Abstract
Stem cells are necessary for the maintenance of many adult tissues. Signals within the stem cell microenvironment, or niche, regulate the self-renewal and differentiation capability of these cells. Misregulation of these signals through mutation or damage can lead to overgrowth or depletion of different stem cell pools. In this review, we focus on the Drosophila testis and ovary, both of which contain well-defined niches, as well as the mouse testis, which has become a more approachable stem cell system with recent technical advances. We discuss the signals that regulate gonadal stem cells in their niches, how these signals mediate self-renewal and differentiation under homeostatic conditions, and how stress, whether from mutations or damage, can cause changes in cell fate and drive stem cell competition.
Keywords: germline stem cells, somatic stem cells, self-renewal, competition, transdifferentiation, niche, oogenesis, spermatogenesis
INTRODUCTION
Stem cells are undifferentiated cells that are important for tissue homeostasis and regeneration through the replacement of lost or damaged cells. Stem cells are capable of producing two types of daughter cells: those that remain stem cells and those that differentiate into more specialized cell types. Maintenance of stem cells is regulated by intrinsic as well as extrinsic signals from the surrounding microenvironment, called the niche. Precise modulation of these signals is vital to ensure proper stem cell number and function. Misregulation of signals resulting from genetic mutations or tissue trauma can cause over- or underrepresentation of one stem cell type versus another, as well as inappropriate changes in cell fate. In this review we focus on gonadal stem cell niches, specifically those in the Drosophila testis and ovary and in the mouse testis. We discuss the signals that control the outcome of stem cell divisions under homeostatic conditions, stem cell competition, and cell fate conversion. Concepts emerging from studies on these topics in gonadal stem cell niches are likely to enhance our understanding of adult stem cells more generally. Understanding the coordination between niche signals, stress, and stem cell dynamics will provide key insight into the mechanisms that drive the progression of diseases such as cancer and allow the development of more effective treatments and preventative care.
GERMLINE STEM CELLS AND THEIR NICHES
The Drosophila Testis Niche
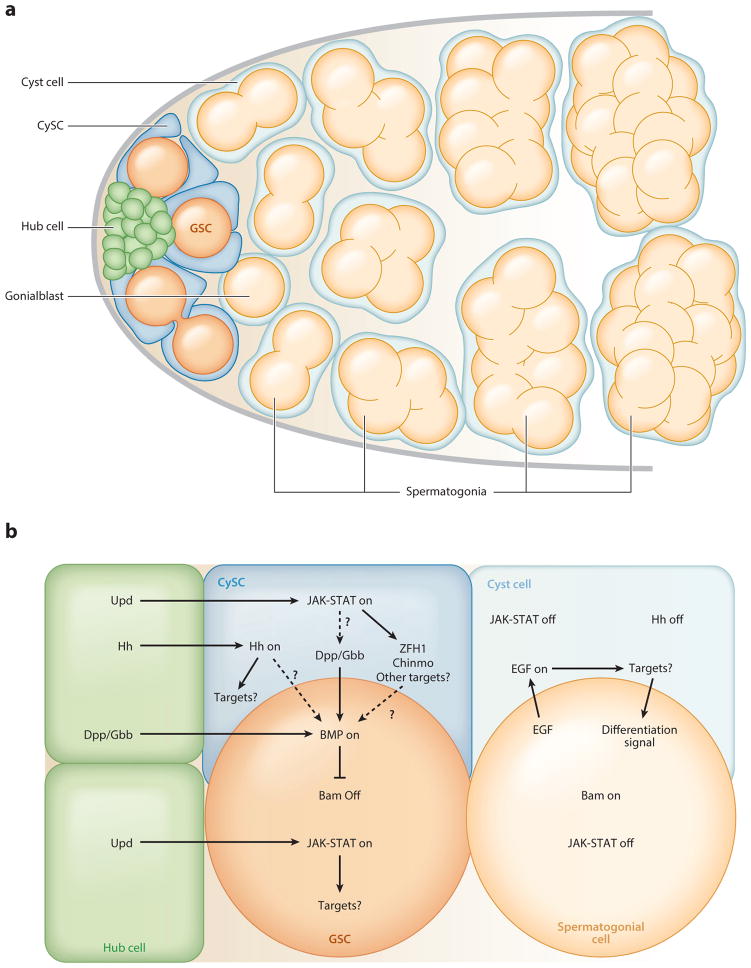
An adult Drosophila male contains a pair of testes, each composed of a blind-ended tubule with a single stem cell niche anchored to the apical end. The signaling center of the niche is a cluster of 10–15 densely packed, terminally differentiated somatic cells called hub cells (Hardy et al. 1979). Two types of stem cells adhere to the hub: sperm-producing germline stem cells (GSCs) and somatic cyst stem cells (CySCs). Each GSC typically divides asymmetrically to produce a daughter GSC and a gonialblast, which is displaced from the hub and undergoes four transit amplifying divisions with incomplete cytokinesis, forming a cluster of 16 interconnected spermatogonia (Figure 1a). As differentiation proceeds, spermatogonia are displaced from the testis apex, maturing into spermatocytes that undergo meiotic divisions to produce 64 haploid spermatids. After spermatid individualization, the mature sperm are released from the testis and stored in the seminal vesicle. For more comprehensive reviews of the testis niche, see de Cuevas & Matunis (2011) and Matunis et al. (2012).
Figure 1.
The architecture and signaling of the Drosophila testis niche. (a) Somatic hub cells ( green) signal to attached germline and somatic cyst stem cells (GSCs, orange, and CySCs, blue, respectively). GSCs asymmetrically divide to produce daughter gonialblasts (light orange), which are displaced from the hub and undergo four mitotic divisions with incomplete cytokinesis, producing interconnected spermatogonia (light orange). CySCs divide asymmetrically to produce daughter cyst cells (light blue); two encase each gonialblast and elongate to accommodate differentiating germ cells throughout spermatogenesis. Panel adapted from de Cuevas & Matunis (2011). (b) Hub cells secrete the ligand Unpaired (Upd), activating Janus kinase–Signal transducer and activator of transcription ( JAK-STAT) signaling in GSCs and CySCs, which is required for their maintenance. The downstream targets of STAT in GSCs are unknown, but two targets of STAT in the CySCs, zinc-finger homeodomain protein 1 (zfh1) and chronologically inappropriate morphogenesis (chinmo), are essential for CySC self-renewal (Leatherman & DiNardo 2008, Flaherty et al. 2010). Hedgehog signaling, which is activated by the ligand Hedgehog secreted from the hub, is also required for CySC but not GSC maintenance. Although its downstream targets are unknown, it may contribute to GSC maintenance by regulating Bone morphogenetic protein (BMP) signaling (Zhang et al. 2013). The hub and CySCs both secrete the ligands Decapentaplegic (Dpp) and Glass bottom boat (Gbb); this secretion is thought to result from activated STAT or one of its downstream targets (Leatherman & DiNardo 2010, Michel et al. 2011). Secretion of these ligands activates the BMP signaling pathway in GSCs. This, in turn, represses the differentiation factor Bag-of-marbles (Bam). Gonialblasts do not receive enough BMP signaling to repress bam expression and thus upregulate bam, initiating the differentiation process. Daughter cells in each lineage are thought to receive fewer niche signals as a result of their displacement from the hub. If these niche signals fall below a certain threshold, they can be overridden by the acquisition of new signals that promote differentiation, such as those triggered by Epidermal growth factor (EGF) signaling within the CySC lineage.
Somatic CySCs contact the hub via long, thin cytoplasmic extensions. Approximately two CySCs fully envelop each GSC, such that GSC-GSC contact does not occur (Hardy et al. 1979). Lineage tracing has revealed that CySCs divide asymmetrically to produce differentiating daughters called cyst cells (Gönczy & DiNardo 1996). Two cyst cells encase each gonialblast, where they no longer divide but instead elongate to accommodate the dividing spermatogonia (Figure 1a) (de Cuevas & Matunis 2011). The cyst cells continue to differentiate throughout spermatogenesis and are ultimately engulfed by epithelial cells at the base of the testis upon spermatid individualization (Fuller 1993). In addition to serving as a source of somatic support cells, CySCs are also an important part of the GSC niche, as described below. For a comprehensive review of the CySC lineage, see Zoller & Schulz (2012).
Hub cells were first implicated as a source of local signals that regulate stem cell maintenance when the Janus kinase–Signal transducer and activator of transcription ( JAK-STAT) pathway was shown to promote self-renewal of both GSCs and CySCs (Kiger et al. 2001, Tulina & Matunis 2001) (Figure 1b). However, the downstream targets of this pathway differ in the two cell types, and the effects may also be lineage specific, as STAT activation is thought to promote self-renewal in CySCs and adhesion to the hub in GSCs (Leatherman & DiNardo 2008, Flaherty et al. 2010, Leatherman & DiNardo 2010). Hedgehog signaling is also important for promoting the self-renewal of CySCs but not GSCs (Michel et al. 2012, Amoyel et al. 2013, Zhang et al. 2013). This pathway is thought to act in parallel to JAK-STAT signaling to mediate self-renewal, although some of the downstream targets of the two pathways may overlap (Amoyel et al. 2013, Zhang et al. 2013). A third signaling pathway that promotes GSC self-renewal is the Bone morphogenetic protein (BMP) pathway. Activation of BMP signaling at adherens junctions between hub cells and GSCs silences transcription of the gene that encodes the differentiation factor Bag-of-marbles (Bam) (Kawase et al. 2004, Michel et al. 2011). Daughter cells that are displaced from the niche are thought to receive insufficient BMP signaling to repress bam expression, leading them to upregulate bam, which enables them to differentiate properly (Kawase et al. 2004). In addition to bam expression, germ cell differentiation requires signals that result from Epidermal growth factor (EGF) signaling within the CySC lineage; without these cyst cell-derived signals, germ cells fail to differentiate (Matunis et al. 1997, Kiger et al. 2000, Tran et al. 2000, Schulz et al. 2002, Lim & Fuller 2012, Hudson et al. 2013). Understanding how multiple signals are integrated to regulate stem cell behavior is a major challenge in most stem cell–based tissues and is best approached via genetics in model organisms such as Drosophila.
The Drosophila Ovarian Niche
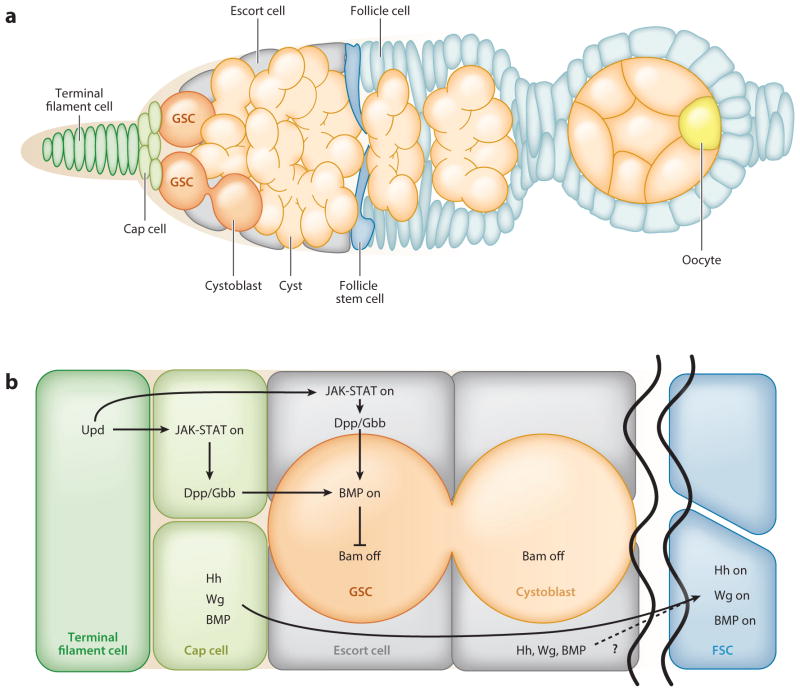
In most female mammals, oocytes form only before birth, during fetal development. GSCs are therefore undetectable in adult mouse ovaries, and single-cell lineage tracing has shown unequivocally that the pool of primordial follicles is maintained without input from GSCs (Zhang et al. 2012, Lei & Spradling 2013, Hanna & Hennebold 2014). By contrast, the Drosophila ovary contains GSCs that remain active and support continuous egg production throughout adulthood. Each ovary is composed of one to two dozen ovarioles, which are the functional units of the ovary and contain chains of developing egg chambers at progressively older stages. The GSCs are located at the anterior tip of each ovariole, in the germarium, where they adhere to the somatic support cells (terminal filament, cap, and escort cells) that comprise the stem cell niche (Figure 2a). As in the testis, GSCs typically divide asymmetrically, producing a new GSC and a daughter cystoblast that is displaced from the niche and differentiates. Each cystoblast undergoes four mitotic divisions with incomplete cytokinesis to produce an interconnected 16-cell cyst; one cell within the cyst will traverse meiosis and become an oocyte, whereas the other 15 will become polyploid nurse cells that support oocyte growth (Huynh & St Johnston 2000, Sahai-Hernandez et al. 2012). At the end of oogenesis, the nurse cells dump their cytoplasm into the oocyte and undergo apoptosis to produce a mature egg (Foley & Cooley 1998, McCall & Steller 1998).
Figure 2.
The architecture and signaling of the Drosophila ovarian niche. (a) The ovarian niche houses two to three germline stem cells (GSCs, orange) at the anterior tip of the germarium, where they adhere to a group of quiescent somatic cap cells (light green), which themselves are adjacent to quiescent somatic terminal filament cells ( green). Each GSC divides asymmetrically to produce a daughter stem cell and a cystoblast that undergoes transit amplification with incomplete cytokinesis to yield a cyst of 16 cells ( pale orange). As female germ cells differentiate, they are displaced posteriorly with the aid of escort cells ( gray) (Morris & Spradling 2011). When cysts reach the midpoint of the germarium, they are surrounded by follicle cells (light blue) originating from follicle stem cells (FSCs, blue). These follicle cells eventually differentiate into a polarized epithelium. One cell within the cyst becomes the oocyte (yellow), and the remainder become nurse cells. Panel adapted from Ma et al. (2014). (b) Janus kinase–Signal transducer and activator of transcription ( JAK-STAT) signaling is activated in the cap cells and possibly the anterior escort cells by the secretion of the ligand Unpaired (Upd) from the terminal filament cells. This leads to the production of the ligand Decapentaplegic (Dpp). Along with Glass bottom boat (Gbb), Dpp triggers a Bone morphogenetic protein (BMP) signaling cascade in the GSCs that results in the repression of the gene that encodes the differentiation factor Bag-of-marbles (Bam). Cystoblasts do not receive enough ligand to activate BMP signaling and thus begin to express bam and differentiate. FSCs reside in a distinct environment from the GSCs and require the Hedgehog (Hh), Wingless (Wg), and BMP signaling pathways for their maintenance. Ligands secreted from cap and escort cells are thought to activate these pathways in FSCs.
As with spermatogenesis, oogenesis is sustained by the cooperative action of GSCs and somatic stem cells, which are called follicle stem cells (FSCs) in the ovary. FSCs produce daughter follicle cells that form a columnar epithelial layer of cells surrounding each germline cyst; the resulting egg chamber buds from the germarium and continues to grow as it matures (Figure 2a). In contrast to the testis, FSCs reside in a distinct location in the germarium and are not adjacent to GSCs. In addition, their progeny are not quiescent but continue to divide as the cyst grows. For more comprehensive reviews of ovarian stem cells, see Eliazer & Buszczak (2011), Spradling et al. (2011), Sahai-Hernandez et al. (2012), and Slaidina & Lehmann (2014).
The signals that maintain stem cells in the ovary are similar to those that act in the testis (Figure 2b). In ovarian GSCs, BMP signaling results in transcriptional repression of bam (Chen & McKearin 2003, Song et al. 2004); as cystoblasts do not receive enough ligand, they begin the differentiation process (Li et al. 2009). JAK-STAT signaling is not directly required in GSCs, but its activation in anterior support cells leads to the production of the BMP ligand Decapentaplegic (Dpp) (Lopez-Onieva et al. 2008, Wang et al. 2008). Additional factors important for GSC self-renewal were recently identified in a large-scale RNA interference (RNAi) screen, and future studies should reveal how these factors are regulated by niche signaling to maintain GSC cell fate (Yan et al. 2014). Although FSCs reside in a distinct environment from the GSCs, the same core signaling pathways regulate both stem cell lineages. Hedgehog, Wingless, BMP, and Epidermal growth factor (EGF) signaling are all required for FSC self-renewal, and the ligands for these signaling pathways are thought to originate from the GSC niche or from adjacent stromal cells (escort cells) (Forbes et al. 1996, Song & Xie 2003, Kirilly et al. 2005, Hartman et al. 2010, Castanieto et al. 2014). Further research is needed to understand how these signaling pathways are coordinated and interact with the GSC niche. For a more in-depth understanding of the signaling pathways that maintain GSCs, see Eliazer & Buszczak (2011); for FSC self-renewal, see Sahai-Hernandez et al. (2012).
The Mammalian Testis Niche
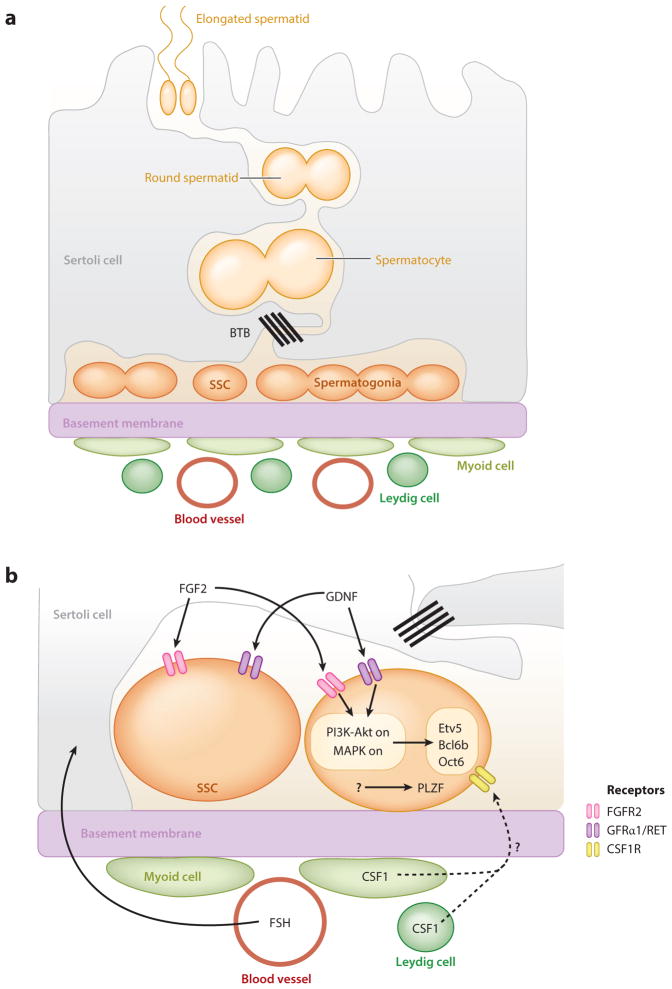
Our understanding of mammalian testis stem cell biology derives largely from studies in mice. In the mouse testis, GSCs [called spermatogonial stem cells (SSCs) in mammals] and their early differentiating progeny reside on the basement membrane inside the seminiferous tubule (Figure 3a). In contrast to the Drosophila testis, where a distinct morphological structure (the hub) marks the niche, SSCs are located along the entire length of the tubule. The nature and arrangement of somatic support cells are also quite different: There is no somatic stem cell population, and the quiescent Sertoli cells that surround the germ cells are large and make contact with multiple germ cells in different stages of development. There are similarities in the behavior of germ cells, however. SSCs and their early progeny divide with incomplete cytokinesis to form clusters of interconnected spermatogonia, which transit into meiotic spermatocytes as they cross the blood-testis barrier to enter the adluminal compartment (Russell 1978). There, they complete meiosis and elongate to form spermatozoa, which exit the epithelium through the center of the tubule (Oatley & Brinster 2012). For comprehensive reviews of mouse spermatogenesis, see Oatley & Brinster (2012), Yoshida (2012), and Kanatsu-Shinohara & Shinohara (2013). For a comparison of rodent and human spermatogenesis, see the review by Hermann et al. (2010).
Figure 3.
The architecture and signaling of the mammalian testis niche. (a) The seminiferous tubule is bounded by a basement membrane, which separates the site of spermatogenesis from the interstitial space, where Leydig cells, myoid cells, and macrophages reside. Quiescent, somatic Sertoli cells adhere to the basal lamina but extend long cytoplasmic processes toward the tubule lumen, simultaneously contacting thousands of germ cells at all stages of differentiation. Sertoli cells meet at specialized tight junctions that comprise the blood-testis barrier (BTB) and polarize the seminiferous epithelium into basal and adluminal compartments (Oatley & Brinster 2012). Spermatogonial stem cells (SSCs) and spermatogonia reside in a single layer on the basement membrane of the seminiferous tubule, where they divide with incomplete cytokinesis to form interconnected clusters of germ cells. As germ cells differentiate, they move toward the tubule lumen. (b) Systemic gonadotropin follicle-stimulating hormone (FSH) regulates the secretion of the glial cell line–derived neurotrophic factor (GDNF) from Sertoli cells. This ligand, along with the secretion of fibroblast growth factor 2 (FGF2) from Sertoli cells, activates the phosphatidylinositol 3-kinase–Akt (PI3K-Akt) and Mitogen-activated protein kinase (MAPK) signaling pathways in SSCs and their daughters. Both pathways in turn upregulate the transcription factors ETS variant 5 (Etv5) and B-cell CLL/lymphoma 6 member B (Bcl6b), which are necessary for SSC self-renewal, though GDNF alone induces POU domain class 3 transcription factor 1 (Oct6) expression (Oatley et al. 2006, Wu et al. 2010, Ishii et al. 2012). Zinc finger and BTB domain containing 16 (Plzf) is another important transcription factor required for SSC self-renewal, but what regulates its expression is unknown (Buaas et al. 2004, Costoya et al. 2004). Colony stimulating factor 1 (CSF1) is secreted from both Leydig and myoid cells and is thought to regulate maintenance specifically in the stem cells (Oatley et al. 2009).
In Drosophila, GSCs can be identified unequivocally by their morphology and location, but this is not true in mammals. In mouse testes, within the population of undifferentiated spermatogonia is a subset of single cells that were long thought to be the SSCs (Tegelenbosch & de Rooij 1993). However, recent studies using lineage tracing and live imaging indicate that the stem cell pool is more heterogeneous, challenging this long-held view (Yoshida 2012, Aloisio et al. 2014). Because all undifferentiated spermatogonia reside in a single layer on the basement membrane of the seminiferous tubule, they are likely to receive signals from a variety of sources, including adjacent Sertoli cells as well as cells that reside in the interstitial region of the testis, such as Leydig cells, myoid cells, and macrophages (Oatley & Brinster 2012, Yoshida 2012). Whether there is heterogeneity in these somatic cell populations remains to be seen. The nerves and capillaries found in the interstitial tissue are also potential sources of systemic signals (Yoshida 2012). How signals from all of these sources are coordinated to regulate the balance between SSC self-renewal and differentiation remains unclear.
As in Drosophila, genetic approaches have uncovered some of the signals that are important for SSC maintenance (Figure 3b). Other potential regulators have been identified through in vitro studies using cultured SSCs. Sertoli cells secrete glial cell line–derived neurotrophic factor (GDNF), which promotes SSC self-renewal, as well as the differentiation factor retinoic acid (Mullaney & Skinner 1992, Tadokoro et al. 2002, Kubota et al. 2004, Hasegawa & Saga 2012). GDNF’s receptors, GDNF family receptor α 1 (GFRα1) and rearranged during transfection (RET) tyrosine kinase, are expressed in both SSCs and early spermatogonia (Meng et al. 2000, Naughton et al. 2006), suggesting that GDNF signaling could function in both cell types (Grisanti et al. 2009, Suzuki et al. 2009). In SSCs, GDNF and fibroblast growth factor 2 (FGF2) activate the Phosphatidylinositol 3-kinase–Akt (PI3K-Akt) and Mitogen-activated protein kinase (MAPK) pathways (Kubota et al. 2004, Hasegawa et al. 2013, Kanatsu-Shinohara & Shinohara 2013), which are thought to promote the transcription of genes necessary for stem cell maintenance (Oatley et al. 2006, Wu et al. 2010, Ishii et al. 2012). Activation of the MAPK pathway in Sertoli cells also leads to the upregulation of GDNF production (Simon et al. 2007, Hasegawa et al. 2013), suggesting an indirect as well as direct role for MAPK activation in SSC maintenance. Other extrinsic factors, such as colony stimulating factor 1, leukemia inhibitory factor, and insulin-like growth factor, regulate self-renewal in cultured SSCs (Kubota et al. 2004, Oatley et al. 2009), but further studies are needed to determine their role in stem cell maintenance in vivo.
MODES OF GERMLINE STEM CELL SELF-RENEWAL
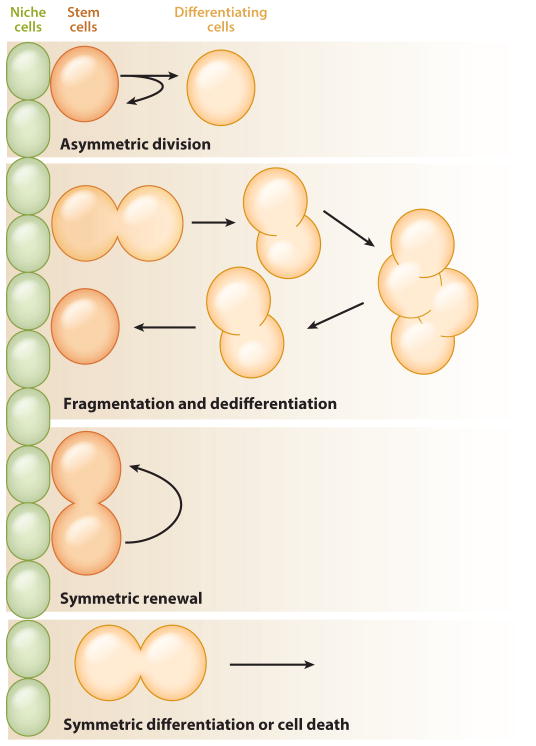
A defining feature of stem cells is their ability to divide asymmetrically, producing one daughter that remains a stem cell (self-renewal) and one that differentiates. However, studies on stem cells in intact tissues have revealed that individual stem cells are far more plastic than previously thought: They modulate the output of their divisions in response to the needs of the tissue. This plasticity enables tissues to maintain a constant stem cell population despite damage or loss of stem cells over time. The different modes of stem cell output are described below (Figure 4).
Figure 4.
Modes of stem cell self-renewal. Stem cells can self-renew using various mechanisms. In asymmetric division, one daughter cell remains a stem cell while the other daughter is displaced from the niche and begins to differentiate. In dedifferentiation, interconnected clusters of germ cells break apart and return to the niche, where they receive signals that promote a stem cell fate. In symmetric renewal, both daughter cells remain stem cells attached to the niche. In symmetric differentiation, both daughter cells leave the niche as a result of differentiation; both cells can also leave the niche as a result of death. During homeostasis, stem cells follow neutral drift dynamics in which the rate of self-renewal equals the rate of differentiation. This ensures that the pool of stem cells in a population remains constant. However, mutations in the stem cells or trauma to the tissue can skew the balance.
Division, Differentiation, and Dedifferentiation in Germline Stem Cells
In the Drosophila testis and ovary, GSCs usually divide asymmetrically to produce one daughter cell that remains anchored to the niche and one that is displaced from the niche and differentiates. This pattern of division results from the precise orientation of spindles in mitotic GSCs: The spindles are aligned perpendicular to the niche-GSC interface, with one pole anchored near the interface (Yamashita & Fuller 2008). Several cell components are asymmetrically segregated during these divisions; in the testis, these include centrosomes, certain histones, and even sex chromosomes, although other chromosomes are segregated at random (Yamashita et al. 2007, Tran et al. 2012, Yadlapalli & Yamashita 2013). Why these asymmetries occur is not known, and they do not have obvious effects on the outcome of stem cell divisions. For example, GSCs lacking centrioles (a main component of centrosomes) still function normally (Riparbelli & Callaini 2011). Understanding the relationship between asymmetric distribution of histones and the segregation of sex chromosomes could be informative. Occasionally, even in unperturbed wild-type testes, GSCs divide with a symmetric outcome and both daughters either remain at the niche (symmetric renewal) or leave the niche and differentiate (symmetric differentiation) (Sheng & Matunis 2011, Salzmann et al. 2013). Further studies are needed to determine whether symmetric renewal or differentiation correlates with changes in the segregation of intracellular components.
In the Drosophila ovary, one asymmetrically inherited component does correlate with cell fate decisions in dividing GSCs: the machinery that regulates ribosomal RNA (rRNA) transcription (Zhang et al. 2014). Higher levels of this machinery are found in GSCs than in their differentiating daughters, and this unequal distribution correlates with higher levels of rRNA transcription in GSCs. Increasing rRNA transcription in stem cell daughters delays their ability to differentiate; conversely, reducing rRNA transcription promotes differentiation and can rescue bam mutant cells, which otherwise fail to differentiate and remain GSC-like. This mode of regulation could be specific for certain proteins that control cell fate decisions, as disrupting rRNA transcription reduces the level of one stem cell factor, the BMP pathway component Mothers against dpp (Mad), but not its binding partner Medea or Histone H2B. Altogether, these data suggest that niche signaling modulates the levels of rRNA transcription in dividing GSCs, which in turn affects cell fate decisions in the daughter cells. A U3snoRNP component that is required for pre-rRNA maturation is also differentially segregated in dividing female GSCs, but whether this asymmetry is important for GSC function is not clear (Fichelson et al. 2009).
Although asymmetric division is the primary mode of GSC division in Drosophila gonads, mouse testes are likely to use a different mechanism. Recent studies using live imaging and pulse labeling have revealed that most SSCs divide symmetrically, as indicated by the expression of the GDNF receptor GFRα1, and that stem cells are renewed by fragmentation of interconnected spermatogonia (dedifferentiation), which break apart and move back into the niche to become stem cells (Hara et al. 2014). However, in 5% of spermatogonial pairs, GFRα1 is asymmetrically distributed, and in many pairs ubiquitin carboxy-terminal hydrolase 1 is enriched in the cell closest to the basement membrane (Grisanti et al. 2009, Luo et al. 2009). The functional significance of these asymmetries is not known. Dedifferentiation of germ cell clusters also occurs in Drosophila testes and ovaries, but this happens primarily in response to trauma such as the genetically induced loss of stem cells, as is discussed further below (Brawley & Matunis 2004, Kai & Spradling 2004, Cheng et al. 2008, Sheng et al. 2009).
Neutral Drift Dynamics in Stem Cells
Whether a stem cell remains in the niche or goes on to differentiate is regulated not just by the signals it receives but also by the physical constraints of the niche in which it resides. Niches contain a limited amount of signals and space, and stem cells are constantly competing with each other for niche occupancy ( Johnston 2009). The stem cell population is therefore regulated by the balance between asymmetric divisions, symmetric renewals, and symmetric differentiation, as well as by competition between neighboring stem cells (Issigonis et al. 2009, Klein & Simons 2011, Sheng & Matunis 2011, Stine & Matunis 2013). Lineage tracing has provided insight into the half-lives of stem cells in many tissues: Stem cells are continually lost from the niche and replaced as a result of natural turnover (Fox et al. 2009). In many vertebrate and invertebrate stem cell systems, turnover occurs in a stochastic manner and is termed neutral competition (Clayton et al. 2007, Doupe et al. 2010, Lopez-Garcia et al. 2010, Snippert et al. 2010, Klein & Simons 2011, de Navascués et al. 2012, Doupe et al. 2012, Stine & Matunis 2013, Teixeira et al. 2013, Vermeulen et al. 2013, Baker et al. 2014, Kronen et al. 2014). This neutral drift dynamic occurs in gonadal stem cell niches (Klein et al. 2010, Sheng & Matunis 2011, Amoyel et al. 2014, Kronen et al. 2014), but whether transient signaling fluctuations control these changes, as seen in the Drosophila eye (Losick & Desplan 2008), remains to be determined. Importantly, the neutral drift dynamics of a stem cell population can be shifted by the acquisition of mutations in stem cells, such that competition between neighboring cells ensues.
STEM CELL COMPETITION
Mutations within stem cells that tip the balance toward symmetric renewal or symmetric differentiation are the defining feature of stem cell competition. Mutations causing a fitness advantage allow mutant cells to take over the niche and outcompete wild-type stem cells. By contrast, mutations causing a disadvantage lead to mutant cells being lost more quickly from the niche, as a result of either cell death or differentiation (Figure 5a). While competition can eliminate defective stem cells from the niche, potentially maintaining stem cell fitness, it can also lead to aberrant tissue function. For example, in multiple myeloma and acute myeloid leukemia, cancer stem cells outcompete normal hematopoietic stem cells (Noll et al. 2012). Changes in adhesion, differentiation ability, or proliferation can all affect stem cell competition (Figure 5b). Thus, understanding these mechanisms is vital for the treatment and prevention of disease as well as for capturing the regenerative capacity of stem cells.
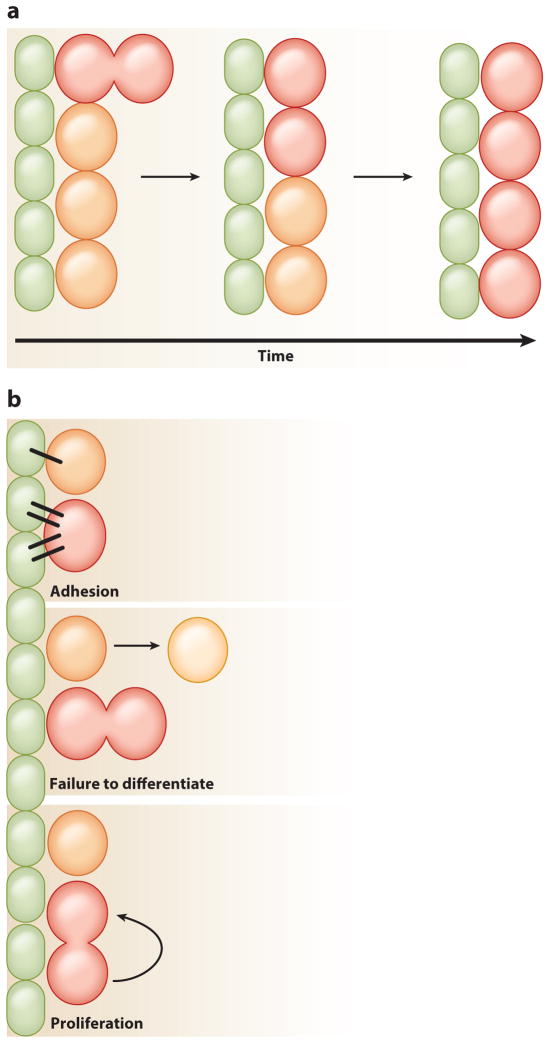
Figure 5.
Mechanisms of stem cell competition. Niche cells ( green) and stem cells with a fitness advantage (red ) or disadvantage (orange) are indicated. (a) When neutral drift dynamics are skewed, stem cells with a fitness advantage take over the niche, whereas those with a disadvantage are lost over time. (b) Three different mechanisms can modulate stem cell competition within the niche: adhesion, failure of daughters to differentiate, and different proliferation rates. Stem cells that adhere better to the niche will remain longer than those that do not. Stem cells that produce daughter cells that fail to differentiate have a greater chance of those daughters staying within the niche and remaining stem cells. Stem cells with a faster proliferation rate will produce more daughters than their neighbors, increasing the likelihood that their daughters will remain stem cells.
Effects of Adhesion on Stem Cell Competition
Adhesion plays a major role in the regulation of stem cell occupancy within many niches (Chen et al. 2013). In the Drosophila ovary, E-cadherin and its intracellular partner β-catenin are necessary to form proper adherens junctions. Both proteins localize along the GSC–cap cell junction and the FSC–inner sheath cell junction and anchor GSCs and FSCs to their respective niches (Song & Xie 2002, Song et al. 2002). Because removal of either protein causes loss of GSCs or FSCs from the niche, these proteins are essential for stem cell maintenance. E-cadherin and β-catenin are also important for niche–stem cell anchorage in the Drosophila testis, where they localize to the hub-GSC and hub-CySC interfaces. Reduction of E-cadherin in either stem cell lineage results in the loss of that lineage from the niche, most likely through differentiation (Yamashita et al. 2003, Voog et al. 2008). However, how niche signals coordinate adhesion levels in stem cells to regulate niche occupancy is poorly understood. Recently, the Slit-Roundabout pathway was shown to modulate adhesion in CySCs by regulating E-cadherin (Stine et al. 2014). The ligand Slit is expressed specifically within the hub, and a reduction in levels of Slit’s receptor Roundabout 2 in CySCs resulted in their loss from the niche. By contrast, loss of the pathway’s downstream effector, Abelson tyrosine kinase, provided CySCs with a competitive advantage. This supports a model in which the Slit-Roundabout pathway attenuates Abelson tyrosine kinase activity, which normally destabilizes β-catenin to allow adherens junction turnover (Stine et al. 2014). The expression of Roundabout 2 in CySCs is also regulated by JAK-STAT signaling, suggesting a mechanism for coordinating niche signaling with modulation of adhesion. As E-cadherin is important for niche–stem cell anchorage in the ovary, it will be interesting to know if the Slit-Roundabout pathway also modulates adhesion in this tissue. Furthermore, Roundabout 4 is expressed in mammalian hematopoietic stem cells and is known to be important for localization to the bone marrow niche; thus, if the same mechanism is involved in controlling adhesion levels within these cells, it would reveal a conserved function for this pathway (Smith-Berdan et al. 2011).
Integrins are another type of adhesion molecule important for stem cell attachment to niches (Chen et al. 2013). In the Drosophila testis, integrins regulate adhesion of hub cells to the extra-cellular matrix but not attachment of stem cells to the hub (Tanentzapf et al. 2007, Lee et al. 2008). However, modulation of integrin levels between the two stem cell populations, GSCs and CySCs, can lead to competition for niche occupancy. Integrin levels are regulated by JAK-STAT signaling, and loss of the JAK-STAT signaling suppressor Suppressor of cytokine signaling 36E (SOCS36E) leads to an increase in integrins within CySCs (Issigonis et al. 2009, Singh et al. 2010). This causes the CySCs to adhere better to the hub and consequently push out neighboring GSCs (Issigonis et al. 2009). Increased integrin levels or JAK-STAT signaling in CySCs does not lead to an overrepresentation of these mutant CySCs in the niche, suggesting that SOCS36E could have additional functions (Amoyel et al. 2014).
SSCs in the mouse testis also express integrins, which could mediate their adhesion to the basement membrane of the seminiferous tubule (Shinohara et al. 1999). When SSCs from β1-integrin knockout mice were transplanted into the seminiferous tubules of recipient mice that lacked endogenous germ cells, the SSCs with reduced β1-integrin were unable to attach to the basement membrane but did not have problems with migration to the niche, adhesion to Sertoli cells, or passage through the blood-testis barrier (Kanatsu-Shinohara et al. 2008). Consequently, the mutant SSCs colonized the recipient testes less successfully than control SSCs, proving that proper attachment to the niche is essential for SSC maintenance.
Stem Cell Proliferation, Differentiation, and Competition
Failure to differentiate or an increase in proliferation can also cause one population of stem cells to outcompete another for space in the niche. In the Drosophila ovary, GSCs lacking Bam fail to differentiate and show a competitive advantage over wild-type neighboring GSCs for space within the niche ( Jin et al. 2008). The mutant cells display increased levels of E-cadherin at the GSC–cap cell junction as well as increased proliferation, suggesting an indirect link between differentiating factors, adhesion, and division rate ( Jin et al. 2008). FSCs lacking the basolateral junction protein Lethal giant larvae or Discs large also outcompete their wild-type neighbors for niche occupancy (Kronen et al. 2014). These proteins establish polarity in early follicle cell daughters, which is necessary for their differentiation. Thus, a reduction in junction proteins causes defects in differentiation. These defects cause immature daughter cells to accumulate within the niche, increasing the likelihood that mutant FSCs will replace lost FSCs.
Another signaling molecule known to modulate general cell competition is dMyc. This phenomenon is best characterized in the Drosophila wing imaginal disc, where knockdown of dMyc in a subset of cells leads to their slow growth and eventually allows wild-type neighbors to take over. By contrast, overexpression of dMyc leads to induction of apoptosis in neighboring cells (de la Cova et al. 2004). Although increasing dMyc levels in GSCs of the Drosophila ovary does give them a competitive advantage over wild-type GSCs, this results from their enhanced sensitivity to niche signaling, which allows them to be retained in the niche longer, and not from induction of apoptosis in neighboring cells (Rhiner et al. 2009). Similarly, individual GSCs containing reduced levels of dMyc are eliminated from the niche more quickly than wild-type GSCs. One study looking at the role of dMyc in GSCs showed that it was not essential for GSC competition; this disparate finding could reflect differences in experimental design, choice of loss of function alleles, and/or overexpression methodologies ( Jin et al. 2008, Rhiner et al. 2009).
Although failure of stem cells to differentiate can lead to their overrepresentation in the niche, an increase in proliferation rate can also cause a competitive advantage. This is seen in the Drosophila testis, in CySCs constitutively expressing either the Hedgehog or Hippo signaling pathways (Michel et al. 2012, Amoyel et al. 2014). Clonal analysis has shown that CySCs homozygous for patched or hippo mutant alleles, which lead to activation of the Hedgehog or Hippo pathways, respectively, can outcompete both wild-type CySCs and GSCs for niche occupancy. This competition is a result of accelerated proliferation, as seen by an increase in the number of cells in S phase or M phase in the patched mutant clones. In addition, the competitive advantage of patched and hippo mutant clones can be rescued by reducing levels of String, an important inducer of mitosis (Amoyel et al. 2014). In contrast, loss of Hedgehog signaling in smoothened mutant CySC clones leads to their rapid loss from the niche, suggesting that mutations that decrease proliferation rates cause a competitive disadvantage. Interestingly, global reduction of Hedgehog signaling leads to a reduced number of CySCs but does not cause a difference in proliferation rate or a complete loss of the mutant cells (Michel et al. 2012). This suggests that a difference in cell signaling and its concomitant effect on proliferation rate between two neighboring cells may cause competition, whereas global changes affecting proliferation in all cells may not. Further supporting this idea, CySC clones overexpressing both String and the G1/S phase–promoting factor Cyclin E are capable of outcompeting wild-type CySCs and a portion of GSCs from the niche (Amoyel et al. 2014). However, overexpression of String in all CySCs leads to an increase in CySC proliferation rate but not a loss of GSCs from the niche (Inaba et al. 2011). Together, these studies provide evidence that changes in proliferation rate are a key factor driving stem cell competition and may represent a mechanism leading to cancer initiation. In support of this idea, loss of the tumor suppressor gene adenomatous polyposis coli (APC ) in intestinal stem cell clones in mice and humans is associated with an expanded stem cell population, resulting from an increase in proliferation (Vermeulen et al. 2013, Baker et al. 2014). As loss of this gene can lead to colon cancer, understanding how changes in stem cell dynamics cause competition is important for preventative intervention and the development of effective treatments (Morrissey & Vermeulen 2014).
Mutations causing a slight advantage in self-renewal without compromising differentiation are thought to impart a competitive advantage to spermatogonial stem cells of the mammalian testis. Although these mutations may lead to an increased division rate, they do not usually affect normal tissue function; instead, they produce mutant sperm that cause congenital disorders in progeny. This is demonstrated by the paternal age effect: older men have a greater chance of fathering children with developmental defects (Goriely & Wilkie 2012). This effect is thought to be driven by rare spontaneous activating mutations, primarily in the Ras-mediated signal transduction pathway, which are hypothesized to promote clonal expansion of mutant stem cells with age, thus leading to the accumulation of mutant sperm over time (Goriely & Wilkie 2012). Although there is evidence indicating an overrepresentation of these mutations in the sperm of older men (Goriely & Wilkie 2012), little is known about the mechanism of competition, which is thought to occur at the stem cell level. It has also been hypothesized that mutations outside of the Ras signaling pathway can confer similar defects.
Recently, a study looking at mouse SSCs with a specific mutation in fibroblast growth factor receptor 2 (FGFR2), which causes Apert syndrome, showed that these mutant stem cells had enhanced sensitivity to growth factors, causing an increase in fitness compared with wild-type stem cells (Martin et al. 2014). In vitro competition assays, in which the wild-type SSC population and the mutant SSC population contained their own fluorescent tags and were grown in the same dish, showed that the mutant SSC population could multiply at a faster rate than the wild-type population, but only in low FGF2 conditions. Furthermore, in transplantation assays in which the differentially labeled SSC populations were mixed in vitro and then transplanted into busulfan-treated mice depleted of germ cells, the mutant SSCs showed greater stem cell activity and produced more colonies than their wild-type counterparts (Martin et al. 2014). Transplantation assays in which SSCs from older mice were transplanted into younger mice have shown that niche function declines with age (Ryu et al. 2006). Thus, it is likely that enhanced growth factor sensitivity confers a greater advantage on mutant SSCs in the changing local microenvironment that is associated with aging. This indicates a link between the quality of the niche and the increased self-renewal capacity of stem cells.
A similar link is seen within stem cells of the colon. Tumor suppressor p53 mutant cells show a competitive advantage only in niches with impaired function resulting from colitis; this environment allows clonal expansion of these mutant cells over time (Vermeulen et al. 2013). This is thought to be how colitis-associated colorectal cancer is initiated. In addition, lower levels of p53 confer a competitive advantage to hematopoietic stem cells under stress conditions (Bondar & Medzhitov 2010). This overrepresentation of stem cells with low p53 levels can easily lead to a complete loss of p53, resulting in the overproliferation of these cells and cancer formation. These examples suggest a common mechanism underlying both paternal age effect disorders and cancer. Thus, understanding how niche signaling can bias the symmetric renewal capacity of stem cells is relevant for understanding the underlying causes of a variety of diseases.
RESPONSE TO TRAUMA
An important goal of regenerative medicine is to replace cells that are lost as a result of aging or tissue damage. In tissues with high rates of cell turnover, such as the skin, blood, or intestines, differentiated cells are continuously replaced by the progeny of tissue-specific stem cells, which divide and differentiate to replenish the lost cells. Tissues with low cell turnover may contain reserve or quiescent stem cells that can be stimulated to divide in response to tissue damage. What happens if stem cells or their niche cells are lost or damaged? As numerous studies have demonstrated, differentiation is not irreversible, and sometimes even fully differentiated cells can be reprogrammed or converted into other cell types to replenish the damaged tissue. Here, we ask how stem cells and their niches respond to trauma, and we review recent studies showing that not just differentiated cells but also stem cells can be regenerated from other cell types.
Differentiated cells can be reprogrammed or converted into other types of cells in vivo in two different ways: they can dedifferentiate into stem-like cells that then redifferentiate, or they can transdifferentiate directly into other cell types without first reverting to a stem-like fate (Figure 6). In plants and some animals, cell fate conversions happen naturally in response to tissue damage; the remarkable ability of hydrozoans, planarians, and salamanders to regenerate amputated body parts is the most striking example of this phenomenon (King & Newmark 2012). Although adult mammals lack the ability to regenerate entire limbs, recent studies have shown that they too can regenerate missing tissue via cell fate conversions in response to damage or induced changes in genetic output. For example, after toxin-induced ablation of pancreatic β-cells in adult mice, the missing cells can be regenerated by the spontaneous transdifferentiation of pancreatic α-cells (Thorel et al. 2010). New β-cells can also be generated from pancreatic exocrine cells, which can be reprogrammed directly into β-cells by re-expressing a set of embryonic transcription factors (Zhou et al. 2008). In both cases, the regenerated β-cells express insulin and are indistinguishable from endogenous β-cells. Studies such as these in a wide variety of tissues and organisms continue to challenge established concepts of what it means to be a differentiated cell (Sánchez Alvarado & Yamanaka 2014).
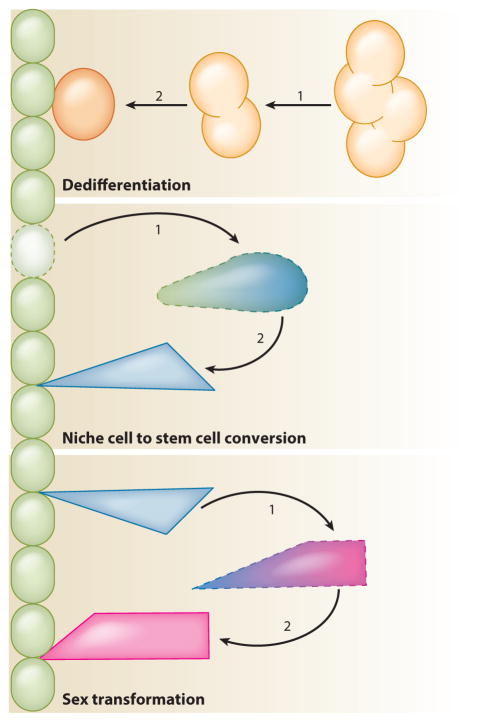
Figure 6.
Stem cell conversion and regeneration. When stem cells are depleted through mutations or tissue trauma, other cells can convert to a stem cell fate to replenish the stem cell pool; stem cells can also convert to other stem cell types. In dedifferentiation, differentiating cells that have moved away from the niche can revert into stem cells and repopulate the niche. In niche cell conversion, quiescent niche cells can re-enter the cell cycle and convert into somatic stem cells. In sex transformation, male somatic stem cells can convert to female somatic stem cells.
Germline Stem Cell Regeneration
Recent work has shown that adult stem cells can also be regenerated by cell fate conversions. Dedifferentiation, in addition to replacing stem cells lost through normal tissue turnover, is one mechanism for replacing populations of stem cells that have been lost through tissue damage. In the adult Drosophila testis, removal of the stem cell maintenance factor STAT or ectopic expression of the differentiation factor Bam induces rapid loss of GSCs (Brawley & Matunis 2004, Sheng et al. 2009). When normal signaling is restored, clusters of interconnected spermatogonia break apart into single cells that can migrate into the niche and revert to fully functional stem cells. The same is true in the adult mouse testis: after γ-irradiation or treatment with the drug busulfan, which is preferentially toxic to SSCs and spermatogonia, both transplanted and remaining endogenous spermatogonia have been shown to revert to SSCs and repopulate the niche (Nakagawa et al. 2007, Barroca et al. 2009, Hara et al. 2014). Female GSCs can also be replaced by dedifferentiation; in the adult Drosophila ovary, interconnected cyst cells (oogonia) can dedifferentiate to replace GSCs lost after removal of the stem cell maintenance factor Dpp (Kai & Spradling 2004). In all of these cases, the reverting cells are connected by stable intercellular bridges that must close to allow the formation of individual stem cells. The signals that mediate closure of the bridges and reversion to a less differentiated state are not known; however, as direct contact with niche cells is not essential for these events to occur, other somatic cells may play a role. In Drosophila, testes that contain spermatocytes but no spermatogonia are not able to regenerate GSCs, suggesting that these more differentiated cells are no longer capable of responding to the signals that mediate dedifferentiation. Therefore, past the spermatogonial stage, it is likely that differentiation is indeed irreversible.
Somatic Stem Cell Regeneration
Somatic cells of the gonad may also be capable of dedifferentiating. In the Drosophila testis, STAT is required for maintenance of somatic stem cells and GSCs. Somatic stem cells are lost upon removal of STAT, and it is likely that they are replaced by the dedifferentiation of cyst cells when STAT expression is restored, although direct evidence for this hypothesis is lacking. Somatic stem cells in the Drosophila testis can also be regenerated from a different and unexpected source: quiescent niche cells. Conditional expression of the proapoptotic gene grim in somatic stem cells and early cyst cells associated with spermatogonia results in the complete ablation of these cells from adult testes, whereas germ cells and older cyst cells associated with spermatocytes remain intact (Hétié et al. 2014). When flies are allowed to recover from ablation, their hub cells re-enter the cell cycle, delaminate from the hub, and convert into CySCs, which repopulate the niche and produce daughter cyst cells that are indistinguishable from normal cyst cells. By contrast, older cyst cells do not re-enter the cell cycle and do not appear to contribute to the new population of CySCs. Hub cell conversion to CySCs is seen only in testes that have lost all CySCs and early cyst cells, not in testes that have lost only some of these cells. However, hub cell conversion can be induced even in testes that have lost none of these cells by either ectopic expression of Cyclin D and Cyclin-dependent kinase 4 in the hub or removal of the transcription factor Escargot or its cofactor, C-terminal binding protein, from hub cells (Hétié et al. 2014, Voog et al. 2014). In these cases, hub cells appear to re-enter the cell cycle before exiting the hub. Mitotic hub cells have never been seen in wild-type adult testes, however, or in testes recovering from CySC loss induced by the removal of STAT in which early cyst cells remain (M. de Cuevas & E. Matunis, unpublished observations). Based on these observations, we speculate that lost CySCs can be regenerated in two ways in adult testes—by dedifferentiation of early cyst cells or by conversion of hub cells—but that hub cell conversion happens only when no early cyst cells remain. Hub cell conversion is not without risks, however. Testes that recover CySCs by hub cell conversion often contain multiple ectopic niches, which could arise from fission of the original hub or from incompletely reprogrammed CySCs that revert to hub cells (Hétié et al. 2014). Whether damage-induced transdifferentiation events are accompanied by uncontrolled niche expansion in mammalian tissues is an important question to ask in future studies.
Germ Cell Fate Conversions
Another intriguing type of stem cell conversion recently documented in the Drosophila testis is the sex transformation of male-to-female somatic stem cells (Ma et al. 2014). The gene chronologically inappropriate morphogenesis (chinmo) is a target of the JAK-STAT signaling pathway and encodes a transcription factor required cell-autonomously for CySC self-renewal (Flaherty et al. 2010). Flies carrying a hypomorphic allele of chinmo, chinmoST, initially develop what look like wild-type testes, but as the flies age, their cyst cells are gradually replaced by cells that resemble ovarian follicle cells. Lineage tracing experiments and cell type-specific knockdown of Chinmo suggest that these follicle-like cells are derived from CySCs that fail to maintain their male sex identity when Chinmo levels are reduced, transforming into female somatic stem cells as a result. Chinmo acts in part through the male sex determination factor DoublesexM, which is expressed in wild-type testes but not in chinmoST testes. This work indicates that the sex of somatic stem cells in the adult gonad must be actively maintained and that chinmo is required directly for this maintenance in CySCs. Somatic cells in vertebrate gonads must also actively maintain their sex. In adult mouse testes, loss of the transcriptional regulator doublesex and mab-3 related transcription factor 1 (DMRT1), which is a homolog of Doublesex, causes the transdifferentiation of Sertoli cells to granulosa cells (Matson et al. 2011), and the reciprocal transdifferentiation of granulosa cells to Sertoli cells can be triggered in adult mouse ovaries by loss of forkhead box L2 (FOXL2) (Uhlenhaut et al. 2009). Sex transformations of adult gonads are a naturally occurring phenomenon in some species of fish (Chan 1970). In other species of fish, sex transformations can be experimentally induced, as in medaka, in which Dmrt1 mutant males develop normal testes that later transform into ovaries (Masuyama et al. 2012), or zebrafish, in which ovaries can retract and functional testis-like organs appear in response to hormone manipulation (Takatsu et al. 2013). Moreover, in several fish species, both oogonia and spermatogonia are capable of reversing sex and giving rise to functional sperm or oocytes, respectively, when transplanted into recipient fish of the opposite sex (Lacerda et al. 2014). Although studies such as these highlight the remarkable sexual plasticity of gonadal cells, whether sex transformations in vertebrates take place in stem cells, differentiated cells, or both remains to be determined.
Besides reversing their sex, germ cells are capable of converting to somatic cells. In the C. elegans gonad, removal of the Polycomb repressor complex 2 (PRC2) paired with ectopic expression of transcription factors that specify somatic cell types, such as specific neurons or muscle cells, causes mitotic germ cells to be reprogrammed into those cell types (Tursun et al. 2011, Patel et al. 2012). PRC2 represses gene expression through its activity as a histone 3 lysine 27 methyltransferase and may define a chromatin state in germ cells that prevents them from converting to other cell fates. Therefore, when PRC2 is removed, germ cells become susceptible to reprogramming in the presence of specific somatic transcription factors. Polycomb proteins may also play a role in preventing germ cell reprogramming in the Drosophila testis (Eun et al. 2014). Knockdown of the Polycomb group gene Enhancer of zeste [E(z)] in adult testes causes germ cells to express the somatic marker Zfh-1. Here, the function of E(z) is non-cell autonomous, as it is required only in cyst lineage cells to prevent germline expression of Zfh-1. Therefore, suppression of Zfh-1 in germ cells must depend on signals from the niche rather than intrinsic factors. It will be interesting to know what happens to other somatic markers in these testes and which cells—stem cells or differentiated cells or both—are misexpressing them.
Lack of Regeneration in Niche Cells
Unlike the stem cells that they support, gonadal niche cells may not be capable of regeneration after damage. Although hub cells in the Drosophila testis can re-enter the cell cycle and convert to CySCs in response to CySC ablation, they are apparently not able to replenish other lost hub cells. Conditional expression of proapoptotic genes in hub cells in adult testes causes some or all hub cells to be ablated, but when flies are allowed to recover from ablation, no new hub cells are generated (P. Hétié & E. Matunis, unpublished manuscript). Somatic stem cells have been proposed to serve as a source of new hub cells (Voog et al. 2008), but contradictory results (DiNardo et al. 2011) and the inability of hub cells to be restored after ablation suggest that this hypothesis is not correct. Hub cells can also be ablated by knockdown of the novel gene headcase, but whether or not lost hub cells can be regenerated after recovery in this case is not known (Resende et al. 2013). Although it lacks the ability to restore itself, the hub is remarkable in its ability to continue supporting stem cells after trauma. After headcase knockdown, even single hub cells can continue to support functional populations of germline and somatic stem cells.
PERSPECTIVES
Proper regulation of adhesion, differentiation, proliferation, and cell fate is important for the maintenance of stem cells within the niche. Mutations affecting any of these factors could cause an increase in symmetric differentiation, leading to stem cell exclusion from the niche, or an increase in symmetric renewal, leading to an overrepresentation of stem cells. In addition, damage to the niche can result in the misregulation of local signals, leading to cell fate changes. Although these mechanisms ensure that only the fittest stem cells occupy the niche, they can also have detrimental consequences for tissue function or, in the case of GSCs, progeny development. Thus, niche signaling modulates adhesion, differentiation, proliferation, and cell fate to ensure a constant supply of stem cells that turnover at a steady rate. Further understanding the mechanisms that control these factors will be essential for the prevention of diseases such as cancer and paternal age effect disorders and may provide targets for future therapies.
Footnotes
DISCLOSURE STATEMENT
The authors are not aware of any affiliations, memberships, funding, or financial holdings that might be perceived as affecting the objectivity of this review.
Contributor Information
Leah Joy Greenspan, Email: lgreens6@jhmi.edu.
Margaret de Cuevas, Email: decuevas@jhmi.edu.
Erika Matunis, Email: ematuni1@jhmi.edu.
LITERATURE CITED
- Aloisio GM, Nakada Y, Saatcioglu HD, Pena CG, Baker MD, et al. PAX7 expression defines germline stem cells in the adult testis. J Clin Investig. 2014;124:3929–44. doi: 10.1172/JCI75943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amoyel M, Sanny J, Burel M, Bach EA. Hedgehog is required for CySC self-renewal but does not contribute to the GSC niche in the Drosophila testis. Development. 2013;140:56–65. doi: 10.1242/dev.086413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amoyel M, Simons BD, Bach EA. Neutral competition of stem cells is skewed by proliferative changes downstream of Hh and Hpo. EMBO J. 2014;33:2295–313. doi: 10.15252/embj.201387500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baker AM, Cereser B, Melton S, Fletcher AG, Rodriguez-Justo M, et al. Quantification of crypt and stem cell evolution in the normal and neoplastic human colon. Cell Rep. 2014;8:940–47. doi: 10.1016/j.celrep.2014.07.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barroca V, Lassalle B, Coureuil M, Louis JP, Le Page F, et al. Mouse differentiating spermatogonia can generate germinal stem cells in vivo. Nat Cell Biol. 2009;11:190–96. doi: 10.1038/ncb1826. [DOI] [PubMed] [Google Scholar]
- Bondar T, Medzhitov R. p53-mediated hematopoietic stem and progenitor cell competition. Cell Stem Cell. 2010;6:309–22. doi: 10.1016/j.stem.2010.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brawley C, Matunis E. Regeneration of male germline stem cells by spermatogonial dedifferentiation in vivo. Science. 2004;304:1331–34. doi: 10.1126/science.1097676. [DOI] [PubMed] [Google Scholar]
- Buaas FW, Kirsh AL, Sharma M, McLean DJ, Morris JL, et al. Plzf is required in adult male germ cells for stem cell self-renewal. Nat Genet. 2004;36:647–52. doi: 10.1038/ng1366. [DOI] [PubMed] [Google Scholar]
- Castanieto A, Johnston MJ, Nystul TG. EGFR signaling promotes self-renewal through the establishment of cell polarity in Drosophila follicle stem cells. eLife. 2014;3:e04437. doi: 10.7554/eLife.04437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chan ST. Natural sex reversal in vertebrates. Philos Trans R Soc B. 1970;259:59–71. doi: 10.1098/rstb.1970.0046. [DOI] [PubMed] [Google Scholar]
- Chen D, McKearin D. Dpp signaling silences bam transcription directly to establish asymmetric divisions of germline stem cells. Curr Biol. 2003;13:1786–91. doi: 10.1016/j.cub.2003.09.033. [DOI] [PubMed] [Google Scholar]
- Chen S, Lewallen M, Xie T. Adhesion in the stem cell niche: biological roles and regulation. Development. 2013;140:255–65. doi: 10.1242/dev.083139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng J, Turkel N, Hemati N, Fuller MT, Hunt AJ, Yamashita YM. Centrosome misorientation reduces stem cell division during ageing. Nature. 2008;456:599–604. doi: 10.1038/nature07386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clayton E, Doupe DP, Klein AM, Winton DJ, Simons BD, Jones PH. A single type of progenitor cell maintains normal epidermis. Nature. 2007;446:185–89. doi: 10.1038/nature05574. [DOI] [PubMed] [Google Scholar]
- Costoya JA, Hobbs RM, Barna M, Cattoretti G, Manova K, et al. Essential role of Plzf in maintenance of spermatogonial stem cells. Nat Genet. 2004;36:653–59. doi: 10.1038/ng1367. [DOI] [PubMed] [Google Scholar]
- de Cuevas M, Matunis EL. The stem cell niche: lessons from the Drosophila testis. Development. 2011;138:2861–69. doi: 10.1242/dev.056242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de la Cova C, Abril M, Bellosta P, Gallant P, Johnston LA. Drosophila Myc regulates organ size by inducing cell competition. Cell. 2004;117:107–16. doi: 10.1016/s0092-8674(04)00214-4. [DOI] [PubMed] [Google Scholar]
- de Navascués J, Perdigoto CN, Bian Y, Schneider MH, Bardin AJ, et al. Drosophila midgut homeostasis involves neutral competition between symmetrically dividing intestinal stem cells. EMBO J. 2012;31:2473–85. doi: 10.1038/emboj.2012.106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dinardo S, Okegbe T, Wingert L, Freilich S, Terry N. lines and bowl affect the specification of cyst stem cells and niche cells in the Drosophila testis. Development. 2011;138:1687–96. doi: 10.1242/dev.057364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doupe DP, Alcolea MP, Roshan A, Zhang G, Klein AM, et al. A single progenitor population switches behavior to maintain and repair esophageal epithelium. Science. 2012;337:1091–93. doi: 10.1126/science.1218835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doupe DP, Klein AM, Simons BD, Jones PH. The ordered architecture of murine ear epidermis is maintained by progenitor cells with random fate. Dev Cell. 2010;18:317–23. doi: 10.1016/j.devcel.2009.12.016. [DOI] [PubMed] [Google Scholar]
- Eliazer S, Buszczak M. Finding a niche: studies from the Drosophila ovary. Stem Cell Res Ther. 2011;2:45. doi: 10.1186/scrt86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eun SH, Shi Z, Cui K, Zhao K, Chen X. A non-cell autonomous role of E(z) to prevent germ cells from turning on a somatic cell marker. Science. 2014;343:1513–16. doi: 10.1126/science.1246514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fichelson P, Moch C, Ivanovitch K, Martin C, Sidor CM, et al. Live-imaging of single stem cells within their niche reveals that a U3snoRNP component segregates asymmetrically and is required for self-renewal in. Drosophila Nat Cell Biol. 2009;11:685–93. doi: 10.1038/ncb1874. [DOI] [PubMed] [Google Scholar]
- Flaherty MS, Salis P, Evans CJ, Ekas LA, Marouf A, et al. chinmo is a functional effector of the JAK/STAT pathway that regulates eye development, tumor formation, and stem cell self-renewal in Drosophila. Dev Cell. 2010;18:556–68. doi: 10.1016/j.devcel.2010.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foley K, Cooley L. Apoptosis in late stage Drosophila nurse cells does not require genes within the H99 deficiency. Development. 1998;125:1075–82. doi: 10.1242/dev.125.6.1075. [DOI] [PubMed] [Google Scholar]
- Forbes AJ, Lin H, Ingham PW, Spradling AC. hedgehog is required for the proliferation and specification of ovarian somatic cells prior to egg chamber formation in Drosophila. Development. 1996;122:1125–35. doi: 10.1242/dev.122.4.1125. [DOI] [PubMed] [Google Scholar]
- Fox DT, Morris LX, Nystul T, Spradling AC. The Stem Cell Research Community, editor. StemBook. Cambridge, MA: Harvard Stem Cell Inst; 2009. Lineage analysis of stem cells. [PubMed] [Google Scholar]
- Fuller MT. Spermatogenesis. In: Bate M, Martinez Arias A, editors. The Development of Drosophila melanogaster. 1. Cold Spring Harbor, NY: Cold Spring Harb. Lab. Press; 1993. pp. 71–147. [Google Scholar]
- Gönczy P, DiNardo S. The germ line regulates somatic cyst cell proliferation and fate during Drosophila spermatogenesis. Development. 1996;122:2437–47. doi: 10.1242/dev.122.8.2437. [DOI] [PubMed] [Google Scholar]
- Goriely A, Wilkie AO. Paternal age effect mutations and selfish spermatogonial selection: causes and consequences for human disease. Am J Hum Genet. 2012;90:175–200. doi: 10.1016/j.ajhg.2011.12.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grisanti L, Falciatori I, Grasso M, Dovere L, Fera S, et al. Identification of spermatogonial stem cell subsets by morphological analysis and prospective isolation. Stem Cells. 2009;27:3043–52. doi: 10.1002/stem.206. [DOI] [PubMed] [Google Scholar]
- Hanna CB, Hennebold JD. Ovarian germline stem cells: an unlimited source of oocytes? Fertil Steril. 2014;101:20–30. doi: 10.1016/j.fertnstert.2013.11.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hara K, Nakagawa T, Enomoto H, Suzuki M, Yamamoto M, et al. Mouse spermatogenic stem cells continually interconvert between equipotent singly isolated and syncytial states. Cell Stem Cell. 2014;14:658–72. doi: 10.1016/j.stem.2014.01.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hardy RW, Tokuyasu KT, Lindsley DL, Garavito M. The germinal proliferation center in the testis of Drosophila melanogaster. J Ultrastruct Res. 1979;69:180–90. doi: 10.1016/s0022-5320(79)90108-4. [DOI] [PubMed] [Google Scholar]
- Hartman TR, Zinshteyn D, Schofield HK, Nicolas E, Okada A, O’Reilly AM. Drosophila Boi limits Hedgehog levels to suppress follicle stem cell proliferation. J Cell Biol. 2010;191:943–52. doi: 10.1083/jcb.201007142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hasegawa K, Namekawa SH, Saga Y. MEK/ERK signaling directly and indirectly contributes to the cyclical self-renewal of spermatogonial stem cells. Stem Cells. 2013;31:2517–27. doi: 10.1002/stem.1486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hasegawa K, Saga Y. Retinoic acid signaling in Sertoli cells regulates organization of the blood-testis barrier through cyclical changes in gene expression. Development. 2012;139:4347–55. doi: 10.1242/dev.080119. [DOI] [PubMed] [Google Scholar]
- Hermann BP, Sukhwani M, Hansel MC, Orwig KE. Spermatogonial stem cells in higher primates: are there differences from those in rodents? Reproduction. 2010;139:479–93. doi: 10.1530/REP-09-0255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hétié P, de Cuevas M, Matunis E. Conversion of quiescent niche cells to somatic stem cells causes ectopic niche formation in the Drosophila testis. Cell Rep. 2014;7:715–21. doi: 10.1016/j.celrep.2014.03.058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hudson AG, Parrott BB, Qian Y, Schulz C. A temporal signature of epidermal growth factor signaling regulates the differentiation of germline cells in testes of Drosophila melanogaster. PLOS ONE. 2013;8:e70678. doi: 10.1371/journal.pone.0070678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huynh JR, St Johnston D. The role of BicD, Egl, Orb and the microtubules in the restriction of meiosis to the Drosophila oocyte. Development. 2000;127:2785–94. doi: 10.1242/dev.127.13.2785. [DOI] [PubMed] [Google Scholar]
- Inaba M, Yuan H, Yamashita YM. String (Cdc25) regulates stem cell maintenance, proliferation and aging in Drosophila testis. Development. 2011;138:5079–86. doi: 10.1242/dev.072579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ishii K, Kanatsu-Shinohara M, Toyokuni S, Shinohara T. FGF2 mediates mouse spermatogonial stem cell self-renewal via upregulation of Etv5 and Bcl6b through MAP2K1 activation. Development. 2012;139:1734–43. doi: 10.1242/dev.076539. [DOI] [PubMed] [Google Scholar]
- Issigonis M, Tulina N, de Cuevas M, Brawley C, Sandler L, Matunis E. JAK-STAT signal inhibition regulates competition in the Drosophila testis stem cell niche. Science. 2009;326:153–56. doi: 10.1126/science.1176817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jin Z, Kirilly D, Weng C, Kawase E, Song X, et al. Differentiation-defective stem cells outcompete normal stem cells for niche occupancy in the Drosophila ovary. Cell Stem Cell. 2008;2:39–49. doi: 10.1016/j.stem.2007.10.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnston LA. Competitive interactions between cells: death, growth, and geography. Science. 2009;324:1679–82. doi: 10.1126/science.1163862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kai T, Spradling A. Differentiating germ cells can revert into functional stem cells in Drosophila melanogaster ovaries. Nature. 2004;428:564–69. doi: 10.1038/nature02436. [DOI] [PubMed] [Google Scholar]
- Kanatsu-Shinohara M, Shinohara T. Spermatogonial stem cell self-renewal and development. Annu Rev Cell Dev Biol. 2013;29:163–87. doi: 10.1146/annurev-cellbio-101512-122353. [DOI] [PubMed] [Google Scholar]
- Kanatsu-Shinohara M, Takehashi M, Takashima S, Lee J, Morimoto H, et al. Homing of mouse spermatogonial stem cells to germline niche depends on β1-integrin. Cell Stem Cell. 2008;3:533–42. doi: 10.1016/j.stem.2008.08.002. [DOI] [PubMed] [Google Scholar]
- Kawase E, Wong MD, Ding BC, Xie T. Gbb/Bmp signaling is essential for maintaining germline stem cells and for repressing bam transcription in the Drosophila testis. Development. 2004;131:1365–75. doi: 10.1242/dev.01025. [DOI] [PubMed] [Google Scholar]
- Kiger AA, Jones DL, Schulz C, Rogers MB, Fuller MT. Stem cell self-renewal specified by JAK-STAT activation in response to a support cell cue. Science. 2001;294:2542–45. doi: 10.1126/science.1066707. [DOI] [PubMed] [Google Scholar]
- Kiger AA, White-Cooper H, Fuller MT. Somatic support cells restrict germline stem cell self-renewal and promote differentiation. Nature. 2000;407:750–54. doi: 10.1038/35037606. [DOI] [PubMed] [Google Scholar]
- King RS, Newmark PA. The cell biology of regeneration. J Cell Biol. 2012;196:553–62. doi: 10.1083/jcb.201105099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirilly D, Spana EP, Perrimon N, Padgett RW, Xie T. BMP signaling is required for controlling somatic stem cell self-renewal in the Drosophila ovary. Dev Cell. 2005;9:651–62. doi: 10.1016/j.devcel.2005.09.013. [DOI] [PubMed] [Google Scholar]
- Klein AM, Nakagawa T, Ichikawa R, Yoshida S, Simons BD. Mouse germ line stem cells undergo rapid and stochastic turnover. Cell Stem Cell. 2010;7:214–24. doi: 10.1016/j.stem.2010.05.017. [DOI] [PubMed] [Google Scholar]
- Klein AM, Simons BD. Universal patterns of stem cell fate in cycling adult tissues. Development. 2011;138:3103–11. doi: 10.1242/dev.060103. [DOI] [PubMed] [Google Scholar]
- Kronen MR, Schoenfelder KP, Klein AM, Nystul TG. Basolateral junction proteins regulate competition for the follicle stem cell niche in the Drosophila ovary. PLOS ONE. 2014;9:e101085. doi: 10.1371/journal.pone.0101085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kubota H, Avarbock MR, Brinster RL. Growth factors essential for self-renewal and expansion of mouse spermatogonial stem cells. PNAS. 2004;101:16489–94. doi: 10.1073/pnas.0407063101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lacerda SM, Costa GM, de Franca LR. Biology and identity of fish spermatogonial stem cell. Gen Comp Endocrinol. 2014;207:56–65. doi: 10.1016/j.ygcen.2014.06.018. [DOI] [PubMed] [Google Scholar]
- Leatherman JL, Dinardo S. Zfh-1 controls somatic stem cell self-renewal in the Drosophila testis and nonautonomously influences germline stem cell self-renewal. Cell Stem Cell. 2008;3:44–54. doi: 10.1016/j.stem.2008.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leatherman JL, Dinardo S. Germline self-renewal requires cyst stem cells, while stat regulates niche adhesion in Drosophila testes. Nat Cell Biol. 2010;12:806–11. doi: 10.1038/ncb2086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee S, Zhou L, Kim J, Kalbfleisch S, Schock F. Lasp anchors the Drosophila male stem cell niche and mediates spermatid individualization. Mech Dev. 2008;125:768–76. doi: 10.1016/j.mod.2008.06.012. [DOI] [PubMed] [Google Scholar]
- Lei L, Spradling AC. Female mice lack adult germ-line stem cells but sustain oogenesis using stable primordial follicles. PNAS. 2013;110:8585–90. doi: 10.1073/pnas.1306189110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Y, Minor NT, Park JK, McKearin DM, Maines JZ. Bam and Bgcn antagonize Nanos-dependent germ-line stem cell maintenance. PNAS. 2009;106:9304–9. doi: 10.1073/pnas.0901452106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lim JG, Fuller MT. Somatic cell lineage is required for differentiation and not maintenance of germline stem cells in Drosophila testes. PNAS. 2012;109:18477–81. doi: 10.1073/pnas.1215516109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lopez-Garcia C, Klein AM, Simons BD, Winton DJ. Intestinal stem cell replacement follows a pattern of neutral drift. Science. 2010;330:822–25. doi: 10.1126/science.1196236. [DOI] [PubMed] [Google Scholar]
- Lopez-Onieva L, Fernandez-Minan A, Gonzalez-Reyes A. Jak/Stat signalling in niche support cells regulates dpp transcription to control germline stem cell maintenance in the Drosophila ovary. Development. 2008;135:533–40. doi: 10.1242/dev.016121. [DOI] [PubMed] [Google Scholar]
- Losick R, Desplan C. Stochasticity and cell fate. Science. 2008;320:65–68. doi: 10.1126/science.1147888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luo J, Megee S, Dobrinski I. Asymmetric distribution of UCH-L1 in spermatogonia is associated with maintenance and differentiation of spermatogonial stem cells. J Cell Physiol. 2009;220:460–68. doi: 10.1002/jcp.21789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma Q, Wawersik M, Matunis EL. The Jak-STAT target Chinmo prevents sex transformation of adult stem cells in the Drosophila testis niche. Dev Cell. 2014;31:474–86. doi: 10.1016/j.devcel.2014.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin LA, Assif N, Gilbert M, Wijewarnasuriya D, Seandel M. Enhanced fitness of adult spermatogonial stem cells bearing a paternal age-associated FGFR2 mutation. Stem Cell Rep. 2014;3:219–26. doi: 10.1016/j.stemcr.2014.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Masuyama H, Yamada M, Kamei Y, Fujiwara-Ishikawa T, Todo T, et al. Dmrt1 mutation causes a male-to-female sex reversal after the sex determination by Dmy in the medaka. Chromosome Res. 2012;20:163–76. doi: 10.1007/s10577-011-9264-x. [DOI] [PubMed] [Google Scholar]
- Matson CK, Murphy MW, Sarver AL, Griswold MD, Bardwell VJ, Zarkower D. DMRT1 prevents female reprogramming in the postnatal mammalian testis. Nature. 2011;476:101–4. doi: 10.1038/nature10239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matunis E, Tran J, Gonczy P, Caldwell K, DiNardo S. punt and schnurri regulate a somatically derived signal that restricts proliferation of committed progenitors in the germline. Development. 1997;124:4383–91. doi: 10.1242/dev.124.21.4383. [DOI] [PubMed] [Google Scholar]
- Matunis EL, Stine RR, de Cuevas M. Recent advances in Drosophila male germline stem cell biology. Spermatogenesis. 2012;2:137–44. doi: 10.4161/spmg.21763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCall K, Steller H. Requirement for DCP-1 caspase during Drosophila oogenesis. Science. 1998;279:230–34. doi: 10.1126/science.279.5348.230. [DOI] [PubMed] [Google Scholar]
- Meng X, Lindahl M, Hyvonen ME, Parvinen M, de Rooij DG, et al. Regulation of cell fate decision of undifferentiated spermatogonia by GDNF. Science. 2000;287:1489–93. doi: 10.1126/science.287.5457.1489. [DOI] [PubMed] [Google Scholar]
- Michel M, Kupinski AP, Raabe I, Bokel C. Hh signalling is essential for somatic stem cell maintenance in the Drosophila testis niche. Development. 2012;139:2663–69. doi: 10.1242/dev.075242. [DOI] [PubMed] [Google Scholar]
- Michel M, Raabe I, Kupinski AP, Perez-Palencia R, Bokel C. Local BMP receptor activation at adherens junctions in the Drosophila germline stem cell niche. Nat Commun. 2011;2:415. doi: 10.1038/ncomms1426. [DOI] [PubMed] [Google Scholar]
- Morris LX, Spradling AC. Long-term live imaging provides new insight into stem cell regulation and germline-soma coordination in the Drosophila ovary. Development. 2011;138:2207–15. doi: 10.1242/dev.065508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morrissey ER, Vermeulen L. Stem cell competition: how speeding mutants beat the rest. EMBO J. 2014;33:2277–78. doi: 10.15252/embj.201489823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mullaney BP, Skinner MK. Basic fibroblast growth factor (bFGF) gene expression and protein production during pubertal development of the seminiferous tubule: follicle-stimulating hormone-induced Sertoli cell bFGF expression. Endocrinology. 1992;131:2928–34. doi: 10.1210/endo.131.6.1446630. [DOI] [PubMed] [Google Scholar]
- Nakagawa T, Nabeshima Y, Yoshida S. Functional identification of the actual and potential stem cell compartments in mouse spermatogenesis. Dev Cell. 2007;12:195–206. doi: 10.1016/j.devcel.2007.01.002. [DOI] [PubMed] [Google Scholar]
- Naughton CK, Jain S, Strickland AM, Gupta A, Milbrandt J. Glial cell-line derived neurotrophic factor-mediated RET signaling regulates spermatogonial stem cell fate. Biol Reprod. 2006;74:314–21. doi: 10.1095/biolreprod.105.047365. [DOI] [PubMed] [Google Scholar]
- Noll JE, Williams SA, Purton LE, Zannettino AC. Tug of war in the haematopoietic stem cell niche: do myeloma plasma cells compete for the HSC niche? Blood Cancer J. 2012;2:e91. doi: 10.1038/bcj.2012.38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oatley JM, Avarbock MR, Telaranta AI, Fearon DT, Brinster RL. Identifying genes important for spermatogonial stem cell self-renewal and survival. PNAS. 2006;103:9524–29. doi: 10.1073/pnas.0603332103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oatley JM, Brinster RL. The germline stem cell niche unit in mammalian testes. Physiol Rev. 2012;92:577–95. doi: 10.1152/physrev.00025.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oatley JM, Oatley MJ, Avarbock MR, Tobias JW, Brinster RL. Colony stimulating factor 1 is an extrinsic stimulator of mouse spermatogonial stem cell self-renewal. Development. 2009;136:1191–99. doi: 10.1242/dev.032243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patel T, Tursun B, Rahe DP, Hobert O. Removal of Polycomb repressive complex 2 makes C. elegans germ cells susceptible to direct conversion into specific somatic cell types. Cell Rep. 2012;2:1178–86. doi: 10.1016/j.celrep.2012.09.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Resende LP, Boyle M, Tran D, Fellner T, Jones DL. Headcase promotes cell survival and niche maintenance in the Drosophila testis. PLOS ONE. 2013;8:e68026. doi: 10.1371/journal.pone.0068026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rhiner C, Diaz B, Portela M, Poyatos JF, Fernandez-Ruiz I, et al. Persistent competition among stem cells and their daughters in the Drosophila ovary germline niche. Development. 2009;136:995–1006. doi: 10.1242/dev.033340. [DOI] [PubMed] [Google Scholar]
- Riparbelli MG, Callaini G. Male gametogenesis without centrioles. Dev Biol. 2011;349:427–39. doi: 10.1016/j.ydbio.2010.10.021. [DOI] [PubMed] [Google Scholar]
- Russell LD. The blood-testis barrier and its formation relative to spermatocyte maturation in the adult rat: a lanthanum tracer study. Anat Rec. 1978;190:99–111. doi: 10.1002/ar.1091900109. [DOI] [PubMed] [Google Scholar]
- Ryu BY, Orwig KE, Oatley JM, Avarbock MR, Brinster RL. Effects of aging and niche microenvironment on spermatogonial stem cell self-renewal. Stem Cells. 2006;24:1505–11. doi: 10.1634/stemcells.2005-0580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sahai-Hernandez P, Castanieto A, Nystul TG. Drosophila models of epithelial stem cells and their niches. Wiley Interdiscip Rev Dev Biol. 2012;1:447–57. doi: 10.1002/wdev.36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salzmann V, Inaba M, Cheng J, Yamashita YM. Lineage tracing quantification reveals symmetric stem cell division in Drosophila male germline stem cells. Cell Mol Bioeng. 2013;6:441–48. doi: 10.1007/s12195-013-0295-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sánchez Alvarado A, Yamanaka S. Rethinking differentiation: stem cells, regeneration, and plasticity. Cell. 2014;157:110–19. doi: 10.1016/j.cell.2014.02.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schulz C, Wood CG, Jones DL, Tazuke SI, Fuller MT. Signaling from germ cells mediated by the rhomboid homolog stet organizes encapsulation by somatic support cells. Development. 2002;129:4523–34. doi: 10.1242/dev.129.19.4523. [DOI] [PubMed] [Google Scholar]
- Sheng XR, Brawley CM, Matunis EL. Dedifferentiating spermatogonia outcompete somatic stem cells for niche occupancy in the Drosophila testis. Cell Stem Cell. 2009;5:191–203. doi: 10.1016/j.stem.2009.05.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheng XR, Matunis E. Live imaging of the Drosophila spermatogonial stem cell niche reveals novel mechanisms regulating germline stem cell output. Development. 2011;138:3367–76. doi: 10.1242/dev.065797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shinohara T, Avarbock MR, Brinster RL. β1- and α6-integrin are surface markers on mouse spermatogonial stem cells. PNAS. 1999;96:5504–9. doi: 10.1073/pnas.96.10.5504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simon L, Ekman GC, Tyagi G, Hess RA, Murphy KM, Cooke PS. Common and distinct factors regulate expression of mRNA for ETV5 and GDNF, Sertoli cell proteins essential for spermatogonial stem cell maintenance. Exp Cell Res. 2007;313:3090–99. doi: 10.1016/j.yexcr.2007.05.002. [DOI] [PubMed] [Google Scholar]
- Singh SR, Zheng Z, Wang H, Oh SW, Chen X, Hou SX. Competitiveness for the niche and mutual dependence of the germline and somatic stem cells in the Drosophila testis are regulated by the JAK/STAT signaling. J Cell Physiol. 2010;223:500–10. doi: 10.1002/jcp.22073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slaidina M, Lehmann R. Translational control in germline stem cell development. J Cell Biol. 2014;207:13–21. doi: 10.1083/jcb.201407102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith-Berdan S, Nguyen A, Hassanein D, Zimmer M, Ugarte F, et al. Robo4 cooperates with CXCR4 to specify hematopoietic stem cell localization to bone marrow niches. Cell Stem Cell. 2011;8:72–83. doi: 10.1016/j.stem.2010.11.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Snippert HJ, van der Flier LG, Sato T, van Es JH, van den Born M, et al. Intestinal crypt homeostasis results from neutral competition between symmetrically dividing Lgr5 stem cells. Cell. 2010;143:134–44. doi: 10.1016/j.cell.2010.09.016. [DOI] [PubMed] [Google Scholar]
- Song X, Wong MD, Kawase E, Xi R, Ding BC, et al. Bmp signals from niche cells directly repress transcription of a differentiation-promoting gene, bag of marbles, in germline stem cells in the Drosophila ovary. Development. 2004;131:1353–64. doi: 10.1242/dev.01026. [DOI] [PubMed] [Google Scholar]
- Song X, Xie T. DE-cadherin-mediated cell adhesion is essential for maintaining somatic stem cells in the Drosophila ovary. PNAS. 2002;99:14813–18. doi: 10.1073/pnas.232389399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song X, Xie T. Wingless signaling regulates the maintenance of ovarian somatic stem cells in Drosophila. Development. 2003;130:3259–68. doi: 10.1242/dev.00524. [DOI] [PubMed] [Google Scholar]
- Song X, Zhu CH, Doan C, Xie T. Germline stem cells anchored by adherens junctions in the Drosophila ovary niches. Science. 2002;296:1855–57. doi: 10.1126/science.1069871. [DOI] [PubMed] [Google Scholar]
- Spradling A, Fuller MT, Braun RE, Yoshida S. Germline stem cells. Cold Spring Harb Perspect Biol. 2011;3:a002642. doi: 10.1101/cshperspect.a002642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stine RR, Greenspan LJ, Ramachandran KV, Matunis EL. Coordinate regulation of stem cell competition by Slit-Robo and JAK-STAT signaling in the Drosophila testis. PLOS Genet. 2014;10:e1004713. doi: 10.1371/journal.pgen.1004713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stine RR, Matunis EL. Stem cell competition: finding balance in the niche. Trends Cell Biol. 2013;23:357–64. doi: 10.1016/j.tcb.2013.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suzuki H, Sada A, Yoshida S, Saga Y. The heterogeneity of spermatogonia is revealed by their topology and expression of marker proteins including the germ cell-specific proteins Nanos2 and Nanos3. Dev Biol. 2009;336:222–31. doi: 10.1016/j.ydbio.2009.10.002. [DOI] [PubMed] [Google Scholar]
- Tadokoro Y, Yomogida K, Ohta H, Tohda A, Nishimune Y. Homeostatic regulation of germinal stem cell proliferation by the GDNF/FSH pathway. Mech Dev. 2002;113:29–39. doi: 10.1016/s0925-4773(02)00004-7. [DOI] [PubMed] [Google Scholar]
- Takatsu K, Miyaoku K, Roy SR, Murono Y, Sago T, et al. Induction of female-to-male sex change in adult zebrafish by aromatase inhibitor treatment. Sci Rep. 2013;3:3400. doi: 10.1038/srep03400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanentzapf G, Devenport D, Godt D, Brown NH. Integrin-dependent anchoring of a stem-cell niche. Nat Cell Biol. 2007;9:1413–18. doi: 10.1038/ncb1660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tegelenbosch RA, de Rooij DG. A quantitative study of spermatogonial multiplication and stem cell renewal in the C3H/101 F1 hybrid mouse. Mutat Res. 1993;290:193–200. doi: 10.1016/0027-5107(93)90159-d. [DOI] [PubMed] [Google Scholar]
- Teixeira VH, Nadarajan P, Graham TA, Pipinikas CP, Brown JM, et al. Stochastic homeostasis in human airway epithelium is achieved by neutral competition of basal cell progenitors. eLife. 2013;2:e00966. doi: 10.7554/eLife.00966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thorel F, Nepote V, Avril I, Kohno K, Desgraz R, et al. Conversion of adult pancreatic α-cells to β-cells after extreme β-cell loss. Nature. 2010;464:1149–54. doi: 10.1038/nature08894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tran J, Brenner TJ, DiNardo S. Somatic control over the germline stem cell lineage during Drosophila spermatogenesis. Nature. 2000;407:754–57. doi: 10.1038/35037613. [DOI] [PubMed] [Google Scholar]
- Tran V, Lim C, Xie J, Chen X. Asymmetric division of Drosophila male germline stem cell shows asymmetric histone distribution. Science. 2012;338:679–82. doi: 10.1126/science.1226028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tulina N, Matunis E. Control of stem cell self-renewal in Drosophila spermatogenesis by JAK-STAT signaling. Science. 2001;294:2546–49. doi: 10.1126/science.1066700. [DOI] [PubMed] [Google Scholar]
- Tursun B, Patel T, Kratsios P, Hobert O. Direct conversion of C. elegans germ cells into specific neuron types. Science. 2011;331:304–8. doi: 10.1126/science.1199082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uhlenhaut NH, Jakob S, Anlag K, Eisenberger T, Sekido R, et al. Somatic sex reprogramming of adult ovaries to testes by FOXL2 ablation. Cell. 2009;139:1130–42. doi: 10.1016/j.cell.2009.11.021. [DOI] [PubMed] [Google Scholar]
- Vermeulen L, Morrissey E, van der Heijden M, Nicholson AM, Sottoriva A, et al. Defining stem cell dynamics in models of intestinal tumor initiation. Science. 2013;342:995–98. doi: 10.1126/science.1243148. [DOI] [PubMed] [Google Scholar]
- Voog J, D’Alterio C, Jones DL. Multipotent somatic stem cells contribute to the stem cell niche in the Drosophila testis. Nature. 2008;454:1132–36. doi: 10.1038/nature07173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Voog J, Sandall SL, Hime GR, Resende LP, Loza-Coll M, et al. Escargot restricts niche cell to stem cell conversion in the Drosophila testis. Cell Rep. 2014;7:722–34. doi: 10.1016/j.celrep.2014.04.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang L, Li Z, Cai Y. The JAK/STAT pathway positively regulates DPP signaling in the Drosophila germline stem cell niche. J Cell Biol. 2008;180:721–28. doi: 10.1083/jcb.200711022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu X, Oatley JM, Oatley MJ, Kaucher AV, Avarbock MR, Brinster RL. The POU domain transcription factor POU3F1 is an important intrinsic regulator of GDNF-induced survival and self-renewal of mouse spermatogonial stem cells. Biol Reprod. 2010;82:1103–11. doi: 10.1095/biolreprod.109.083097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yadlapalli S, Yamashita YM. Chromosome-specific nonrandom sister chromatid segregation during stem-cell division. Nature. 2013;498:251–54. doi: 10.1038/nature12106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamashita YM, Fuller MT. Asymmetric centrosome behavior and the mechanisms of stem cell division. J Cell Biol. 2008;180:261–66. doi: 10.1083/jcb.200707083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamashita YM, Jones DL, Fuller MT. Orientation of asymmetric stem cell division by the APC tumor suppressor and centrosome. Science. 2003;301:1547–50. doi: 10.1126/science.1087795. [DOI] [PubMed] [Google Scholar]
- Yamashita YM, Mahowald AP, Perlin JR, Fuller MT. Asymmetric inheritance of mother versus daughter centrosome in stem cell division. Science. 2007;315:518–21. doi: 10.1126/science.1134910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yan D, Neumuller RA, Buckner M, Ayers K, Li H, et al. A regulatory network of Drosophila germline stem cell self-renewal. Dev Cell. 2014;28:459–73. doi: 10.1016/j.devcel.2014.01.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshida S. Elucidating the identity and behavior of spermatogenic stem cells in the mouse testis. Reproduction. 2012;144:293–302. doi: 10.1530/REP-11-0320. [DOI] [PubMed] [Google Scholar]
- Zhang H, Zheng W, Shen Y, Adhikari D, Ueno H, Liu K. Experimental evidence showing that no mitotically active female germline progenitors exist in postnatal mouse ovaries. PNAS. 2012;109:12580–85. doi: 10.1073/pnas.1206600109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Q, Shalaby NA, Buszczak M. Changes in rRNA transcription influence proliferation and cell fate within a stem cell lineage. Science. 2014;343:298–301. doi: 10.1126/science.1246384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Z, Lv X, Jiang J, Zhang L, Zhao Y. Dual roles of Hh signaling in the regulation of somatic stem cell self-renewal and germline stem cell maintenance in Drosophila testis. Cell Res. 2013;23:573–76. doi: 10.1038/cr.2013.29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou Q, Brown J, Kanarek A, Rajagopal J, Melton DA. In vivo reprogramming of adult pancreatic exocrine cells to β-cells. Nature. 2008;455:627–32. doi: 10.1038/nature07314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zoller R, Schulz C. The Drosophila cyst stem cell lineage: Partners behind the scenes? Spermatogenesis. 2012;2:145–57. doi: 10.4161/spmg.21380. [DOI] [PMC free article] [PubMed] [Google Scholar]