Abstract
MET and its ligand HGF are involved in many biological processes, both physiological and pathological, making this signaling pathway an attractive therapeutic target in oncology. Downstream signaling effects are transmitted via mitogen-activated protein kinase (MAPK), PI3K (phosphoinositide 3-kinase protein kinase B)/AKT, signal transducer and activator of transcription proteins (STAT), and nuclear factor-κB. The final output of the terminal effector components of these pathways is activation of cytoplasmic and nuclear processes leading to increases in cell proliferation, survival, mobilization and invasive capacity. In addition to its role as an oncogenic driver, increasing evidence implicates MET as a common mechanism of resistance to targeted therapies including EGFR and VEGFR inhibitors. In the present review, we summarize the current knowledge on the role of the HGF-MET signaling pathway in cancer and its therapeutic targeting (HGF activation inhibitors, HGF inhibitors, MET antagonists and selective/nonselective MET kinase inhibitors). Recent advances in understanding the role of this pathway in the resistance to current anticancer strategies used in lung, kidney and pancreatic cancer are discussed.
Keywords: MET, HGF, drug resistance, targeted therapy, NSCLC, renal cancer, pancreatic cancer
Introduction
Receptor tyrosine kinases (RTKs) are high-affinity cell surface receptors for polypeptide growth factors, cytokines, and hormones. RTKs regulate many intracellular signal transduction pathways involved in multiple normal and pathological biological processes, including cancer initiation, progression, invasiveness, metastasis and resistance to therapy.1 The Mesenchymal Epithelial Transition (MET) receptor belongs to a family of RTKs. Dysregulation of the MET signaling pathway occurs in a wide range of human cancers.1,2
Increasing evidence indicated that MET may be a common mechanism of resistance to anticancer treatment. In the present paper we review the mechanisms underlying this resistance and possible solutions to restore the sensitivity to several anticancer therapies, including targeted therapy (EGFR inhibitors, VEGFR inhibitors, anti-HER2 drugs, B-RAF inhibitor, ALK inhibitors), chemotherapy (gemcitabine, taxanes, cisplatin, capecitabine) and radiotherapy. The above mentioned drugs are currently used in the treatment of the most frequent solid tumors, including lung, renal and pancreatic cancer. For these tumors the mechanisms of resistance are specifically discussed.
Discovery, Structure and Function of MET and HGF/SF
The MET proto-oncogene is located on chromosome 7q21–31 and encodes the receptor tyrosine kinase MET. In 1984, TPR-MET oncogenic fusion protein from human osteosarcoma tumor cells was first discovered.3 The MET receptor is a 190 kDa glycoprotein heterodimer consisting of an extracellular α-subunit linked to transmembrane β-subunit by a disulfide bond. The extracellular portion includes the semaphorin (Sema) domain, the PSI domain (Plexin, Semaphorin and Integrin cysteine-rich) and four IPT domains (immunoglobulin plexins transcription).4 The intracellular domain includes a juxtamembrane sequence, a catalytic region and a carboxy-terminal multifunctional docking site. The juxtamembrane domain contains both Ser975 and Tyr1003 residues, that are involved in MET downregulation.5 A catalytic region positively modulates the kinase activity. Finally, a carboxy-terminal multifunctional docking site is responsible for the recruitment of many intracellular transducers and adaptors (Fig. 1).5,6
Figure 1.
Structure of the c-MET receptor. Extracellular portion include SEMA domain, PSI domain and IPT domain. Intracellular portion include juxtamembrane sequence, catalytic domain and C-terminal region with multifunctional docking site.
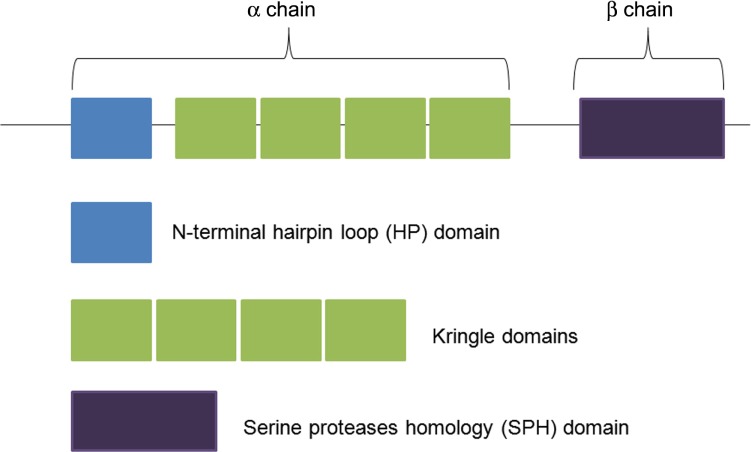
The MET receptor is expressed on the surface of epithelial and endothelial cells, where it can be bound specifically by its only known ligand, the hepatocyte growth factor (HGF), also called the scatter factor (SF). HGF/SF is a protein belonging to the serine protease family and produced in cells of mesenchymal origin. It is secreted as a single chain, biologically inactive, and converted into its mature form by a cleavage process catalyzed by extracellular proteases.6–8 Its biologically active form consists of a disulfide-bond heterodimer containing an α-chain and a β-chain. The first contains an amino-terminal hairpin loop (HL) domain followed by four peculiar domains, known as kringle domains; the latter contains a serine proteases homology (SPH) domain that lacks proteolytic activity,6–8 as shown in Figure 2. MET has two binding sites for HGF. Firstly, the IPT3 and IPT4 domains that bind the N domain of HGF/SF with high affinity, independently of HGF maturation. Secondly, the SEMA domain will bind the SPH domain of HGF/SF with low affinity but only when HGF is active.9–10
Figure 2.
Structure of HGF. Its biologically active form consists of a disulfide-bond heterodimer containing an α-chain and a β-chain.
MET and its ligand HGF are involved in many normal and pathological biological processes, such as fetal development where it plays an important role in liver, placenta and muscle formation, as well as development of the nervous system.11–13 After birth, activation of the HGF-MET pathway appears to be involved in epithelial-mesenchymal transition (EMT),14 as well as hepatic, renal and epidermis regeneration.11 Furthermore, MET signaling is also involved in tumor growth, invasion, resistance to therapy, angiogenesis and specially in the generation and maintenance of cancer stem cells (CSCs).15–17
Activation of MET Signaling Pathway
Activation of the MET signaling pathway can result from various molecular mechanisms, including germline or somatic mutations, chromosomal rearrangement, MET amplification, increased MET protein expression, increased HGF expression or by alteration of other pathways affecting MET activation.2 These modifications can be observed in a tissue-specific manner alone or combined in different proportions.1
The initiation of MET signaling begins with the binding of HGF to the MET receptor at the plasma membrane leading to the stable dimerization of two molecules of MET. Subsequent activation of its intracellular domain is through a process of trans-phosphorylation of the two tyrosine residues in the catalytical regions Y1234 and Y1235, followed by trans-phosphorylation of two docking tyrosines (Y1349 and Y1356) in the carboxy-terminal site. These two tyrosines form the multifunctional docking site which is unique to members of the MET subfamily and essential for MET signaling. It enables MET to bind to multiple substrates and activate a variety of signaling pathways either through direct interaction with signaling molecules or through adaptors such as growth factor receptor-bound protein 2 (Grb2) and Grb2-associated-binding protein 1 (Gab1). Subsequent activation of different intracellular signaling pathways (MAPK, PI3K-AKT cascades, STAT and NF-κB signaling pathways) is responsible for driving proliferation, cell survival, migration and invasiveness (Fig. 3).18–22
Figure 3.
HGF-MET signaling pathway with its downstream effector components (MAPK, STAT, PI3K-AKT cascades and NF-κB) leading to increases in cell survival, motility and proliferation.
MAPK signaling pathways
The Mitogen-activated protein kinases (MAPK)s are a group of serine/threonine protein kinases that are activated in response to a variety of extracellular stimuli and mediate signal transduction from the cell surface to the nucleus. They are involved in the regulation of normal cell proliferation, survival and differentiation.23
These cascades consist of three protein kinases: a MAPK kinase kinase (MAPKKK), a MAPK kinase (MAPKK) and a MAPK that are partially controlled by protein phosphorylation.20,24 The MAPKs represent the final effectors of the cascade. There are several groups of MAPKs in mammalian cells: the Extracellular signal-regulated kinases (ERKs), the p38MAPKs and the c-Jun NH2-terminal kinases (JNKs).7 The ERK pathway is the best studied of the mammalian MAPK pathway, and is deregulated in approximately one-third of all human cancers.25 The ERKs are mainly triggered by tyrosine kinase-dependent stimulation of RAS.7 MET activates RAS through the GRB2–SOS complex which can interact directly with carboxy-terminal multifunctional docking site of MET or can be associated indirectly through the SHC adaptor protein.18,26 Moreover, the tyrosine phosphatase SHP2 dephosphorylates the binding site on the adaptor protein GAB1; this will also result in RAS-ERK activation.27,28 In this pathway, ligand-mediated activation of receptor tyrosine kinases triggers guanosine triphosphate (GTP) loading of the RAS GTPase which can then recruit RAF kinases to the plasma membrane for activation.25 When RAF translocates to the membrane, it becomes activated which leads to phosphorylation of the dual specificity kinases MEK1 and MEK2. Finally, the activated MEKs phosphorylate the terminal effectors ERK1/ERK2.7
PI3K–AKT signaling pathway
The PI3K-AKT pathway is highly conserved and its activation is tightly controlled via a multistep process.29 PI3K can be activated directly by MET and/or indirectly by RAS.18 In response to the activation of the MET tyrosine kinases domain, the lipid kinase PI3K phosphorylates phosphatidylinositol (4,5)-bisphosphate (PIP2) to synthesize the second messenger phosphatidylinositol (3,4,5)-triphosphate (PIP3). PIP3 recruits AKT to the plasma membrane where it is phosphorylated and activated by phosphoinositide-dependent kinase-1 (PDK-1). Activated AKT subsequently phosphorylates numerous substrates that promote tumor genesis, including the mammalian target of rapamycin (mTOR), pro-apoptotic protein BAD, anti-apoptotic protein BCL2, E3 ubiquitin-protein ligase MDM2 (which promotes the degradation of the pro-apoptotic protein p53)7 and glycogen synthase kinase 3β (GSK3β). These targets interfere with important cell cycle regulators such as myc, cyclin D1.7
STAT signaling pathway
The Janus kinase (JAK)–signal transducer and activator of transcription (STAT) pathway consists of the seven members of the STAT protein family from which STAT3 and STAT5 have been demonstrated to be the most important for cancer progression.30–32 Boccaccio et al found that phosphorylation of the docking site of MET is followed by phosphorylation of STAT3 which causes STATs to dissociate from the receptors.33 STAT3 is mainly considered to be a direct transcription factor, though many other functions have been described, including gene expression regulation via epigenetic mechanisms, mitochondrion functions regulation, angiogenesis and CSCs regulation. This signaling pathway is able to promote tumor cell proliferation, survival and tumor invasion which highlights its function in cancer.30
NF-κB signaling
Nuclear factor-kappaB (NF-κB) is present in almost all cell types and tissues where it regulates gene expression by binding to promoters/enhancers of a host of genes. The NF-κB family consists of five proteins, p65 (RelA), RelB, c-Rel, p105/p50 (NF-κB1) and p100/52 (NF-κB2) that form homo- and heterodimeric complexes by associating with each other to transcriptionally regulate target genes.34 The NF-κB system is inactive in the cytoplasm due to the action of the inhibitor of κB (IκB).35 NF-κB activation generally occurs through either classical or alternative pathways.34 In the classical pathway, c-MET stimulation activates the inhibitor of nuclear factor kappa-B kinase (IKK) complex resulting in the phosphorylation of two N-terminal serine residues of IκB proteins leading to ubiquitin-mediated degradation of IκB and NF-κB activation. In the alternative pathway, IKKα is phosphorylated by NF-κB inducing kinase, which phosphory lates p100 leading to polyubiquitination and degradation of the inhibitory molecules by the proteasome. The result of both pathways is the destruction of IκBs which unmask the nucleus localization signal (NLS) resulting in the release of NF-κB. NF-κB translocates to the nucleus and transactivates target genes by binding to gene promoter/enhancer regions.21,22,36,37
In conclusion, the final output of the terminal effector components of these pathways is the activation of cytoplasmic and nuclear processes leading to increases in cell proliferation, survival, mobilization and invasive capabilities.
HGF and MET Dysregulation in Cancer
In human malignancies, genetic alterations of the MET proto-oncogene are relatively rare. In a recent study MET amplification was detected in 2.5% of 1,115 patients with advanced solid cancers. The prevalence was highest in renal cell (RCC, 14%) followed by adrenocortical tumors (15%), gastroesophageal (6%), breast (5%) and ovarian cancers (4%).38 Generally, in genetically altered MET tumors, MET oncogene behaves as a cell-autonomous selectable driver of tumor growth.17 The role of MET in tumors is not only restricted to relatively rare genetic alterations but relies on the frequent overexpression of the wild-type gene.4,7,39 In the CSCs, the wild-type form of MET helps to maintain the phenotype ‘inherent’ in the stem/progenitor cell of origin.17
Indeed, activation of the HGF-MET pathway is currently associated with aggressive pathologic features, poor prognosis, and treatment resistance in several different tumor types.38,40,41 Lorenzato et al noted that activating somatic MET mutations were infrequent in primary tumors but commonly present at metastatic sites, suggesting that MET mutations are associated with progression rather than initiation of tumorigenesis.42 Cancers of unknown origin (CUPs) are characterized by upfront metastatic dissemination, a highly undifferentiated, stem-like phenotype and lacking histological markers of the tissue of origin.43 Interestingly, in these tumors MET activating mutations are present more frequently (30%) in comparison to other solid tumors.43 A more aggressive biologic behavior of tumors overexpressing MET is also reflected in the inferior survival outcomes found in some studies,40,41 however, there are some contradictory results on this issue.44 The frequency of MET dysregulation is higher in adenocarcinomas compared to squamous cell tumors. In gastro esophageal tumors, MET amplification has been reported in only 1% of esophageal squamous cell carcinoma, whereas it ranges from 2% to 10% in adenocarcinomas.41,45,46 Moreover, some interesting association of MET dysregulation and BRAF mutation or PTEN loss have been described.47 Finally, MET overexpression has been associated with treatment resistance in radiotherapy, anti-EGFR TKI, VEGFR/BRAF/mTOR inhibitors and anti-HER2 therapy.48–51
In summary, alterations in MET and/or HGF are frequently observed in a wide range of cancers and their presence appears to confer an increased propensity for a more aggressive clinical behavior manifested by invasion, metastasis and resistance to the therapy. However, it is possible, that in some specific histological types and in the presence of some molecular aberrations, there is no correlation between MET overexpression and survival outcome.
HGF and MET Inhibitors for Cancer Therapy
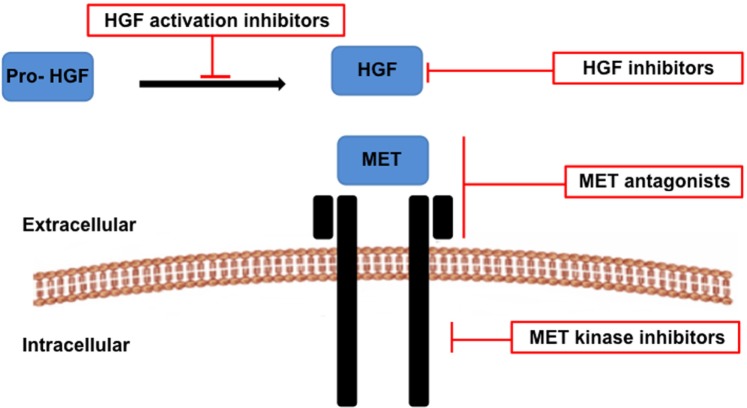
RTK pathways have proven to be attractive drug targets allowing the development of novel treatment strategies. One such promising target is the HGF-MET pathway. The HGF and MET targeting agents can be categorized into those that target the HGF ligand or those that target the MET receptor. Agents targeting the ligand can further be categorized into either HGF activation inhibitors (preventing the cleavage of pro-HGF into the active form) or HGF inhibitors (blocking the direct binding of HGF to the MET receptor).4 Agents targeting the MET receptor can further be categorized into either MET antagonists (binding the receptor) or MET tyrosine kinase inhibitors (TKIs) targeting MET intracellu-larly4 (Fig. 4, Table 1).
Figure 4.
Therapeutic strategies targeting the HGF-MET signaling pathway in cancer include HGF activation inhibitors, HGF inhibitors, MET antagonists and MET kinase inhibitors.
Table 1.
Drugs targeting HGF-MET signaling pathway.
| DRUG | MOLECULAR TARGETS | |
|---|---|---|
| HGF activation inhibitors | HGFAs (activators) | Pro-HGF |
| HaIs (inhibitors) | Pro-HGF | |
| HGF inhibitors | Rilotumumab (AMG102) | HGF |
| Ficlatuzumab (AV-299) | HGF | |
| TAK701 | HGF | |
| MET antagonists | Onartuzumab | MET |
| CE-355621 | MET | |
| DN-30 | MET | |
| LA480 | MET | |
| MET kinase inhibitors | ||
| Selective | Tivantinib | MET |
| Savolitinib | MET | |
| AMG 337 | MET | |
| INC 280 | MET | |
| Nonselective | Crizotinib | MET, ALK, ROS, RON |
| Cabozantinib | MET, VEGFR2, KIT, RET, AXL, FLT3 |
HGF and MET-targeting agents can be classified as either monoclonal antibodies or small molecules and they differ in terms of pharmacological properties as well as their underlying mechanism of action. They can be used either as a monotherapy, in combination with chemotherapy or in combination with other targeted therapy.15 Most of the HGF and MET inhibitors showed promising results when used in drug combinations, most likely due to the complexity of tumor biology. Regarding side effects, HGF and MET inhibitors have demonstrated few adverse effects when administered to patients and any combination treatment has been well tolerated. The most frequent adverse events seen in clinical trials included fatigue, anorexia, nausea, vomiting, fever, hypersensitivity reactions, peripheral edema, proteinuria, hematuria, hypertension and bleeding.1,15
Inhibition of HGF activation
The activation of HGF from its inactive precursor pro-HGF is a critical step in HGF function52 and depends on the balance between the activators (HGFAs) and the inhibitors (HAIs).53,54 Hu et al.55 investigated the role of HAI-1 in patients with prostate cancer and benign prostate hyperplasia. It was observed that a high level of HAI-1 protein and mRNA expression was present in patients affected by benign disease compared to the more aggressive prostate cancer specimens. Low HAI-1 correlated with a high Gleason score, a more advanced pathological stage and was a predictor for poor prognosis. Therefore, high HAI-1 might be used as a favorable prognostic marker for prostate cancer and a therapeutic target for the treatment of this malignancy.55 In another study, Tsai et al.56 investigated the role of HAI-2 in human prostate cancer progression. In this study, a human prostate cancer progression model consisting of cancer cells with increasing invasion capability was used, as well as xenograft models. The expression of HAI-2 decreased throughout the progression in cell invasion capability, and was accompanied by an increased activation of matriptase, an extracellular matrix (ECM)-modulating protease which contributed to tumor genesis and metastasis.56 Therefore, HAI-1 and HAI-2 might represent novel prognostic markers as well as therapeutic targets. The development of HAIs in cancer is still in the early stages and up to date, no available clinically tested HAIs are available.
Inhibition of HGF binding to the MET receptor
HGF inhibitors bind to HGF and block binding to the MET receptor, effecting downstream activation of the pathway. Several monoclonal antibodies against HGF have been tested preclinically, such as rilotumumab, ficlatuzumab and TAK701. Rilotumumab is a fully humanized IgG2 monoclonal antibody.57 Preclinical data showed synergistic cytotoxicity when acting in combination with temozolomide and docetaxel.58,59 The efficacy of rilotumumab was evaluated in a phase 2 study in patients affected by gastric or oesophagogastric junction tumors. The patients were randomized to receive rilotumumab plus capecitabine or placebo plus capecitabine. Interestingly, improved median progression-free survival (PFS) and overall survival (OS) in patients treated with rilotumumab plus capecitabine was seen only among the patients with MET overexpression60 which underlines the growing importance of stratifying the patients for target treatment. Ficlatuzumab is a humanized anti-HGF IgG1 monoclonal antibody that was evaluated in a phase I study in solid tumors as a single agent or in combination with erlotinib.61 TAK701 is a humanized monoclonal antibody directed against HGF.62 Okamoto et al described that the addition of TAK-701 to gefitinib treatment is a promising strategy to overcome EGFR TKI resistance induced by HGF in non-small-cell lung cancer (NSCLC) with an activating EGFR mutation.63
MET antagonists
MET antagonists compete with HGF for MET binding, resulting in the degradation of MET and subsequent inactivation.1 Several MET antagonists have been synthesized, including onartuzumab, CE-355621, DN-30, and LA480.
Sano et al.64 engineered the human NSCLC cell line PC-9, resulting in variants with MET/HGF overexpression and EGFR mutations (exon 19). The combination of onartuzumab and erlotinib was tested in vitro and in vivo in xenograft models. A PC-9 cell line with HGF overexpression was less sensitive to erlotinib than the parental PC-9 cell line without HGF overexpression; the addition of onartuzumab to erlotinib suppressed the proliferation of parental cells in vitro. In PC-9/HGF xenograft tumors, onartuzumab or erlotinib alone minimally inhibited tumor growth; however, combining onartuzumab and erlotinib markedly increased tumor suppression. The authors concluded that patients with NSCLC with EGFR mutations who express high levels of HGF may benefit from onartuzumab and erlotinib combination therapy while HGF expression could be a potential novel biomarker for patient selection.64 In clinical practice, onartuzumab showed significant survival benefits in combination with erlotinib in NSCLC patients with MET overexpression in a randomized phase II study.65
MET kinase inhibitors
Another approach for inhibiting the MET pathway is through MET kinase inhibitors which target intracellular MET. Several small molecule MET kinase inhibitors have entered clinical development over the past decade, including selective MET kinase inhibitors, such as tivantinib (ARQ 197), savolitinib (AZD6094, HMPL-504; volitinib), AMG 337, INC 280 and nonselective MET kinase inhibitors (crizotinib (PF02341066), cabozantinib (XL 184) and foretinib.
Tivantinib (ARQ 197) is a selective, oral, small molecule inhibitor of the MET RTK.66 It has demonstrated antitumor activity in a wide range of human tumor cell lines and in human xenograft models.66,67 Recently, a phase I study investigating the combination of tivantinib and sorafenib in patients with advanced solid tumors has been published.67 Preliminary evidence of anticancer activity was observed in patients with renal cancer, hepatocellular cancer and melanoma, including patients refractory to sorafenib and/or other anti-VEGF pathway therapies. Therefore this combination treatment was considered to have therapeutic potential treatment a variety of solid tumors. The combination of tivantinib plus erlotinib has been investigated in a phase 3 trial for the treatment of patients with advanced or metastatic NSCLC68 and in a phase 2 trial of tivantinib as a single agent for the treatment of hepatocellular carcinoma.69 The combination treatment in NSCLC patients did not show statistically significant differences in OS between the arm of tivantinib plus erlotinib compared to the erlotinib alone arm,68 although a significant improvement in PFS was observed in the group of patients with KRAS-mutant tumors (P = 0.006). Similarly, in HCC patients, a significant improvement in OS was observed in patients with high tumor MET expression.69
Savolitinib (AZD6094, HMPL-504) is a novel, selective MET inhibitor.70 In preclinical studies, savolitinib displayed nanomolar in vitro activity against MET and its downstream signaling targets. In vivo, savolitinib induced antitumor activity, particularly in tumor models with high MET gene amplification, including papillary renal cell carcinoma xenografts.71 In an early clinical dose escalation study, savolitinib demonstrated partial responses in 3 papillary renal cell cancer patients, with a fourth patient still on study reaching 27% tumor reduction. Analysis of pre-treatment tumor samples showed that the responders had either a high MET gene copy number or a high MET protein expression.72
Crizotinib is a small molecule inhibitor of the anaplastic lymphoma kinase (ALK) with additional activity against the MET, ROS, and RON receptors. Crizotinib was approved for use in ALK-rearranged advanced NSCLC in Europe and US.73–75 The efficacy of crizotinib has been demonstrated in two randomized trials limited to patients whose tumors had the ALK rearrangement. The first study is a phase 3 trial which randomly assigned 347 pre-treated patients (all one prior platinum-based chemotherapy regimen) to either crizotinib or single agent pemetrexed or docetaxel.76 In this study, PFS, the primary endpoint of the trial was significantly increased with crizotinib compared to traditional chemotherapy (median 7.7 versus 3 months), the objective response rate (ORR) was also significantly increased (65 versus 20 percent). Responses were achieved more rapidly than with chemotherapy (median time to response 6.3 versus 12.6 weeks) and were of longer duration (32 versus 24 weeks). In this study, no significant difference was observed in OS (median 20.3 versus 22.8 months). The absence of an OS benefit presumably reflects subsequent treatment since 64 percent of chemotherapy-treated patients had crossed over to crizotinib after progressing on chemotherapy. The second study is a phase 2 trial which randomly assigned 343 chemotherapy naïve patients to crizotinib or chemotherapy with pemetrexed plus either cisplatin or carboplatin.77 PFS, the primary endpoint of the trial, was prolonged with crizotinib compared to chemotherapy (median 10.9 versus 7 months, HR 0.45, 95% CI 0.35–0.60). The ORR was also increased (74 versus 45 percent), although again OS was not significantly different. However, a recently published meta-analysis of six clinical trials revealed extended survival and improved response rates in NSCLC patients treated with crizotinib.75 The ORR, partial response and complete response rates were 61.2%, 59.8% and 1.5%, respectively. The proportion of patients achieving stable disease was 42.6%.78
Cabozantinib is a small molecule inhibitor of MET, VEGFR2, KIT and RET followed by AXL and FLT379 and is approved by the US Food and Drug Administration for the treatment of progressive, metastatic medullary thyroid cancer. In a randomized trial, 330 patients with progressive, metastatic or unresectable locally advanced medullary thyroid cancer were randomly assigned to receive either cabozantinib or placebo.80 A significant prolongation in PFS was observed for cabozantinib treatment compared to placebo (11.2 versus 4.0 months). Partial responses were observed in 28 versus 0 percent. Currently, multiple phase 3 trials in a variety of solid tumors are undergoing.
Targeting HGF-MET in Solid Tumors to Overcome the Drug Resistance
In addition to its role as an oncogenic driver, increasing evidence implicates MET as a common mechanism of resistance to targeted therapies including approved EGFR and VEGFR inhibitors.48,49 Moreover, MET is involved in resistance to anti-HER2 therapies (trastuzumab and lapatinib)51,81 and a BRAF inhibitor (vemurafenib)82 has been described. In the following paragraphs, we describe how targeting of the HGF-MET signaling pathway can overcome the drug resistance in NSCLC, RCC and pancreatic cancer (PDAC) and we summarize clinical studies (Table 2). The expression of MET and HGF is high in these tumors: in NSCLC 40% and 50%, in RCC 70% and 60% and in PDAC >70% and >35%.83
Table 2.
The frequency of the most common MET alterations in selected human cancers.
Targeting HGF-MET in NSCLC to overcome drug resistance
Lung cancer is the leading cause of cancer-related death for both men and women worldwide. The most prevalent mutated or rearranged oncogenes identified in NSCLC are KRAS, epidermal growth factor receptor (EGFR) and ALK. This knowledge has been translated into clinical practice with the introduction of targeted therapies that have led to the improvement of NSCLC patients’ outcome: EGFR TKIs (erlotinib, gefitinib) for NSCLC patients harboring activating mutations in the EGFR TK domain and crizotinib for NSCLC patients carrying ALK translocations.84–86
Nevertheless, many patients acquire resistance to anti-EGFR therapy after 6–12 months.87 Acquired resistance to EGFR TKIs can occur as a result of secondary EGFR mutations or parallel activation of downstream signaling pathways, including MET. Approximately 5%–22% of NSCLC patients with secondary resistance to EGFR TKIs had evidence of amplification of the MET oncogene.88,89
Functional crosstalk of MET with EGFR has been reported in lung and colorectal cancer85,87 and has emerged as a major mechanism for cancer progression as well as resistance to anti-EGFR targeted therapy (see Fig. 5). The therapy of lung cancer with EGFR TKI leads to MET amplification which subsequently activates PI3K–AKT signaling.88,89 In this way, MET signaling can compensate for EGFR inhibition. The resistance can be prevented by combined inhibition of EGFR and MET, as has been shown in human lung tumor xenografts.90,91 Conversely, the treatment of tumor cells with MET TKIs may lead to the selection of tumor cell populations that escape growth inhibition via the EGFR or SRC kinases.92
Figure 5.
Functional crosstalk of HGF-MET signaling pathway with EGFR and VEGFR signaling pathway.
Notes: The anticancer treatment with EGFR antibodies (cetuximab, panitumumab) or EGFR TKIs (gefitinib, erlotinib) leads to MET amplification with subsequent activation of PI3K-AKT signaling. The resistance can be prevented by dual inhibition of EGFR and MET. Similarly, MET-HGF signaling pathway activation help evade VEGFR inhibition induced by bevacizumab, sunitinib or pazopanib and dual inhibition with VEGFR and MET inhibitor might overcome the resistance to anticancer treatment.
Several MET inhibitors have been tested in combination with EGFR inhibitors. However, no difference in OS was observed with the combination of either tivantinib with erlotinib,68 or onartuzumab with erlotinib.65 When analyzing the subgroups of the patients, a significant improvement in PFS was observed in the group of patients with KRAS-mutant tumors (P = 0.006) treated with tivantinib and erlotinib. Similarly, subgroup analysis of NSCLC patients revealed that tumors which overexpressed MET, the combination of onartuzumab plus erlotinib was associated with a significant improvement in both PFS and OS (P = 0.04 and 0.002, respectively).
Another type of MET-targeted therapy available in clinical practice is crizotinib, the first clinically available ALK inhibitor for ALK-rearranged NSCLC in the world.93,94 This drug was initially designed as a MET inhibitor and indeed, Ou and colleagues showed that patients with NSCLC with MET amplification, but without ALK rearrangement, experienced a rapid and durable response to crizotinib. This demonstrated its therapeutic role also as a MET inhibitor.95 Kogita et al investigated the role of the MET signal in ALK-positive NSCLC demonstrating that HGF mediated resistance to alectinib (selective ALK inhibitor), but not to crizotinib.96 It was observed that alectinib activated the MET signal even in the absence of HGF and that the inhibition of the MET signal enhanced the efficacy of alectinib.96 Moreover, MET expression was significantly increased in ALK- rearranged NSCLC.97
Crizotinib showed remarkable responses in NSCLC patients harboring CD74-ROS1 rearrangement; however, crizotinib resistance eventually developed due to acquired mutations such as G2032R in ROS1. As the result of high-throughput drug screening, the authors found that the cabozantinib effectively inhibited the survival of CD74-ROS1-mutant Ba/F3 cells and crizotinib-resistant patient-derived cancer cells (MGH047) harboring G2032R-mutated CD74-ROS1. Cabozantinib was therefore identified as a potential therapeutic strategy to overcome this form of resistance to crizotinib.98,99
Actually, several clinical trials targeting HGF-MET signaling in NSCLC are ongoing, using several different monotherapy drugs targeting HGF-MET, such as cabozantinib (NCT01639508) or PF-02341066 (NCT00585195) and combinations with EGFR TKI (gefitinib, erlotinib) (NCT01610336, NCT01911507, NCT01982955, NCT01822496, NCT01887886), nivolumab (NCT02323126) or pemetrexed (NCT02134912).
Therefore, in conclusion, HGF-MET signaling plays an important role in acquired resistance to EGFR TKIs in NSCLC and studies demonstrated that combined inhibition of EGFR and MET can overcome resistance to EGFR inhibitors. Therefore, it seems reasonable to prefer combination therapies that target both signalling pathways that are primarily responsible for the cancer phenotype. In this way, the rescue pathways are targeted simultaneously.
Targeting HGF-MET in renal cancer carcinoma to overcome the drug resistance
RCC is the third most frequent cancer originating from the genitourinary organs.100 It originates from either the proximal tubule of the kidney or the collecting duct and is classified into four major histological types: clear cell (ccRCC, 75–85% of tumors), papillary (pRCC, 10–15% of tumors), chromophobe (5–10%), and collecting duct tumor (rare). pRCC can be further divided into two morphological subtypes; type 1 consists of predominantly basophilic cells and type 2 of mostly eosinophilic cells. In general, metastatic pRCC has a worse prognosis than ccRCC.98 Moreover, type I and type 2 of pRCC have different clinical features. Type I is characterized by an indolent clinical course, and type II by a more aggressive clinical behavior.101
In ccRCC tumors, mutations or functional inactivation of the von Hippel-Lindau (VHL) gene occur in the majority of cases, resulting in the loss of function or reduced levels of the VHL protein.102 The subsequent transcriptional hyperactivation of HIF-targeted genes, such as VEGF, PDGF, TGFa, HGF, MET drives tumor progression and hypervascularization.103,104 Albiges and colleagues105 reported that MET expression was higher in pRCC than in ccRCC, and higher in type I pRCC compared to type II pRCC. In pRCC, several activating missense mutations of the MET gene have been described, both in sporadic and hereditary forms.106 The trisomy of chromosome 7, in which MET is located, has been seen to be a common occurrence in pRCC.107
Currently, first-line treatment of patients affected by RCC include several anti-VEGF agents (sunitinib, pazopanib and bevacizumab) and an mTOR inhibitor (temsirolimus).108 VEGF-targeted therapies have a survival benefit in RCC patients, but fail to produce enduring clinical responses in most patients. Inevitably, the disease progresses following a transient 9–11 month period of clinical benefit.108 It is now well established that crosstalk between the MET and VEGFR pathways supports tumor vascularization and progression but is also implicated in the resistance to anti-VEGFR therapies (Fig. 5). Gene expression studies comparing primary glioblastoma to bevacizumab-treated tumors revealed MET as one of the most upregulated genes.109 Thus, MET activation is implicated in the upregulation of alternate pathways that help evade VEGFR inhibition.110,111
Recently, Ciamporcero et al.103 evaluated the effects of either monotherapy or combination strategy targeting the VEGF (axitinib) and MET pathways (crizotinib) in ccRCC models (SCID mice). The authors tested these drugs in a human ccRCC patient–derived xenograft, in both sunitinib-sensitive and -resistant models. The combination therapy increased the antitumor effect of both drugs, independently from MET expression. It was concluded that, clinical testing of a combined VEGF and HGF-MET pathway blockade could improve the outcome of patients affected by RCC. Similarly, Shojaei et al.49 observed increased HGF expression in mouse models resistant to sunitinib. The addition of a MET inhibitor was able to overcome sunitinib resistance.49 Choueiri and colleagues112 conducted a phase II clinical trial to investigate the role of foretinib, a dual inhibitor of MET and VEGF in pRCC. Interestingly, pRCC harboring the germline MET mutations were highly predictive of foretinib response, with 50% of patients with mutations having an objective response compared with 9% of patients without mutations.112 Another MET inhibitor, savolitinib, is currently in clinical development for various indications, including pRCC.72,113 In a recent study pharmacodynamic and antitumor activity of savolitinib were tested using a dose response up to 25 mg/kg daily, representing clinically achievable exposures and comparable with the activity of sunitinib or crizotinib.71 Savolitinib treatment resulted in tumor regressions, whereas sunitinib or crizotinib resulted in unsustained growth inhibition. Evaluation of the pharmacodynamic effects of savolitinib showed that this drug can suppress the MET signalling pathway and the duration of target inhibition is dose related. Interestingly, continuous dosing of savolitinib for approximately 5 weeks showed antitumor activities with no signs of developing resistance, in contrast to sunitinib,114 which suggested that savolitinib could be of therapeutic potential in sunitinib-resistant pRCC patients.71
Temsirolimus is an mTOR inhibitor and is used in RCC patients with poor prognosis.108 mTOR, a serine/threonine kinase, is a downstream target of the PI3K and AKT pathways, similar to EGFR, MET and VEGF. It plays a critical role in cell survival and proliferation.115,116 Ishikawa et al.115 demonstrated that mTOR inhibitors (temsirolimus and everolimus) can overcome HGF-dependent resistance to EGFR-TKIs in EGFR mutant lung cancer cells. Moreover suppression of tumor angiogenesis has been observed.115
Actually, several clinical trials targeting HGF-MET in RCC are ongoing, both as a monotherapy (crizotinib in NCT01524926, AZD6094 in NCT02127710, INC280 in NCT02019693) or in combination therapy (axitinib and crizotinib in NCT01999972).
Targeting the MET and VEGFR pathways simultaneously represents a promising approach for RCC treatment since this will target multiple pathways involved in angiogenesis, tumor survival and metastasis.
Targeting HGF-MET in pancreatic ductal adenocar-cinoma to overcome the drug resistance
PDAC is a highly aggressive malignancy and fourth leading cause of cancer-related death in developed countries.117 The median survival after diagnosis is 2–8 months and only 3–6% of all patients with PDAC survive 5 years after diagnosis. Surgical resection remains the cornerstone of management of PDAC, but this is only feasible for a limited number of patients. The average survival of successfully resected patients is between 12 to 20 months, with a high probability of relapse. Since symptoms are not very clear in early stages, 80% of PDACs are diagnosed when already advanced, and no curative therapy is currently available.117 Chemotherapy prolongs life by only a few months, and PDAC chemoresistance renders most drugs ineffective. Nowadays, there are three different therapeutic options for PDAC in the metastatic setting, gemcitabine as monotherapy, gemcitabine in combinations with nab-paclitaxel or the combination of 5-FU, leucovorin, irinotecan, and oxaliplatin (FOLFIRINOX).117–118
MET overexpression was significantly associated with TNM stage and lymph node invasion, poor tumor differentiation, increased abnormal angiogenesis and poor OS of PDAC patients.119–121 There are different mechanisms by which MET overexpression confers chemoresistance in PDAC. MET has been identified as a marker of pancreatic CSCs which have been associated with PDAC aggressiveness, metastatic behavior and intrinsic resistance to chemotherapy.122 Another mechanism involves the mesenchymal support network. Stroma is the predominant source of HGF, suggesting MET activation is, at least in part, a result of paracrine signaling.123 Interestingly, HGF has been shown to not only increase VEGF production by stromal cells, but also to act synergistically with VEGF to induce endothelial cell tube formation and proliferation.124 Inhibition of HGF could therefore represent a potentially useful antiangiogenic approach in PDAC. Additionally, preclinical studies have demonstrated that overexpression of MET has also been associated with EMT-like changes in acquired-gemcitabine-resistant PDAC cells.125
Avan et al.126 showed the ability of crizotinib to specifically target CSC-like-subpopulations, interfere with cell- proliferation, induce apoptosis, reduce migration and synergistically interact with gemcitabine, supporting further studies on this novel therapeutic approach for PDAC. Moreover, the same group showed that crizotinib decreased tumor dimension, prolonged survival, promoted gemcitabine uptake but reduced gemcitabine catabolism. These effects were mediated by an increased activity of the human equilibrative and concentrative nucleoside transporters (hENT1 and hCNT1), and a decreased cytidine deaminase (CDA). The synergism of gemcitabine and crizotinib in an orthotopic mouse model of primary PDAC warrants clinical evaluation for PDAC treatment.127
Also cabozantinib enhanced the effect of gemcitabine in a human pancreatic cancer model growing orthotopically in NOD SCID mice. Alone and in combination the tumor growth was inhibited and the population of CSCs was decreased.119 Hage et al.128 demonstrated that cabozantinib altered the expression of apoptosis molecules and shifted the balance to antiapoptotic signaling. In parallel, cabozantinib inhibited SOX2, MET and CD133 expression in addition to the self-renewal potential. Most importantly, cabozantinib increased efficacy of gemcitabine even in high-gemcitabine-resistant PDAC, suggesting that cabozantinib can overcome gemcitabine resistance.
Several clinical trials in advanced cancers, including PDAC are currently ongoing including combinations with crizotinib (dasatinib, pazopanib/pemetrexed, vemurafenib, axitinib) or the combination cabozantinib and gemcitabine (NCT01744652, NCT01548144, NCT01531361, NCT01999972, NCT01663272).
Targeting HGF-MET in other tumors
Aberrant MET signaling is a hallmark of multiple cancer types. For metastatic medullary thyroid cancer, cabozantinib is approved by the US Food and Drug Administration.129 The MET pathway is frequently aberrantly activated in colorectal cancer, esophagogastric cancer, hepatocellular carcinoma, prostate cancer, sarcomas and several studies have shown promising activity of HGF-MET targeting drugs in these tumor types.130–138 Moreover, targeting the HGF-MET axis opens new therapeutic possibilities for liquid cancers. For example, HGF expression is critical in acute myeloid leukemia (AML) pathogenesis. Kentsis et al.139 demonstrated that leukemic cells treated with crizotinib can develop resistance due to compensatory upregulation of HGF expression, leading to restoration of HGF signaling. In cases of AML where MET is coactivated with other tyrosine kinases, such as fibroblast growth factor receptor 1 (FGFR1), concomitant inhibition of FGFR1 and MET blocked compensatory HGF upregulation resulting in sustained logarithmic cell kill both in vitro and in vivo xenograft models in vivo.139
Biomarkers for MET Inhibitors
In the era of personalized therapy with more frequent use of targeted agents, it is important to identify biomarkers which can predict response to a specific class of agents, including MET inhibitors. A number of predictive biomarkers to HGF-MET inhibitors are currently being evaluated, such as circulating HGF and MET, MET protein overexpression, MET gene amplification and mutation. However, none of them has been validated yet.
Circulating HGF and MET
Treatment with HGF inhibitors (ficlatuzumab and rilotumumab) can result in an increase in plasma total HGF and soluble MET concentrations from baseline, but no association with clinical outcomes was demonstrated.140–144 In another clinical trial, administration of the MET antagonist onartuzumab resulted in an increase in HGF, but this elevation was independent of dose, drug exposure, dose duration or tumor type. In addition, the evaluation of the relationship between changes in HGF levels and clinical outcomes in MET-positive patients was inconclusive.145 In contrast, MET kinase inhibitors (tivantinib) did not show obvious changes in HGF in phase I clinical studies.146
MET protein overexpression
Currently, high MET protein expression levels in tumor tissues may be associated with poor prognosis in selected cancer types. In a phase 2 trial with patients that had positive MET expression in tumor tissues, a longer survival was found in patients who received the HGF inhibitor rilotumumab plus ECX (epirubicin, cisplatin, capecitabine) than patients who received ECX alone.147 However, the results could not be reproduced in the confirmatory phase 3 RILOMET-1 study148 and OS were statistically significantly worse with rilotumumab (P = 0.016). Exploratory biomarker analyses performed on patients with advanced NSCLC receiving the MET antagonist onartuzumab in combination with erlotinib demonstrated an OS benefit in MET-positive patients (P = 0.002).149 A subgroup analysis suggested that MET expression by IHC was a more sensitive predictor than MET amplification measured by FISH.149 However, mRNA expression of MET did not predict survival in patients treated with onartuzumab.149 Furthermore, phosphorylated MET, a marker for MET pathway activation, and serum MET did not appear to be a suitable biomarker for NSCLC.151
A multicenter Phase II randomized controlled trial, investigated the role of tivantinib in the second-line setting in patients with unresectable hepatocellular carcinoma.152 The patients were randomized 2:1 to receive either oral tivantinib in two different drug dosages (360 mg twice-daily or 240 mg twice-daily) or to receive placebo. Better PFS was observed in the tivantinib arm in comparison with the placebo arm (HR 0.45, P = 0.02) but no detrimental effect in MET-expression-negative patients wasfound.152,153 A retrospective analysis of patients with NSCLC tumors treated with tivantinib plus erlotinib failed to demonstrate a predictive value for MET protein overexpression.154 In subgroup analyses, it was shown that MET expression could have predictive potential only in MET-positive patients with nonsquamous histology, suggesting that the clinical relevance of MET biomarker for tivantinib may vary among tumor types.155
MET gene amplification and mutation
Amplification of the MET gene locus with overexpression of the receptor on the cell surface is a well-characterized aberrancy as well as MET gene mutations. The latter leads in most cases to a constitutively active form of the molecule. The frequency of these molecular changes differs among tumor types (see Table 2).
In preclinical studies MET amplification predicted sensitivity to multiple MET targeted agents including crizotinib; this was also found in several clinical case reports.156–157 In a subset of esophagogastric adenocarcinoma patients, a high level of MET amplification as measured by FISH, correlated with responsiveness to crizotinib, although this response was transient.156 A durable response to crizotinib was observed in a NSCLC patient with de novo MET amplification.157 In a Phase II trial of foretinib in pRCC, germline mutations of MET were predictive of clinical response.112 Patients carrying a MET germline mutation experienced partial response (5/10) or stable disease (5/10), compared to only 9% (5/57) of patients with no MET mutations.112 Moreover, in newly diagnosed multiple myeloma, patients carrying four MET gene copies (9.8%) had a short PFS.159
MET phosphorylation/activation
In clinical pre- and post treatment patients’ biopsies, changes in MET phosphorylation and activation of downstream signaling effectors were observed in response to several anti-MET agents.160–161 Treatment with foretinib or tivantinib led to a decrease in the level of MET phosphorylation and activation of ERK and AKT pathways in post treatment biopsies, although it remains unclear whether changes in these markers are predictive of clinical responses.153,162
Conclusions
Circulating HGF and MET were evaluated as a pharmacodynamic biomarker of MET inhibition in different clinical trials and it seems that their potential as predictive biomarkers to response depends on the type of MET inhibition (HGF inhibitors, MET antagonists, MET kinase inhibitors). To date their predictive value for clinical response has not been shown. MET protein expression levels in tumor tissues may be prognostic biomarkers of survival in selected cancer types with specific molecular aberrations. MET gene amplification, copy number, and mutations appear to be relatively conservative biomarkers, but they may be associated with rare events in cancer development.
Future Directions
The HGF-MET axis seems to be one of the most functional signaling pathways involved in tumor genesis, progression and resistance to anticancer treatment.
Several studies demonstrated both in vitro and in xenografts that the inhibition of the MET signaling pathway by using selective MET inhibitors correlated with cell growth arrest and apoptosis. Furthermore, the combination strategy resulted in an additive antitumor effect and also restored chemosensitivity in MET-overexpressed resistant cells. Unfortunately, there is a discrepancy between preclinical and clinical studies, which can often be attributed to inappropriate patients’ selection and missing circulating biomarkers.2 In fact, one of the key challenges in the development of targeted therapeutics is the identification of patients likely to obtain clinical benefit by having potentially sensitive tumors. It is essential to develop biomarker assays that have good specificity and sensitivity, and should be included in both early stage and registration-enabled clinical trials to determine their clinical utility.163 In accordance with Hack et al.163, possible biomarker strategies to identify MET-driven tumors might be at the DNA level (MET gene amplifications/mutations), at the RNA level (MET-RNA overexpression, microRNA associated with response/resistance to therapy), or at the protein level (MET protein overexpression, MET posttranslational modifications ie, phosphorylation).163
Molecular heterogeneity exists both between patients and intra-patient. These molecular changes occur throughout the disease process, in response to treatment and as a consequence of resistance to therapy. Protein markers are often used to assess and predict disease progression and drug response, but gene expression markers may provide more reliable results.163 MicroRNAs may also represent novel markers of MET activity. For example, three candidate microRNAs that are involved in the modulation of MET expression, miR-449a, miR-340, and miR-409–3p are down regulated in NSCLC, aggressive breast cancer cell lines, and bladder cancer cells, respectively.164–165 In breast cancer cell lines, expression of miR-340 was inversely correlated with MET expression.166 Hence, the identification of small sets of gene signatures based on mRNA expression profiles may be helpful in predicting drug response.167
Thus, in future clinical trials emphasis on biomarker identification and development should be prioritized, which will allow a better prediction of response to MET inhibitors.
Another challenge in the effective use of HGF/MET-targeted agents for cancer treatment, is the identification and testing of rational drug combinations. Since solid malignancies are comprised of highly heterogeneous groups of cells, the use of targeted inhibitors may select a malignant clone of cells, which are inherently resistant to blockage of the HGF-MET pathway. Acquired resistance to HGF/MET inhibition in cancer cells can develop by point mutations, by increased amplification of MET or by activation of a compensatory signaling pathway which can bypass the effects of targeted agents. Strategies to overcome HGF-MET resistance would involve the targeting of multiple compensatory pathways simultaneously either by using multitargeted agents such as cabozantinib (MET, VEGF), crizotinib (MET, ALK), or by combining targeted agents such as onartuzumab or ficlatuzumab with erlotinib/gefitinib (MET/HGF and EGFR inhibitors, respectively).
Inhibitors of heat shock protein 90 (HSP90), a molecular chaperone to MET, and of other key cellular proteins may offer another approach to overcome resistance to MET inhibition.168
A better understanding of the toxicities associated with HGF-MET pathway inhibition is necessary. Peripheral edema due to an attenuation of HGF-mediated signaling in the vascular endothelium, has been associated in multiple tumor types after treatment with all monoclonal antibodies targeting HGF or MET, combined either with various cytotoxic or targeted therapies.2 This could also explain the increased incidence of venous thromboembolism with HGF/MET inhibition. HGF/MET signaling has been implicated in physiological processes such as tissue growth/repair, hematopoesis and glucose metabolism; therefore it is possible to expect additional toxicity signals such as myelosuppression, mucosal injury, wound healing complications or disturbances in glucose homeostasis.143–144
Another challenging topic includes the potential of gene therapy for c-MET overexpression. As a potential therapeutic strategy to inhibit tumor growth Stabile et al.169 constructed U6 expression plasmids for delivery of sense or antisense sequences into lung tumor cells. These should target the translation start site of the human c-MET gene.169 These constructs have been examined both in vitro and an in vivo tumor xenograft model. The c-MET protein was downregulated by 50–60% in two lung cancer cell lines that were transiently transfected with the c-MET antisense versus U6 control. Tumor cells treated with the c-MET antisense construct also showed decreased phosphorylation of c-MET and MAP kinase when exposed to exogenous HGF. The treatment of patient with lung tumors with c-MET antisense versus U5 control plasmid resulted in the downregulation of the c-MET protein expression, a 50% decrease in tumor growth over a 5-week treatment period and an increased rate of apoptosis.169
Conclusions
c-MET encodes a versatile RTK involved in many physiological, and pathological and biological processes. The downstream effectors activated by HGF-MET signaling pathway are involved in cell survival, motility and proliferation. The HGF-MET axis is frequently dysregulated in cancer, especially in advanced or metastatic disease and is responsible for tumor growth, invasion and resistance to anticancer therapy.
Clinical trials of MET-targeted drug monotherapy have shown promising results in terms of antitumor efficacy and improvement of clinical outcomes in various tumor types (Table 3). However, in our opinion, the potential of MET targeted agents might be in combination approaches whereby both molecular drivers and mechanisms of resistance are inhibited (Table 4).
Table 3.
An overview of cancer patients’ clinical outcome after treatment with c-MET inhibitors within clinical studies.
| TUMOR TYPE | PHASE | DRUG | RESPONSE RATE | PFS | OS | REF. |
|---|---|---|---|---|---|---|
| NSCLC | III | Tivantinib + erlotinib vs. placebo + erlotinib | 8.4% vs. 6.5% | 2.9 mo vs. 2 mo | 12.9 mo vs. 11.2 mo | 68 |
| NSCLC | III | Onartuzumab + erlotinib vs. placebo + erlotinib | NA | 2.6 mo vs. 2.7 mo | 6.8 mo vs. 9.1 mo | 65 |
| NSCLC | III | Crizotinib vs. CT (pemetrexed or docetaxel) | 65% vs. 20% | 7.7 mo vs. 3 mo | 20.3 mo vs. 22.8 mo | 90 |
| NSCLC | III | Crizotinib vs. CT (pemetrexed + cisplatin or carboplatin) | 74 vs. 45% | 10.9 mo vs. 7 mo | NA | 94 |
| NSCLC | II | Erlotinib vs. cabozantinib vs. erlotinib + cabozantinib | NA | 1.9 mo vs. 3.9 mo vs. 4.1 mo | 4.0 mo vs. na vs. na | 96 |
| NSCLC | II | Ficlatuzumab + geftinib vs. geftinib | 43% vs. 40% | 5.6 mo vs. 4.7 mo | NA | 170 |
| RCC | II | Foretinib | 13.5% | 9.3 mo | NA | 106 |
| RCC | II | Rilotumumab 10 mg vs. 20 mg | 2.5% vs. 0% | 3.7 mo vs. 2.0 mo | 14.9 mo vs. 17.6 mo | 171 |
| RCC | I | Cabozantinib | PR 28%, SD 52% | 12.9 mo | 15 mo | 172 |
| RCC | III | Cabozantinib + rosiglitazone | 28% | 14.7 mo | NA | 173 |
| Solid tumors (mainly RCC and colorectal cancer) | I | Savolitinib | 3 RCC pts achieved PR, 1 pt CRC achieved PR | NA | NA | 72 |
| Prostate cancer | III | Cabozantinib vs. prednisone | 41% vs. 3% | 5.5 mo vs. 2.8 mo | 11 mo vs. 9.8 mo | 174 |
| Prostate cancer | II | Mitoxantrone + prednisone + rilotumumab vs. mitoxantrone + prednisone + placebo | 11% vs. 14% | 3.0 mo vs. 2.9 mo | 12.2 mo vs. 11.1 mo | 175 |
| Colorectal cancer | I/II | Cetuximab + irinotecan + tivantinib vs. cetuximab + irinotecan + placebo | 45% vs. 33% | 8.3 mo vs. 7.3 mo | NA | 176 |
| Colorectal cancer | I/II | Panitumumab + rilotumumab vs. panitumumab + ganitumumab vs. panitumumab + placebo | 31% vs. 22% vs. 21% | 5.2 mo vs. 5.3 mo vs. 3.7 mo | NA | 177 |
| Esophagogastric cancer | II | Epirubicin + cisplatin + xeloda + rilotumumab vs. epirubicin + cisplatin + xeloda + placebo | 38% vs. 24% | 5.6 mo vs. 4.2 mo | 11.1 mo vs. 8.9 mo | 178 |
| Gatric cancer | II | Forentinib (intermittent vs. daily cohort) | 0% vs. 0% | 1.7 mo vs. 1.8 mo | 7.4 mo vs. 4.3 mo | 179 |
| Hepatocellular cancer | II | Tivantinib vs. placebo | 1% vs. 0% | 1.5 mo vs. 1.4 mo | 6.6 vs. 6.2 mo | 153 |
| Hepatocellular cancer | II | Cabozantinib | 5% | 4.4 mo | 15.1 mo | 180 |
| Hepatocellular cancer | I/II | Foretinib | 24% | 4.2 mo | NA | 181 |
| Uveal melanoma | II | Cabozantinib | NA | 4.8 mo | 12.6 mo | 182 |
| Melanoma | II | Cabozantinib | PR 5%, SD 57% | 4.2 mo | NA | 183 |
| Breast cancer | II | Cabozantinib | PR 14%, SD 57% | 4.3 mo | NA | 184 |
| HNSCC | II | Foretinib | NA | 3.65 mo | 5.59 mo | 185 |
| Germ cell tumors | II | Tivantinib | PR 0%, SD 20% | 1.0 mo | 6.0 mo | 186 |
| Glioblastoma | II | Rilotumumab (10 vs. 20 mg/kg) | 0% vs. 0% | 4.1 mo vs. 4.3 mo | 6.5 mo vs. 5.4 mo | 144 |
| Sarcoma | II | Crizotinib | PR 0%, SD 58.3%, PD 41.7% | 5.25 mo | NA | 187 |
| Thyroid cancer | III | Cabozantinib vs. placebo | 28% vs. 0% | 11.2 mo vs. 4.0 mo | NA | 188 |
Abbreviations: HNSCC, head and neck squamous cell carcinoma; mo, months; NA, not available; NSCLC, non small cell lung cancer; OS, overall survival; PFS, progression free survival; PR, partial response; RCC, renal cancer carcinoma; SD, stable disease.
Table 4.
MET inhibitors combination therapy to overcome the drug resistance.
| DRUG | CANCER | POSSIBILLTY TO OVERCOME RESISTANCE TO ANTICANCER TREATMENT | REF. |
|---|---|---|---|
| TARGETED THERAPY | |||
| EGFR inhibitors | |||
| Erlotinib | NSCLC, K-RAS mut | Erlotinib and tivantinib | 68 |
| NSCLC, MET overexpression | Erlotinib and onartuzumab | 65 | |
| NSCLC, EGFR mut | mTOR inhibitor | 115 | |
| Geftinib | NSCLC | Butein (dual EGFR and MET inhibitor) | 150 |
| NSCLC, EGFR mut | Gefitinib and TAK701 | 63 | |
| Cetuximab | CRC, MET overexpression | Cetuximab and MET inhibitor | 198 |
| Panitumumab | CRC, MET overexpression | Panitumumab and MET inhibitor | 198 |
| VEGFR inhibitors | |||
| Sunitinib | ccRCC | Axitib and crizotinib | 103 |
| pRCC | Savolitinib | 71 | |
| Foretinib (dual MET and VEGF inhibition) | 112 | ||
| Sorafenib | RCC, HCC, melanoma | Sorafenib and tivantinib | 67 |
| HCC, MET overexpression | Tivantinib | 69 | |
| Anti-HER2 therapies | |||
| Trastuzumab | Breast cancer, HER2 positive | Trastuzumab and MET inhibitor | 51 |
| Lapatinib | Esophageal squamous cell carcinoma | Lapatinib and MET inhibitor | 81 |
| B-RAF inhibitor | |||
| Vemurafenib | Melanoma, B-RAF activating mutation V600E | Vemurafenib and MET inhibitor | 82 |
| Selective ALK inhibitors | |||
| Alectinib | NSCLC with ALK rearrangement and MET overexpression | Crizotinib | 93–97 |
| Non-selective ALK inhibitors | |||
| Crizotinib | NSCLC, G2032R-mutated CD74-ROS1 | Cabozantinib | 98,99 |
| CHEMOTHERAPY | |||
| Gemcitabine | PDAC | Gemcitabine and crizotinib | 126 |
| PDAC | Gemcitabine and cabozantinib | 127 | |
| Capecitabine | Gastric or oesofagogastric junction, MET overxpression | Capecitabine and rilotumab | 60 |
| Cisplatin | Head and neck cancer, MET overexpression | Cisplatin and MET inhibitor | 199 |
| cervical cancer | Cisplatin and MET inhibitor | 200 | |
| Taxanes | Ovarian cancer, MET overexpression | Taxane and MET inhibitor | 201 |
| RADIOTHERAPY | Cancer cell lines | Radiation and MET inhibitor | 202 |
In future, an accurate stratification of patients’ population and rational mechanism-based treatment combinations are critical for success of MET targeted therapy in the clinical practice.
Short Overview of Anticancer Therapy
VEGFR inhibitors
Bevacizumab, axitinib, sunitinib, pazopanib.
EGFR inhibitors
EGF receptor inhibitors (cetuximab, panitumumab) or EGFR TKIs (erlotinib, gefitinib).
B-RAF inhibitor
Vemurafenib.
Anti-HER2 therapy
Trastuzumab, lapatinib.
mTOR inhibitor
Temsirolimus.
Gemcitabine
Nucleoside analog used as chemotherapy.
Pemetrexed
Folate antimetabolite used as chemotherapy.
FOLFIRINOX
Chemotherapy schedule that consist of 5-fluorouracil, leucovorin, irinotecan and oxaliplatin.
Acknowledgments
We would like to thank Dr. Richard J Honeywell for the careful reading and correction of the manuscript.
Abbreviations
- ALK
anaplastic lymphoma kinase
- CDA
cytidine deaminase
- CUP
cancer of unknown origin
- Gab1
Grb2-associated-binding protein 1
- Grb2
growth factor receptor bound protein 2
- HGF
hepatocyte growth factor
- HL domain
hairpin loop domain
- IPT1-4 domain
four immunoglobulin plexins transcription domains
- ccRCC
clear cell renal cancer carcinoma
- CSCs
cancer stem cells
- EGFR
epidermal growth factor receptor
- EMT
epithelial-mesenchymal transition
- ERK
extracellular signal-related kinase
- GSK3β
glycogen synthase kinase
- 3β GTP
guanosine triphosphate
- HAI
HGF inhibitor
- HGFA
HGF activator
- JAK
Janus kinase
- JNKs
c-Jun NH2-terminal kinase
- MAPK
mitogen-activated protein kinase
- MET
mesenchymal epithelial transition receptor
- mTOR
mammalian target of rapamycin
- NF-κB
nuclear factor kappaB
- NSCLC
non small cell lung cancer
- OS
overal survival
- PDK-1
phsphoinositide-dependent-kinase-1
- PDAC
pancreatic ductal adenocarcinoma
- PIP2
(4,5)-bisphosphate
- PIP3
(3,4,5)-triphosphate
- PFS
progression-free survival
- pRCC
papillary cell renal cancer carcinoma
- PSI domain
plexin, semaphorin and integrin cystein-rich domain
- RCC
renal cancer carcinoma
- RTKs
receptor tyrosine kinases
- SEMA domain
semaphorin domain
- SF
scatter factor
- SPH
serine proteases homology domain
- TKI
tyrosine kinase inhibitor
- VEGFR
vascular endothelial growth factor
Footnotes
ACADEMIC EDITOR: Todd W. Miller, Editor in Chief
PEER REVIEW: Four peer reviewers contributed to the peer review report. Reviewers’ reports totaled 760 words, excluding any confidential comments to the academic editor.
FUNDING: Authors disclose no funding sources.
COMPETING INTERESTS: Authors disclose no potential conflicts of interest.
Paper subject to independent expert blind peer review. All editorial decisions made by independent academic editor. Upon submission manuscript was subject to anti-plagiarism scanning. Prior to publication all authors have given signed confirmation of agreement to article publication and compliance with all applicable ethical and legal requirements, including the accuracy of author and contributor information, disclosure of competing interests and funding sources, compliance with ethical requirements relating to human and animal study participants, and compliance with any copyright requirements of third parties. This journal is a member of the Committee on Publication Ethics (COPE).
AuthorContributions
Wrote the first draft of the manuscript: GP, IG. Contributed to the writing of the manuscript: EG, GB. Agree with manuscript results and conclusions: EG, GB. Jointly developed the structure and arguments for the paper: GP, IG. Made critical revisions and approved final version: GP, EG, GB. All authors reviewed and approved of the final manuscript.
REFERENCES
- 1.Peters S, Adjei AA. MET: a promising anticancer therapeutic target. Nat Rev Clin Oncol. 2012;9(6):314–26. doi: 10.1038/nrclinonc.2012.71. [DOI] [PubMed] [Google Scholar]
- 2.Maroun CR, Rowlands T. The Met receptor tyrosine kinase: a key player in oncogenesis and drug resistance. Pharmacol Ther. 2014;142(3):316–38. doi: 10.1016/j.pharmthera.2013.12.014. [DOI] [PubMed] [Google Scholar]
- 3.Cooper CS, Park M, Blair DG, et al. Molecular cloning of a new transforming gene from a chemically transformed human cell line. Nature. 1984;311(5981):29–33. doi: 10.1038/311029a0. [DOI] [PubMed] [Google Scholar]
- 4.Gherardi E, Birchmeier W, Birchmeier C, et al. Targeting MET in cancer: rationale and progress. Nat Rev Cancer. 2012;12(2):89–103. doi: 10.1038/nrc3205. [DOI] [PubMed] [Google Scholar]
- 5.Peschard P, Fornier TM, Lamorte L, et al. Mutation of the c-Cbl TKB domain binding site on the Met receptor tyrosine kinase converts it into a transforming protein. Mol Cell. 2001;8(5):995–1004. doi: 10.1016/s1097-2765(01)00378-1. [DOI] [PubMed] [Google Scholar]
- 6.Gelsomino F, Rossi G, Tiseo M. MET and Small-Cell Lung Cancer. Cancers (Basel) 2014;6(4):2100–15. doi: 10.3390/cancers6042100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Trusolino L, Bertotti A, Comoglio PM. MET signalling: principles and functions in development, organ regeneration and cancer. Nat Rev Mol Cell Biol. 2010;11(12):834–48. doi: 10.1038/nrm3012. [DOI] [PubMed] [Google Scholar]
- 8.Furlan A, Kherrouche Z, Montagne R, et al. Thirty years of research on met receptor to move a biomarker from bench to bedside. Cancer Res. 2014;74(23):6737–44. doi: 10.1158/0008-5472.CAN-14-1932. [DOI] [PubMed] [Google Scholar]
- 9.Basilico C, Arnesano A, Galluzzo M, et al. A high affinity hepatocyte growth factor-binding site in the immunoglobulin-like region of Met. J Biol Chem. 2008;283(30):21267–77. doi: 10.1074/jbc.M800727200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Stamos J, Lazarus RA, Yao X, et al. Crystal structure of the HGF beta-chain in complex with the Sema domain of the Met receptor. EMBO J. 2004;23(12):2325–35. doi: 10.1038/sj.emboj.7600243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Parikh RA, Wang P, Beumer JH, et al. The potential roles of hepatocyte growth factor (HGF)-MET pathway inhibitors in cancer treatment. Onco Targets Ther. 2014;7:969–83. doi: 10.2147/OTT.S40241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Uehara Y, Monowa O, Mori C, et al. Placental defect and embryonic lethality in mice lacking hepatocyte growth factor/scatter factor. Nature. 1995;373(6516):702–5. doi: 10.1038/373702a0. [DOI] [PubMed] [Google Scholar]
- 13.Schmidt C, Bladt F, Goedecke S, et al. Scatter factor/hepatocyte growth factor is essential for liver development. Nature. 1995;373(6516):699–702. doi: 10.1038/373699a0. [DOI] [PubMed] [Google Scholar]
- 14.Tsarfaty I, Rong S, Resau JH, et al. The Met proto-oncogene mesenchymal to epithelial cell conversion. Science. 1994;263(5143):98–101. doi: 10.1126/science.7505952. [DOI] [PubMed] [Google Scholar]
- 15.Zhang Y, Jain RK, Zhu M. Recent Progress and Advances in HGF/MET-Targeted Therapeutic Agents for Cancer Treatment Biomedicines. 2015;3(1):149–181. doi: 10.3390/biomedicines3010149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bussolino F, Di Renzo MF, Ziche M, et al. Hepatocyte growth factor is a potent angiogenic factor which stimulates endothelial cell motility and growth. J Cell Biol. 1992;119(3):629–41. doi: 10.1083/jcb.119.3.629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Boccaccio C, Comoglio PM. MET, a driver of invasive growth and cancer clonal evolution under therapeutic pressure. Curr Opin Cell Biol. 2014;31:98–105. doi: 10.1016/j.ceb.2014.09.008. [DOI] [PubMed] [Google Scholar]
- 18.Ponzetto C, Bardelli A, Zhen Z, et al. A multifunctional docking site mediates signaling and transformation by the hepatocyte growth factor/scatter factor receptor family. Cell. 1994;77(2):261–71. doi: 10.1016/0092-8674(94)90318-2. [DOI] [PubMed] [Google Scholar]
- 19.Furge KA, Zhang YW, Vande Woude GF. Met receptor tyrosine kinase: enhanced signaling through adapter proteins. Oncogene. 2000;19(49):5582–9. doi: 10.1038/sj.onc.1203859. [DOI] [PubMed] [Google Scholar]
- 20.Johnson GL, Lapadat R. Mitogen-activated protein kinase pathways mediated by ERK, JNK, and p38 protein kinases. Science. 2002;298(5600):1911–2. doi: 10.1126/science.1072682. [DOI] [PubMed] [Google Scholar]
- 21.Fan S, Gao M, Meng Q, et al. Role of NF-kappaB signaling in hepatocyte growth factor/scatter factor-mediated cell protection. Oncogene. 2005;24(10):1749–66. doi: 10.1038/sj.onc.1208327. [DOI] [PubMed] [Google Scholar]
- 22.Muller M, Morotti A, Ponzetto C. Activation of NF-kappaB is essential for hepatocyte growth factor-mediated proliferation and tubulogenesis. Mol Cell Biol. 2002;22(4):1060–72. doi: 10.1128/MCB.22.4.1060-1072.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Roberts PJ, Der CJ. Targeting the Raf-MEK-ERK mitogen-activated protein kinase cascade for the treatment of cancer. Oncogene. 2007;26(22):3291–310. doi: 10.1038/sj.onc.1210422. [DOI] [PubMed] [Google Scholar]
- 24.Kesavan K, Lobel-Rice K, Sun W, et al. MEKK2 regulates the coordinate activation of ERK5 and JNK in response to FGF-2 in fibroblasts. J Cell Physiol. 2004;199(1):140–8. doi: 10.1002/jcp.10457. [DOI] [PubMed] [Google Scholar]
- 25.Dhillon AS, Hagan S, Rath O, et al. MAP kinase signalling pathways in cancer. Oncogene. 2007;26(22):3279–90. doi: 10.1038/sj.onc.1210421. [DOI] [PubMed] [Google Scholar]
- 26.Pelicci G, Giordano S, Zhen Z, et al. The motogenic and mitogenic responses to HGF are amplified by the Shc adaptor protein. Oncogene. 1995;10(8):1631–8. [PubMed] [Google Scholar]
- 27.Maroun CR, Naujokas MA, Holgado-Madruga M, et al. The tyrosine phosphatase SHP-2 is required for sustained activation of extracellular signal-regulated kinase andepithelial morphogenesis downstream from the met receptor tyrosine kinase. Mol Cell Biol. 2000;20(22):8513–25. doi: 10.1128/mcb.20.22.8513-8525.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Montagner A, Yart A, Dance M, et al. A novel role for Gab1 and SHP2 in epidermal growth factor-induced Ras activation. J Biol Chem. 2005;280(7):5350–60. doi: 10.1074/jbc.M410012200. [DOI] [PubMed] [Google Scholar]
- 29.Hemmings BA, Restuccia DF. PI3K-PKB/Akt pathway. Cold Spring Harb Perspect Biol. 2012;4(9):a011189. doi: 10.1101/cshperspect.a011189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Yu H, Lee H, Herrmann A, et al. Revisiting STAT3 signalling in cancer: new and unexpected biological functions. Nat Rev Cancer. 2014;14(11):736–46. doi: 10.1038/nrc3818. [DOI] [PubMed] [Google Scholar]
- 31.Yu H, Jove R. The STATs of cancer – new molecular targets come of age. Nat Rev Cancer. 2004;4(2):97–105. doi: 10.1038/nrc1275. [DOI] [PubMed] [Google Scholar]
- 32.Haura EB, Turkson J, Jove R. Mechanisms of disease: Insights into the emerging role of signal transducers and activators of transcription in cancer. Nat Clin Prat Oncol. 2005;2(6):315–24. doi: 10.1038/ncponc0195. [DOI] [PubMed] [Google Scholar]
- 33.Boccaccio C, Ando M, Tamagnone L, et al. Induction of epithelial tubules by growth factor HGF depends on the STAT pathway. Nature. 1998;391(6664):285–8. doi: 10.1038/34657. [DOI] [PubMed] [Google Scholar]
- 34.Mukherjee N, Houston TJ, Cardenas E, et al. To be an ally or an adversary in bladder cancer: the NF-κB story has not unfolded. Carcinogenesis. 2015;36(3):299–306. doi: 10.1093/carcin/bgu321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Karin M, Cao Y, Greten FR, et al. NF-kappaB in cancer: from innocent bystander to major culprit. Nat Rev Cancer. 2002;2(4):301–10. doi: 10.1038/nrc780. [DOI] [PubMed] [Google Scholar]
- 36.Proto JD, Tang Y, Lu A, et al. NF-kappaB inhibition reveals a novel role for HGF during skeletal muscle repair. Cell Death Dis. 2015;6:e1730. doi: 10.1038/cddis.2015.66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Wang W, Du Z, Yan J, et al. Mesenchymal stem cells promote liver regeneration and prolong survival in small-for-size liver grafts: involvement of C-Jun N- terminal kinase, cyclin D1, and NF-kappaB. PLoS One. 2014;9(12):e112532. doi: 10.1371/journal.pone.0112532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Jardim DL, Tang C, Gagliato D, et al. Analysis of 1,115 patients tested for MET amplification and therapy response in the MD Anderson Phase I Clinic. Clin Cancer Res. 2014;20(24):6336–45. doi: 10.1158/1078-0432.CCR-14-1293. [DOI] [PubMed] [Google Scholar]
- 39.Comoglio PM, Giordano S, Trusolino L. Drug development of MET inhibitors: targeting oncogene addiction and expedience. Nat Rev Drug Discov. 2008;7(6):504–16. doi: 10.1038/nrd2530. [DOI] [PubMed] [Google Scholar]
- 40.Lengyel E, Prechtel D, Resau JH, et al. C-Met overexpression in node-positive breast cancer identifies patients with poor clinical outcome independent of Her2/neu. Int J Cancer. 2005;113(4):678–82. doi: 10.1002/ijc.20598. [DOI] [PubMed] [Google Scholar]
- 41.Lennerz JK, Kwak EL, Ackerman A, et al. MET amplification identifies a small and aggressive subgroup of esophagogastric adenocarcinoma with evidence of responsiveness to crizotinib. J Clin Oncol. 2011;29(36):4803–10. doi: 10.1200/JCO.2011.35.4928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Lorenzato A, Olivero M, Patane S, et al. Novel somatic mutations of the MET oncogene in human carcinoma metastases activating cell motility and invasion. Cancer Res. 2002;62(23):7025–30. [PubMed] [Google Scholar]
- 43.Stella GM, Benvenuti S, Gramaglia D, et al. MET mutations in cancers of unknown primary origin (CUPs) Hum Mutat. 2011;32(1):44–50. doi: 10.1002/humu.21374. [DOI] [PubMed] [Google Scholar]
- 44.Lee SJ, Lee J, Sohn I, et al. A survey of c-MET expression and amplification in 287 patients with hepatocellular carcinoma. Anticancer Res. 2013;33(11):5179–86. [PubMed] [Google Scholar]
- 45.Kato H, Arao T, Matsumoto K, et al. Gene amplification of EGFR, HER2, FGFR2 and MET in esophageal squamous cell carcinoma. Int J Oncol. 2013;42(4):1151–8. doi: 10.3892/ijo.2013.1830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Graziano F, Galluccio N, Lorenzini P, et al. Genetic activation of the MET pathway and prognosis of patients with high-risk, radically resected gastric cancer. J Clin Oncol. 2011;29(36):4789–95. doi: 10.1200/JCO.2011.36.7706. [DOI] [PubMed] [Google Scholar]
- 47.Straussman R, Morikawa T, Shee K, et al. Tumour micro-environment elicits innate resistance to RAF inhibitors through HGF secretion. Nature. 2012;487(7408):500–4. doi: 10.1038/nature11183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Engelman JA, Zejnullahu K, Mitsudomi T, et al. MET amplification leads to gefitinib resistance in lung cancer by activating ERBB3 signaling. Science. 2007;316(5827):1039–43. doi: 10.1126/science.1141478. [DOI] [PubMed] [Google Scholar]
- 49.Shojaei F, Lee JH, Simmons BH, et al. HGF/c-Met acts as an alternative angiogenic pathway in sunitinib-resistant tumors. Cancer Res. 2010;70(24):10090–100. doi: 10.1158/0008-5472.CAN-10-0489. [DOI] [PubMed] [Google Scholar]
- 50.Ishikawa D, Takeuchi S, Nakagawa T, et al. mTOR inhibitors control the growth of EGFR mutant lung cancer even after acquiring resistance by HGF. PLoS One. 2013;8(5):e62104. doi: 10.1371/journal.pone.0062104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Minuti G, et al. Increased MET and HGF gene copy numbers are associated with trastuzumab failure in HER2-positive metastatic breast cancer. Br J Cancer. 2012;107(5):793–9. doi: 10.1038/bjc.2012.335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Gak E, Taylor WG, Chan AM, et al. Processing of hepatocyte growth factor to the heterodimeric form is required for biological activity. FEBS Lett. 1992;311(1):17–21. doi: 10.1016/0014-5793(92)81356-q. [DOI] [PubMed] [Google Scholar]
- 53.Naldini L, Vigna E, Bardelli A, et al. Biological activation of pro-HGF (hepatocyte growth factor) by urokinase is controlled by a stoichiometric reaction. J Biol Chem. 1995;270(2):603–11. doi: 10.1074/jbc.270.2.603. [DOI] [PubMed] [Google Scholar]
- 54.Parr C, Sanders AJ, Jiang WG. Hepatocyte growth factor activation inhibitors–therapeutic potential in cancer. Anticancer Agents Med Chem. 2010;10(1):47–57. doi: 10.2174/1871520611009010047. [DOI] [PubMed] [Google Scholar]
- 55.Hu C, Jiang N, Wang G, et al. Expression of hepatocyte growth factor activator inhibitor-1 (HAI-1) gene in prostate cancer: clinical and biological significance. J BUON. 2014;19(1):215–20. [PubMed] [Google Scholar]
- 56.Tsai CH, Teng CH, Tu YT, et al. HAI-2 suppresses the invasive growth and metastasis of prostate cancer through regulation of matriptase. Oncogene. 2014;33(38):4643–52. doi: 10.1038/onc.2013.412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Giordano S. Rilotumumab, a mAb against human hepatocyte growth factor for the treatment of cancer. Curr Opin Mol Ther. 2009;11(4):448–55. [PubMed] [Google Scholar]
- 58.Buchanan IM, Scott T, Tandle AT, et al. Radiosensitization of glioma cells by modulation of Met signalling with the hepatocyte growth factor neutralizing antibody, AMG102. J Cell Mol Med. 2011;15(9):1999–2006. doi: 10.1111/j.1582-4934.2010.01122.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Jun HT, Sun J, Rex K, et al. AMG 102, a fully human anti-hepatocyte growth factor/scatter factor neutralizing antibody, enhances the efficacy of temozolomide or docetaxel in U-87 MG cells and xenografts. Clin Cancer Res. 2007;13(22 Pt 1):6735–42. doi: 10.1158/1078-0432.CCR-06-2969. [DOI] [PubMed] [Google Scholar]
- 60.Zhu M, Tang R, Doshi S, et al. Exposure-response analysis of rilotumumab in gastric cancer: the role of tumour MET expression. Br J Cancer. 2015;112(3):429–37. doi: 10.1038/bjc.2014.649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Patnaik A, Weiss GJ, Papadopoulos K, et al. Phase I study of SCH 900105 (SC), an anti-hepatocyte growth factor (HGF) monoclonal antibody (MAb), as a single agent and in combination with erlotinib (E) in patients (pts) with advanced solid tumors. J Clin Oncol (Meeting abstracts) 2010;28(15):2525. [Google Scholar]
- 62.Hori A, et al. Monotherapeutic and combination antitumor activities of TAK701, a humanized anti-hepatocyte growth factor neutralizing antibody, against multiple types of cancer. Proceedings of the American Association of Cancer Res. 2009;1244 [Google Scholar]
- 63.Okamoto W, Okamoto I, Tanaka K, et al. TAK-701, a humanized monoclonal antibody to hepatocyte growth factor, reverses gefitinib resistance induced by tumor-derived HGF in non-small cell lung cancer with an EGFR mutation. Mol Cancer Ther. 2010;9(10):2785–92. doi: 10.1158/1535-7163.MCT-10-0481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Sano Y, Hashimoto E, Nakatani N, et al. Combining onartuzumab with erlotinib inhibits growth of non-small cell lung cancer with activating EGFR mutations and HGF overexpression. Mol Cancer Ther. 2015;14(2):533–41. doi: 10.1158/1535-7163.MCT-14-0456. [DOI] [PubMed] [Google Scholar]
- 65.Spigel DR, Ervin TJ, Ramlau RA, et al. Randomized phase II trial of Onartuzumab in combination with erlotinib in patients with advanced non-small-cell lung cancer. J Clin Oncol. 2013;31:4105–14. doi: 10.1200/JCO.2012.47.4189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Munshi N, Jeay S, Li Y, et al. ARQ 197, a novel and selective inhibitor of the human c-Met receptor tyrosine kinase with antitumor activity. Mol Cancer Ther. 2010;9(6):1544–53. doi: 10.1158/1535-7163.MCT-09-1173. [DOI] [PubMed] [Google Scholar]
- 67.Puzanov I, Sosman J, Santoro A, et al. Phase 1 trial of tivantinib in combination with sorafenib in adult patients with advanced solid tumors. Invest New Drugs. 2015;33(1):159–68. doi: 10.1007/s10637-014-0167-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Azuma K, Yoshioka H, Yamamoto N, et al. Tivantinib plus erlotinib versus placebo plus erlotinib in Asian patients with previously treated nonsquamous NSCLC with wild-type EGFR: First report of a phase III ATTENTION trial. J Clin Oncol (Meeting abstracts) 2014;32:5s, a8044. [Google Scholar]
- 69.Trojan J, Zeuzem S. Tivantinib in hepatocellular carcinoma. Expert Opin Investig Drugs. 2013;22(1):141–7. doi: 10.1517/13543784.2013.741586. [DOI] [PubMed] [Google Scholar]
- 70.Jia H, Dai G, Weng J, et al. Discovery of (S)-1-(1- (Imidazo[1,2-a]pyridin-6-yl)ethyl)-6-(1-methyl-1H-pyrazol-4-yl)-1H- [1,2, 3]triazolo[4,5-b]pyrazine (volitinib) as a highly potent and selective mesenchymal-epithelial transition factor (c-Met) inhibitor in clinical development for treatment of cancer. J Med Chem. 2014;57:7577–89. doi: 10.1021/jm500510f. [DOI] [PubMed] [Google Scholar]
- 71.Schuller AG, Barry ER, Jones RD, et al. The MET Inhibitor AZD6094 (Savolitinib, HMPL-504) Induces Regression in Papillary Renal Cell Carcinoma Patient-Derived Xenograft Models. Clin Cancer Res. 2015;21(12):2811–9. doi: 10.1158/1078-0432.CCR-14-2685. [DOI] [PubMed] [Google Scholar]
- 72.Hui Kong, Gan JL, Michael Millward, et al. First-in-human phase I study of a selective c-Met inhibitor volitinib (HMP504/AZD6094) in patients with advanced solid tumors (Meeting abstracts) J Clin Oncol. 2014;32:11111. [Google Scholar]
- 73.Cappuzzo F, Moro-Sibilot D, Gautschi O, et al. Management of crizotinib therapy for ALK-rearranged non-small cell lung carcinoma: an expert consensus. Lung Cancer. 2015;87(2):89–95. doi: 10.1016/j.lungcan.2014.12.010. [DOI] [PubMed] [Google Scholar]
- 74.Reck M, Popat S, Reinmuth N, et al. Metastatic non-small-cell lung cancer (NSCLC): ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2014;25(3):iii27–39. doi: 10.1093/annonc/mdu199. [DOI] [PubMed] [Google Scholar]
- 75.Ettinger DS, Akerley W, Borghaei H, et al. Non-small cell lung cancer. J Natl Compr Cancer Netw. 2012;10:1236–71. doi: 10.6004/jnccn.2012.0130. [DOI] [PubMed] [Google Scholar]
- 76.Shaw AT, Kim DW, Nakagawa K, et al. Crizotinib versus chemotherapy in advanced ALK-positive lung cancer. N Engl J Med. 2013;368(25):2385–94. doi: 10.1056/NEJMoa1214886. [DOI] [PubMed] [Google Scholar]
- 77.Solomon BJ, Mok T, Kim DW, et al. First-line crizotinib versus chemotherapy in ALK-positive lung cancer. N Engl J Med. 2014;371(23):2167–77. doi: 10.1056/NEJMoa1408440. [DOI] [PubMed] [Google Scholar]
- 78.Qian H, Gao F, Wang H, et al. The efficacy and safety of crizotinib in the treatment of anaplastic lymphoma kinase-positive non-small cell lung cancer: a meta-analysis of clinical trials. BMC Cancer. 2014;14:683. doi: 10.1186/1471-2407-14-683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Viola D, Cappagli V, Elisei R. Cabozantinib (XL184) for the treatment of locally advanced or metastatic progressive medullary thyroid cancer. Future Oncol. 2013;9(8):1083–92. doi: 10.2217/fon.13.128. [DOI] [PubMed] [Google Scholar]
- 80.Schoffski P, Elisei R, Muller S, et al. An international, double-blind, randomized, placebo-controlled phase III trial (EXAM) of cabozantinib (XL184) in medullary thyroid carcinoma (MTC) patients (pts) with documented RECIST progression at baseline. J Clin Oncol (Meeting abstracts) 2012;30:5508. [Google Scholar]
- 81.Saito S, Morishima K, Ui T, et al. The role of HGF/MET and FGF/FGFR in fibroblast-derived growth stimulation and lapatinib-resistance of esophageal squamous cell carcinoma. BMC Cancer. 2015;15(1):1065. doi: 10.1186/s12885-015-1065-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Chapman PB, Hauschild A, Robert C, et al. Improved survival with vemurafenib in melanoma with BRAF V600E mutation. N Engl J Med. 2011;364(26):2507–16. doi: 10.1056/NEJMoa1103782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Sierra JR, Tsao MS. c-MET as a potential therapeutic target and biomarker in cancer. Ther Adv Med Oncol. 2011;3(1):S21–35. doi: 10.1177/1758834011422557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Chan BA, Hughes BG. Targeted therapy for non-small cell lung cancer: current standards and the promise of the future. Transl Lung Cancer Res. 2015;4(1):36–54. doi: 10.3978/j.issn.2218-6751.2014.05.01. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Van Der Steen N, Pauwels P, Gil-Bazo I, et al. cMET in NSCLC: Can We Cut off the Head of the Hydra? From the Pathway to the Resistance. Cancers (Basel) 2015;7(2):556–73. doi: 10.3390/cancers7020556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Melosky B. Treatment algorithms for patients with metastatic non-small cell, non-squamous lung cancer. Front Oncol. 2014;4:256. doi: 10.3389/fonc.2014.00256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Yano S, Nakagawa T. The current state of molecularly targeted drugs targeting HGF/Met. Jpn J Clin Oncol. 2014;44(1):9–12. doi: 10.1093/jjco/hyt188. [DOI] [PubMed] [Google Scholar]
- 88.Engelman JA, Zejnullahu K, Mitsudomi T, et al. MET amplification leads to gefitinib resistance in lung cancer by activating ERBB3 signaling. Science. 2007;316(5827):1039–43. doi: 10.1126/science.1141478. [DOI] [PubMed] [Google Scholar]
- 89.Sequist LV, Waltman BA, Dias-Santagata D, et al. Genotypic and histological evolution of lung cancers acquiring resistance to EGFR inhibitors. Sci Transl Med. 2011;3(75):75ra26. doi: 10.1126/scitranslmed.3002003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Song N, Liu S, Zhang J, et al. Cetuximab-induced MET activation acts as a novel resistance mechanism in colon cancer cells. Int J Mol Sci. 2014;15(4):5838–51. doi: 10.3390/ijms15045838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Zhang YW, Staal B, Essenburg C, et al. MET kinase inhibitor SGX523 synergizes with epidermal growth factor receptor inhibitor erlotinib in a hepatocyte growth factor-dependent fashion to suppress carcinoma growth. Cancer Res. 2010;70(17):6880–90. doi: 10.1158/0008-5472.CAN-10-0898. [DOI] [PubMed] [Google Scholar]
- 92.Qi J, McTigue MA, Rogers A, et al. Multiple mutations and bypass mechanisms can contribute to development of acquired resistance to MET inhibitors. Cancer Res. 2011;71(3):1081–91. doi: 10.1158/0008-5472.CAN-10-1623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Shaw AT, Kim DW, Nakagawa K, et al. Crizotinib versus chemotherapy in advanced ALK-positive lung cancer. N Engl J Med. 2013;368(25):2385. doi: 10.1056/NEJMoa1214886. [DOI] [PubMed] [Google Scholar]
- 94.Solomon BJ, Mok T, Kim DW, et al. First-line crizotinib versus chemotherapy in ALK-positive lung cancer. N Engl J Med. 2014;371(23):2167–77. doi: 10.1056/NEJMoa1408440. [DOI] [PubMed] [Google Scholar]
- 95.Ou SH, Kwak EL, Siwak-Tapp C, et al. Activity of crizotinib (PF02341066), a dual mesenchymal-epithelial transition (MET) and anaplastic lymphoma kinase (ALK) inhibitor, in a non-small cell lung cancer patient with de novo MET amplification. J Thorac Oncol. 2011;6(5):942–6. doi: 10.1097/JTO.0b013e31821528d3. [DOI] [PubMed] [Google Scholar]
- 96.Kogita A, Togashi Y, Hayashi H, et al. Activated MET acts as a salvage signal after treatment with alectinib, a selective ALK inhibitor, in ALK-positivenon-small cell lung cancer. Int J Oncol. 2015;46(3):1025–30. doi: 10.3892/ijo.2014.2797. [DOI] [PubMed] [Google Scholar]
- 97.Feng Y, Minca EC, Lanigan C, et al. High MET receptor expression but not gene amplification in ALK 2p23 rearrangement positive non-small-cell lung cancer. J Thorac Oncol. 2014;9(5):646–53. doi: 10.1097/JTO.0000000000000145. [DOI] [PubMed] [Google Scholar]
- 98.Katayama R, Kobayashi Y, Friboulet L, et al. Cabozantinib overcomes crizotinib resistance in ROS1 fusion-positive cancer. Clin Cancer Res. 2015;21(1):166–74. doi: 10.1158/1078-0432.CCR-14-1385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Neal JW, Dahlberg SE, Wakelee HA, et al. Cabozantinib (C), erlotinib (E) or the combination (E+C) as second- or third-line therapy in patients with EGFR wild-type (wt) non-small cell lung cancer (NSCLC): A randomized phase 2 trial of the ECOG-ACRIN Cancer Research Group. J Clin Oncol (Meeting abstracts) 2015;33:8003. [Google Scholar]
- 100.Miyata Y, Asai A, Mitsunari K, et al. Met in urological cancers. Cancers (Basel) 2014;6(4):2387–403. doi: 10.3390/cancers6042387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Delahunt B, Eble JN. Papillary renal cell carcinoma: a clinicopathologic and immunohistochemical study of 105 tumors. Mod Pathol. 1997;10(6):537–44. [PubMed] [Google Scholar]
- 102.Gnarra JR, Tory K, Weng Y, et al. Mutations of the VHL tumour suppressor gene in renal carcinoma. Nat Genet. 1994;7(1):85–90. doi: 10.1038/ng0594-85. [DOI] [PubMed] [Google Scholar]
- 103.Ciamporcero E, Miles KM, Adelaiye R, et al. Combination strategy targeting VEGF and HGF/c-met in human renal cell carcinoma models. Mol Cancer Ther. 2015;14(1):101–10. doi: 10.1158/1535-7163.MCT-14-0094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Pennacchietti S, Michieli P, Galluzzo M, et al. Hypoxia promotes invasive growth by transcriptional activation of the met protooncogene. Cancer Cell. 2003;3(4):347–61. doi: 10.1016/s1535-6108(03)00085-0. [DOI] [PubMed] [Google Scholar]
- 105.Albiges L, Guegan J, Le Formal A, et al. MET is a potential target across all papillary renal cell carcinomas: result from a large molecular study of pRCC with CGH array and matching gene expression array. Clin Cancer Res. 2014;20(13):3411–21. doi: 10.1158/1078-0432.CCR-13-2173. [DOI] [PubMed] [Google Scholar]
- 106.Giubellino A, Linehan WM, Bottaro DP. Targeting the Met signaling pathway in renal cancer. Expert Rev Anticancer Ther. 2009;9(6):785–93. doi: 10.1586/era.09.43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Balint I, Szponar A, Jauch A, et al. Trisomy 7 and 17 mark papillary renal cell tumours irrespectively of variation of the phenotype. J Clin Pathol. 2009;62(10):892–5. doi: 10.1136/jcp.2009.066423. [DOI] [PubMed] [Google Scholar]
- 108.Escudier B, Porta C, Schmidinger M, et al. Renal cell carcinoma: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2014;25(3):iii49–56. doi: 10.1093/annonc/mdu259. [DOI] [PubMed] [Google Scholar]
- 109.Jahangiri A, De Lay M, Miller LM, et al. Gene expression profile identifies tyrosine kinase c-Met as a targetable mediator of antiangiogenic therapy resistance. Clin Cancer Res. 2013;9(7):1773–83. doi: 10.1158/1078-0432.CCR-12-1281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Ebos JM, Lee CR, Cruz-Munoz W, et al. Accelerated metastasis after short-term treatment with a potent inhibitor of tumor angiogenesis. Cancer Cell. 2009;15(3):232–9. doi: 10.1016/j.ccr.2009.01.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Pàez-Ribes M, Allen E, Hudock J, et al. Antiangiogenic therapy elicits malignant progression of tumors to increased local invasion and distant metastasis. Cancer Cell. 2009;15(3):220–31. doi: 10.1016/j.ccr.2009.01.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Choueiri TK, Vaishampayan U, Rosenberg JE, et al. Phase II and biomarker study of the dual MET/VEGFR2 inhibitor foretinib in patients with papillary renal cell carcinoma. J Clin Oncol. 2013;31(2):181–6. doi: 10.1200/JCO.2012.43.3383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Gavine PR, Ren Y, Han L, et al. Volitinib, a potent and highly selective c-Met inhibitor, effectively blocks c-Met signaling and growth in c-MET amplified gastric cancer patient-derived tumor xenograft models. Mol Oncol. 2015;9:323–33. doi: 10.1016/j.molonc.2014.08.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Houk BE, Bello CL, Poland B, et al. Relationship between exposure to sunitinib and efficacy and tolerability end- points in patients with cancer: results of a pharmacokinetic/pharmacody- namic meta-analysis. Cancer Chemother Pharmacol. 2010;66:357–71. doi: 10.1007/s00280-009-1170-y. [DOI] [PubMed] [Google Scholar]
- 115.Ishikawa D, Takeuchi S, Nakagawa T. mTOR inhibitors control the growth of EGFR mutant lung cancer even after acquiring resistance by HGF. mTOR PLoS One. 2013;8(5):e62104. doi: 10.1371/journal.pone.0062104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Pópulo H, Lopes JM, Soares P. The mTOR signalling pathway in human cancer. Int J Mol Sci. 2012;13(2):1886–918. doi: 10.3390/ijms13021886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Mohammed S, Van Buren G, 2nd, Fisher WE. Pancreatic cancer: advances in treatment. World J Gastroenterol. 2014;20(28):9354–60. doi: 10.3748/wjg.v20.i28.9354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Garajová I, Le Large TY, Frampton AE, et al. Molecular mechanisms underlying the role of microRNAs in the chemoresistance of pancreatic cancer. Biomed Res Int. 2014;2014:678401. doi: 10.1155/2014/678401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Neuzillet C, Couvelard A, Tijeras-Raballand A, et al. High c-Met expression in stage I–II pancreatic adenocarcinoma: proposition of an immunostaining scoring method and correlation with poor prognosis. Histopathology. 2015;67:664–76. doi: 10.1111/his.12691. [DOI] [PubMed] [Google Scholar]
- 120.Patel MB, Pothula SP, Xu Z, et al. The role of the hepatocyte growth factor/c-MET pathway in pancreatic stellate cell-endothelial cell interactions: antiangiogenic implications in pancreatic cancer. Carcinogenesis. 2014;35(8):1891–900. doi: 10.1093/carcin/bgu122. [DOI] [PubMed] [Google Scholar]
- 121.Zhu GH, Huang C, Qiu ZJ, et al. Expression and prognostic significance of CD151, c-Met, and integrin alpha3/alpha6 in pancreatic ductal adenocarcinoma. Dig Dis Sci. 2011;56(4):1090–8. doi: 10.1007/s10620-010-1416-x. [DOI] [PubMed] [Google Scholar]
- 122.Li C, Wu JJ, Hynes M, et al. c-Met is a marker of pancreatic cancer stem cells and therapeutic target. Gastroenterology. 2011;141(6):2218–27.e5. doi: 10.1053/j.gastro.2011.08.009. [DOI] [PubMed] [Google Scholar]
- 123.Neesse A, Michl P, Frese KK, et al. Stromal biology and therapy in pancreatic cancer. Gut. 2011;60(6):861–8. doi: 10.1136/gut.2010.226092. [DOI] [PubMed] [Google Scholar]
- 124.Xin X, Yang S, Ingle G, et al. Hepatocyte growth factor enhances vascular endothelial growth factor-induced angiogenesis in vitro and in vivo. Am J Pathol. 2001;158(3):1111–20. doi: 10.1016/S0002-9440(10)64058-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Shah AN, Summy JM, Zhang J, et al. Development and characterization of gemcitabine-resistant pancreatic tumor cells. Ann Surg Oncol. 2007;14(12):3629–37. doi: 10.1245/s10434-007-9583-5. [DOI] [PubMed] [Google Scholar]
- 126.Avan A, Quint K, Nicolini F, et al. Enhancement of the antiproliferative activity of gemcitabine by modulation of c-Met pathway in pancreatic cancer. Curr Pharm Des. 2013;19(5):940–50. [PubMed] [Google Scholar]
- 127.Avan A, Caretti V, Funel N, et al. Crizotinib inhibits metabolic inactivation of gemcitabine in c-Met-driven pancreatic carcinoma. Cancer Res. 2013;73(22):6745–56. doi: 10.1158/0008-5472.CAN-13-0837. [DOI] [PubMed] [Google Scholar]
- 128.Hage C, Rausch V, Giese N, et al. The novel c-Met inhibitor cabozantinib overcomes gemcitabine resistance and stem cell signaling in pancreatic cancer. Cell Death Dis. 2013 May 9;4:e627. doi: 10.1038/cddis.2013.158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Elisei R, Schlumberger MJ, Müller SP, et al. Cabozantinib in progressive medullary thyroid cancer. J Clin Oncol. 2013;31(29):3639–46. doi: 10.1200/JCO.2012.48.4659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Senetta R, Duregon E, Sonetto C, et al. YKL-40/c-Met Expression in Rectal Cancer Biopsies Predicts Tumor Regression following Neoadjuvant Chemoradiotherapy: A Multi-Institutional Study. PLoS One. 2015;10(4):e0123759. doi: 10.1371/journal.pone.0123759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Liu Y, Yu XF, Zou J, et al. Prognostic value of c-Met in colorectal cancer: A meta-analysis. World J Gastroenterol. 2015;21(12):3706–10. doi: 10.3748/wjg.v21.i12.3706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Jardim DL, de Melo Gagliato D, Falchook GS, et al. MET aberrations and c-MET inhibitors in patients with gastric and esophageal cancers in a phase I unit. Oncotarget. 2014;5(7):1837–45. doi: 10.18632/oncotarget.1828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Song EK, Tai WM, Messersmith WA, et al. Potent antitumor activity of cabozantinib, a c-MET and VEGFR2 inhibitor, in a colorectal cancer patient- derived tumor explant model. Int J Cancer. 2015;136(8):1967–75. doi: 10.1002/ijc.29225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Xiang Q, Chen W, Ren M, et al. Cabozantinib suppresses tumor growth and metastasis in hepatocellular carcinoma by a dual blockade of VEGFR2 and MET. Clin Cancer Res. 2014;20(11):2959–70. doi: 10.1158/1078-0432.CCR-13-2620. [DOI] [PubMed] [Google Scholar]
- 135.Eswaraka J, Giddabasappa A, Han G, et al. Axitinib and crizotinib combination therapy inhibits bone loss in a mouse model of castration resistantprostate cancer. BMC Cancer. 2014;14:742. doi: 10.1186/1471-2407-14-742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Smith DC, Smith MR, Sweeney C, et al. Cabozantinib in patients with advanced prostate cancer: results of a phase II randomized discontinuation trial. J Clin Oncol. 2013;31(4):412–9. doi: 10.1200/JCO.2012.45.0494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Schmitz K, Koeppen H, Binot E, et al. MET Gene Copy Number Alterations and Expression of MET and Hepatocyte Growth Factor Are Potential Biomarkers in Angiosarcomas and Undifferentiated Pleomorphic Sarcomas. PLoS One. 2015;10(4):e0120079. doi: 10.1371/journal.pone.0120079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Schöffski P, Cornillie J, Wozniak A, et al. Soft tissue sarcoma: an update on systemic treatment options for patients with advanced disease. Oncol Res Treat. 2014;37(6):355–62. doi: 10.1159/000362631. [DOI] [PubMed] [Google Scholar]
- 139.Kentsis A, Reed C, Rice KL, et al. Autocrine activation of the MET receptor tyrosine kinase in acute myeloid leukemia. Nat Med. 2012;18(7):1118–22. doi: 10.1038/nm.2819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Amgen A Phase 3, Multicenter, Randomized, Double-Blind, Placebo Controlled Study of Rilotumumab (AMG102) With Epirubicin, Cisplatin, and Capecitabine (ECX) as First-line Therapy in Advanced MET-Positive Gastric or Gastroesophageal Junction Adenocarcinoma. Available from: http://clinicaltrials.gov/show/NCT01697072. NLM Identifier: NCT01697072.
- 141.Tan EP, Park K, Lim WT, et al. Phase Ib study of ficlatuzumab (formerly AV-299), an anti-hepatocyte growth factor (HGF) monoclonal antibody (MAb) in combination with gefitinib (G) in Asian patients (pts) with NSCLC. J Clin Oncol (Meeting abstracts) 2011;29(15):7571. [Google Scholar]
- 142.Ryan CJ, Rosenthal M, Ng S, et al. Targeted MET inhibition in castration-resistant prostate cancer: a randomized phase II study and biomarker analysis with rilotumumab plus mitoxantrone and prednisone. Clin Cancer Res. 2013;19(1):215–24. doi: 10.1158/1078-0432.CCR-12-2605. [DOI] [PubMed] [Google Scholar]
- 143.Schoffski P, Garcia JA, Stadler, et al. A phase II study of the efficacy and safety of AMG 102 in patients with metastatic renal cell carcinoma. BJU Int. 2011;108(5):679–86. doi: 10.1111/j.1464-410X.2010.09947.x. [DOI] [PubMed] [Google Scholar]
- 144.Wen PY, Schiff D, Cloughesy TF, et al. A phase II study evaluating the efficacy and safety of AMG 102 (rilotumumab) in patients with recurrent glioblastoma. Neuro Oncol. 2011;13(4):437–46. doi: 10.1093/neuonc/noq198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Penuel E, Li C, Parab V, et al. HGF as a circulating biomarker of onartuzumab treatment in patients with advanced solid tumors. Mol Cancer Ther. 2013;12:1122–30. doi: 10.1158/1535-7163.MCT-13-0015. [DOI] [PubMed] [Google Scholar]
- 146.Rosen LS, Senzer N, Mekhail T, et al. A phase I dose-escalation study of Tivantinib (ARQ 197) in adult patients with metastatic solid tumors. Clin Cancer Res. 2011;17:7754–64. doi: 10.1158/1078-0432.CCR-11-1002. [DOI] [PubMed] [Google Scholar]
- 147.Iveson T, Donehower RC, Davidenko I, et al. Rilotumumab in combination with epirubicin, cisplatin, and capecitabine as first-line treatment for gastric or oesophagogastric junction adenocarcinoma: An open-label, dose de-escalation phase 1b study and a double-blind, randomised phase 2 study. Lancet Oncol. 2014;15:1007–18. doi: 10.1016/S1470-2045(14)70023-3. [DOI] [PubMed] [Google Scholar]
- 148.Cunningham D, Tebbutt NC, Davidenko I, et al. Phase III, randomized, double-blind, multicenter, placebo (P)-controlled trial of rilotumumab (R) plus epirubicin, cisplatin and capecitabine (ECX) as first-line therapy in patients (pts) with advanced MET-positive (pos) gastric or gastroesophageal junction (G/GEJ) cancer: RILOMET-1 study. J Clin Oncol (Meeting abstracts) 2015;33:4000. [Google Scholar]
- 149.Yu W, Pandita A, Penuel E, et al. Exploratory biomarker analyses from OAM4558 g: A placebo-controlled phase II study of erlotinib with or without MetMAb in patients with advanced non-small cell lung cancer (NSCLC) J Clin Oncol (Meeting abstracts) 2011;29:7529. [Google Scholar]
- 150.Jung SK, Lee MH, Lim do Y, et al. Butein, a novel dual inhibitor of MET and EGFR, overcomes gefitinib-resistant lung cancer growth. Mol Carcinog. 2015;54(4):322–31. doi: 10.1002/mc.22191. [DOI] [PubMed] [Google Scholar]
- 151.Mai E, Zheng Z, Chen Y, et al. Nonclinical evaluation of the serum pharmacodynamic biomarkers HGF and shed MET following dosing with the anti-MET monovalent monoclonal antibody onartuzumab. Mol Cancer Ther. 2014;13:540–52. doi: 10.1158/1535-7163.MCT-13-0494. [DOI] [PubMed] [Google Scholar]
- 152.Rimassa LPC, Borbath I, Daniele B, et al. Tivantinib (ARQ 197) versus placebo in patients (Pts) with hepatocellular carcinoma (HCC) who failed one systemic therapy: Results of a randomized controlled phase II trial (RCT) J Clin Oncol (Meeting abstracts) 2012:4006. [Google Scholar]
- 153.Santoro A, Rimassa L, Borbath I, et al. Tivantinib for second-line treatment of advanced hepatocellular carcinoma: a randomised, placebo-controlled phase 2 study. Lancet Oncol. 2013;14(1):55–63. doi: 10.1016/S1470-2045(12)70490-4. [DOI] [PubMed] [Google Scholar]
- 154.Zahir H, Rodig S, Sequist L, et al. Relationship between tumor MET expression and clinical outcomes in cancer patients treated with tivantinib. EJC supplements. 2012;48(6):149. [Google Scholar]
- 155.Rodig S, Sequist L, Schiller JH, et al. An exploratory biomarker analysis evaluating the effect of the c-Met inhibitor tivantinib (ARQ 197) and erlotinib in NSCLC patients in a randomized, double-blinded phase 2 study. Proceedings of the American Association of Cancer Res. 2012;72:1729. [Google Scholar]
- 156.Lennerz JK, Kwak EL, Ackerman A, et al. MET amplification identifies a small and aggressive subgroup of esophagogastric adenocarcinoma with evidence of responsiveness to crizotinib. J Clin Oncol. 2011;29(36):4803–10. doi: 10.1200/JCO.2011.35.4928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Tanizaki J, Okamoto I, Okamoto K, et al. MET tyrosine kinase inhibitor crizotinib (PF-02341066) shows differential antitumor effects in non-small cell lung cancer according to MET alterations. J Thorac Oncol. 2011;6(10):1624–31. doi: 10.1097/JTO.0b013e31822591e9. [DOI] [PubMed] [Google Scholar]
- 158.Ou SH, Kwak EL, Siwak-Tapp C, et al. Activity of crizotinib (PF02341066), a dual mesenchymal–epithelial transition (MET) and anaplastic lymphoma kinase (ALK) inhibitor, in a non-small cell lung cancer patient with de novo MET amplification. J Thorac Oncol. 2011;6(5):942–6. doi: 10.1097/JTO.0b013e31821528d3. [DOI] [PubMed] [Google Scholar]
- 159.Rocci A, Gambella M, Aschero S, et al. MET dysregulation is a hallmark of aggressive disease in multiple myeloma patients. Br J Haematol. 2014;164:841–50. doi: 10.1111/bjh.12719. [DOI] [PubMed] [Google Scholar]
- 160.Eder JP, Shapiro GI, Appleman LJ, et al. A phase I study of foretinib, a multi-targeted inhibitor of c-Met and vascular endothelial growth factor receptor 2. Clin Cancer Res. 2010;16(13):3507–16. doi: 10.1158/1078-0432.CCR-10-0574. [DOI] [PubMed] [Google Scholar]
- 161.Klotz M, Schmid E, Steiner-Hahn K, et al. Preclinical evaluation of biomarkers for response monitoring to the MET inhibitor BAY−853474. Biomarkers. 2012;17(4):325–35. doi: 10.3109/1354750X.2012.670865. [DOI] [PubMed] [Google Scholar]
- 162.Feldman DR, Einhorn LH, Quinn DI, et al. A phase 2 multicenter study of tivantinib (ARQ 197) monotherapy in patients with relapsed or refractory germ cell tumors. Invest New Drugs. 2013;31(4):1016–22. doi: 10.1007/s10637-013-9934-y. [DOI] [PubMed] [Google Scholar]
- 163.Hack SP, Bruey JM, Koeppen H. HGF/MET-directed therapeutics in gastroesophageal cancer: a review of clinical and biomarker development. Oncotarget. 2014;5(10):2866–80. doi: 10.18632/oncotarget.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Giampieri R, Scartozzi M, Del PM, et al. Molecular biomarkers of resistance to anti-EGFR treatment in metastatic colorectal cancer, from classical to innovation. Crit Rev Oncol Hematol. 2013;88(2):272–83. doi: 10.1016/j.critrevonc.2013.05.008. [DOI] [PubMed] [Google Scholar]
- 165.Luo W, Huang B, Li Z, Li, et al. MicroRNA-449a is downregulated in non-small cell lung cancer and inhibits migration and invasion by targeting c-Met. PLoS One. 2013;8(5):e64759. doi: 10.1371/journal.pone.0064759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Wu ZS, Wu Q, Wang CQ, et al. miR-340 inhibition of breast cancer cell migration and invasion through targeting of oncoprotein c-Met. Cancer. 2011;117(13):2842–52. doi: 10.1002/cncr.25860. [DOI] [PubMed] [Google Scholar]
- 167.Itadani H, Hasako S, Yonekura K, et al. Identification of gene expression signature to predict anti-tumor efficacy of MET/VEGFR inhibitor, TAS-115. EJC supplements. 2012;48(6):136. [Google Scholar]
- 168.Bachleitner-Hofmann T, Sun MY, Chen CT, et al. Antitumor activity of SNX-2112, a synthetic heat shock protein-90 inhibitor, in MET amplified tumor cells with or without resistance to selective MET Inhibition. Clin Cancer Res. 2011;17(1):122–33. doi: 10.1158/1078-0432.CCR-10-0253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Stabile LP, Lyker JS, Huang L, et al. Inhibition of human non-small cell lung tumors by a c-Met antisense/U6 expression plasmid strategy. Gene Ther. 2004;11(3):325–35. doi: 10.1038/sj.gt.3302169. [DOI] [PubMed] [Google Scholar]
- 170.Mok TSK, Park K, Geater SL, et al. A randomized phase 2 study with exploratory biomarker analysis of ficlantuzumab a humanized hepatocyte growth factor inhibitory MAB in combination with gefitinib versus gefitinib in Asian patients with lung adenocarcinoma. Ann Oncol (Meeting abstracts) 2012;23:1198P. [Google Scholar]
- 171.Schoffski P, Garcia JA, Stadler WM, et al. A phase II study of the efficacy and safety of AMG 102 in patients with metastatic renal cell carcinoma. BJU Int. 2011;108(5):679–86. doi: 10.1111/j.1464-410X.2010.09947.x. [DOI] [PubMed] [Google Scholar]
- 172.Choueiri TK, Pal SK, McDermott DF, et al. A phase I study of cabozantinib (XL184) in patients with renal cell cancer. Ann Oncol. 2014;25(8):1603–8. doi: 10.1093/annonc/mdu184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Choueiri TK, Pal SK, McDermott DF, et al. Activity of cabozantinib (XL184) in patients (pts) with metastatic, refractory renal cell carcinoma (RCC) J Clin Oncol. 2012;30(5):364. [Google Scholar]
- 174.Smith MR, De Bono JS, Sternberg CN, et al. Final analysis of COMET-1: Cabozantinib (Cabo) versus prednisone (Pred) in metastatic castration-resistant prostate cancer (mCRPC) patients (pts) previously treated with docetaxel (D) and abiraterone (A) and/or enzalutamide (E) J Clin Oncol. 2015;33(7):139. [Google Scholar]
- 175.Ryan CJ, Rosenthal M, Ng S, et al. Targeted MET inhibition in castration-resistant prostate cancer: a randomized phase II study and biomarker analysis with rilotumumab plus mitoxantrone and prednisone. Clin Cancer Res. 2013;19(1):215–24. doi: 10.1158/1078-0432.CCR-12-2605. [DOI] [PubMed] [Google Scholar]
- 176.Eng C, Hart LL, Severtsev A, et al. A randomized, placebo-controlled, phase I/II study of tivantinib (ARQ 197) in combination with cetuximab and irinotecan in patients (pts) with KRAS wild-type (WT) metastatic colorectal cancer (CRC) who had received previous front-line systemic therapy. J Clin Oncol. 2013;31:3508. doi: 10.1002/ijc.30049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Eng C, Van Cutsem E, Nowara E, et al. A randomized, phase Ib/II trial of rilotumumab (AMG 102; ril) or ganitumab (AMG 479; gan) with panitumumab (pmab) versus pmab alone in patients (pts) with wild- type (WT) KRAS metastatic colorectal cancer (mCRC): Primary and biomarker analyses. J Clin Oncol. 2011;29:3500. [Google Scholar]
- 178.Oliner KS, Tang R, Anderson A, et al. Evaluation of MET pathway bio- markers in a phase II study of rilotumumab (R, AMG 102) or placebo (P) in combination with epirubicin, cisplatin, and capecitabine (ECX) in patients (pts) with locally advanced or metastatic gastric (G) or esoph- agogastric junction (EGJ) cancer. J Clin Oncol. 2012;30:4005. [Google Scholar]
- 179.Shah MA, Wainberg ZA, Catenacci DV, et al. Phase II study evaluating 2 dosing schedules of oral foretinib (GSK1363089), cMET/VEGFR2 inhibitor, in patients with metastatic gastric cancer. PLoS One. 2013;8(3):e54014. doi: 10.1371/journal.pone.0054014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Verslype C, Cohn AL, Kelley RK, et al. Activity of cabozantinib (XL184) in hepatocellular carcinoma: results from a phase II randomized discontinuation trial (RDT) J Clin Oncol. 2012;30:4007. [Google Scholar]
- 181.Yau TC, Sukeepaisarnjaroen W, Chao Y, et al. A phase I/II study of foretinib, an oral multikinase inhibitor targeting MET, RON, AXL, TIE-2, and VEGFR in advanced hepatocellular carcinoma (HCC) J Clin Oncol. 2012;30:4108. [Google Scholar]
- 182.Daud A, Kluger HM, Edelman G, et al. Activity of cabozantinib in metastatic uveal melanoma: updated results from a phase II randomized discontinuation trial (RDT) J Clin Oncol (Meeting abstracts) 2013;31(15):9094. [Google Scholar]
- 183.Gordon MS, Kluger HM, Shapiro G, et al. Activity of cabozantinib (XL184) in metastatic melanoma: Results from a phase II randomized discontinuation trial (RDT) J Clin Oncol (Meeting abstracts) 2012;30:8531. [Google Scholar]
- 184.Winer EP, Tolaney S, Nechushtan H, et al. Activity of cabozantinib (XL184) in metastatic breast cancer (MBC): Results from a phase II randomized discontinuation trial (RDT) J Clin Oncol (Meeting abstracts) 2012;30:535. [Google Scholar]
- 185.Seiwert T, Swann S, Kurz H, et al. A phase II study of the efficacy and safety of foretinib, a novel receptor tyrosine kinase inhibitor, given on an intermittent 5-days-on/9-days-off (5/9) schedule in patients with recurrent or metastatic squamous cell cancer of the head and neck. Mol Cancer Ther. 2009;2009;8:12(1):B6. [Google Scholar]
- 186.Feldman DR, Einhorn LH, Quinn DI, et al. A phase 2 multicenter study of tivantinib (ARQ 197) monotherapy in patients with relapsed or refractory germ cell tumors. Invest New Drugs. 2013;31(4):1016–22. doi: 10.1007/s10637-013-9934-y. [DOI] [PubMed] [Google Scholar]
- 187.Schoffski P, Wozniak A, Stacchiotti S, et al. Activity of crizotinib (C) in patients (pts) with clear cell sarcoma (CCSA) in EORTC phase II trial 90101 “CREATE”. J Clin Oncol (Meeting abstracts) 2015;33:10542. [Google Scholar]
- 188.Elisei R, Schlumberger MJ, Müller SP, et al. Cabozantinib in progressive medullary thyroid cancer. J Clin Oncol. 2013;31(29):3639–46. doi: 10.1200/JCO.2012.48.4659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Ludovini V, Bianconi F, Pistola L, et al. Optimization of patient selection for EGFR-TKIs in advanced non-small cell lung cancer by combined analysis of KRAS, PIK3CA, MET, and non-sensitizing EGFR mutations. Cancer Chemother Pharmacol. 2012;69(5):1289–99. doi: 10.1007/s00280-012-1829-7. [DOI] [PubMed] [Google Scholar]
- 190.Cappuzzo F, Marchetti A, Skokan M, et al. Increased MET gene copy number negatively affects survival of surgically resected non-small-cell lung cancer patients. J Clin Oncol. 2009;27(10):1667–74. doi: 10.1200/JCO.2008.19.1635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Park S, Choi YL, Sung CO, et al. High MET copy number and MET overexpression: poor outcome in non-small cell lung cancer patients. Histol Histopathol. 2012;27(2):197–207. doi: 10.14670/HH-27.197. [DOI] [PubMed] [Google Scholar]
- 192.Okuda K, Sasaki H, Yukiue H, et al. Met gene copy number predicts the prognosis for completely resected non-small cell lung cancer. Cancer Sci. 2012;99(11):2280–5. doi: 10.1111/j.1349-7006.2008.00916.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Albiges L, Guegan J, Le Formal A, et al. MET is a potential target across all papillary renal cell carcinomas: result from a large molecular study of pRCC with CGH array and matching gene expression array. Clin Cancer Res. 2014;20(13):3411–21. doi: 10.1158/1078-0432.CCR-13-2173. [DOI] [PubMed] [Google Scholar]
- 194.Giordano S, Columbano A. Met as a therapeutic target in HCC: facts and hopes. J Hepatol. 2014;60(2):442–52. doi: 10.1016/j.jhep.2013.09.009. [DOI] [PubMed] [Google Scholar]
- 195.Christensen JG, Burrows J, Salgia R. c-Met as a target for human cancer and characterization of inhibitors for therapeutic intervention. Cancer Lett. 2005;225(1):1–26. doi: 10.1016/j.canlet.2004.09.044. [DOI] [PubMed] [Google Scholar]
- 196.Di Renzo MF, Olivero M, Giacomini A, et al. Overexpression and amplification of the met/HGF receptor gene during the progression of colorectal cancer. Clin Cancer Res. 1995;1(2):147–54. [PubMed] [Google Scholar]
- 197.Seiwert TY, Jagadeeswaran R, Faoro L, et al. The MET receptor tyrosine kinase is a potential novel therapeutic target for head and neck squamous cell carcinoma. Cancer Res. 2009;69(7):3021–31. doi: 10.1158/0008-5472.CAN-08-2881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198.Bardelli A, Corso S, Bertotti A, et al. Amplification of the MET receptor drives resistance to anti-EGFR therapies in colorectal cancer. Cancer Discov. 2013;3(6):658–73. doi: 10.1158/2159-8290.CD-12-0558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199.Sun S, Wang Z. Head neck squamous cell carcinoma c-Met+ cells display cancer stem cell properties and are responsible for cisplatin-resistance and metastasis. Int J Cancer. 2011;129(10):2337–48. doi: 10.1002/ijc.25927. [DOI] [PubMed] [Google Scholar]
- 200.Kina S, Phonaphonh T, Liang F, et al. PDGF alpha receptor is a mediator for Cisplatin-induced Met expression. Eur J Pharmacol. 2013;699(1–3):227–32. doi: 10.1016/j.ejphar.2012.11.014. [DOI] [PubMed] [Google Scholar]
- 201.Mitamura T, Watari H, Wang L, et al. Downregulation of miRNA-31 induces taxane resistance in ovarian cancer cells through increase of receptor tyrosine kinase MET. Oncogenesis. 2013;2:e40. doi: 10.1038/oncsis.2013.3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 202.Ganapathipillai SS, Medova M, Aebersold DM, et al. Coupling of mutated Met variants to DNA repair via Abl and Rad51. Cancer Res. 2008;68(14):5769–77. doi: 10.1158/0008-5472.CAN-08-1269. [DOI] [PubMed] [Google Scholar]