Abstract
Neurons and glial cells in the retina contribute to neovascularization, or the formation of abnormal new blood vessels, in proliferative retinopathy, a condition that can lead to vision loss or blindness. We identified a mechanism by which suppressor of cytokine signaling 3 (SOCS3) in neurons and glial cells prevents neovascularization. We found that Socs3 expression was increased in the retinal ganglion cell and inner nuclear layers after oxygen-induced retinopathy. Mice with Socs3 deficiency in neuronal and glial cells had substantially reduced vaso-obliterated retinal areas and increased pathological retinal neovascularization in response to oxygen-induced retinopathy, suggesting that loss of neuronal/glial SOCS3 increased both retinal vascular regrowth and pathological neovascularization. Furthermore, retinal expression of Vegfa (which encodes vascular endothelial growth factor A) was higher in these mice than in Socs3 flox/flox controls, indicating that neuronal and glial Socs3 suppressed Vegfa expression during pathological conditions. Lack of neuronal and glial SOCS3 resulted in greater phosphorylation and activation of STAT3, which led to increased expression of its gene target Vegfa, and increased endothelial cell proliferation. In summary, SOCS3 in neurons and glial cells inhibited the STAT3-mediated secretion of VEGF from these cells, which suppresses endothelial cell activation, resulting in decreased endothelial cell proliferation and angiogenesis. These results suggest that neuronal and glial cell SOCS3 limits pathological retinal angiogenesis by suppressing VEGF signaling.
Introduction
Neurovascular interactions are important in the maintenance of the nervous system, and defects in this relationship can lead to disease, such as stroke (1), Alzheimer’s disease (2), and epilepsy (3). In the retina, which is part of the central nervous system, accumulating evidence suggests that dysregulated cross talk between the vasculature and retinal neuroglia, photoreceptors, and other neural cells in diabetes might contribute to the pathogenesis of diabetic retinopathy (4–6). Similarly, the immature retinas of preterm neonates are susceptible to insults that disrupt both neural and vascular growth, leading to proliferative retinopathy of prematurity (7). While many factors have been suggested to mediate neurovascular crosstalk, including growth factors, hydrogen, potassium, neurotransmitters, adenosine, arachidonic acid metabolites, nitric oxide, neurotrophins and glutamate (4), we specifically investigated suppressor of cytokine signaling 3 (SOCS3) in neurons and glial cells, which inhibits inflammation and growth factor signaling (8), as a potential regulator of pathological retinal vessel growth.
SOCS3 inhibits the cytoplasmic effectors Janus kinase/signal transducers and activators of transcription (JAK/STAT), thereby deactivating tyrosine kinase receptor signaling (9). It increases endothelial cell apoptosis (10). Neuronal SOCS3 deletion promotes optic nerve regeneration in adult mice (11). We have previously shown that vascular SOCS3 deletion (Tie2-Cre driven) inhibits pathological angiogenesis (12). To investigate the role of neuronal SOCS3 in controlling neurovascular coupling-mediated pathological retinal angiogenesis in vivo, we generated a conditional knockout of Socs3 in retinal neurons and glia using a Cre/loxP sites-pecific DNA recombination fate mapping strategy, because systemic deletion of Socs3 is embryonically lethal (13). We deleted Socs3 in neuronal/glial cells by crossing mice expressing the Cre recombinase transgene under the control of the nestin promoter with mice carrying Socs3/loxP (Socs3flox/flox;NestinCre/+, Socs3 cKO). We studied pathological retinal angiogenesis in these mice after oxygen-induced retinopathy (OIR) (14). In the OIR model, mice are exposed to 75% oxygen to induce vessel loss (phase I) followed by exposure to room air from P12–17 when the now avascular retina becomes hypoxic, stimulating pathological neovascularization (phase II). When OIR was induced, Socs3 cKO mice showed significantly greater pathological retinal neovascularization than littermate flox/flox controls (Socs3f/f); however, normal retinal vascular growth during development was unaffected. These results suggested that neuronal/glial SOCS3 suppressed retinal angiogenesis in pathological conditions but was dispensable in physiologic vascular development. Lack of SOCS3 in retinal neuronal and glial cells increased neuronal STAT3 activation and promoted vascular endothelial growth factor (VEGF) production in neuronal cells to increase signaling in endothelial cells, leading to proliferative retinopathy. Therefore, neuronal/glial SOCS3 controls the release of growth factors, which mediate vascular growth specifically in pathological contexts.
Results
Socs3 mRNA was localized in neuronal layers in the retinas
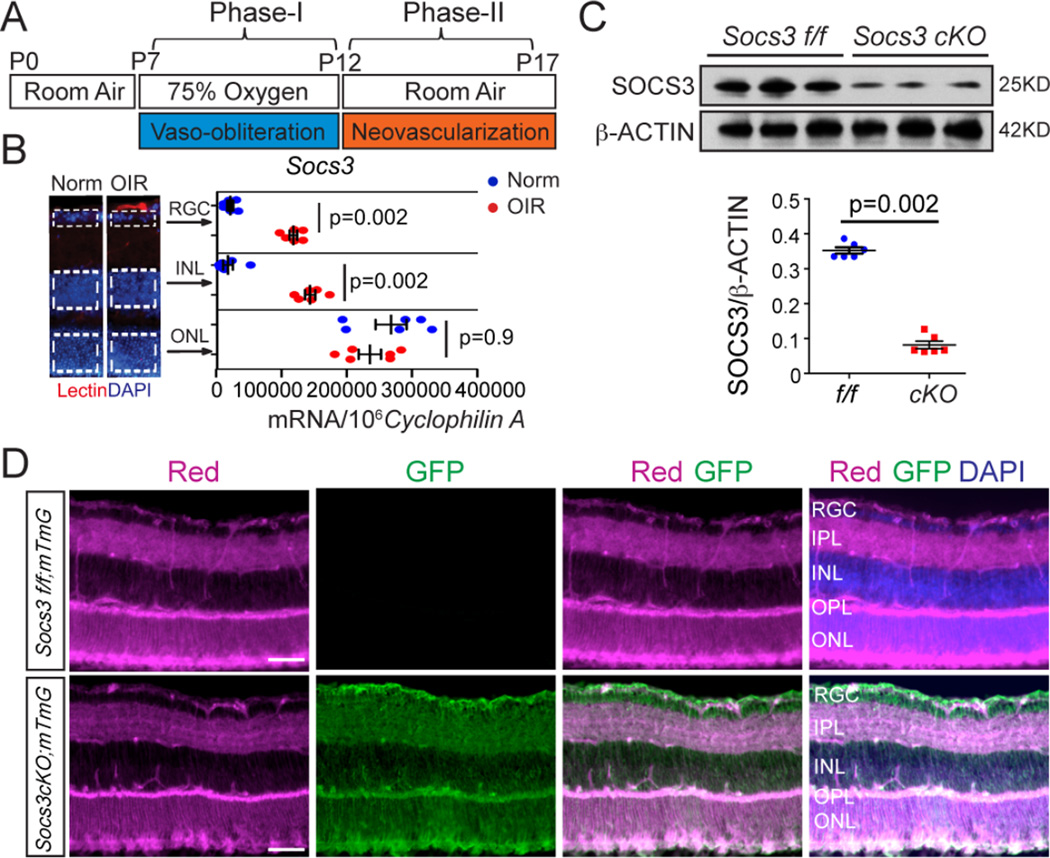
To examine the role of neuronal Socs3 in controlling pathological retinal angiogenesis, we generated pathological neovascularization in a mouse model of OIR (Figure 1A). To localize Socs3 expression in mouse retinas with OIR, the retinal layers were laser-capture microdissected (Figure 1B) and each isolated layer assessed for specific mRNA expression with qPCR. In P17 retinas with OIR, Socs3 mRNA expression is highly increased in proliferative vessels (12). We showed that Socs3 mRNA expression was also highly increased in the retinal ganglion cell (RGC) layer and inner nuclear layer (INL) without any change in the outer nuclear layer (ONL) compared with age-matched controls exposed to room air,.
Figure 1. Socs3 mRNA expression was induced in neuronal layers in the OIR model.
(A) Schematic diagram of OIR. Neonatal mice were exposed to 75% oxygen from postnatal day (P) 7–12 to induce vessel loss and returned to room air from P12–P17 to induce maximum pathological neovascularization at P17. (B) Socs3 mRNA expression in P17 retinal layers from laser-capture-micro-dissection from retinas with OIR compared to age-matched normoxia (Norm) control retinas (n=6 retinas per group). Socs3 mRNA was increased in RGC and INL, but not in ONL in retinas with OIR compared with normoxia control retinas. Images on the left show representative retinal cross sections from normoxia and retinas with OIR stained with isolectin B4 (Lectin, red) for endothelial cells and DAPI (blue), with dotted lines highlighting the areas for laser-capture-micro-dissection. (C) Socs3 was decreased by 80% in the retinas of mice that were generated by crossing Socs3 flox/flox (Socs3 f/f) mice with nestin-Cre driven mice (Socs3 cKO). Decreased SOCS3 abundance at the protein level was confirmed in whole retinas by Western blot (n= 6 retinas per group). (D) P17 retinal cross sections from Socs3 cKO;mTmG retinas with OIR show that Nestin-driven Cre recombinase present in all the neuronal layers (labeled with GFP) (n= 3 mice with 3 retinas per group). Scale bar: 100 µm.
Neuronal/glial Socs3 attenuated pathological neovascularization in OIR
The nestin Cre recombination appears in most neural and glial cells within the retina (15–17). To explore the role of neuronal/glial Socs3 in pathological retinal angiogenesis, we generated conditional Socs3 knockout mice driven by nestin Cre (Socs3 cKO). Decreased retinal abundance of Socs3 was confirmed by Western blot in Socs3 cKO retinas (Figure 1C). To confirm the knockdown of Socs3 in INL and RGC layers in Socs3 cKO retinas, we crossed Socs3 cKO mice with ROSAmT/mG reporter mice. ROSAmT/mG is a cell membrane-targeted, two-color fluorescent Cre reporter allele, expressing cell membrane-localized tomato red fluorescence in widespread cells and tissues prior to Cre recombinase exposure, and cell membrane-localized green fluorescence in Cre recombinase expressing cells (and future cell lineages derived from these cells). We found nestin Cre recombination, as indicated by green fluorescence, appeared in most neural/glial cells, especially in the INL and RGC layers, indicating that Socs3 was knocked out in the INL and RGC layers in Socs3 cKO retinas (Figure 1D).
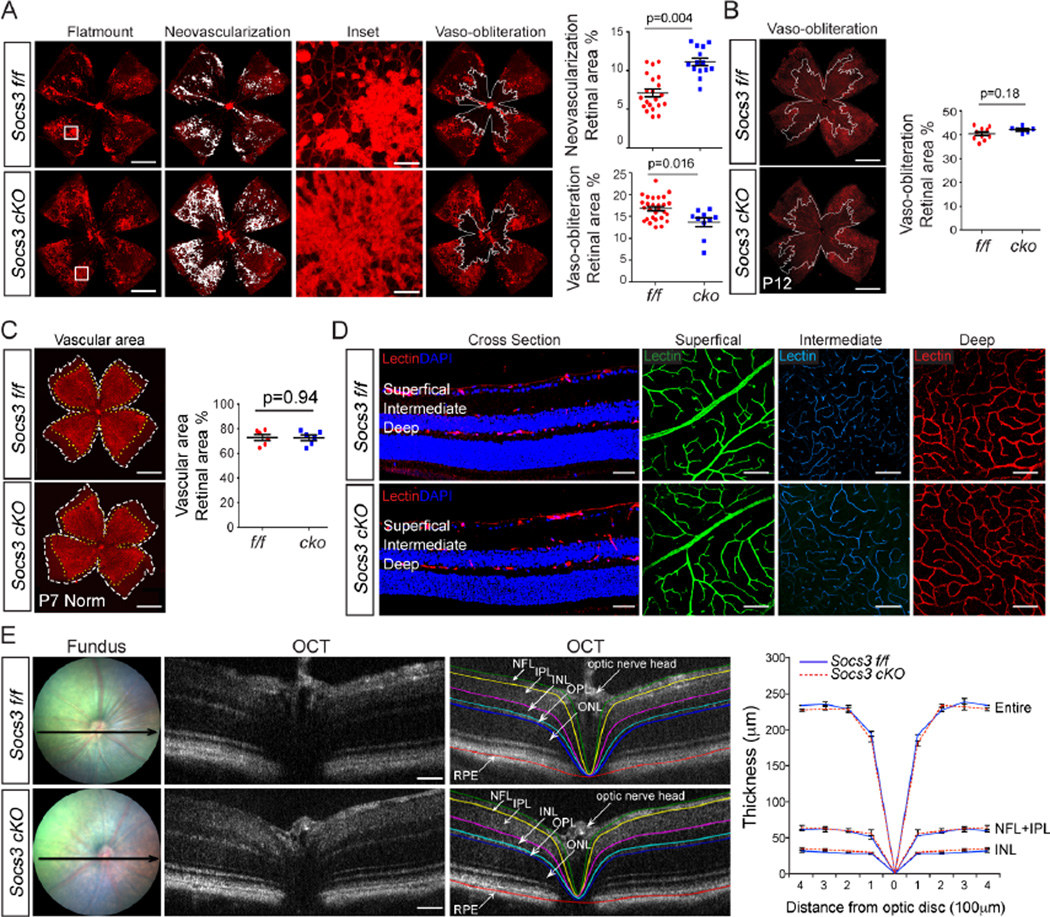
We have previously shown (17) that in mice, expression of Cre recombinase in nestin-expressing cells does not influence vaso-obliteration and neovascularization in retinas with OIR. Socs3 cKO retinas with OIR showed ~40% more retinal neovascularization than littermate Socs3 f/f control retinas with OIR at P17 (Figure 2A). There was also significantly less vaso-obliterated retinal area, suggesting increased vascular regrowth into vaso-obliterated areas from P12 to P17 (Figure 2A). Socs3 cKO and Socs3 f/f mice had comparable amounts of vaso-obliterated retinal area (Figure 2B) at P12 in response to OIR indicating that lack of Socs3 did not influence vessel loss during hyperoxia. To investigate whether lack of Socs3 in the INL and RGC layers influences normal developmental vascular growth, we compared the whole superficial vascular area on flat mounts at P7 (Figure 2C) and compared the three vascular layers (superficial, intermediate and deep) in cross sections and with confocal microscopy in whole retinas at P30 after full vascular development (Figure 2D). There was no difference in the superficial vascular area at P7 during development nor any differences in any of the three vascular layers in adult mice between Socs3 f/f and Socs3 cKO groups. The thickness of each retinal layers in live adult mice as measured with optical coherence tomography (OCT) was comparable in Socs3 f/f and Socs3 cKO retinas (Figure 2E). These data suggested that loss of neuronal/glial Socs3 attenuated pathological neovascularization in OIR but did not affect developmental vascular growth.
Figure 2. Neuronal/glial Socs3 attenuated pathological neovascularization in mouse model with OIR.
(A) Representative retinal flat-mounts (A, left panel) from P17 Socs3 f/f and Socs3 cKO retinas with OIR stained with isolectin B4 (red). The areas of neovascularization and vaso-obliteration were highlighted for quantification (white). Insets show enlarged pathological neovessels. Quantification of pathological neovascularization (A, right panel) showed that Socs3 attenuated pathological neovascularization (n=14–27 retinas per group) and decreased the area of central vaso-obliteration in P17 retinas with OIR (n=10–27 retinas per group). (B) Between Socs3 f/f and Socs3 Nes-ko retinas, the retinal vaso-obliteration areas were comparable at P12 (n=6–10 retinas per group). (C and D) There was no significant difference in normal developmental retina areas at P7 (n=5–6 retinas per group) (C), or in vessel layers in adults (D), or in layer thicknesses of entire retina, INL and nerve fiber layer (NFL)+IPL layer in live Socs3 f/f and Socs3 Nes-ko mice (n=6 retinas per group) (E) Black arrows in fundus images indicate the position of OCT. Scale bar for flat mounts in A–C: 1000 µm; for inset in A: 100 µm; for cross section in D: 50 µm; for layers in D: 25 µm; for OCT in E: 50 µm. NV, neovascularization; VO, vaso-obliteration.
Neuronal/glial Socs3 deficiency increased retinal VEGF mRNA and protein abundance
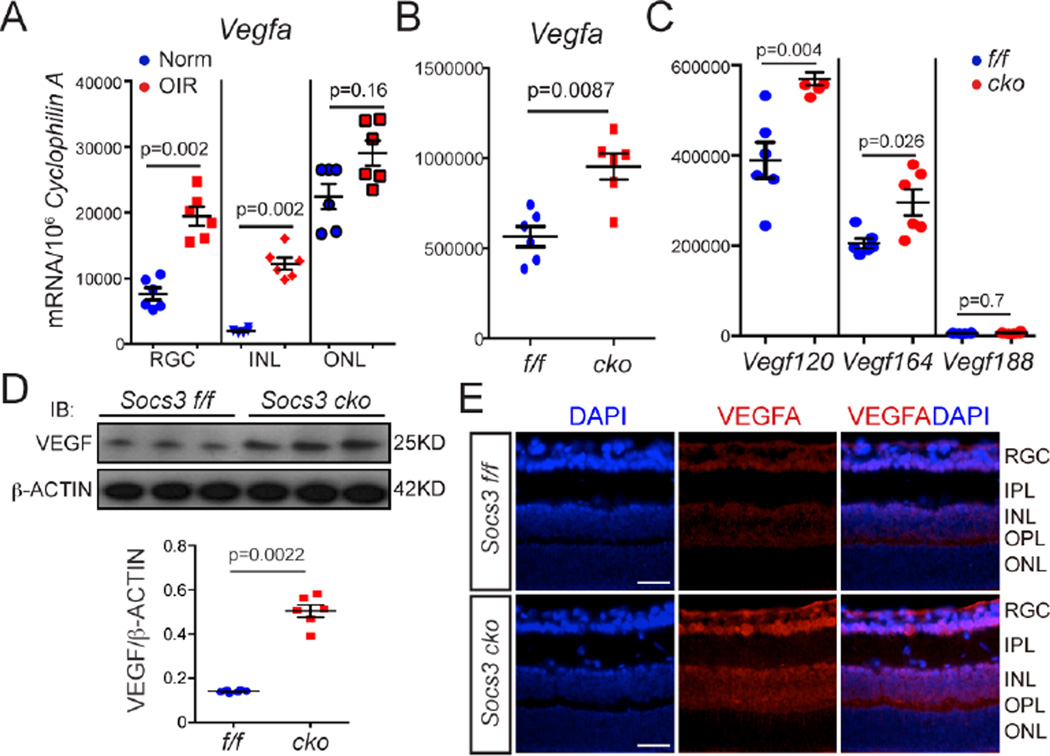
In the OIR model, mice are exposed to 75% oxygen to induce vessel loss, then to room air from P12–17 when the retina becomes relatively hypoxic and pathological neovascularization occurs. During the hypoxic and proliferative phase from P12–P17, Vegfa mRNA abundance increases mostly in Müller cells of the inner retina and contributes to pathological neovascularization (18–20). We confirmed that Vegfa expression was higher in neuronal and glial cells including RGC and INL layers in retinas with OIR than in age-matched normoxic controls (Figure 3A). Next we explored whether neuronal/glial SOCS3 regulated VEGF abundance at the mRNA and protein levels. In the P17 mice with OIR, retinal Vegfa mRNA expression was significantly higher in Socs3 cKO than that in Socs3 f/f mice with OIR (Figure 3B). There are at least three distinct isoforms of murine VEGFA (120, 164 and 188 amino acids), which are generated by alternative transcription from a single gene locus (21). Real time PCR analysis of retinas with OIR (Figure 3C) showed that neuronal/glial Socs3 deficiency promoted expression of the Vegfa mRNAs that correspond to isoforms 120 and 164, but not that corresponding to isoform 188. Increased protein abundance of VEGF-A isoform 164 was confirmed by Western blot (Figure 3D) and immunohistochemistry staining showed that neuronal/glial Socs3 deficiency was associated with increased abundance of VEGF-A isoform 164 mainly in the RGC and INL layers in retinas with OIR (Figure 3E).
Figure 3. Neuronal/glial Socs3 deficiency enhanced Vegfa expression.
(A) Vegfa mRNA expression in retinal layers from laser-capture-micro-dissection from P17 retinas with OIR compared to age-matched normoxia (Norm) control retinas. Vegfa mRNA expression was increased in RGC and INL, but not in ONL in retinas layers from P17 retinas with OIR compared with age-matched normoxic control retinas (n=6 retinas per group). Socs3 suppression in retinal neurons and glia increased Vegfa mRNA expression (n= 6 retinas per group) (B) and Vegfa isoform 120 and 164, but not 188 (C), and VEGFA-164 protein abundance (~3.5 fold, n= 6 retinas per group) (D) were increased in P17 Socs3 cKO retinas with OIR compared with control retinas. (E) Immunohistochemistry staining showed increased VEGFA (VEGFA-164) mainly in the INL and RGC layers. Representative images were from 3 mice per group. Scale bar: 50 µm.
Neuronal/glial Socs3 deficiency feedback enhanced STAT3 activation
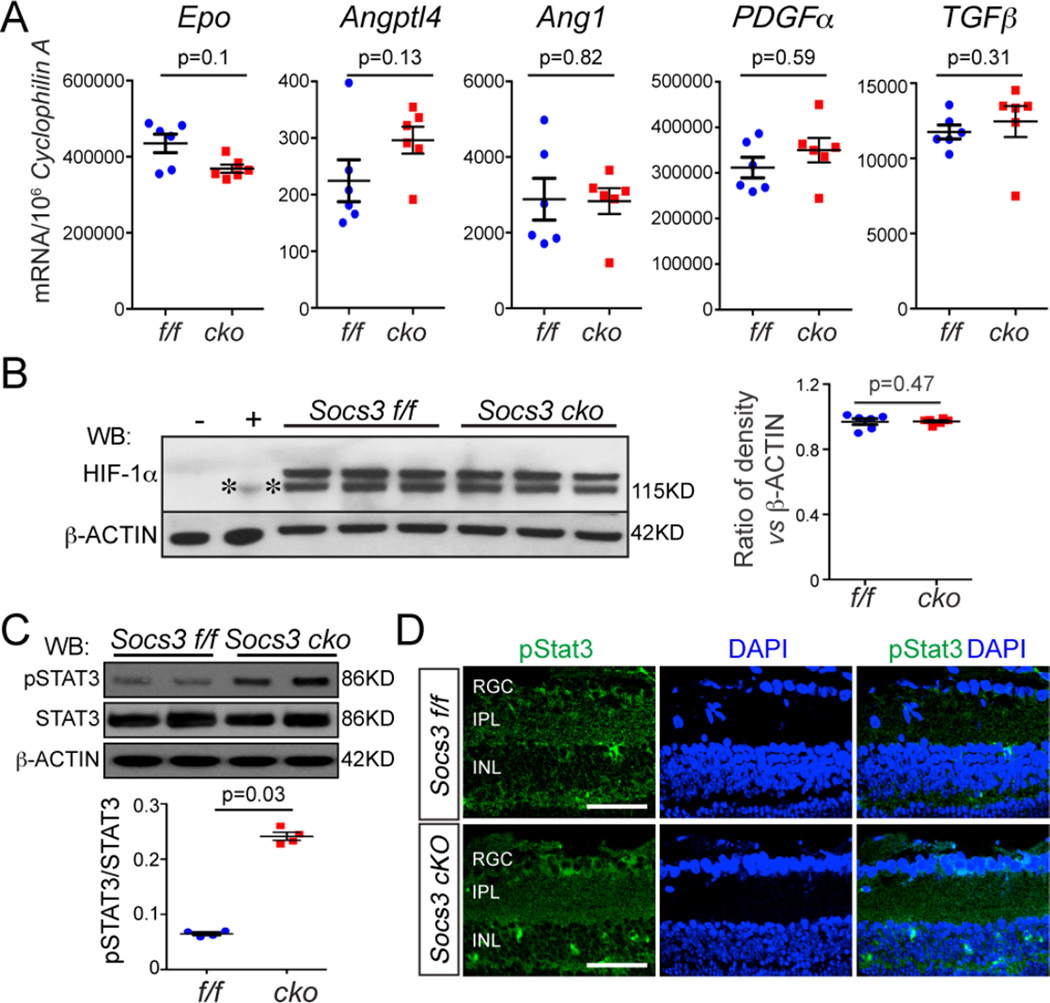
Our data suggested that in OIR, neuronal/glial SOCS3 partially controls the increased production of VEGF, a key angiogenic protein, that is secreted from neuronal and glial cells and that acts on vascular endothelial cells resulting in retinal neovascularization. Hypoxia-inducible factor 1-alpha (HIF-1α) is stabilized by hypoxia and transcriptionally activates Vegf expression (22). However, in retinas with OIR from Socs3 cKO and littermate Socs3 f/f controls, the mRNA expression of other HIF-1α target genes involved in angiogenesis including Epo (which encodes erythropoietin), Angptl4 (which encodes angiopoietin-like 4), Ang1 (which encodes angiopoietin 1), PDGFα (which encodes platelet-derived growth factors alpha) and TGFβ (which encodes transforming growth factor beta) (Figure 4A), and HIF-1α protein abundance (Figure 4B) were similar, indicating that HIF-1α might not primarily regulate the increased Vegf expression that occurs in neuronal/glial Socs3-deficient retinas in response to OIR.
Figure 4. Knocking out Socs3 in neurons and glia increased STAT3 activity.
(A, B) In P17 retinas with OIR, the mRNA expression of HIF-1α target genes Epo, Angptl4, Ang1, PDGFα and TGFβ and HIF-1α protein abundance were comparable between Socs3 cKO retinas and littermate Socs3 f/f retinas (n=6 retinas per group). (C) In P17 retinas with OIR, representative Western blots show that phosphorylated STAT3 (pSTAT3) was highly increased in Socs3 cKO retinas compared Socs3 f/f retinas. Band densities for Western blots were quantified using Image J in the bottom panel (n=4 retinas per group). (D) In P17 retinas with OIR, phosphorylated STAT3 (pSTAT3) was increased mainly in the INL and RGC layers. Cross-sections were stained with pSTAT3 (green) and DAPI (blue). Representative images were from 3 mice per group. Scale bar: 50 µm.
SOCS3 binds to both the kinase JAK and the interleukin-6 receptor, which inhibits the activation of STAT3 (23). STAT3 activation induces the expression of Vegf (24, 25). We examined the activation of STAT3 in response to neuronal/glial Socs3 expression. Phosphorylated STAT3, the active form of STAT3, was 3.5 fold higher in P17 Socs3 cKO retinas with OIR than in Socs3 f/f controls with OIR (Figure 4C) and was increased mainly in the INL and RGC layers (Figure 4D). These results indicated that STAT3 activation was increased by neuronal/glial SOCS3 deficiency, which may lead to the observed increase in Vegfa. Therefore, neuronal/glial Socs3 is an important regulator of pathological angiogenesis that may act through STAT3-mediated Vegf expression.
In addition to VEGF, nerve growth factor (which is encoded by Ngf) contributes to retinal neovascularization in the OIR mouse model through TrkA activation, which is attenuated by inhibition of Trk receptors (26). There was no significant difference in Ngfβ expression between Socs3 cKO and Socs3 f/f control retinas with OIR at P17 (Figure S1) suggesting that differences in neovascularization were not attributable to Ngf regulation.
Socs3 transcription is regulated by the Foxo family including Foxo3a and this regulation is feedback controlled by cytokines (27, 28). We found that the expression of mRNAs encoding Foxo1 and Foxo3a were similar in Socs3 cKO and Socs3 f/f control retinas with OIR at P17, suggesting that the Socs3/Foxo pathway was not affected by neuronal/glial Socs3 deficiency (Figure S2).
SOCS3 is a major regulator of inflammation. mRNAs encoding the inflammatory factors IL-6 and TNFα, but not Il-1β, all of which are involved in retinal neovascularization, showed increased expression in Socs3 cKO retinas with OIR compared with Socs3 f/f controls (Figure S3). Since cytokines like IL-6 also can induce VEGF production (29), increases in cytokines induced by neuronal/glial Socs3 deficiency might also contribute to increased VEGF expression in Socs3 cKO retinas with OIR.
Neuronal/glial Socs3 deficiency increased endothelial proliferation and ERK phosphorylation
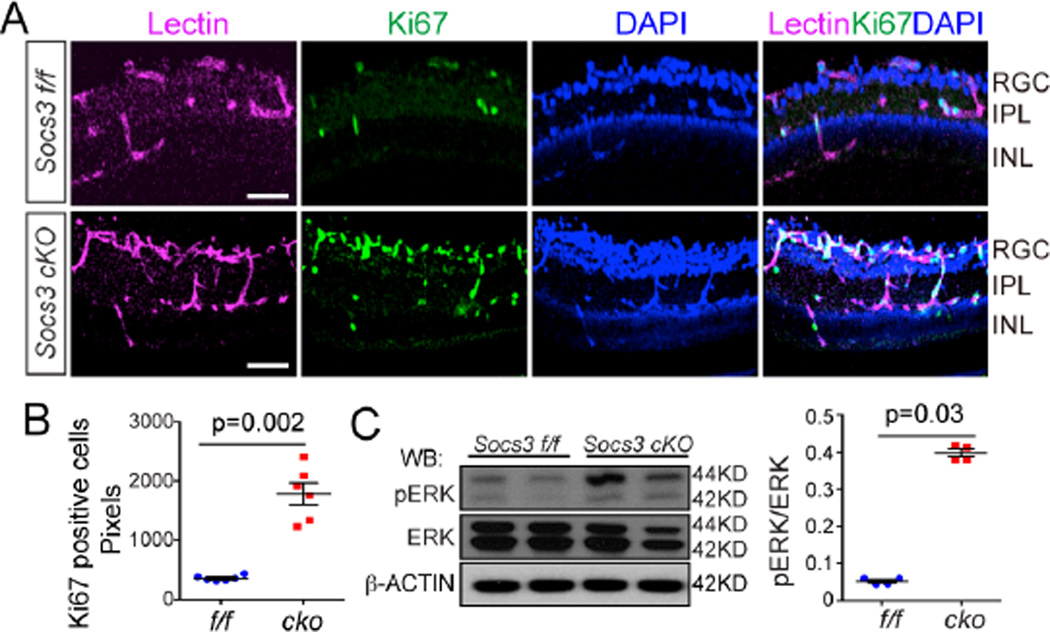
VEGF stimulates endothelial cell proliferation to promote angiogenesis. We found that a proliferation marker Ki67 co-localized with the endothelial cell marker isolectin IB4, and was significantly increased in Socs3 cKO retinas with OIR compared with Socs3 f/f controls with OIR indicating that neuronal/glial Socs3 deficiency increased vascular endothelial cell proliferation (Figure5A and 5B). Next, we explored whether the VEGF could induce activation of ERK (30) in neuronal/glial Socs3 deficient retinas with OIR. Phosphorylated ERK was higher in Socs3 cKO P17 retinas with OIR than in Socs3 f/f retinas with OIR (Figure 5C), consistent with proliferative endothelium and increased neovascularization. These results suggested that neuronal/glial Socs3 suppressed the increased pathological VEGF-induced vascular endothelial cell proliferation.
Figure 5. Neuronal/glial Socs3 deficiency increased endothelial cell proliferation and ERK phosphorylation.
(A and B) In P17 retinas with OIR, Ki67 positive (which denotes proliferation) cells were increased in Socs3 cKO retinas compared to Socs3 f/f control retinas. Cross-sections from P17 retinas with OIR were stained for proliferative cells with Ki67 (green, proliferative cells), isolectin B4 (magenta, endothelial cells) and cell nuclei with DAPI (blue) (A). Scale bar: 25 µm. Quantification of Ki67 pixels is shown in (B) (n=6 retinas per group). (C) In P17 retinas with OIR, representative Western blots and quantification show that phosphorylated ERK (pERK) was increased in Socs3 cKO retinas compared to Socs3 f/f OIR retinas (n=4 retinas per group).
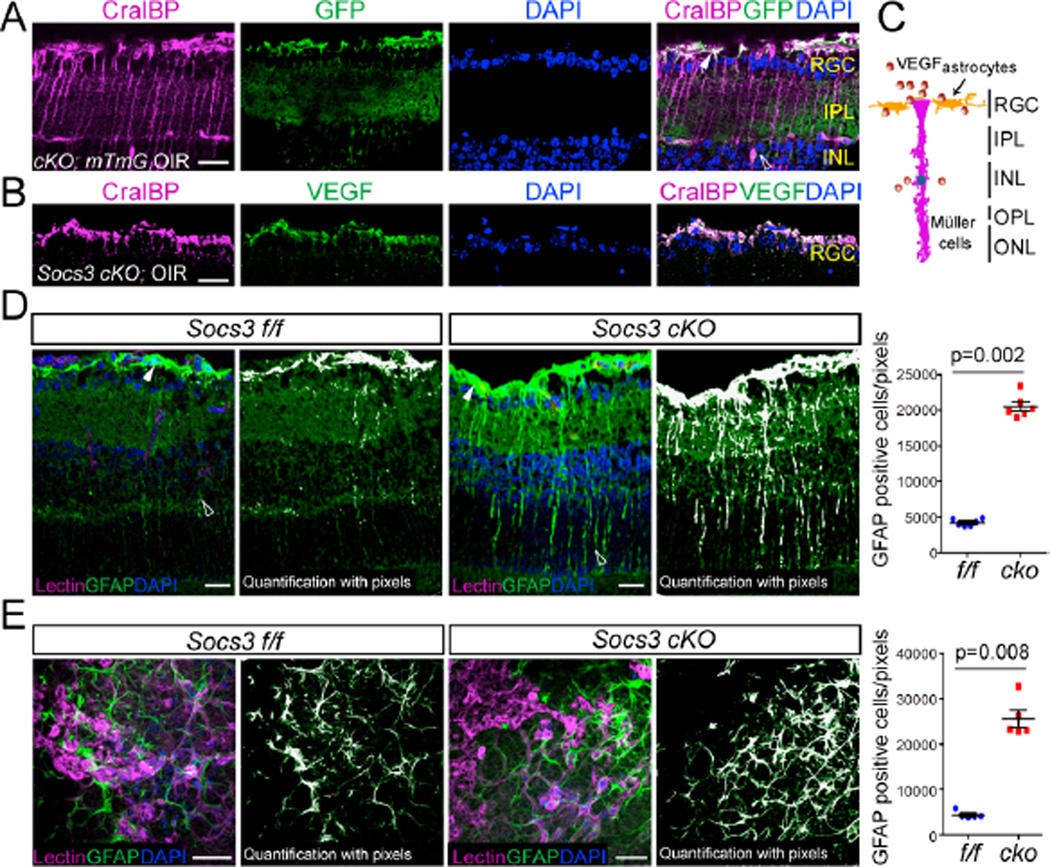
To better understand how neuronal/glial Socs3 controls VEGF production, we investigated the cell types that could be potential sources of VEGF. The nestin Cre recombination occurs in most neural and glial cells within the retina (15–17). We previously have demonstrated that in retinas with OIR, Vegfa mRNA is localized to cell bodies identified morphologically as Müller cells (19). In addition, astrocyte-derived VEGF is essential for hypoxia-induced neovascularization (31). First we showed that cellular retinaldehyde binding protein (CralBP), a marker for Müller cells and astrocytes (32), co-localized with GFP which labels cells where nestin Cre recombination has occurred in Socs3 cKO; mTmG reporter retinas with OIR (Figure 6A) indicating that Socs3 was deleted in both Müller cells and astrocytes. Immunohistochemstry staining in Socs3 cKO retinas with OIR showed that VEGF and CralBP were colocalized in the RGC layer indicating that VEGF could be produced by CralBP-positive cells including Müller cells (end-feet) and astrocytes (Figure6B and 6C). Glial fibrillary acidic protein (GFAP), a 51-kDa intermediate filament protein, is another marker for astrocytes, and activated Müller cell end-feet and processes (33). In this study we found that in Socs3 cKO retinas with OIR, Müller cells were highly activated, as indicated by intense GFAP immunofluorescence (Figure 6D). Consistently, we found more GFAP-positive astrocytes in the RGC layer in Socs3 cKO retinas with OIR compared with littermate Socs3 f/f controls with OIR (Figure 6E). These data suggest that Müller cells and astrocytes might produce VEGF in addition to other neurons in RGC and INL layers.
Figure 6. Neuronal/glial deficiency of Socs3 results in activated Müller glial cells and astrocytes.
(A) In P17 Socs3 cKO;mTmG reporter retinas with OIR, CralBP, labeled Müller cells and astrocytes colocalized with GFP. Cross-sections were stained for Müller cells and astrocytes with anti-CralBP (magenta) and cell nuclei with DAPI (blue). White arrow: astrocytes or end-feet of activated Müller cells. Open arrowheads: activated Müller cells. Scale bar: 10 µm. Images are representative of 3 mice per group. (B) Cross-sections from P17 Socs3 cKO retinas with OIR were stained with CralBP (magenta), VEGF (green) and DAPI (blue). VEGF colocalized with CralBP positive cells. Scale bar: 10 µm. (C) A diagram showing VEGF in the RGC and INL layers including activated astrocytes and Müller cells. (D) In P17 retinas with OIR, GFAP-labeled activated Müller cells in Socs3 cKO retinas were increased compared to Socs3 f/f control retinas. Cross-sections from P17 Socs3 cKO and Socs3 f/f control retinas with OIR were stained for endothelial cells with isolectin B4 (magenta), activated Müller cells and astrocytes with anti-GFAP (green) and cell nuclei with DAPI (blue). Scale bar: 25 µm. (E) GFAP-labeled astrocytes and end-feet of activated Müller cells in flat-mounted P17 Socs3 cKO and Socs3 f/f retinas with OIR. Representative images in A, B, D, and E were from 6 mice per group. White arrow: astrocytes or end-feet of activated Müller cells. Open arrowheads: activated Müller cells. Scale bar: 50µm.
Discussion
In this study we identified a neurovascular crosstalk pathway governing the progression of retinopathy. Specifically, neuronal/glial Socs3 deficiency promoted pathological retinal angiogenesis in retinopathy. Socs3 expression was increased in the RGC and INL layers in wild-type retinas with OIR. Neuronal/glial Socs3 deficient mice with OIR had increased pathological retinal neovascularization, but there was no effect on normal retinal vascular development (Figure 2) suggesting that neuronal/glial SOCS3 controls the pathological vascular response in retinopathy. Neuronal/glial Socs3 loss suppressed inhibition of JAK, resulting in increased phosphorylation of STAT3 (Figure 4C and D) leading to Vegfa overexpression (Figure 3), increased endothelial cell proliferation and ERK activation (Figure 5C). These results suggest that neuronal/glial Socs3 suppresses pathological vascular endothelial cell proliferation by controlling VEGF secretion mediated by STAT3 activation.
In OIR, hyperoxia-induced retinal vessel loss is followed by hypoxia-induced increase in VEGF (34), which contributes to pathological neovascularization (18–20). Typically, the larger the area of vaso-obliteration, the greater the area of neovascularization that is stimulated by hypoxia-regulated factors. In Socs3 cKO with OIR compared to Socs3 f/f mice with OIR at P17, the vaso-obliterated retinal area was reduced (though pathological neovascularization was increased). This result is consistent with hypoxia-independent growth factor stimulation of endothelial cell proliferation (like VEGF production mediated by phosophorylated STAT3 in our study), resulting in more revascularization and less vaso-obliteration, yet also resulting in more neovascularization, despite less hypoxia-driven neovascular growth. VEGF is a soluble factor that interacts with neurons, neuroglia and the vasculature affecting vascular development and neovascularization (35). In OIR retinal neovascularization, Vegf mRNA has been localized to Müller cells in the INL layer of the retina (19), but the oxygen-dependent and independent regulation of VEGF in Müller cells has not been well studied. Hypoxic condition stabilize HIF-1α, allowing it to translocate from the cytosol to the nucleus (36), where it transcriptionally activates Vegf expression (22). Consistent with HIF-1α not being the only regulatory pathways for Vegf regulation in OIR, in Socs3 cKO retinas with OIR, we found similar HIF-1α protein abundance in both Socs3 cKO and Socs3f/f retinas with OIR and similar mRNA expression of other HIF-1α target genes that are involved in OIR and angiogenesis, such as Epo (37, 38), Anptl4 (39, 40), Ang1, PDGFα and TGFβ (Figure4A and 4B).
We found that the increased phosphorylation of STAT3 was correlated with increased Vegf in neuronal/glial Socs3 deficient retinas (Figure 4), suggesting that neuroglial cell-secreted VEGF could be regulated by the SOCS3-JAK-STAT3 pathway. STAT3 and HIF-1α can cooperatively activate HIF-1α target genes including Vegf in tumor cells (41). In our study, increased Vegfa in neuronal/glial Socs3 deficient retinas appeared to be activated primarily by STAT3 and perhaps to a much lesser extent by HIF-1α.
Identifying the molecular mechanisms underlying neurovascular cross talk is an important step in understanding pathological proliferative retinopathy. Neuronal/glial SOCS3 suppresses neuronal/glial VEGF production to decrease vascular endothelial cell proliferation in the context of retinopathy, but not in retinal vascular development.
Materials and Methods
Animals
All animal studies were approved by the Institutional Animal Care and Use Committee at the Boston Children’s Hospital. Nestin Cre expressing C57Bl/6 mice (Jackson Laboratory, stock # 003771) were crossed with Socs3 flox/flox (Socs3 f/f) mice (kind gift of Dr. A. Yoshimura) to generate Socs3 cKO and littermate control flox mice. ROSAmTmG reporter mice were from the Jackson Laboratory (stock# 007576). C57Bl/6 mice were from the Jackson Laboratory (stock # 000664).
Oxygen-induced retinopathy (OIR) and vessel quantification
OIR was carried out in neonatal mice as described previously (14). Briefly, mouse pups with their nursing mothers were exposed to 75% oxygen from postnatal day (P) 7 to 12, then returned to room air until P17 (Figure 1A). The retinas were collected at P17 followed by retina dissection, staining overnight with fluorescent Griffonia Simplicifolia Isolectin IB4 (Invitrogen) and flat mounting. The avascular (vaso-obliteration) and neovascularized areas were quantified (42) by using Adobe Photoshop (Adobe Systems) and Image J (National Institutes of Health, http://imagej.nih.gov/ij/). Mice that weighed less than 5 grams at P17 were excluded from the study (43).
In vivo imaging using Optical Coherence Tomography (OCT)
Mice were anesthetized with a mixture of xylazine (6 mg/kg) and ketamine (100 mg/kg), and pupils were dilated with a topical drop of Cyclomydril (Alcon Laboratories, Fort Worth, TX). Two minutes after pupil dilation, lubricating eye drops (Alcon Laboratories) were applied to the cornea. Spectral domain optical coherence tomography (SD-OCT) with guidance of bright-field live fundus image was performed using the image-guided OCT system (Micron IV, Phoenix Research Laboratories) according to the manufacturer’s instruction and using the vendor’s image acquisition software to generate fundus images and OCT scans. The vendor’s software Insight was used to accurately measure the thickness of retinal layers (Nerve fiber layer (NFL)+IPL, INL) and entire retinas. The thickness of retinal layers was plotted with 4 distances from the optic nerve head (100, 200, 300, 400 µm).
Laser capture microdissection, RNA Isolation and quantitative RT-PCR
Cross-sectional retinal layers were laser microdissected according to manufacturer’s instructions (Leica LMD6000). Briefly, eyes were enucleated from C57Bl/6J wild type mice at P17 in either OIR or normoxic conditions and embedded. 8 µm sections were isolated using cryostat, mounted on ribonuclease (RNase)-free polyethylene naphthalate glass slides (Leica Microsystems; Wetzlar, Germany), followed by fixation in 50% ethanol for 15 seconds, and 30 seconds in 75% ethanol, before being washed with diethyl pyrocarbonate-treated water for 15 seconds. Sections were treated with RNase inhibitor (Roche) at 25 °C for 3 minutes. Retinal layers were then lasercapture microdissected with the Leica LMD 6000 system (Leica Microsystems) and collected directly into lysis buffer from the RNeasy Micro kit (Qiagen) followed by RNA isolation. Isolated RNA from whole retinas or laser-captured retinal layers using RNeasy kit (Qiagen) was reverse transcribed with M-MLV reverse transcriptase (Invitrogen) to generate cDNA. Quantitative RT-PCR was performed using a 7300 system (Applied Biosystems) with KAPA SYBR FAST qPCR Kits (Kapa Biosystems). Cyclophilin A was used as internal control.
Immunohistochemistry
Immunostaining in retinas was performed as described (17) Briefly, eyes were isolated from P17 mice with OIR, fixed and permeabilized. The flat-mounted retinas or cross sections were stained with isolectin IB4 (Invitrogene, 121413), anti-GFAP (Abcam, ab4674), anti-CralBP (Thermo, MA1-813), anti-phosphorylated STAT3 (Cell Signaling, M9C6, 4113), anti-VEGFA (R&D System, AF-493-NA) and DAPI (Invitrogene, D3571), and imaged using a confocal laser scanning microscope (FV1000; Olympus).
Immunoblot
A standard immunoblotting protocol was used. Briefly, 300 mM NaCl, 0.5% NP-40, 50mM Tris.HCl pH7.4, 0.5 mM EDTA was used to lyse the retinas. Proteinase and phosphatase inhibitor cocktails were added. The antibodies used were: anti-phosphorylated ERK (Cell Signaling, 4376), anti-ERK (Cell Signaling, 4695), anti-phosphorylated STAT3 (Cell Signaling, 9131S), anti-STAT3 (Cell Signaling, 9132), β-ACTIN (Sigma, A1978).
Statistical analysis
Statistical analyses were performed with GraphPad Prism (v6.0) (GraphPad Software, Inc., San Diego, CA) and the results were compared using the Mann-Whitney test. P values <0.05 were considered statistically significant.
Supplementary Material
Acknowledgements
The authors thank Dr. A. Yoshimura for the Socs3 flox/flox mice and thank Hurst C, Juan A, Pei D and Cui R for their excellent technical assistance. We thank the Harvard Catalyst Biostatistical Consulting Program and Dr. Catherine Stamoulis for reviewing the raw data and selecting appropriate statistical methods used in the manuscript. Funding: This work was supported by the National Institutes of Health/National Eye Institute (EY022275, EY017017, P01 HD18655), Lowy Family Foundation, European Commission FP7 project 305485 PREVENT-ROP for LEHS; JC was supported by National Institutes of Health/National Eye Institute (R01 EY024963), Boston Children's Hospital (BCH) Career Development Award, BrightFocus Foundation, BCH Ophthalmology Foundation, Mass Lions Eye Research Fund Inc. and Alcon Research Institute. AS was supported by Deutsche Forschungsgemeinschaft (DFG STA 1102/5-1), Deutsche Ophthalmologische Gesellschaft (DOG). JSJ was supported by Burroughs Wellcome Fund Career Awards for Medical Scientists (CAMS), Foundation Fighting Blindness, Canadian Child Health Clinician Scientist Program and Fonds de recherche du Québec - Santé.
Footnotes
Supplementary Materials
Figure S1. Ngfβ mRNA expression in retinas with OIR from P17 Socs3 f/f and Socs3 cKO mice
Figure S2 Foxo1 and Foxo3a mRNA expression in retinas with OIR from P17 Socs3 f/f and Socs3 cKO mice
Figure S3 Expression of mRNAs encoding inflammatory factors in retinas with OIR from P17 Socs3 f/f and Socs3 cKO mice
Author contributions: Y.S., J.M., A.S., J.S.J., J.C., W.P., L.E.H.S., contributed to designing the experiments and interpreting the results. Y.S., J.M., Z.L., T.F., L.E., K.T., N.S., P.M. performed the experiments. Y.S., J.C., L.E.H.S., wrote the manuscript.
Competing interests: The authors declare that they have no competing interests.
References and Notes
- 1.Guo S, Kim WJ, Lok J, Lee SR, Besancon E, Luo BH, Stins MF, Wang X, Dedhar S, Lo EH. Neuroprotection via matrix-trophic coupling between cerebral endothelial cells and neurons. Proceedings of the National Academy of Sciences of the United States of America. 2008;105:7582–7587. doi: 10.1073/pnas.0801105105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Iadecola C. Neurovascular regulation in the normal brain and in Alzheimer's disease. Nature reviews. Neuroscience. 2004;5:347–360. doi: 10.1038/nrn1387. [DOI] [PubMed] [Google Scholar]
- 3.Nishijima T, Piriz J, Duflot S, Fernandez AM, Gaitan G, Gomez-Pinedo U, Verdugo JM, Leroy F, Soya H, Nunez A, Torres-Aleman I. Neuronal activity drives localized blood-brain-barrier transport of serum insulin-like growth factor-I into the CNS. Neuron. 2010;67:834–846. doi: 10.1016/j.neuron.2010.08.007. [DOI] [PubMed] [Google Scholar]
- 4.Kern TS. Interrelationships between the Retinal Neuroglia and Vasculature in Diabetes. Diabetes & metabolism journal. 2014;38:163–170. doi: 10.4093/dmj.2014.38.3.163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Nakahara T, Mori A, Kurauchi Y, Sakamoto K, Ishii K. Neurovascular interactions in the retina: physiological and pathological roles. Journal of pharmacological sciences. 2013;123:79–84. doi: 10.1254/jphs.13r03cp. [DOI] [PubMed] [Google Scholar]
- 6.Qian H, Ripps H. Neurovascular interaction and the pathophysiology of diabetic retinopathy. Experimental diabetes research. 2011;2011:693426. doi: 10.1155/2011/693426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hellstrom A, Smith LE, Dammann O. Retinopathy of prematurity. Lancet. 2013;382:1445–1457. doi: 10.1016/S0140-6736(13)60178-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Starr R, Willson TA, Viney EM, Murray LJ, Rayner JR, Jenkins BJ, Gonda TJ, Alexander WS, Metcalf D, Nicola NA, Hilton DJ. A family of cytokine-inducible inhibitors of signalling. Nature. 1997;387:917–921. doi: 10.1038/43206. [DOI] [PubMed] [Google Scholar]
- 9.Lebel E, Vallieres L, Rivest S. Selective involvement of interleukin-6 in the transcriptional activation of the suppressor of cytokine signaling-3 in the brain during systemic immune challenges. Endocrinology. 2000;141:3749–3763. doi: 10.1210/endo.141.10.7695. [DOI] [PubMed] [Google Scholar]
- 10.Jiang Y, Zhang Q, Soderland C, Steinle JJ. TNFalpha and SOCS3 regulate IRS-1 to increase retinal endothelial cell apoptosis. Cellular signalling. 2012;24:1086–1092. doi: 10.1016/j.cellsig.2012.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Smith PD, Sun F, Park KK, Cai B, Wang C, Kuwako K, Martinez-Carrasco I, Connolly L, He Z. SOCS3 deletion promotes optic nerve regeneration in vivo. Neuron. 2009;64:617–623. doi: 10.1016/j.neuron.2009.11.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Stahl A, Joyal JS, Chen J, Sapieha P, Juan AM, Hatton CJ, Pei DT, Hurst CG, Seaward MR, Krah NM, Dennison RJ, Greene ER, Boscolo E, Panigrahy D, Smith LE. SOCS3 is an endogenous inhibitor of pathologic angiogenesis. Blood. 2012;120:2925–2929. doi: 10.1182/blood-2012-04-422527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Marine JC, McKay C, Wang D, Topham DJ, Parganas E, Nakajima H, Pendeville H, Yasukawa H, Sasaki A, Yoshimura A, Ihle JN. SOCS3 is essential in the regulation of fetal liver erythropoiesis. Cell. 1999;98:617–627. doi: 10.1016/s0092-8674(00)80049-5. [DOI] [PubMed] [Google Scholar]
- 14.Smith LE, Wesolowski E, McLellan A, Kostyk SK, D'Amato R, Sullivan R, D'Amore PA. Oxygen-induced retinopathy in the mouse. Investigative ophthalmology & visual science. 1994;35:101–111. [PubMed] [Google Scholar]
- 15.Arnold TD, Ferrero GM, Qiu H, Phan IT, Akhurst RJ, Huang EJ, Reichardt LF. Defective retinal vascular endothelial cell development as a consequence of impaired integrin alphaVbeta8-mediated activation of transforming growth factor-beta. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2012;32:1197–1206. doi: 10.1523/JNEUROSCI.5648-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Michan S, Juan AM, Hurst CG, Cui Z, Evans LP, Hatton CJ, Pei DT, Ju M, Sinclair DA, Smith LE, Chen J. Sirtuin1 over-expression does not impact retinal vascular and neuronal degeneration in a mouse model of oxygen-induced retinopathy. PloS one. 2014;9:e85031. doi: 10.1371/journal.pone.0085031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Chen J, Michan S, Juan AM, Hurst CG, Hatton CJ, Pei DT, Joyal JS, Evans LP, Cui Z, Stahl A, Sapieha P, Sinclair DA, Smith LE. Neuronal sirtuin1 mediates retinal vascular regeneration in oxygen-induced ischemic retinopathy. Angiogenesis. 2013;16:985–992. doi: 10.1007/s10456-013-9374-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Alon T, Hemo I, Itin A, Pe'er J, Stone J, Keshet E. Vascular endothelial growth factor acts as a survival factor for newly formed retinal vessels and has implications for retinopathy of prematurity. Nature medicine. 1995;1:1024–1028. doi: 10.1038/nm1095-1024. [DOI] [PubMed] [Google Scholar]
- 19.Pierce EA, Avery RL, Foley ED, Aiello LP, Smith LE. Vascular endothelial growth factor/vascular permeability factor expression in a mouse model of retinal neovascularization. Proceedings of the National Academy of Sciences of the United States of America. 1995;92:905–909. doi: 10.1073/pnas.92.3.905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Stone J, Chan-Ling T, Pe'er J, Itin A, Gnessin H, Keshet E. Roles of vascular endothelial growth factor and astrocyte degeneration in the genesis of retinopathy of prematurity. Investigative ophthalmology & visual science. 1996;37:290–299. [PubMed] [Google Scholar]
- 21.Ferrara N, Houck K, Jakeman L, Leung DW. Molecular and biological properties of the vascular endothelial growth factor family of proteins. Endocrine reviews. 1992;13:18–32. doi: 10.1210/edrv-13-1-18. [DOI] [PubMed] [Google Scholar]
- 22.Forsythe JA, Jiang BH, Iyer NV, Agani F, Leung SW, Koos RD, Semenza GL. Activation of vascular endothelial growth factor gene transcription by hypoxia-inducible factor 1. Molecular and cellular biology. 1996;16:4604–4613. doi: 10.1128/mcb.16.9.4604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wei X, Wang G, Li W, Hu X, Huang Q, Xu K, Lou W, Wu J, Liang C, Lou Q, Qian C, Liu L. Activation of the JAK-STAT3 pathway is associated with the growth of colorectal carcinoma cells. Oncology reports. 2014;31:335–341. doi: 10.3892/or.2013.2858. [DOI] [PubMed] [Google Scholar]
- 24.Wei D, Le X, Zheng L, Wang L, Frey JA, Gao AC, Peng Z, Huang S, Xiong HQ, Abbruzzese JL, Xie K. Stat3 activation regulates the expression of vascular endothelial growth factor and human pancreatic cancer angiogenesis and metastasis. Oncogene. 2003;22:319–329. doi: 10.1038/sj.onc.1206122. [DOI] [PubMed] [Google Scholar]
- 25.Niu G, Wright KL, Huang M, Song L, Haura E, Turkson J, Zhang S, Wang T, Sinibaldi D, Coppola D, Heller R, Ellis LM, Karras J, Bromberg J, Pardoll D, Jove R, Yu H. Constitutive Stat3 activity up-regulates VEGF expression and tumor angiogenesis. Oncogene. 2002;21:2000–2008. doi: 10.1038/sj.onc.1205260. [DOI] [PubMed] [Google Scholar]
- 26.Liu X, Wang D, Liu Y, Luo Y, Ma W, Xiao W, Yu Q. Neuronal-driven angiogenesis: role of NGF in retinal neovascularization in an oxygen-induced retinopathy model. Investigative ophthalmology & visual science. 2010;51:3749–3757. doi: 10.1167/iovs.09-4226. [DOI] [PubMed] [Google Scholar]
- 27.Honda M, Takehana K, Sakai A, Tagata Y, Shirasaki T, Nishitani S, Muramatsu T, Yamashita T, Nakamoto Y, Mizukoshi E, Sakai Y, Yamashita T, Nakamura M, Shimakami T, Yi M, Lemon SM, Suzuki T, Wakita T, Kaneko S G. Hokuriku Liver Study. Malnutrition impairs interferon signaling through mTOR and FoxO pathways in patients with chronic hepatitis C. Gastroenterology. 2011;141:128–140. 140 e121–140 e122. doi: 10.1053/j.gastro.2011.03.051. [DOI] [PubMed] [Google Scholar]
- 28.Barclay JL, Anderson ST, Waters MJ, Curlewis JD. Regulation of suppressor of cytokine signaling 3 (SOC3) by growth hormone in pro-B cells. Molecular endocrinology. 2007;21:2503–2515. doi: 10.1210/me.2006-0498. [DOI] [PubMed] [Google Scholar]
- 29.Cohen T, Nahari D, Cerem LW, Neufeld G, Levi BZ. Interleukin 6 induces the expression of vascular endothelial growth factor. The Journal of biological chemistry. 1996;271:736–741. doi: 10.1074/jbc.271.2.736. [DOI] [PubMed] [Google Scholar]
- 30.Kuriyama M, Taniguchi T, Shirai Y, Sasaki A, Yoshimura A, Saito N. Activation and translocation of PKCdelta is necessary for VEGF-induced ERK activation through KDR in HEK293T cells. Biochemical and biophysical research communications. 2004;325:843–851. doi: 10.1016/j.bbrc.2004.10.102. [DOI] [PubMed] [Google Scholar]
- 31.Weidemann A, Krohne TU, Aguilar E, Kurihara T, Takeda N, Dorrell MI, Simon MC, Haase VH, Friedlander M, Johnson RS. Astrocyte hypoxic response is essential for pathological but not developmental angiogenesis of the retina. Glia. 2010;58:1177–1185. doi: 10.1002/glia.20997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Xu XL, Lee TC, Offor N, Cheng C, Liu A, Fang Y, Jhanwar SC, Abramson DH, Cobrinik D. Tumor-associated retinal astrocytes promote retinoblastoma cell proliferation through production of IGFBP-5. The American journal of pathology. 2010;177:424–435. doi: 10.2353/ajpath.2010.090512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Chang ML, Wu CH, Jiang-Shieh YF, Shieh JY, Wen CY. Reactive changes of retinal astrocytes and Muller glial cells in kainate-induced neuroexcitotoxicity. Journal of anatomy. 2007;210:54–65. doi: 10.1111/j.1469-7580.2006.00671.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ozaki H, Yu AY, Della N, Ozaki K, Luna JD, Yamada H, Hackett SF, Okamoto N, Zack DJ, Semenza GL, Campochiaro PA. Hypoxia inducible factor-1alpha is increased in ischemic retina: temporal and spatial correlation with VEGF expression. Investigative ophthalmology & visual science. 1999;40:182–189. [PubMed] [Google Scholar]
- 35.Ferrara N, Gerber HP, LeCouter J. The biology of VEGF and its receptors. Nature medicine. 2003;9:669–676. doi: 10.1038/nm0603-669. [DOI] [PubMed] [Google Scholar]
- 36.Kallio PJ, Okamoto K, O'Brien S, Carrero P, Makino Y, Tanaka H, Poellinger L. Signal transduction in hypoxic cells: inducible nuclear translocation and recruitment of the CBP/p300 coactivator by the hypoxia-inducible factor-1alpha. The EMBO journal. 1998;17:6573–6586. doi: 10.1093/emboj/17.22.6573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Jiang BH, Rue E, Wang GL, Roe R, Semenza GL. Dimerization, DNA binding, and transactivation properties of hypoxia-inducible factor 1. The Journal of biological chemistry. 1996;271:17771–17778. doi: 10.1074/jbc.271.30.17771. [DOI] [PubMed] [Google Scholar]
- 38.Chen J, Connor KM, Aderman CM, Smith LE. Erythropoietin deficiency decreases vascular stability in mice. The Journal of clinical investigation. 2008;118:526–533. doi: 10.1172/JCI33813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Xin X, Rodrigues M, Umapathi M, Kashiwabuchi F, Ma T, Babapoor-Farrokhran S, Wang S, Hu J, Bhutto I, Welsbie DS, Duh EJ, Handa JT, Eberhart CG, Lutty G, Semenza GL, Montaner S, Sodhi A. Hypoxic retinal Muller cells promote vascular permeability by HIF-1-dependent up-regulation of angiopoietin-like 4. Proceedings of the National Academy of Sciences of the United States of America. 2013;110:E3425–E3434. doi: 10.1073/pnas.1217091110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Perdiguero EG, Galaup A, Durand M, Teillon J, Philippe J, Valenzuela DM, Murphy AJ, Yancopoulos GD, Thurston G, Germain S. Alteration of developmental and pathological retinal angiogenesis in angptl4-deficient mice. The Journal of biological chemistry. 2011;286:36841–36851. doi: 10.1074/jbc.M111.220061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Pawlus MR, Wang L, Hu CJ. STAT3 and HIF1alpha cooperatively activate HIF1 target genes in MDA-MB-231 and RCC4 cells. Oncogene. 2014;33:1670–1679. doi: 10.1038/onc.2013.115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Connor KM, Krah NM, Dennison RJ, Aderman CM, Chen J, Guerin KI, Sapieha P, Stahl A, Willett KL, Smith LE. Quantification of oxygen-induced retinopathy in the mouse: a model of vessel loss, vessel regrowth and pathological angiogenesis. Nat Protoc. 2009;4:1565–1573. doi: 10.1038/nprot.2009.187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Stahl A, Chen J, Sapieha P, Seaward MR, Krah NM, Dennison RJ, Favazza T, Bucher F, Lofqvist C, Ong H, Hellstrom A, Chemtob S, Akula JD, Smith LE. Postnatal weight gain modifies severity and functional outcome of oxygen-induced proliferative retinopathy. The American journal of pathology. 2010;177:2715–2723. doi: 10.2353/ajpath.2010.100526. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.