastid DNA minicircle as described by Avila et al (13). Subsequently in order to confirm T. cruzi infection and characterize the parasite's genotype, we conducted a novel simple strain typing assay from a single amplification product using primers TcSC5D-fwd 5'- GGACGTGGCGTTTGATTTAT-3' and TcSC5D-rev 5'-TCCCATCTTCTTCGTTGACT-3' which amplify a 832 bp fragment of the TcSC5D gene (16). All PCR reactions were ran in parallel with a positive control of known T. cruzi DNA, and a negative control excluding DNA. Additionally, we analyzed the triatomines' genetic material to identify the insect species by sequencing a PCR amplification product of mitochondrial DNA using primers CYT BF 5'-GGA CAA ATA TCA TTT TGA GGA GCA ACA G-3' and CYT BR 5'-ATT ACT CCT CCT AGC TTA TTA GGA ATT G-3' according to Lyman et al. (15).
Fig. 1.
Map from UTEP Indio Mountains Research Station (UTEP-IMRS). Location: appx. 25 miles (40 km) south of Van Horn, Texas (30.776667°N, 105.015833°W).
3. Results
In total, 25 of the 39 triatomines collected, which represent 64.1 %, were identified to be infected with T. cruzi using the PCR method described by Avila et al (13). Confirmatory PCR test, using TcSC5D primers, showed 61.5% infection rate (n=39). From the confirmed infections, all 24(100%) T. cruzi parasites were characterized to belong to the TcI discrete typing unit. The characterization of the triatomine bug specie showed that all 39 triatomines were identified to be Triatoma rubida. The results are summarized in table 1.
Table 1.
Trypanosoma cruzi infection results of 39 triatomines collected at Indio Mountains Research Station.
| Results of Triatomines |
Collected | Specie | Infected |
T. cruzi DTU |
| 39 |
Triatoma rubida |
24 (61.5%) |
TcI |
4. Conclusions
The prevalence of Trypanosoma cruzi in the United States has been recently studied in the past few decades. Autochthonous cases, serological studies, and ecological reports propose that American Trypanosomiasis is an emerging infectious disease in the United States. Most of these studies have been conducted in the southern part of the country mainly in the states of Texas, Arizona, and New Mexico (8). In our study, we found that 61.5 % of the collected bugs were infected with T. cruzi which is consistent with the infection rates reported in other systematic studies. For instance two of the most recent studies, one conducted in Arizona and the other in Texas, reported 41.5 % and 51% infection rates respectively (11).Though, it is important to note that the infection rate reported in our study is higher than the ones previously reported which may suggest an increase on the risk of infection.
The literature indicates that there is a lack of information on local parasite genotypes from epidemiological studies. Therefore, such studies need to report specific genotypes since the parasite's genetic variability is implicated in the pathogenesis of the disease (12). It is found that past studies report infection rates, but rarely report the parasites' genotypes. This is mainly due to the lack of simple and fast typing strategies. However in our study, we used a novel typing method proposed by Cosentino and Aguero (10) that allows the quick discrimination of all discrete typing units by direct sequencing of a single PCR amplification. As a result, we identified that only TcI genotype was present among our infected specimens. Accordingly, in the United States only two genotypes have been reported from mammals and vector isolates, TcI and TcIV (6,8).The presence of only two genotypes is suggested to be caused by a low diversity of natural reservoir hosts and the low infectivity of local triatomines (6).
In addition, we characterized the specie of the triatomines collected. A total of 39 of 39 belonged to the Triatoma rubida specie. This specie has been found from western Texas to southern California (8), and it was the prevalent triatomine specie reported in the Arizona study (11). It can be distinguished from other species by its morphology which presents the first antennal segment to reach or surpass the tip of the head (8). Also, it has low transmission efficiency due in part to delayed defection after blood ingestion (15).Triatoma rubida might be the only specie present in our study because the specimens were collected from the same location.
Currently, there are no vaccines against Chagas nor effective treatment for the chronic disease. Therefore, improved knowledge of the local epidemiology and ecology is needed to develop a more comprehensive assessment of the magnitude of local transmission risk that will lead to more efficient efforts to prevent transmission (8). Future studies will try to assess the incidence of T. cruzi infection in the city of El Paso, Texas. Also, in our laboratory there is an ongoing study assessing the prevalence of T. cruzi infection on street dogs and cats from El Paso, Texas.
Highlights.
We conducted the first epidemiological study on T. cruzi at Indio Mountains Research Station.
The incidence of T. cruzi infection was of 64.1% among the triatomines collected.
T. cruzi population was represented only by TcI strain.
We identified the triatomines to belong to the Triatoma rubida specie.
Acknowledgements
MHB was supported by RISE-NIH grant #R25GM069621-11 from the National Institutes of General Sciences. SG was supported by Summer Program in Chihuahuan Desert Biodiversity REU-NSF grant # DBI-1263089. We are thankful to the Biomolecule Analysis Core Facility (BACF) and the Genomic Analysis Core facility(GACF) at the Border Biomedical Research Center (BBRC), UTEP, supported by NIH-NIMHD-RCMI grant # 5G12MD007592.We thank Dr. Jerry Johnson and Jose Orozco for field assistance in the collection of the study specimens on Indio Mountains Research Station. Also, we would like to thank Jonathan Gutierrez for assisting on the molecular diagnosis.
Dr. Rosa Maldonado is an associate professor in the Biological Sciences department in the College of Science at the University of Texas at El Paso. Her research focuses on developing novel immune- and chemotherapy approaches to treat Chagas disease and Leishmaniasis.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Pan American Health Organization. Estimación cuantitativa de la enfermedad de Chagas en las Américas. 2006 Retrieved from: www.bvsops.org.uy/pdf/chagas19.pdf.
- 2.Center of Disease Control and Prevention. Chagas Disease Epidemiology and Risk Factors. 2013 Retrieved from: http://www.cdc.gov/parasites/chagas/epi.html.
- 3.Woody NC, Woody HB. American trypanosomiasis (Chagas’s disease): first indigenous case in the United States. JAMA. 1955;159:676–677. doi: 10.1001/jama.1955.02960240042010a. [DOI] [PubMed] [Google Scholar]
- 4.Herwaldt BL, Grijalva M, Newsome A, McGhee C, Powell M, Nemec D, et al. Use of polymerase chain reaction to diagnose the fifth reported US case of autochthonous transmission of Trypanosoma cruzi, in Tennessee, 1998. J Infect Dis. 2000;181:395–399. doi: 10.1086/315212. [DOI] [PubMed] [Google Scholar]
- 5.Dorn PL, Perniciaro L, Yabsley MJ, Roellig DM, Balsamo G, Diaz J, et al. Autochthonous transmission of Trypanosoma cruzi, Louisiana. Emerg Infect Dis. 2007;13:605–607. doi: 10.3201/eid1304.061002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kjos SA, Snowden KF, Olson JK. Biogeography and Trypanosoma cruzi infection prevalence of Chagas disease vectors in Texas, USA. Vector Borne Zoonotic Dis. 2009;9:41–50. doi: 10.1089/vbz.2008.0026. [DOI] [PubMed] [Google Scholar]
- 7.Centers for Disease Control and Prevention. Blood donor screening for Chagas disease, United States. MMWR Morb Mortal Wkly Rep. 2006–2007;23:141–143. [PubMed] [Google Scholar]
- 8.Bern C, Kjos S, Yabsley MJ, Montgomery SP. Trypanosoma cruzi and Chagas' disease in the United States. Clinical microbiology reviews. 2011;24(4):655–681. doi: 10.1128/CMR.00005-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Zingales B, Miles MA, Campbell DA, Tibayrenc M, Macedo AM, et al. The revised Trypanosoma cruzi subspecific nomenclature: rationale, epidemiological relevance and research applications. Infect Genet Evol. 2012;12:240–253. doi: 10.1016/j.meegid.2011.12.009. [DOI] [PubMed] [Google Scholar]
- 10.Roellig DM, Brown EL, Barnabé C, Tibayrenc M, Steurer FJ, Yabsley MJ. Molecular typing of Trypanosoma cruzi isolates, United States. Emerging infectious diseases. 2008;14(7):1123. doi: 10.3201/eid1407.080175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Reisenman CE, Lawrence G, Guerenstein PG, Gregory T, Dotson E, Hildebrand JG. Infection of kissing bugs with Trypanosoma cruzi, Tucson, Arizona, USA. Emerg Infect Dis. 2010;16(3):400–405. doi: 10.3201/eid1603.090648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Macedo AM, Pena SD. Genetic Variability of Trypanosoma cruzi: Implications for the Pathogenesis of Chagas Disease. Parasitol Today. 1998;14:119–124. doi: 10.1016/s0169-4758(97)01179-4. [DOI] [PubMed] [Google Scholar]
- 13.Avila H, Goncalves AM, Nehme NS, Morel CM, Simpson L. Schizodeme analysis of Trypanosoma cruzi stocks from South and Central America by analysis of PCR-amplified minicircle variable region sequences. Molecular and biochemical parasitology. 1990;42(2):175–187. doi: 10.1016/0166-6851(90)90160-n. [DOI] [PubMed] [Google Scholar]
- 14.Moser DR, Kirchhoff LV, Donelson JE. Detection of Trypanosoma cruzi by DNA amplification using the polymerase chain reaction. Journal of Clinical Microbiology. 1989;27(7):1477–1482. doi: 10.1128/jcm.27.7.1477-1482.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lyman DF, Monteiro FA, Escalante AA, Cordon-Rosales C, Wesson DM, Dujardin JP, Beard CB. Mitochondrial DNA sequence variation among triatomine vectors of Chagas' disease. The American journal of tropical medicine and hygiene. 1999;60(3):377–386. doi: 10.4269/ajtmh.1999.60.377. [DOI] [PubMed] [Google Scholar]
- 16.Cosentino RO, Agüero F. A simple strain typing assay for Trypanosoma cruzi: discrimination of major evolutionary lineages from a single amplification product. PLoSneglected tropical diseases. 2012;6(7):e1777. doi: 10.1371/journal.pntd.0001777. [DOI] [PMC free article] [PubMed] [Google Scholar]