Abstract
Muscarinic acetylcholine receptors (mAChRs) have long been viewed as viable targets for novel therapeutic agents for the treatment of Alzheimer's disease and other disorders involving impaired cognitive function. In an attempt to identify orthosteric and allosteric modulators of the muscarinic acetylcholine receptor M4 (M4), we developed a homogenous, multiparametric, 1,536 well assay to measure M4 receptor agonism, positive allosteric modulation (PAM) and antagonism in a single well. This assay yielded a Z’ of 0.85 ± 0.05 in the agonist, 0.72 ± 0.07 in PAM, and 0.80 ± 0.06 in the antagonist mode. Parallel screening of the M1 and M5 subtypes using the same multiparametric assay format revealed chemotypes that demonstrate selectivity and/or promiscuity between assays and modalities. This identified 503 M4 selective primary agonists, 1,450 PAMs and 2,389 antagonist hits. Concentration response analysis identified 25 selective agonists, 4 PAMs and 41 antagonists. This demonstrates the advantages of this approach to rapidly identify selective receptor modulators while efficiently removing assay artifacts and undesirable compounds.
Introduction
G-protein-coupled receptors (GPCRs) are implicated in a multitude of human disorders that have been linked to associated mutations and polymorphisms.1,2 These receptors are encoded by more than 1,000 genes, yet synthetic ligands exist for only a small fraction of the receptor superfamily.3 Another approach to discovering ligands that act on the orthosteric site of GPCRs is the development of selective modulators that bind at an alternatively located binding site (allosteric site) to either potentiate or inhibit the activation of the receptor by its natural ligand.4 This approach has proven particularly fruitful for identifying metabotropic glutamate receptor (mGlu) ligands and there is mounting evidence that the same may hold true for muscarinic acetylcholine receptors (mAChR).5
To date, five mAChR subtypes have been identified (M1–M5) which play important roles in mediating the actions of acetylcholine (ACh) in the peripheral and central nervous systems.6 Of these, M1 and M4 are the most heavily expressed in the central nervous system (CNS) and represent attractive therapeutic targets for cognitive illnesses such as Alzheimer's disease (AD) and schizophrenia.7 In contrast, the adverse effects of cholinergic agents are thought to be primarily due to activation of peripheral M2 and M3 mAChRs. Due to the high sequence homology and conservation of the orthosteric ACh binding site among the mAChR subtypes, development of selective chemical agents for a single subtype has been largely unsuccessful. Specifically, the absence of highly selective activators of M4 has made it impossible to test the role of selective M4 activation.8 On the other hand, novel compound scaffolds that behave as selective agonists, PAMs, or antagonists of any muscarinic receptor may have significant value as chemical probes.9
In past literature, the term multiparametric is used to describe assays with multiple end-points from the same experiment.10 Here, multiparametric is used to describe an assay where each well provides readouts for multiple modes of pharmacology, in this case agonist, antagonism and positive allosteric modulation. Multiparametric assays are an increasingly popular approach to efficiently investigate receptor pharmacology in a variety of targets; recently, these types of HTS assays have involved mAChRs.9 These reports, however, use different parallel processes requiring multiple compound additions, to find selective, confirmed inhibitors or activators of M4, but not PAMs.
Here, we describe a homogenous, single compound addition, multiparametric, 1,536 well screening assay to measure M4 receptor agonism, positive allosteric modulation and antagonism in the same well. We utilized well known control compounds to validate each mode; acetylcholine for the agonist mode, ML108 for the PAM mode and atropine for the antagonist mode.11 The performance of this assay in an HTS campaign against a diverse, public domain compound collection is documented. Further, a methodological approach based on parallel HTS efforts is presented to better understand the behaviors of compounds demonstrating M4 specific pharmacology in contrast to those appearing to be artifacts associated with this assay format.
Materials and Methods
Cell lines
Human M1 (hM1) cDNA in pcDNA3.1 (+) was purchased from www.cDNA.org. hM1 was transfected into CHO cells purchased from the ATCC (www.atcc.org), and single neomycin-resistant clones were isolated and screened for M1-mediated calcium mobilization. hM1/CHO cells were cultured in Ham’s F-12; 10% FBS, 20mM HEPES, 50µg/mL G418 (Mediatech, Inc., Herndon, VA). The human M4 (hM4) cDNA in pcDNA3.1 (+) was purchased from www.cDNA.org. CHO cells purchased from the ATCC (http://www.atcc.org), were stably transfected with hM4 cDNA along with the chimeric G protein Gqi512 to facilitate measurement of receptor function via intracellular calcium in pIREShygro (Invitrogen, Carlsbad, CA) and single hygromycin- and neomycin-resistant clones were isolated and screened for M4-mediated calcium mobilization. hM4/CHO-Gqi5 cells were cultured in Ham’s F-12; 10% FBS, 20mM HEPES, 50µg/mL G418 (Mediatech, Inc., Herndon, VA), 500 µg/ml Hygromycin. Human M5 (hM5) CHO cells were a gift from the laboratory of Allan Levey (Emory University). hM5/CHO cells were cultured in Ham’s F-12; 10% FBS, 20mM HEPES, 50µg/mL G418 (Mediatech, Inc., Herndon, VA). Parental CHO-K1 cells were purchased from ATCC (Manassas, VA).
Cell culture
Cells were cultured in T-175 cm2 flasks (Corning, Corning NY) at 37 °C and 95% relative humidity. Unless indicated otherwise, all cell culture reagents were sourced through Life Technologies, Carlsbad, USA. The growth media consisted of Ham's F-12 nutrient media supplemented with 10% v/v heat-inactivated qualified fetal bovine serum (Hyclone Laboratories Inc., Logan, UT), 20 mM HEPES, 500 micrograms/mL Geneticin, 200 micrograms/mL Hygromycin and 1X penicillin and streptomycin mix. The parental CHO-K1 cell line was cultured in the same growth media lacking Geneticin and Hygromycin.
FLIPR assay
The assay media consisted of Ham’s F-12 nutrient media supplemented with 10% v/v heat-inactivated qualified fetal bovine serum, and 20 mM HEPES. All assays were run in Corning 1,536 well clear bottom plates (part 7338, Corning). Fluo-8 detection kits were used for all FLIPR assays (part 36316, AAT Bioquest, Sunnyvale, CA), which included addition of 250 mM probenicid (pH 8) (Sigma, St. Louis, MO) while reading on the fluorescence imaging plate reader (FLIPR, Molecular Devices, Sunnyvale, CA). Agonist control acetylcholine (part A6625), antagonist control Atropine (part A0132) and PAM control ML108 (also known as VU0152100, part V5015) were purchased from Sigma. All additions of compound and controls were accomplished by an integrated 1,536 pintool mounted on the FLIPR Tetra. While all three readouts occur with only one compound addition, the system does not read the well continuously but, rather separates the reads by the amount of time required for the instruments manipulation of the source plates required for the next ligand and control addition. Cell and reagent additions were performed on the Kalypsys/GNF robotic screening platform at the Scripps Research Institute Molecular Screening Center (The Scripps Research Institute, Jupiter, FL). The final protocol is summarized in Table 1. Detailed protocols for every assay mentioned in this manuscript can be retrieved on the PubChem website using its respective PubChem AID shown in Table 2.
Table 1.
M4 uHTS assay protocol in 1,536 well plate format for all 3 modes
| Order | Step | Condition | Comments |
|---|---|---|---|
| 1 | Cell dispensing | 3µL/well | 750 cells/well final concentration |
| 2 | Primary incubation time | 17 hours | 37°C, 95% R.H., 5% CO2 |
| 3 | Fluo8 addition | 2µL/well | 1250X concentrated Fluo-8 |
| 4 | Secondary incubation time | 1 hour | 37°C, 95% R.H., 5% CO2 |
| 5 | Tertiary incubation time | 30 min | Room temperature |
| 6 | Agonist mode baseline read | 5 sec | Baseline read on FLIPR |
| 7 | Compound addition | 15nL/well | 3µM compound, EC100 and DMSO (EC0) controls added by FLIPR 0.3% DMSO final concentration |
| 8 | Agonist mode kinetic read | 140 sec | Kinetic read on FLIPR |
| 9 | PAM mode baseline read | 5 sec | Baseline read on FLIPR |
| 10 | EC20 agonist stimulus addition | 15nL/well | EC20, EC100, & DMSO (EC0) controls added by FLIPR |
| 11 | PAM mode kinetic read | 140 sec | Kinetic read on FLIPR |
| 12 | Antagonist mode baseline read | 5 sec | Baseline read on FLIPR |
| 13 | EC80 agonist stimulus addition | 15nL/well | EC80, EC100, & DMSO (EC0) controls added by FLIPR |
| 14 | Antagonist mode kinetic read | 140 sec | Kinetic read on FLIPR |
Table 2.
uHTS primary campaign summary and results
| Step | Screen type |
Target | Number of compounds tested (3uM) |
Selection criteria |
Number of selected compounds |
PubChem AIDd |
Assay statistics | |
|---|---|---|---|---|---|---|---|---|
| Z’ | S/B | |||||||
| 1a | Primary screen Agonist | M4 | 364,131 | 31.95%a | 503/281c | 624127 | 0.85 ±0.05 | 2.77 ±0.47 |
| 1b | Primary screen PAM | M4 | 364,131 | 22.06%b | 1,450/1,414c | 624126 | 0.72 ±0.07 | 2.09 ±0.30 |
| 1c | Primary screen Antagonist | M4 | 364,131 | 26.39 %a | 2640/2389c | 624125 | 0.80 ±0.06 | 2.59 ±0.35 |
| 2a | Primary Counter screen Agonist | CHOK1 | 362,351 | 15.08% | 1,732 | 602248 | 0.89 ±0.03 | 3.37 ± 0.25 |
| 2b | Primary Counter screen PAM | CHOK1 | 362,351 | 39.90% | 78 | 602247 | 0.61 ±0.07 | 2.57 ±0.30 |
| 2c | Primary Counter screen Antagonist | CHOK1 | 362,351 | 23.66% | 2,170 | 602250 | 0.81 ±0.05 | 2.96 ± 0.18 |
The primary screen hit-cutoff was calculated at the average percent activity of all test compounds plus three times the standard deviation across the entire screen.
The primary screen hit-cutoff was calculated at the average percent activation of all test compounds plus three times the standard deviation for each plate.
281 agonist, 1,411 PAMs and 389 antagonists compounds were found to be specific to their respective mode.
PubChem AIDs are accessible on-line at http://www.ncbi.nlm.nih.gov/sites/entrez?db=pcassay&term=xxxx, where xxxx represents the PubChem AID number listed in the table.
N.A., not applicable
Screening Data
Kinetic data was acquired by the FLIPR at the rate of 1 Hz. Agonist, PAM and antagonist mode kinetic data were saved as separate data files. Individual well activity is interpreted as the ratio of the maximum signal divided by the minimum signal from the basal read or Max/ baseline. All data files were uploaded into the Scripps database for plate QC and hit identification. Activity for the agonist and PAM modes was normalized on a per-plate basis using the following equation:
| Eq (1) |
For all three modes, Test well refers to those that contain test compounds. Low Control wells for the agonist mode contain DMSO only. For the PAM mode, Low Control wells contain acetylcholine at EC20. High Control wells for both PAM and agonist mode contain acetylcholine at EC100. Activity for the antagonist mode was normalized on a per-plate basis using the following equation:
| Eq (2) |
In this case, Low Control is defined as wells that contain EC80 of acetylcholine. High Control wells contain DMSO only.
A Z’ value greater than 0.5 was required for a plate to be consider valid.13 During the primary screen, test compounds from the library were screened in singlicate at a final nominal test concentration of 3M (final DMSO concentration of 0.3%). For each mode, the hit-cutoff used to qualify active compounds was calculated as the average percentage activity (agonist, PAM modes) or inhibition (antagonists) of all compounds tested plus three times the standard deviation.14
Dose-response curves were fitted using a four parameter equation describing a sigmoidal curve without constraining parameters within the Assay Explorer software (Symyx Technologies). Where appropriate, data have been represented using Prism version 4.03 (GraphPad Software, San Diego, CA). In those cases EC50 or IC50 determinations were done using the variable slope sigmoidal curve analysis tool.
A 4-way Venn diagram generator was used to compare the parental CHO-K1, M1 and M5 multiparametric assay data vs. the M4 data described here. Each of these assays was run at the SRIMSC and previously published in PubChem (AID#s 588814, 588819, 588852, 624037, 624038, and 624040). The tool used is freely available at http://www.pangloss.com/seidel/Protocols/venn4.cgi.
Screening Library
The Molecular Libraries Small Molecule Repository (MLSMR) library was provided by BioFocus DPI (South San Francisco, CA) through the NIH’s Roadmap Initiative. Details can be found online at http://mli.nih.gov/mli/compound-repository/mlsmr-compounds/. The MLSMR library is a collection of over 360,000 synthetic and natural small molecules from both commercial and academic sources, that can be grouped into the following four categories: 1) specialty sets of known bioactive compounds such as drugs and toxins (0.65%), (2) focused libraries aimed at specific target classes (2.85%), (3) non-commercial sources (7.4%) and (4) diversity sets covering a large area of the chemical space (89.1%).
Results
Assay principle and screening strategy
As we had great interest in efficiently identifying M4 agonists, antagonists and PAMs for this research program, a miniaturized multiparametric FLIPR-based assay was developed. Using methods similar to those described previously but, in 1536-well format, it measures receptor agonism, positive allosteric modulation and antagonism from a single well, following a single addition of test compound.15,16,17,18,19 As developed, a fully automated robotic platform accomplishes the dispensing of cells and detection reagent to the microtiter plate, as well as necessary incubation prior to the intracellular calcium assay. Intracellular calcium measurements, including test compound and control addition, are performed by the FLIPR. To enable continuous HTS, the originally separated assays were concatenated into a single assay protocol with a specific order of pharmacologic test modes: agonism was measured first, followed by PAM, then antagonism.
Each mode of detection relied on a specific set of plate controls. These control were used either for response normalization or to verify appropriate agonist, PAM or antagonist pharmacologic response in that mode. Figure 1 illustrates the performance of various controls in each mode of the resulting assay detection workflow that was applied to the discovery of modulators of M4. We used three relevant controls: acetylcholine, atropine and ML108 as references for M4 receptor agonism, antagonism and PAM, respectively. Figure 1 shows examples of typical FLIPR kinetic response traces obtained for each of these reference compounds in all three modes. The first kinetic measurement, which is utilized for the detection of potential agonists, was initiated by the addition of test compound to cells loaded with the intracellular calcium probe. Agonists were identified as compounds that show a response three standard deviations greater than cells challenged with vehicle (DMSO). The second measurement, for detection of PAMs, was performed in the presence of EC20 acetylcholine stimulation. PAMs were identified as compounds in wells that exhibited a response three standard deviations greater than that of the EC20 stimulation alone. The third and final measurement was used to detect antagonists in the presence of an EC80 challenge of acetylcholine. Antagonists were identified as compounds in wells that had a response three standard deviations lower than that of wells challenged with the EC80.
Figure 1.
(A.) Illustration of FLIPR kinetic trace data for each mode, agonist, PAM or antagonist, for each control compound. The compound trace, as tested at 3uM, in comparison to the controls for each mode is shown in blue. Red=acetylcholine at EC100, purple=acetylcholine at EC80, green=acetylcholine at EC20, black=DMSO only. (B.) CRC graphs for control compounds of each screening mode. The y-axis represents the ratio of the maximum signal divided by the minimum signal from the basal read of the particular mode. The x-axis represents the log of the molar concentration of the control compound used for that mode. The curves represent the mean and standard deviation for 16 points. pEC50 and pIC50 values for each control are shown (N=>10 experiments).
Multiparametric, 1536-well assay optimization
The first step in 1,536 well assay development was to optimize the cell number per well and then Fluo8 incubation time using methods previously described.20 The signal to basal ratio (S/B) in the agonist mode, as calculated by the high control response divided by low control response, was not significantly affected by the number of cells plated per well. However, increasing the cell concentration resulted in sub-optimal Z’ values (Supplemental Figure 1A and B). Hence, a cell density of 750 cells per well was chosen.
Fluo8 incubation time was also optimized. The length of incubation with Fluo8 had little effect on Z’ but longer incubation times increased the S/B (Supplemental Figure 1C). The stability of the S/B between 60 and 90 minutes allows for some flexibility during robotic screening, and a 60-minute incubation time was chosen for all future experiments.
Characterization of controls
Critical to the success of the multiparametric HTS assay was the utilization of dedicated pharmacologic control wells specific to each measurement mode, as well as control wells that indicated the cells were competent throughout the assay (Supplemental Figure 2). As expected, having wells in each mode for the “High” and “Low” responses facilitated the efficient identification of hits, as each well response was normalized to synchronous responses in control wells. Further, each assay mode could be treated as a unique entity in our HTS database, streamlining the plate-based QC. Unique to the multiparametric assay format, we found that additional controls, viz. an EC0 (DMSO) in the PAM mode and an EC100 of acetylcholine in the antagonist mode, also aided in the efficient QC of assay quality. Specifically, we used the responses from these wells to verify the M4 cells were fully capable of eliciting basal and full calcium responses throughout the entire assay. Kinetic traces for control compounds tested in each mode are shown in Figure 1.
The M4 multiparametric FLIPR assay, as outlined in Table 1, was run in homogeneous format. All three controls as shown in Figure 1B, acetylcholine (pEC50=7.45±7.65), ML108 (pEC50=6.59±6.91), and atropine (pIC50=9.65±10.22), yielded pEC50 or pIC50 values similar to previously published in the agonist, PAM, and antagonist modes respectively.11 Notably, ML108 was also found to be active in the agonist assay mode.
Data deconvolution and compound triage
Successful execution of the multiparametric assay was dependent upon reliable identification of hits within a mode as well as identification of a compound’s pharmacologic activity across all measurement modes. For demonstration purposes, kinetic traces from a set of M4 HTS hits are shown in Figure 2. Within the agonist mode, a compound was defined as an agonist if it elicited a response greater than that of a vehicle (DMSO) challenge (Figure 2, compound I). Since the same cells are used for subsequent measurements, the magnitude of the response in the following two modes was highly dependent on the level of the response in the agonist mode. For example, an EC100 challenge of the full agonist acetylcholine elicits a large calcium response in the agonist mode, which persists through the PAM mode. In the antagonist mode, upon re-stimulation of the cells with an EC80 of acetylcholine, the compound fails to elicit a significant calcium response (Figure 1A, top panel). This phenomenon is attributed to the depletion of intracellular calcium stores by acetylcholine in the agonist mode, leaving less calcium available for the cells to respond to the EC80 challenge in the antagonist mode.21 In the case of compound I in Figure 2, the “partial” response in the agonist mode was insufficient to deplete intracellular calcium stores, allowing for the compound to be identified as a moderate agonist hit with no PAM or antagonist activity. In contrast, compound IV behaves similarly to acetylcholine in Figure 1A, indicating that it may be a full agonist. In this multiparametric method, an agonist that behaves similarly to acetylcholine will be inappropriately recorded as a hit in the antagonist mode. That is, the lack of a calcium response in the antagonist mode would be similar to that of an antagonist compound and therefore incorrectly scored as a hit. This potential “false positive” artifact is a strength of the multiparametric format, as it distinguished efficacious agonists (i.e. those that liberate the same calcium stores as a muscarinic receptor agonist) from partial agonists or assay artifact.
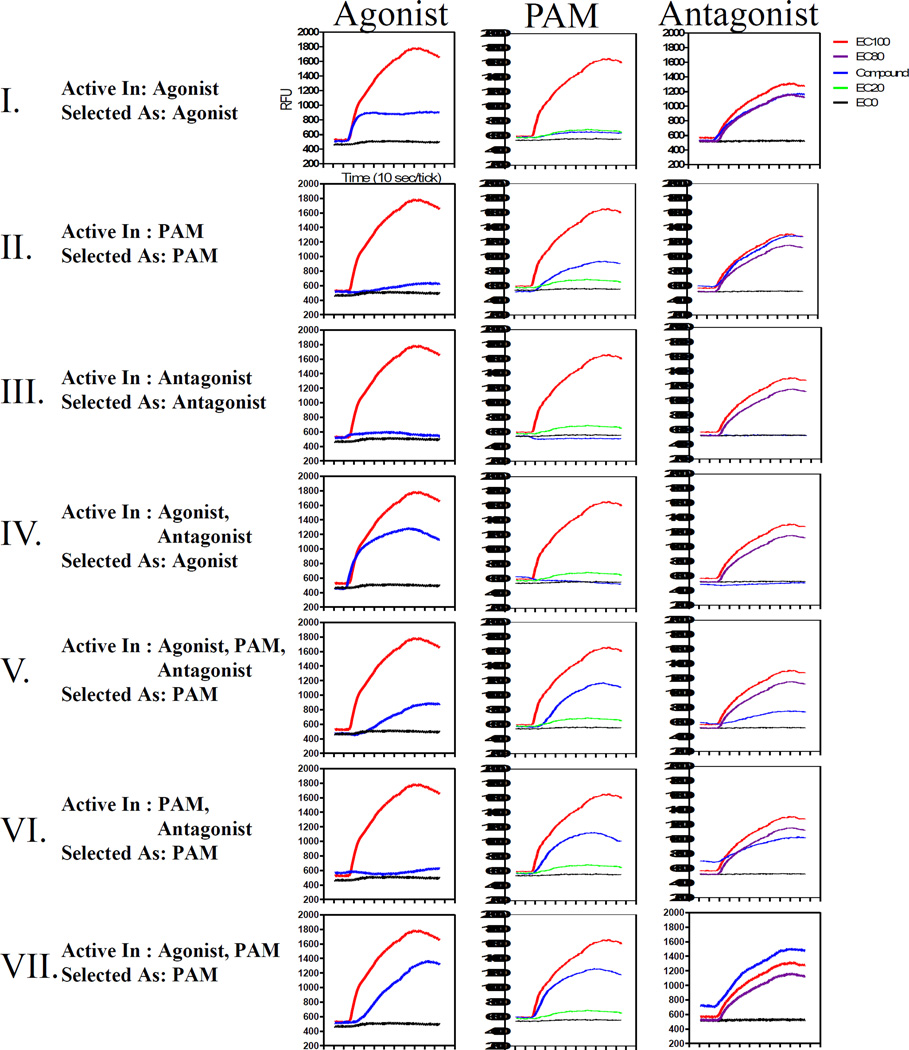
Figure 2.
Typical trace files for compounds shown as an example of responses observed in each screening mode (I–VII). The compound trace, as tested at 3µM, in comparison to the controls for each mode is shown in blue. Red=acetylcholine at EC100, purple=acetylcholine at EC80, green=acetylcholine at EC20, black=DMSO only. The analysis of the traces is described in two respects: the mode/s the compound was found “active in” and then which mode the compound was progressed forward as or “selected as”.
For the M4 HTS, antagonists were defined as compounds that elicited a response less than that of the EC80. An example of complete antagonism is the HTS control, atropine, shown in Figure 1A. As expected, a putative antagonist would be inactive in the agonist mode and abrogate the EC20 response in the PAM mode. Compound III in Figure 2 is an HTS antagonist mode hit that behaves similarly to atropine in all three test modes: In the agonist mode this compound has no response; in the PAM mode, the same compound blocks the response of the EC20 acetylcholine challenge; in the antagonist mode, the compound completely abrogates the EC80 response of the acetylcholine challenge. In this case the behavior of the hit in all three test modes of the multiparametric assay adds validity to its identification as an M4 antagonist.
Critical to a compound’s identification as PAM hit was its behavior within the PAM mode itself, however the identification of putative PAMs also benefitted from the multiparametric HTS approach. Ideally, a PAM would not elicit a calcium response by itself, but rather in the presence of an orthosteric agonist. An example of this type of response profile is exhibited by compound II in Figure 2, as it yields an insignificant response in the agonist mode, but a significantly higher response than the acetylcholine control EC20 and EC80 responses in the PAM and antagonist modes, respectively. In recombinant systems, agonist behavior of PAMs has been observed, presumably due to the overexpression of the target receptor and/or signaling mechanism components.4,22 This type of behavior was observed in the multiparametric HTS format for the PAM control ML108 (Figure 1A, middle panel) as well as compounds V and VII in Figure 2; all yielded responses in the agonist mode as well as in the PAM mode and were classified as PAMs.
Interestingly, PAM hits yielded different magnitudes of kinetic responses in the antagonist mode, appearing to have peak responses indistinguishable (cf. compound II), below (cf. compounds V,VI), or elevated (cf. compound VII) in reference to the EC20 acetylcholine control response. For example, compound V generated a moderate agonist and a strong PAM response, which was unable to decay to basal levels before the EC20 and EC80 challenges were added. However, the method for calculating the antagonist mode HTS response, i.e. the ratio of the peak response divided by the same well’s baseline level response obtained just prior to the EC80 of Ach addition, made all these compounds appear as hits in the antagonist mode. All PAM hits that displayed these patterns of responses in multiparametric HTS assay were selected for follow up, as they could be distinguished from compounds displaying classical agonism (cf. compounds I) or antagonism (cf. compound III). However, to validate their PAM pharmacology, more detailed dose-response studies are necessary.23
Multiparametric screen for the identification of M4 modulators
The M4 multiparametric FLIPR assay described above was run against the MLPCN library of 364,131 compounds. These data and the outcome of the primary counterscreen are summarized in Table 2.
During the course of the campaign, monitoring the range of receptor stimulation and Z’ showed that, compared to basal and EC100 controls, the PAM EC20 response yielded an average value of 27.2±1.6%, while the antagonist mode EC80 response demonstrated an average value of 79.8±2.2%, compared to the EC0 (DMSO only) for an N=302 plates. Note, a typical EC20 response determined acceptable for screening was 10–30% of EC100 and the EC80 response range was acceptable between 70–90% of EC100. For this effort, the average Z’ of each mode always exceeded 0.5 as listed in Table 2.
The results of the primary multiparametric screen yielded 503 M4 agonist, 1,450 PAM and 2,640 antagonist mode hits (Table 2). Each mode’s hits were reanalyzed based upon the hit compound’s activity across the entire multiparametric assay. The results of this reanalysis method are shown in Table 2. Of the 503 agonist hits, 281 or 55.86% were active only in that mode, while 97.52% or 1414 of the 1,450 PAM hits acted solely in the PAM mode. Of the 2,640 antagonist hits, 2,389 or 90.4% had activity only in the antagonist mode. When comparing the overlap between agonist and PAM hits, only six hits acted as both an agonist and a PAM of which five of those were also found as antagonists. All primary hits were assessed for promiscuity against the multiparametric data generated from a parental CHO-K1 cell FLIPR counterscreen (Table 2) and parallel executed M1, M5 HTS assays described in detail elsewhere (PubChem AIDs: 588814, 588819, 588852, 624037, 624038, and 624040) and summarized below.9,24
Parallel assays for selectivity and promiscuity
With the goal of identifying selective M4 modulators, we performed counterscreens against two cell lines, one expressing the M1 receptor and the other expressing M5 receptor. This was done in parallel to the M4 HTS, and each cell line was screened against the entire MLPCN compound collection. Although not described here, hit identification for the M1 and M5 multiparametric HTS campaigns was done similar to that illustrated in Figure 2.
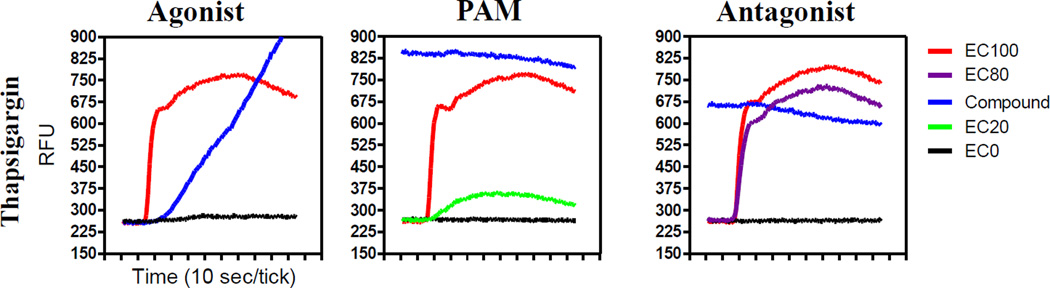
Using this approach, we found M4 HTS hits that non-specifically modulated the release of calcium, as evidenced by their activity in the parallel HTS assays across multiple screening modes. The method used for triaging non-specific activators is illustrated in Figure 3. In this example, addition of 3 µM thapsigargin, which is known to intrinsically block the ability of cells to pump calcium into the endoplasmic reticulum, results in an increase in intracellular calcium and ultimately would be identified as a hit in all modes of the M4 multiparametric assay as well as all counterscreens run in parallel to the M4 HTS campaign. Compounds that were inactive in the parental cell HTS campaign but active in the M4, M1 and/or M5 HTS, were similarly identified and triaged.
Figure 3.
The effects of thapsigargin on Fluo8 response in all 3 modes of the M4 multiparametric assay. The y-axis represents the relative fluorescence units (RFUs). The x-axis represents the kinetic read time in seconds for each mode which was the same in each case. The controls for each mode are color coded.
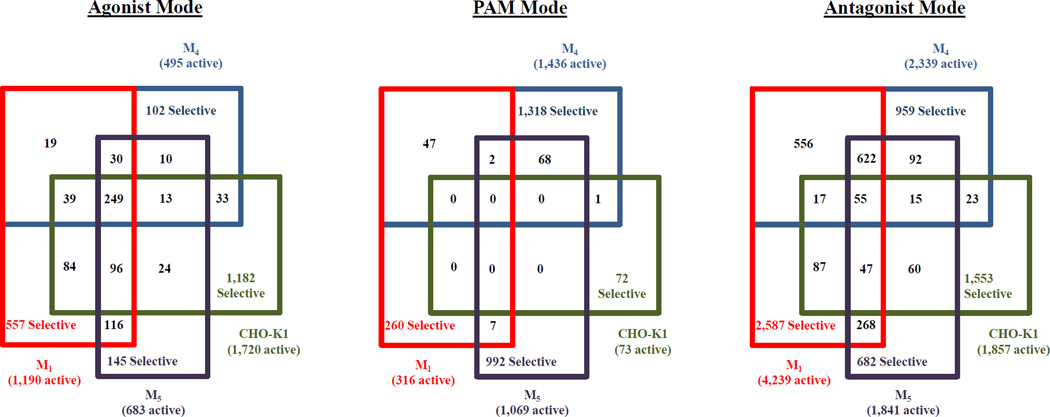
Venn comparison of results from the M4, parental, M1 and M5 primary HTS assays identified M4 HTS hits that exhibited non-specific pharmacology or were assay artifact. Specifically, 102 agonist hits, 1,318 PAM hits, and 959 antagonist hits exhibit selectivity to the M4 receptor (Figure 4). Of the 2,379 combined total M4 selective hits, in-silico triage reduced the list to 2,185 compounds of interest of which 2,103 were available and retested as concentration response curves (CRC) across the three modes as a 10 point, 1:3 dilution titration in triplicate. These same compounds were tested in multiparametric M4, M1, M5, and CHO-K1 assay formats. Following CRC assays, compounds were considered a hit only if they yielded graded dose response curves with EC50 or IC50 values less than 10 µM. From these titration assays, 25 were found to be M4 selective agonists, 4 compounds were found to be M4 selective PAMs, while 41 compounds were found to be M4 selective antagonists. Some of these were followed up at M2 and M3 when selectivity studies were performed lower in the tier, but it was not deemed that primary screening for these targets was resource and time efficient. Of the 21 compounds that progressed through M2 and M3 selectivity assays, only 2 were active as M3 agonists. The individual hit-cutoff applied to each of the 12 primary campaigns was low, averaging only 24.30±7.68%. When combined with our method to qualify dose response hits, this lead to a small but, potent and selective group of compounds being identified. In addition, similar to the triage of the primary HTS data, all CRC data was filtered to remove hits that overlapped in the other assays. Examples of the CRC data generated for the three most potent compounds for each mode of the M4 assay are shown in Supplemental Figure 3. These compounds are specific to the M4 assay described here and were not found active in the others.
Figure 4.
4-way Venn diagram for muscarinic screens in the three modes. Data from the primary assays for M1, M4, M5 and parental CHO-K1 cells are shown here. The numbers in parenthesis next to the target are the number of hits specific for that screening mode. The numbers in the various boxes represent the number of compounds found to respond in those overlapping assays.
Following this triage there is still the potential of finding compounds that trigger a native Gi-coupled receptor in the CHO-M4-Gqi5 cell line and signal through the Gqi5 protein to activate calcium. Since the parental cell line does not have Gqi5 then it is possible that non-M4 receptor mediated agonist activity could be seen in the M4 cell line and not picked up in the CHO-parental counterscreen. We also did not include Gqi5 in the M1 and M5 cell lines and hence compounds could slip past here too. This is likely not so much an issue with PAMs or antagonists as the activity of these compound relies upon its ability to modulate ACh responses. We recognize that additional follow up of the 503 putative agonist hits will be required in an assay in which M4 activity is monitored using a native Gi/o-dependent assay, such as M4 coupling through potassium (GIRK) channels.25 Similar to the M1 and M5 specific modulators described elsewhere, the details of the efficacy found for the M4 selective compounds including further mode of action studies, SAR and pharmaceutical development will be the topic for future publication.24,26
Discussion
In this report, we present a homogeneous, 1,536-well HTS assay that screens for a compound’s agonist, PAM, and antagonist activity on the same set of cells expressing the M4 receptor. This multiparametric assay has the benefit of reducing overall screening costs: a multiparametric HTS campaign consumes ~1/3 of reagents and cells compared to running HTS campaigns separately for each type of pharmacology. An unexpected advantage of the multiparametric format is the savings of screening campaign duration time. In our lab, an average single detection mode HTS campaign of the MLPCN collection takes 5 business days of robot time per target; in addition, scale up of cells takes an additional 10 days per screen. Here, we were able to generate three pharmacologically distinct data sets against 364,131 compounds in 8 business days of robot time with 10 days for cell preparation for a 2.5 fold reduction in time (18 days vs. 45).
The ability to assess a compound’s behavior across the three different test modes is another advantage of the multiparametric assay format. Behavior of a hit across all three modes of the HTS assay facilitated the rapid identification of hits that were “classical” agonists, PAMs or antagonists. The identification of agonists that exhibited more complex pharmacology was also aided by the multiparametric approach. Careful analysis of a hit’s response across all modes allowed the identification of agonists that holistically desensitized the M4 intracellular calcium response, and PAMs that demonstrated agonist activity. Triage of selective hits was also aided by comparison of the M4 primary hits with hits from three other multiparametric HTS campaigns.
Knowing that adverse effects of cholinergic agents are thought to be primarily due to activation of peripheral M2 and M3 mAChRs raises the question why we chose to triage against M1 and M5. We were initially interested in M1, M4 and M5 as targets and felt this was the most efficient way to find compounds for each target and to get an idea of selectivity. Finally, the antagonist component of the screen could potentially identify negative allosteric modulators or NAMs. One would anticipate these would be further elucidated in secondary assays when tested in the presence of a suboptimal concentration of a known antagonist such as atropine while looking for further diminishment of receptor signaling.
While we successfully reduced timelines and increased output one must be aware that assay development and implementation may take longer. Crucially, there must be pharmacological controls available for each mode in order to optimize each assay, help classify compounds and determine their fate. Even with excellent controls caution must be exercised. For instance, many PAM hits will not reach 100% of maximal Ach activity. Here, we set the high control as an EC100 of Ach which seems prudent but, may have unrealistically biased us toward finding and or normalizing data that demonstrates low activity for PAMs. In addition, data management becomes more daunting. Software tools must be put in place that allows compilation of concatenated data to yield percent activity or inhibition for each mode. Finally, algorithms need to be established to quickly triage compounds that would qualify as agonists, PAMs and antagonists.
Muscarinic acetylcholine receptors (mAChRs) play a vital role in cognitive function making them important targets for drug discovery. Several types of modulators have been the focus of pharmaceutical companies to target mAChRs. Some of these areas include: agonists, antagonists, and allosteric modulators. Efforts to develop subtype-selective, mAChR agonists have been hampered by difficulty in achieving high selectivity for individual mAChRs important for CNS function (M1 and M4) while reducing adverse effects due to activation of peripheral mAChRs (especially M2 and M3). Major advances have now been achieved in the discovery of allosteric agonists and positive allosteric modulators of M1 and M4 that show greater selectivity for individual mAChR subtypes than do previous mAChR activators. Early studies indicate that these allosteric mAChR agonists and PAMs have properties needed for optimization as potential clinical candidates and have robust effects in animal models that predict efficacy in the treatment of AD, schizophrenia and related disorders.4
To address the need for subtype selective mAchR modulators, save time, and reduce cost, we have developed a rapid miniaturized method to detect the three aforementioned modulator types, successively, in the same well during a single assay requiring only one compound addition. Not only does this expedite the discovery of agonists, PAMs, and antagonists, but it also allows for more robust results as the variability in assay to assay discrepancies, day to day cell handling and other environmental conditions is removed. This method has proven to be successful as indicated by the discovery of specific probes for M1, M4, and M5 and is the focus of follow-up research here and at other institutes.27,28
Supplementary Material
Acknowledgements
We thank Pierre Baillargeon and Lina DeLuca (Lead Identification, Scripps Florida) for compound management.
Funding
The authors disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This work was supported by the National Institutes of Health’s Roadmap Initiative through grants 1X01 MH077607-01 awarded to Professor Colleen Niswender and U54MH084512 for all authors.
Footnotes
Declaration of Conflicting Interests
CMN, MRW, PJC, and CWL receive research support and royalties from AstraZeneca for the development of M4 positive allosteric modulators. The remaining authors declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
References
- 1.George SR, O'Dowd BF, Lee SP. G-Protein-coupled receptor oligomerization and its potential for drug discovery. Nat Rev Drug Discov. 2002;1:808–820. doi: 10.1038/nrd913. [DOI] [PubMed] [Google Scholar]
- 2.Rana BK, Shiina T, Insel PA. GENETIC VARIATIONS AND POLYMORPHISMS OF G PROTEIN-COUPLED RECEPTORS: Functional and Therapeutic Implications. Annual Review of Pharmacology and Toxicology. 2001;41:593–624. doi: 10.1146/annurev.pharmtox.41.1.593. [DOI] [PubMed] [Google Scholar]
- 3.Howard AD, McAllister G, Feighner SD, et al. Orphan G-protein-coupled receptors and natural ligand discovery. Trends in Pharmacological Sciences. 2001;22:132–140. doi: 10.1016/s0165-6147(00)01636-9. [DOI] [PubMed] [Google Scholar]
- 4.Jeffrey Conn P, Christopoulos A, Lindsley CW. Allosteric modulators of GPCRs: a novel approach for the treatment of CNS disorders. Nat Rev Drug Discov. 2009;8:41–54. doi: 10.1038/nrd2760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Conn PJ, Lindsley CW, Jones CK. Activation of metabotropic glutamate receptors as a novel approach for the treatment of schizophrenia. Trends in pharmacological sciences. 2009;30:25–31. doi: 10.1016/j.tips.2008.10.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ishii M, Kurachi Y. Muscarinic acetylcholine receptors. Current pharmaceutical design. 2006;12:3573–3581. doi: 10.2174/138161206778522056. [DOI] [PubMed] [Google Scholar]
- 7.Langmead CJ, Watson J, Reavill C. Muscarinic acetylcholine receptors as CNS drug targets. Pharmacology & Therapeutics. 2008;117:232–243. doi: 10.1016/j.pharmthera.2007.09.009. [DOI] [PubMed] [Google Scholar]
- 8.Katzenschlager R, Sampaio C, Costa J, Lees A. Anticholinergics for symptomatic management of Parkinson's disease. Cochrane Database of Systematic Reviews. 2002;3 doi: 10.1002/14651858.CD003735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gentry PR, Kokubo M, Bridges TM, et al. Discovery, synthesis and characterization of a highly muscarinic acetylcholine receptor (mAChR)-selective M5-orthosteric antagonist, VU0488130 (ML381): a novel molecular probe. ChemMedChem. 2014;9:1677–1682. doi: 10.1002/cmdc.201402051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Heyse S, Bruttger O, Duerr O, et al. Quantifying Bioactivity on a Large Scale: Quality Assurance and Analysis of Multiparametric Ultra-HTS Data. Journal of Laboratory Automation. 2005;10:207–212. [Google Scholar]
- 11.Lewis LM, Bridges TM, Niswender CM, et al. Discovery of a Highly Selective in vitro and in vivo M4 Positive Allosteric Modulator (PAM) Probe Reports from the NIH Molecular Libraries Program. 2010 [PubMed] [Google Scholar]
- 12.Conklin BR, Farfel Z, Lustig KD, et al. Substitution of three amino acids switches receptor specificity of Gq alpha to that of Gi alpha. Nature. 1993;363:274–276. doi: 10.1038/363274a0. [DOI] [PubMed] [Google Scholar]
- 13.Zhang JH, Chung T, Oldenburg K. A Simple Statistical Parameter for Use in Evaluation and Validation of High Throughput Screening Assays. J Biomol Screen. 1999;4:67–73. doi: 10.1177/108705719900400206. [DOI] [PubMed] [Google Scholar]
- 14.Spicer T, Fernandez-Vega V, Chase P, et al. Identification of Potent and Selective Inhibitors of the Plasmodium falciparum M18 Aspartyl Aminopeptidase (PfM18AAP) of Human Malaria via High-Throughput Screening. J Biomol Screen. 2014;19:1107–1115. doi: 10.1177/1087057114525852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Burford NT, Wehrman T, Bassoni D, et al. Identification of selective agonists and positive allosteric modulators for micro- and delta-opioid receptors from a single high-throughput screen. J Biomol Screen. 2014;19:1255–1265. doi: 10.1177/1087057114542975. [DOI] [PubMed] [Google Scholar]
- 16.Liu K, Southall N, Titus S, et al. A Multiplex Calcium Assay for Identification of GPCR Agonists and Antagonists. Assay Drug Dev Technol. 2010;8:367–369. doi: 10.1089/adt.2009.0245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Jacoby E, Bouhelal R, Gerspacher M, et al. The 7 TM G-protein-coupled receptor target family. ChemMedChem. 2006;1:761–782. doi: 10.1002/cmdc.200600134. [DOI] [PubMed] [Google Scholar]
- 18.Rodriguez AL, Grier MD, Jones CK, et al. Discovery of novel allosteric modulators of metabotropic glutamate receptor subtype 5 reveals chemical and functional diversity and in vivo activity in rat behavioral models of anxiolytic and antipsychotic activity. Molecular pharmacology. 2010;78:1105–1123. doi: 10.1124/mol.110.067207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Noblin DJ, Bertekap RL, Jr, Burford NT, et al. Development of a high-throughput calcium flux assay for identification of all ligand types including positive, negative, and silent allosteric modulators for G protein-coupled receptors. Assay Drug Dev Technol. 2012;10:457–467. doi: 10.1089/adt.2011.443. [DOI] [PubMed] [Google Scholar]
- 20.Brothers SP, Saldanha SA, Spicer TP, et al. Selective and brain penetrant neuropeptide y y2 receptor antagonists discovered by whole-cell high-throughput screening. Molecular pharmacology. 2010;77:46–57. doi: 10.1124/mol.109.058677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hodder P, Cassaday J, Peltier R, et al. Identification of metabotropic glutamate receptor antagonists using an automated high-throughput screening system. Anal Biochem. 2003;313:246–254. doi: 10.1016/s0003-2697(02)00608-5. [DOI] [PubMed] [Google Scholar]
- 22.Noetzel MJ, Rook JM, Vinson PN, et al. Functional impact of allosteric agonist activity of selective positive allosteric modulators of metabotropic glutamate receptor subtype 5 in regulating central nervous system function. Molecular pharmacology. 2012;81:120–133. doi: 10.1124/mol.111.075184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Conn PJ, Jones CK, Lindsley CW. Subtype-selective allosteric modulators of muscarinic receptors for the treatment of CNS disorders. Trends Pharmacol Sci. 2009;30:148–155. doi: 10.1016/j.tips.2008.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Han C, Chatterjee A, Noetzel MJ, et al. Discovery and SAR of muscarinic receptor subtype 1 (M) allosteric activators from a molecular libraries high throughput screen. Part 1: 2,5-Dibenzyl-2H-pyrazolo[4,3-c]quinolin-3(5H)-ones as positive allosteric modulators. Bioorg Med Chem Lett. 2015;25:384–388. doi: 10.1016/j.bmcl.2014.11.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Niswender CM, Johnson KA, Luo Q, et al. A novel assay of Gi/o-linked G protein-coupled receptor coupling to potassium channels provides new insights into the pharmacology of the group III metabotropic glutamate receptors. Molecular pharmacology. 2008;73:1213–1224. doi: 10.1124/mol.107.041053. [DOI] [PubMed] [Google Scholar]
- 26.Gentry PR. Thesis submission for PhD. to the Department of Chemical and Physical Biology. Vanderbilt University; 2014. Discovery, Optimization, and Characterization of Novel Subtype-selective M5 Muscarinic Acetylcholine Receptor Ligands. [Google Scholar]
- 27.Reid PR, Bridges TM, Sheffler DJ, et al. Discovery and optimization of a novel, selective and brain penetrant M1 positive allosteric modulator (PAM): The development of ML169, an MLPCN probe. Bioorganic & Medicinal Chemistry Letters. 2011;21:2697–2701. doi: 10.1016/j.bmcl.2010.12.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Gentry PR, Kokubo M, Bridges TM, et al. Discovery, Synthesis and Characterization of a Highly Muscarinic Acetylcholine Receptor (mAChR)-Selective M5-Orthosteric Antagonist, VU0488130 (ML381): A Novel Molecular Probe. ChemMedChem. 2014;9:1677–1682. doi: 10.1002/cmdc.201402051. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.