Abstract
Acyl-CoA:cholesterol acyltransferase 1 (ACAT1) is a key enzyme exclusively using free cholesterols as the substrates in cell and is involved in the cellular cholesterol homeostasis. In this study, we used human neuroblastoma cell line SK-N-SH as a model and first observed that inhibiting ACAT1 can decrease the amyloid precursor protein (APP)-α-processing. Meanwhile, the transfection experiments using the small interfering RNA and expression plasmid of ACAT1 indicated that ACAT1 can dependently affect the APP-α-processing. Furthermore, inhibiting ACAT1 was found to increase the free cholesterols in plasma membrane (PM-FC), and the increased PM-FC caused by inhibiting ACAT1 can lead to the decrease of the APP-α-processing, indicating that ACAT1 regulates the dynamics of PM-FC, which leads to the alteration of the APP-α-processing. More importantly, further results showed that under the ACAT1 inhibition, the alterations of the PM-FC and the subsequent APP-α-processing are not dependent on the cellular total cholesterol level, confirming that ACAT1 regulates the dynamics of PM-FC. Finally, we revealed that even when the Niemann–Pick-Type C-dependent pathway is blocked, the ACAT1 inhibition still obviously results in the PM-FC increase, suggesting that the ACAT1-dependent pathway is responsible for the shuttling of PM-FC to the intracellular pool. Our data provide a novel insight that ACAT1 which enzymatically regulates the dynamics of PM-FC may play important roles in the human neuronal cells.
Keywords: ACAT1, free cholesterol, plasma membrane, APP-α-processing, human neuroblastoma cell line SK-N-SH
Introduction
Acyl-CoA:cholesterol acyltransferase (ACAT) is an exclusively intracellular enzyme that can utilize free cholesterols as the substrates to produce cholesteryl esters and plays important roles in maintaining cellular cholesterol homeostasis [1–3]. In mammals, two ACAT genes encoding ACAT1 and ACAT2 have been identified [4,5]. Unlike ACAT2 that is specifically expressed in liver and intestine cells, ACAT1 is expressed in all the cells examined [6–8]. The regulatory expression and functional mechanisms of human ACAT1 have been studied [9–20]. It is involved in certain serious human diseases including atherosclerosis and Alzheimer's disease (AD) and has been recognized as a potential therapeutic target for these diseases [21–24].
Amyloid precursor protein (APP) is a transmembrane protein and can be cleaved through α- or β-processing, which competes with each other [25]. In the α-processing, which is the major processing, APP is cleaved by α-secretase to produce a neurotrophic and neuroprotective fragment sAPPα [26,27], which has been demonstrated to have many beneficial functions, such as promoting neurite extension, enhancing neural cell survival, and protecting neurons from excitotoxin [28–32], whereas in the β-processing, which is the minor processing, APP is sequentially cleaved by β- and γ-secretases to produce amyloid β-peptides (Aβs) that deposit in brain as a neuropathologic hallmark of AD [33,34]. So far, the mechanistic understanding on the APP-α-processing is much less than that on the APP-β-processing.
The regulation of cellular cholesterol homeostasis in the neuronal cells has been considered to affect the APP processing, and the dysfunction of cholesterol metabolism has been recognized as an important risk factor for the AD formation [35–37]. As an exclusive ACAT enzyme expressed in the neuronal cells, ACAT1 plays an essential role in the cellular cholesterol homeostasis and is also correlated with the APP processing or the AD formation [38,39]. The treatment with an isotype-nonselective ACAT inhibitor CP-113,818 (CP) in primary neurons from transgenic mice can remarkably suppress Aβ generation [40]. In mouse model of AD, both treatment of CP and knockdown of ACAT1 can reduce amyloid pathology [12,13]. However, in a human neuroglioma cell line H4APP751, ACAT1 RNA interference (RNAi) not only reduces APP-β-processing by 48.4% but also decreases APP-α-processing by 27.4% [41]. This suggests that the effect of ACAT1 on the APP-α-processing may be significant in neuronal cells because APP-α-processing takes >90% of the total APP processing in these cells and definitely happens on the plasma membrane where free cholesterols mainly function [42,43]. But the mechanistic effect of ACAT1 on the APP-α-processing is still unclear.
In the present study, we use human neuroblastoma cell line SK-N-SH to study the role of ACAT1, and reveal that ACAT1 regulates the dynamics of free cholesterols in plasma membrane (PM-FC), which leads to the alteration of the APP-α-processing.
Materials and Methods
Reagents
RPMI 1640 and fetal bovine serum (FBS) were from Gibco-BRL (Grand Island, USA). Methyl-β-cyclodextrin (MCD) was from Cyclodextrin Technologies Development (Gainesville, USA). Lipoprotein-deficient serum (LPDS) was prepared from the FBS by ultracentrifugation [44]. Low density lipoprotein (LDL) was produced as described [10,45]. Lovastatin (Lova), mevalonate (Mev), and U18666A were provided by Dr Baoliang Song. ACAT inhibitor CP and anti-ACAT1 (DM10) antibody were provided by Dr Tayuan Chang. Filipin, cholesterol oxidase from Streptomyces sp., and anti-β-actin (AC-15) antibody were from Sigma (St Louis, USA). Anti-sAPPα (6E10) antibody was from Abcam (Cambridge, England). Anti-APP (22C11) antibody was from Chemicon International (Temecura, USA). Horseradish peroxidase (HRP)-conjugated anti-rabbit and anti-mouse immunoglobulin G secondary antibodies were from Pierce (Rockford, USA) and Jackson Immuno Research Laboratories (Baltimore, USA), respectively.
siRNA and expression plasmid of human ACAT1
The sequence of human ACAT1 small interfering RNA (siRNA) is 5′-GAGUUCUCAUCCGCUGAUC-3′. The expression plasmid of human ACAT1 (pACAT1) was constructed as described in our previous work [10].
Cell culture and transfection
Human neuroblastoma cell line SK-N-SH (ATCC, Manassas, USA) was maintained in RPMI 1640 supplemented with 10% FBS, 100 µg/ml ampicillin, 50 U/ml streptomycin, and 2 g/l sodium bicarbonate at 37°C in a humid atmosphere of 5% CO2 and 95% air. Transfection of plasmid was performed using FuGENE6™ transfection reagent (Roche, Basel, Switzerland) according to the manufacturer's instructions. Transfection of siRNA was performed using Lipofectamine 2000 transfection reagent (Invitrogen, Carlsbad, USA) according to the manufacturer's instructions.
Western blotting
The cultured media were collected to test the secreted protein sAPPα, and then cells were washed two times, scraped in ice-cold phosphate buffered saline (PBS), and extracted on ice for 30 min with radioimmunoprecipitation assay (RIPA) lysis buffer (50 mM Tris–HCl, 0.1% sodium dodecyl sulfate (SDS), 150 mM NaCl, 2 mM MgCl2, 1.5% NP-40, 0.5% deoxycholate, 50 mM DL-Dithiothreitol, pH 8.0) containing protease inhibitor mixture (Sigma). The protein concentrations of cell lysates after removing cell debris by a spin at 16,000 g were determined using the bicinchoninic acid (BCA) protein assay kit (Bio-Rad, Hercules, USA). For western blot analysis, the cultured medium (30 µl after normalizing to the lowest protein concentration of cell lysates) or cell lysate (30 µg protein) per lane was resolved by 8% or 12% SDS-polyacrylamide gel electrophoresis (SDS-PAGE). After gel separation, the proteins were transferred to nitrocellulose membranes. The membranes were blocked with 5% milk in Tris-buffered saline with Tween-20 (TBST; 50 mM Tris–HCl, pH 7.6, 0.15 M NaCl, and 0.05% Tween-20) for 2 h at the room temperature and then incubated with anti-sAPPα, anti-APP, anti-β-actin, or anti-ACAT1 antibody for 3 h, respectively. After incubation with HRP-conjugated secondary antibodies for 1 h, the membranes were rinsed extensively with TBST and Tris-buffered saline (50 mM Tris–HCl, pH 7.6, and 0.15 M NaCl), respectively. The signals were developed using electrochemiluminescence western blotting detection reagent (Santa Cruz Biotechnology, Santa Cruz, USA). The protein intensities were quantified using the UVP Labwork software (UVP Inc., Upland, USA) for densitometry analysis.
Determination of the PM-FC by filipin staining
Cells cultured on coverslips were fixed with 4% paraformaldehyde for 30 min, then washed with PBS and stained with staining solution (50 µg/ml filipin and 10% FBS in PBS) at the room temperature for 30 min. The coverslips were mounted in Fluosave (Polysciences Inc., Warrington, USA). Filipin signals from the PM-FC of the stained cells were analyzed with the LSM 510 two-photon confocal microscope (Zeiss, Oberkochen, Germany) and the software Image-Pro Plus 5.02.
Determination of the PM-FC by cholesterol assay
Cells were extracted with chloroform/methanol (2 : 1; v/v), and then the chloroform phase was collected and dried with N2. The extracted cholesterols were dissolved in the reaction buffer (100 mM potassium phosphate, 50 mM NaCl, 5 mM cholic acid, 0.1% Triton X-100, pH 7.4), and the cellular total cholesterols (TC) were determined using Amplex Red Cholesterol Assay kit (Invitrogen) according to the manufacturer's instructions. The cellular total proteins (TP) were determined using the BCA protein assay kit (Bio-Rad).
The determination of the PM-FC of the adherent cells was performed using cholesterol oxidase assay [46,47] with slight modifications. Briefly, the adherent cells were washed two times with assay buffer (310 mM sucrose, 0.5 mM sodium phosphate, 1 mM MgSO4, pH 7.4), treated with or without 1 U/ml cholesterol oxidase in the assay buffer at the room temperature for 3 min, and then the cholesterol oxidase reaction was stopped by adding methanol to a final concentration of 30%. The treated cells were washed two times with PBS containing 30% methanol, harvested with RIPA lysis buffer containing 30% methanol, and extracted with chloroform/methanol (final 2 : 1; v/v). The TC and TP were determined as described above. PM-FC was calculated as follows: PM-FC = TC/TP − TC-e/TP-e, where TC and TP are the total cholesterols and total proteins of the cells treated without enzyme, and TC-e and TP-e are the total cholesterols and total proteins of the enzyme-treated cells.
Statistical analysis
Experimental data were analyzed as indicated in figures 3–6. Statistical significance was evaluated with the Student's t-test. P values of <0.05 were considered as statistically significant.
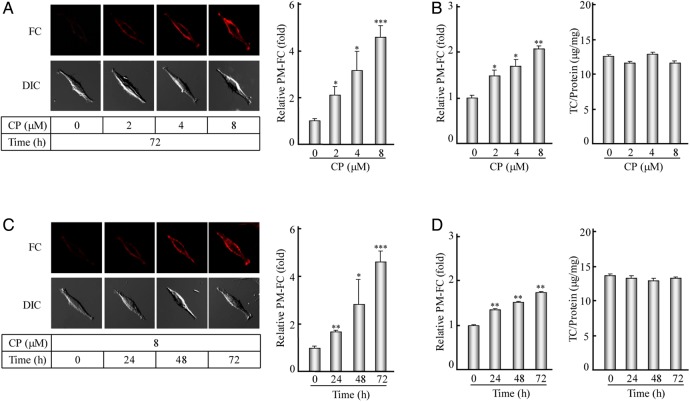
Figure 3.
Filipin staining and determination of the PM-FC after the ACAT1 inhibition (A) The human SK-N-SH cells were treated with the CP at different concentrations (0, 2, 4, or 8 µM) for 72 h. The filipin staining and the corresponding quantification of the PM-FC were performed according to the procedures described in the ‘Materials and Methods’. The data were the mean ± SD from filipin signals of 50 stained cells randomly. The relative PM-FC were expressed as fold to the control without the CP treatment. (B) The PM-FC and TC of the cells treated as indicated in (A) were determined according to the cholesterol assay procedures described in the ‘Materials and Methods’. The data were the mean ± SD from two independent experiments. The relative PM-FC were expressed as fold to the control without the CP treatment. (C,D) The human SK-N-SH cells were treated with 8 µM CP for different time intervals (0, 24, 48, or 72 h). The data were obtained by the same methods as indicated in (A) and (B), respectively. ***P < 0.001; **P < 0.01; *P < 0.05.
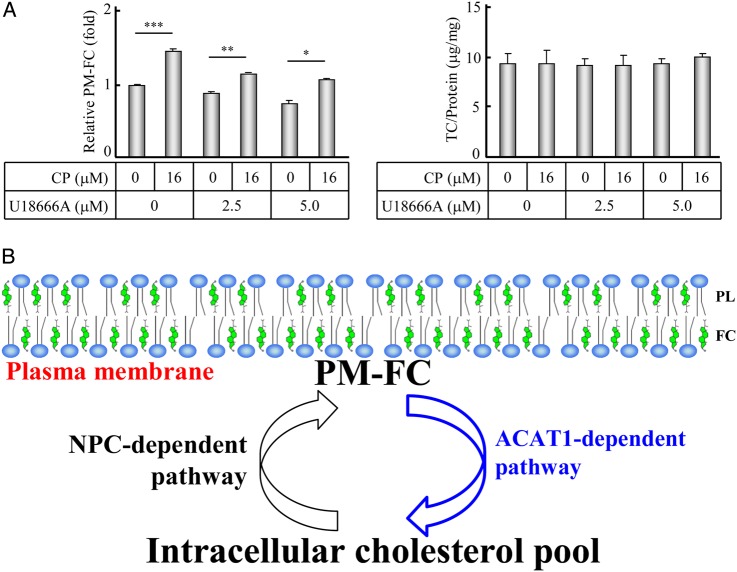
Figure 6.
Working model for the dynamics of PM-FC (A) The human SK-N-SH cells were incubated in media with 10% FBS for 12 h, followed by the incubation in the fresh media with or without 16 µM CP and 2.5 or 5.0 µM U18666A for another 12 h. The PM-FC and TC of the treated cells were determined according to the procedures described in the ‘Materials and Methods’. The data were the mean ± SD from three independent experiments. The relative PM-FC were expressed as fold to the control without the CP and U18666A treatment. (B) Working model. PL, phospholipid; FC, free cholesterol. ***P < 0.001; **P < 0.01; *P < 0.05.
Results
Inhibiting ACAT1 can decrease the APP-α-processing
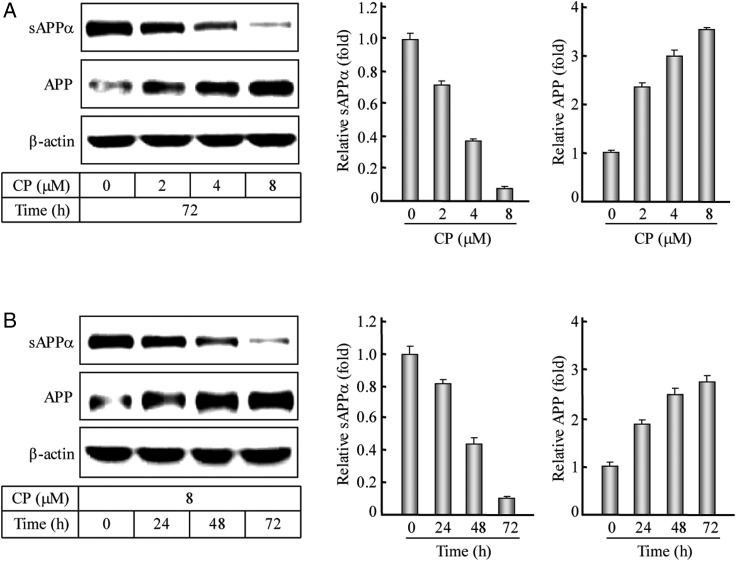
It has been reported that ACAT1 RNAi not only reduces APP-β-processing by 48.4% but also decreases APP-α-processing by 27.4% in a human neuroglioma cell line H4APP751 [41]. Because the APP-α-processing takes >90% of the total APP processing and happens on the plasma membrane where free cholesterols mainly function [42,43], we are very interested in testing whether and how ACAT1 affects the APP-α-processing in neuronal cells. First, we treated human neuroblastoma cell line SK-N-SH, which only expresses ACAT1, with an isotype-nonselective ACAT inhibitor CP, and used western blotting to test the APP-α-processing product sAPPα in media and its precursor APP in the human SK-N-SH cells after the CP treatments. Western blotting (Fig. 1A,B, left panel) and the corresponding quantification (Fig. 1A,B, middle and right panels) showed that under the ACAT1 inhibition, the sAPPα was decreased and its precursor APP was increased evidently after 72 h of treatment with different concentrations (0, 2, 4, or 8 µM) of CP or treatment with 8 µM CP for the different time intervals (0, 24, 48, or 72 h). These results indicate that inhibiting ACAT1 in the human SK-N-SH cells can decrease APP-α-processing in dose- and time-dependent manners.
Figure 1.
Western blotting and quantification of the APP-α-processing after the ACAT1 inhibition (A) The human SK-N-SH cells were treated with the CP at different concentrations (0, 2, 4, or 8 µM) for 72 h. The immunoblotting of the cultured media and cell lysates as well as the quantification of the sAPPα or APP protein were performed according to the procedures described in the ‘Materials and Methods’. The data were the mean ± SD from three quantifications. The relative levels of proteins were expressed as fold to the controls without the CP treatment. (B) The human SK-N-SH cells were treated with 8 µM CP for different time intervals (0, 24, 48, or 72 h). The data were obtained by the same methods as indicated in (A).
ACAT1 affects the APP-α-processing
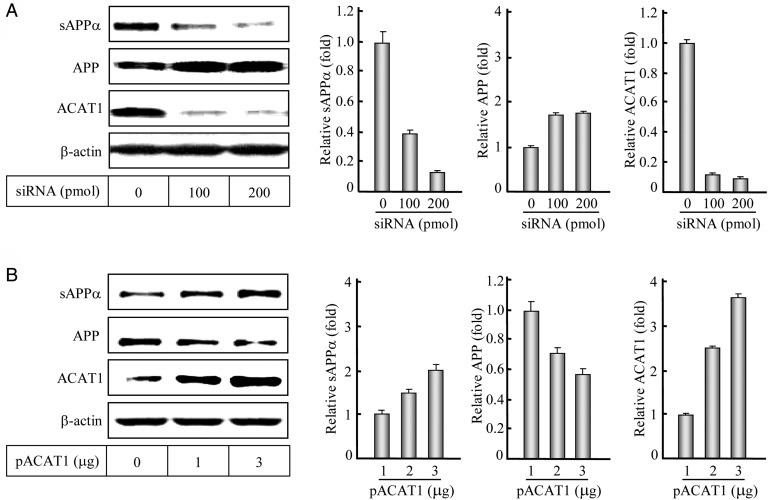
Meanwhile, we performed RNAi or overexpression of ACAT1 in the human SK-N-SH cells to further study the effect of ACAT1 on the APP-α-processing. Western blotting and the corresponding quantification showed that the reduction of ACAT1 with siRNA could obviously decrease the APP-α-processing product sAPPα and increase its precursor APP (Fig. 2A), when compared with the control without the siRNA transfection. Conversely, when ACAT1 was overexpressed, the increase in sAPPα and the decrease in APP were obviously seen, when compared with the control without the plasmid transfection (Fig. 2B). These results illustrate that ACAT1 affects the APP-α-processing in human SK-N-SH cells.
Figure 2.
Western blotting and quantification of the APP-α-processing after RNAi or overexpression of ACAT1 (A) The human SK-N-SH cells were transiently transfected with duplexes of siRNA targeting ACAT1 mRNA. After a 48-h transfection, the immunoblotting of the cultured media and cell lysates as well as the quantification of the sAPPα, APP, or ACAT1 protein were performed according to the procedures described in the ‘Materials and Methods’. The data were the mean ± SD from three quantifications. The relative levels of proteins were expressed as fold to the controls without transfection. (B) The human SK-N-SH cells were transiently transfected with the expression plasmid pACAT1. After a 24-h transfection, the data were obtained by the same methods as indicated in (A).
Inhibiting ACAT1 obviously increases the PM-FC
As a key enzyme exclusively using free cholesterols as the substrates in cell and involving in the cellular cholesterol homeostasis, ACAT1 may control the dynamics of PM-FC. To test this hypothesis, we also used human neuroblastoma cell line SK-N-SH as a model and induced ACAT1 inhibition as described above. As shown in Fig. 3A, filipin staining (left panel) and statistical analysis (right panel) displayed that the PM-FC were dramatically increased with the treatments of 2, 4, or 8 µM of CP for 72 h. Furthermore, cholesterol assay confirmed that while the cellular TC/protein were not obviously changed (Fig. 3B, right panel) with the CP treatments, the PM-FC were significantly increased (Fig. 3B, left panel). However, the fold of increase was lower than that obtained in the filipin staining, mainly due to the partial PM-FC detected in the treatment of adherent cells with the cholesterol oxidase reaction. Similar results were obtained when the human SK-N-SH cells were treated with 8 µM CP for 24, 48, or 72 h (Fig. 3C,D). These data demonstrate that inhibiting ACAT1 in the human SK-N-SH cells can significantly increase the PM-FC in dose- and time-dependent manners.
The increased PM-FC leads to the decrease of the APP-α-processing under the ACAT1 inhibition
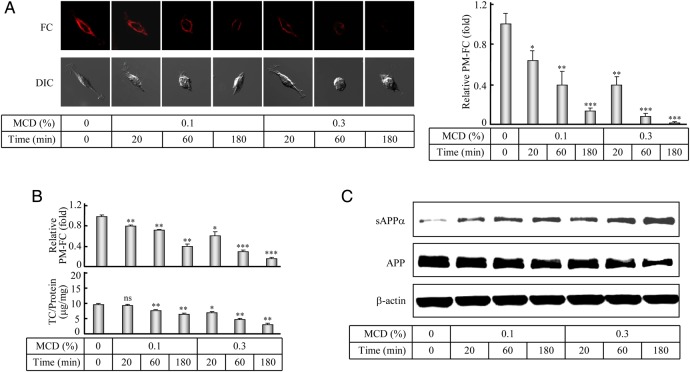
The regulation of cellular cholesterol homeostasis has been considered to affect the APP processing [35]. According to our results from the above experiments, we further supposed that the decreased APP-α-processing happened on the plasma membrane might be related to the increased PM-FC under the ACAT1 inhibition. To verify this correlation, we pretreated the human SK-N-SH cells with 8 µM CP for 72 h, then added MCD to lower cholesterols [48] for a short period of time. Both filipin staining (Fig. 4A) and cholesterol assay (Fig. 4B, upper panel) showed that under the ACAT1 inhibition, both 0.1% and 0.3% MCD could lower the increased PM-FC, while western blot analysis (Fig. 4C) indicated that the decreased sAPPα was obviously resumed. Interestingly, under the treatment with 0.1% MCD for 20 min, the increased PM-FC were evidently decreased (Fig. 4A, right panel, and Fig. 4B, upper panel, comparing bar 2 with 1) and the decreased sAPPα was inversely increased (Fig. 4C, comparing lane 2 with 1), while the cellular TC were not obviously changed (Fig. 4B, bottom panel, comparing bar 2 with 1). These results demonstrated that the decreased APP-α-processing could be obviously restored by the MCD treatment, which can lower the PM-FC for a very short period of time, indicating that ACAT1 regulates the dynamics of PM-FC that leads to the APP-α-processing alteration in the human SK-N-SH cells.
Figure 4.
Filipin staining and determination of the PM-FC and western blotting of the APP-α-processing after the ACAT1 inhibition and the MCD treatment (A) The human SK-N-SH cells were treated with 8 µM CP for 72 h, followed by the treatment with 0.1% or 0.3% MCD for different time intervals (0, 20, 60, or 180 min). The filipin staining and the corresponding quantification of the PM-FC were performed according to the procedures described in the ‘Materials and Methods’. The data were the mean ± SD from filipin signals of 50 stained cells randomly. The relative PM-FC were expressed as fold to the control without the MCD treatment. (B) The PM-FC and TC of the cells treated with the CP and MCD as indicated in (A) were determined according to the cholesterol assay procedures described in the ‘Materials and Methods’. The data were the mean ± SD from two independent experiments. The relative PM-FC were expressed as fold to the control without the MCD treatment. (C) The human SK-N-SH cells were treated with the CP and MCD as indicated in (A). The immunoblotting of the cultured media and cell lysates were performed according to the procedures described in the ‘Materials and Methods’. ***P < 0.001; **P < 0.01; *P < 0.05; ns, not significant.
Alterations of PM-FC and the subsequent APP-α-processing are not dependent on the cellular TC level under the ACAT1 inhibition
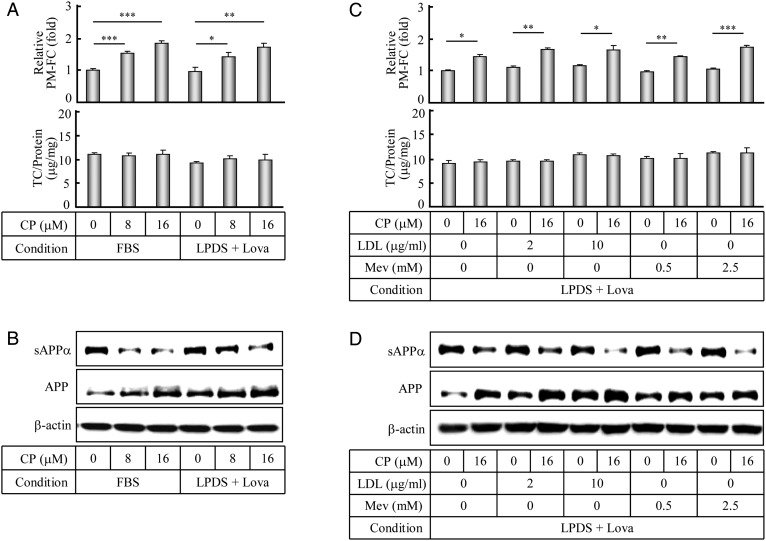
In order to analyze whether other cellular cholesterol metabolic pathways could affect the PM-FC and APP-α-processing, the human SK-N-SH cells were cultured with lipid-depleting (LPDS) media to block the influx and efflux of cholesterols and treated with Lova to inhibit the synthesis of cholesterols. Cholesterol assay and western blot analysis showed that under these experimental conditions together with the CP treatment, the PM-FC were evidently increased (Fig. 5A, upper panel, bars 4–6) with a slight reduction of the cellular TC (Fig. 5A, bottom panel, bars 4–6), and at the same time, the decrease of the APP-α-processing product sAPPα and the inverse increase of its precursor APP were also clearly observed (Fig. 5B, lanes 4–6). Moreover, under the experimental conditions mentioned above, LDL or Mev, which is a precursor of cholesterol synthesis without the feedback inhibition, was added to enhance the influx and synthesis of cholesterols. Similar results (Fig. 5C,D, upper panel, bars 3–10) were obtained with a slight increase of the cellular TC (Fig. 5C, bottom panel, bars 3–10). These data show that the ACAT1 inhibition contributes to the increase of the PM-FC which leads to the decrease of the APP-α-processing product sAPPα and the increase of its precursor APP in different cellular TC levels, and also illustrates that the alterations of the PM-FC and the subsequent APP-α-processing are not dependent on the cellular TC level under the ACAT1 inhibition, confirming that ACAT1 regulates the dynamics of PM-FC in the human SK-N-SH cells.
Figure 5.
Determinations of the PM-FC and the APP-α-processing after culture under different conditions (A) The human SK-N-SH cells were incubated in normal media with 10% FBS or cholesterol-depleting media with 5% LPDS plus Lova (1 µM) for 12 h, followed by the incubation in fresh individual media with CP at different concentrations for another 12 h. The PM-FC and TC of the treated cells were determined according to the procedures described in the ‘Materials and Methods’. The data were the mean ± SD from three independent experiments. The relative PM-FC were expressed as fold to the control cultured in FBS without the CP treatment. (B) The human SK-N-SH cells were cultured and treated as indicated in (A). The immunoblotting of the cultured media and cell lysates was performed according to the procedures described in the ‘Materials and Methods’. (C,D) The human SK-N-SH cells were incubated in cholesterol-depleting media with 5% LPDS plus Lova (1 µM) for 12 h, followed by changing to the fresh media with or without 16 µM CP under different concentrations of the LDL (2 and 10 μg/ml) or Mev (0.5 and 2.5 mM) for another 12 h. The data were obtained by the same methods as indicated in (A) and (B), respectively. The relative PM-FC were expressed as fold to the control without the CP, LDL, and Mev treatment. ***P < 0.001; **P < 0.01; *P < 0.05.
ACAT1 inhibition consistently increases the PM-FC even when the Niemann–Pick-Type C-dependent pathway was blocked
It has been reported that the compound U18666A can specifically reduce the PM-FC by blocking the Niemann–Pick-Type C (NPC)-dependent pathway that is responsible for shuttling endocytotic cholesterols to the plasma membrane [49,50]. Significantly, when treated together with U18666A, inhibiting ACAT1 was found to consistently raise the PM-FC (Fig. 6A, left panel, comparing bars 4 and 6 with 3 and 5, respectively). It clearly shows that the inhibition of ACAT1, which is located at the endoplasmic reticulum, can suppress the shuttling of free cholesterols from the plasma membrane to the intracellular pool. Accordingly, we postulate a working model that the ACAT1-dependent pathway is responsible for shuttling the PM-FC to the intracellular pool (Fig. 6B).
Discussion
ACAT1, as a key enzyme exclusively using free cholesterol as the substrates in cell and involving in the cellular cholesterol homeostasis, may play essential roles in the APP processing, which includes α- and β-processing [38]. So far, the mechanistic studies on the APP-α-processing are much less than those on the APP-β-processing. In this study, we found that ACAT1 can affect the APP-α-processing in the human SK-N-SH cells. We then prove that inhibiting ACAT1 can increase the PM-FC, which leads to the decrease of the APP-α-processing, indicating that ACAT1 regulates the dynamics of PM-FC that leads to the alteration of the APP-α-processing in the human SK-N-SH cells. Our further experiments show that under the ACAT1 inhibition, the alterations of PM-FC and the subsequent APP-α-processing are not dependent on the cellular TC level, confirming that ACAT1 regulates the dynamics of PM-FC. We finally reveal that even when the NPC-dependent pathway was blocked, the ACAT1 inhibition consistently increases the PM-FC and, thus, postulate that the ACAT1-dependent pathway is responsible for shuttling PM-FC to the intracellular pool. Our data provide a novel insight that ACAT1 which enzymatically regulates the dynamics of PM-FC may play important roles in the neuronal cells. In addition, by using the APP-α-processing as a model, a system of ACAT1 regulating the dynamics of PM-FC has been established, which will be useful in studies on the PM-FC-dependent functions of the proteins or transmembrane events.
So far, it is believed that the APP-α-processing that constitutionally happens on the plasma membrane takes >90% of the APP processing [42,43]. In this study, we found that ACAT1 regulates the dynamics of PM-FC and subsequently affects the APP-α-processing in the human SK-N-SH cells. However, under the inhibition or RNAi of ACAT1, it seems contradictory that the major APP-α-processing is decreased to suppress the neurotrophic and neuroprotective effects of sAPPα [27], whereas the minor APP-β-processing is also decreased to reduce the generation of Aβ and ameliorate AD [40,41]. It has been hypothesized that the reduced ACAT1 affects all the three secretases (α, β, and γ) for the APP processing or disrupts the trafficking of APP in the early secretory pathway to limit its availability for the secretases [41,51]. The detail mechanisms are to be clarified by further studies.
According to the results that under the inhibition or RNAi of ACAT1, the decreases in both the APP-α- and APP-β-processing were observed with the increase of the PM-FC; we believe that the dysfunction of the PM-FC dynamics is the primary trigger for the abnormal minor APP-β-processing, which competes with the major APP-α-processing under physiological conditions [25]. Evidently, under the conditions of the ACAT1 inhibition or RNAi, the elevated PM-FC lower the fluidity of plasma membrane, which may block the α-secretases contacting with the cleavage sites, leading to the decrease of the APP-α-processing, and meanwhile suppress the endocytosis of the precursor APP, also leading to the decrease of APP-β-processing which happens in the endosome [25,42]. On the contrary, under the pathological conditions, ACAT1 may be dramatically enhanced to reduce the PM-FC, which would further boost the endocytosis of APP and the APP-β-processing. We hypothesized that the regulation of the enzymatic activity of ACAT1 may be responsible predominantly for the dynamics of PM-FC and the fluidity of plasma membrane and suggested that the enzymatic activity of ACAT1 which regulates the dynamics of PM-FC needs to be precisely regulated to prevent and treat certain neurological diseases and nonresolving inflammation.
Funding
This work was supported by the grants from the Ministry of Science and Technology of China (No. 2011CB910900 to B.L.) and the National Natural Science Foundation of China (Nos. 31271377 and 30571057 to B.L.) and by National Institutes of Health (No. AG 037609 to T.C. and C.C.Y.C.).
References
- 1.Chang TY, Chang CC, Cheng D. Acyl-coenzyme A:cholesterol acyltransferase. Annu Rev Biochem 1997, 66: 613–638. [DOI] [PubMed] [Google Scholar]
- 2.Yu C, Chen J, Lin S, Liu J, Chang CC, Chang TY. Human acyl-CoA:cholesterol acyltransferase-1 is a homotetrameric enzyme in intact cells and in vitro. J Biol Chem 1999, 274: 36139–36145. [DOI] [PubMed] [Google Scholar]
- 3.Chang TY, Li BL, Chang CC, Urano Y. Acyl-coenzyme A:cholesterol acyltransferases. Am J Physiol Endocrinol Metab 2009, 297: E1–E9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chang CC, Huh HY, Cadigan KM, Chang TY. Molecular cloning and functional expression of human acylcoenzyme A:cholesterol acyltransferase cDNA in mutant Chinese hamster ovary cells. J Biol Chem 1993, 268: 20747–20755. [PubMed] [Google Scholar]
- 5.Song BL, Wang CH, Yao XM, Yang L, Zhang WJ, Wang ZZ, Zhao XN et al. . Human acyl-CoA:cholesterol acyltransferase 2 gene expression in intestinal Caco-2 cells and in hepatocellular carcinoma. Biochem J 2006, 394: 617–626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lee O, Chang CC, Lee W, Chang TY. Immunodepletion experiments suggest that acyl-coenzyme A:cholesterol acyltransferase-1 (ACAT-1) protein plays a major catalytic role in adult human liver, adrenal gland, macrophages, and kidney, but not in intestines. J Lipid Res 1998, 39: 1722–1727. [PubMed] [Google Scholar]
- 7.Chang CC, Sakashita N, Ornvold K, Lee O, Chang ET, Dong R, Lin S et al. . Immunological quantitation and localization of ACAT-1 and ACAT-2 in human liver and small intestine. J Biol Chem 2000, 275: 28083–28092. [DOI] [PubMed] [Google Scholar]
- 8.Chang TY, Chang CC, Lin S, Yu C, Li BL, Miyazaki A. Roles of acyl-coenzyme A:cholesterol acyltransferase-1 and -2. Curr Opin Lipidol 2001, 12: 289–296. [DOI] [PubMed] [Google Scholar]
- 9.Yang JB, Duan ZJ, Yao W, Lee O, Yang L, Yang XY, Sun X et al. . Synergistic transcriptional activation of human acyl-coenzyme A:cholesterol acyltransterase-1 gene by interferon-gamma and all-trans-retinoic acid THP-1 cells. J Biol Chem 2001, 276: 20989–20998. [DOI] [PubMed] [Google Scholar]
- 10.Yang L, Yang JB, Chen J, Yu GY, Zhou P, Lei L, Wang ZZ et al. . Enhancement of human ACAT1 gene expression to promote the macrophage-derived foam cell formation by dexamethasone. Cell Res 2004, 14: 315–323. [DOI] [PubMed] [Google Scholar]
- 11.Li BL, Li XL, Duan ZJ, Lee O, Lin S, Ma ZM, Chang CC et al. . Human acyl-CoA:cholesterol acyltransferase-1 (ACAT-1) gene organization and evidence that the 4.3-kilobase ACAT-1 mRNA is produced from two different chromosomes. J Biol Chem 1999, 274: 11060–11071. [DOI] [PubMed] [Google Scholar]
- 12.Yang L, Lee O, Chen J, Chen J, Chang CC, Zhou P, Wang ZZ et al. . Human acyl-coenzyme A:cholesterol acyltransferase 1 (acat1) sequences located in two different chromosomes (7 and 1) are required to produce a novel ACAT1 isoenzyme with additional sequence at the N terminus. J Biol Chem 2004, 279: 46253–46262. [DOI] [PubMed] [Google Scholar]
- 13.Chen J, Zhao XN, Yang L, Hu GJ, Lu M, Xiong Y, Yang XY et al. . RNA secondary structures located in the interchromosomal region of human ACAT1 chimeric mRNA are required to produce the 56-kDa isoform. Cell Res 2008, 18: 921–936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zhao X, Chen J, Lei L, Hu G, Xiong Y, Xu J, Li Q et al. . The optional long 5′-untranslated region of human ACAT1 mRNAs impairs the production of ACAT1 protein by promoting its mRNA decay. Acta Biochim Biophys Sin 2009, 41: 30–41. [DOI] [PubMed] [Google Scholar]
- 15.Hu GJ, Chen J, Zhao XN, Xu JJ, Guo DQ, Lu M, Zhu M et al. . Production of ACAT1 56-kDa isoform in human cells via trans-splicing involving the ampicillin resistance gene. Cell Res 2013, 23: 1007–1024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Xu J, Hu G, Lu M, Xiong Y, Li Q, Chang CC, Song B et al. . MiR-9 reduces human acyl-coenzyme A:cholesterol acyltransferase-1 to decrease THP-1 macrophage-derived foam cell formation. Acta Biochim Biophys Sin 2013, 45: 953–962. [DOI] [PubMed] [Google Scholar]
- 17.Hongo S, Watanabe T, Arita S, Kanome T, Kageyama H, Shioda S, Miyazaki A. Leptin modulates ACAT1 expression and cholesterol efflux from human macrophages. Am J Physiol Endocrinol Metab 2009, 297: E474–E482. [DOI] [PubMed] [Google Scholar]
- 18.Kanome T, Watanabe T, Nishio K, Takahashi K, Hongo S, Miyazaki A. Angiotensin II upregulates acyl-CoA:cholesterol acyltransferase-1 via the angiotensin II Type 1 receptor in human monocyte-macrophages. Hypertens Res 2008, 31: 1801–1810. [DOI] [PubMed] [Google Scholar]
- 19.Ge J, Zhai W, Cheng B, He P, Qi B, Lu H, Zeng Y et al. . Insulin induces human acyl-coenzyme A: cholesterol acyltransferase1 gene expression via MAP kinases and CCAAT/enhancer-binding protein α. J Cell Biochem 2013, 114: 2188–2198. [DOI] [PubMed] [Google Scholar]
- 20.Cheng B, Wan J, Wang Y, Mei C, Liu W, Ke L, He P. Ghrelin inhibits foam cell formation via simultaneously down-regulating the expression of acyl-coenzyme A:cholesterol acyltransferase 1 and up-regulating adenosine triphosphate-binding cassette transporter A1. Cardiovasc Pathol 2010, 19: e159–e166. [DOI] [PubMed] [Google Scholar]
- 21.Chang C, Dong R, Miyazaki A, Sakashita N, Zhang Y, Liu J, Guo M et al. . Human acyl-CoA:cholesterol acyltransferase (ACAT) and its potential as a target for pharmaceutical intervention against atherosclerosis. Acta Biochim Biophys Sin 2006, 38: 151–156. [DOI] [PubMed] [Google Scholar]
- 22.Hutter-Paier B, Huttunen HJ, Puglielli L, Eckman CB, Kim DY, Hofmeister A, Moir RD et al. . The ACAT inhibitor CP-113,818 markedly reduces amyloid pathology in a mouse model of Alzheimer's disease. Neuron 2004, 44: 227–238. [DOI] [PubMed] [Google Scholar]
- 23.Murphy SR, Chang CC, Dogbevia G, Bryleva EY, Bowen Z, Hasan MT, Chang TY. Acat1 knockdown gene therapy decreases amyloid-β in a mouse model of Alzheimer's disease. Mol Ther 2013, 21: 1497–1506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Li BL, Chang TY, Chen J, Chang CC, Zhao XN. Human ACAT1 gene expression and its involvement in the development of atherosclerosis. Future Cardiol 2006, 21: 93–99. [DOI] [PubMed] [Google Scholar]
- 25.Yang X, Sun GY, Eckert GP, Lee JC. Cellular membrane fluidity in amyloid precursor protein processing. Mol Neurobiol 2014, 50: 119–129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Esch FS, Keim PS, Beattie EC, Blacher RW, Culwell AR, Oltersdorf T, McClure D et al. . Cleavage of amyloid beta peptide during constitutive processing of its precursor. Science 1990, 248: 1122–1128. [DOI] [PubMed] [Google Scholar]
- 27.Thornton E, Vink R, Blumbergs PC, Van Den Heuvel C. Soluble amyloid precursor protein alpha reduces neuronal injury and improves functional outcome following diffuse traumatic brain injury in rats. Brain Res 2006, 1094: 38–46. [DOI] [PubMed] [Google Scholar]
- 28.Jin LW, Ninomiya H, Roch JM, Schubert D, Masliah E, Otero DA, Saitoh T. Peptides containing the RERMS sequence of amyloid (beta)/A4 protein precursor bind cell surface and promote neurite extension. J Neurosci 1994, 14: 5461–5470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Yamamoto K, Miyoshi T, Yae T, Kawashima K, Araki H, Hanada K, Otero DA et al. . The survival of rat cerebral cortical neurons in the presence of trophic APP peptides. J Neurobiol 1994, 25: 585–594. [DOI] [PubMed] [Google Scholar]
- 30.Mattson MP, Cheng B, Culwell AR, Esch FS, Lieberburg I, Rydel RE. Evidence for excitoprotective and intraneuronal calcium-regulating roles for secreted forms of the beta-amyloid precursor protein. Neuron 1993, 10: 243–254. [DOI] [PubMed] [Google Scholar]
- 31.Smith-Swintosky VL, Pettigrew LC, Craddock SD, Culwell AR, Rydel RE, Mattson MP. Secreted forms of beta-amyloid precursor protein protect against ischemic brain injury. J Neurochem 1994, 63: 781–784. [DOI] [PubMed] [Google Scholar]
- 32.Bodovitz S, Klein WL. Cholesterol modulates alpha-secretase cleavage of amyloid precursor protein. J Biol Chem 1996, 271: 4436–4440. [DOI] [PubMed] [Google Scholar]
- 33.Vassar R. BACE1: the beta-secretase enzyme in Alzheimer's disease. J Mol Neurosci 2004, 23: 105–114. [DOI] [PubMed] [Google Scholar]
- 34.De Strooper B. Proteases and proteolysis in Alzheimer disease: a multifactorial view on the disease process. Physiol Rev 2010, 90: 465–494. [DOI] [PubMed] [Google Scholar]
- 35.Burns MP, Rebeck GW. Intracellular cholesterol homeostasis and amyloid precursor protein processing. Biochim Biophys Acta 2010, 1801: 853–859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Puglielli L, Tanzi RE, Kovacs DM. Alzheimer's disease: the cholesterol connection. Nat Neurosci 2003, 6: 345–351. [DOI] [PubMed] [Google Scholar]
- 37.Wolozin B. Cholesterol and the biology of Alzheimer's disease. Neuron 2004, 41: 7–10. [DOI] [PubMed] [Google Scholar]
- 38.Bhattacharyya R, Kovacs DM. ACAT inhibition and amyloid beta reduction. Biochim Biophys Acta 2010, 8: 960–965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Huttunen HJ, Kovacs DM. ACAT as a drug target for Alzheimer's disease. Neurodegener Dis 2008, 5: 212–214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Puglielli L, Konopka G, Pack-Chung E, Ingano LA, Berezovska O, Hyman BT, Chang TY et al. . Acyl-coenzyme A:cholesterol acyltransferase modulates the generation of the amyloid beta-peptide. Nat Cell Biol 2001, 3: 905–912. [DOI] [PubMed] [Google Scholar]
- 41.Huttunen HJ, Greco C, Kovacs DM. Knockdown of ACAT-1 reduces amyloidogenic processing of APP. FEBS Lett 2007, 581: 1681–1692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Plácido AI, Pereira CM, Duarte AI, Candeias E, Correia SC, Santos RX, Carvalho C et al. . The role of endoplasmic reticulum in amyloid precursor protein processing and trafficking: implications for Alzheimer's disease. Biochim Biophys Acta 2014, 1842: 1444–1453. [DOI] [PubMed] [Google Scholar]
- 43.Parvathy S, Hussain I, Karran EH, Turner AJ, Hooper NM. Cleavage of Alzheimer's amyloid precursor protein by alpha-secretase occurs at the surface of neuronal cells. Biochemistry 1999, 38: 9728–9734. [DOI] [PubMed] [Google Scholar]
- 44.Goldstein JL, Basu SK, Brown MS. Receptor-mediated endocytosis of low-density lipoprotein in cultured cells. Methods Enzymol 1983, 98: 241–260. [DOI] [PubMed] [Google Scholar]
- 45.Havel RJ, Eder HA, Bragdon JH. The distribution and chemical composition of ultracentrifugally separated lipoproteins in human serum. J Clin Invest 1955, 34: 1345–1353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Lange Y, Ramos BV. Analysis of the distribution of cholesterol in the intact cell. J Biol Chem 1983, 258: 15130–15134. [PubMed] [Google Scholar]
- 47.Brasaemle DL, Attie AD. Rapid intracellular transport of LDL-derived cholesterol to the plasma membrane in cultured fibroblasts. J Lipid Res 1990, 31: 103–112. [PubMed] [Google Scholar]
- 48.Rothblat GH, de la Llera-Moya M, Atger V, Kellner-Weibel G, Williams DL, Phillips MC. Cell cholesterol efflux: integration of old and new observations provides new insights. J Lipid Res 1999, 40: 781–796. [PubMed] [Google Scholar]
- 49.Blanchette-Mackie EJ. Intracellular cholesterol trafficking: role of the NPC1 protein. Biochim Biophys Acta 2000, 1486: 171–183. [DOI] [PubMed] [Google Scholar]
- 50.Sparrow SM, Carter JM, Ridgway ND, Cook HW, Byers DM. U18666A inhibits intracellular cholesterol transport and neurotransmitter release in human neuroblastoma cells. Neurochem Res 1999, 24: 69–77. [DOI] [PubMed] [Google Scholar]
- 51.Huttunen HJ, Peach C, Bhattacharyya R, Barren C, Pettingell W, Hutter-Paier B, Windisch M et al. . Inhibition of acyl-coenzyme A:cholesterol acyl transferase modulates amyloid precursor protein trafficking in the early secretory pathway. FASEB J 2009, 23: 3819–3828. [DOI] [PMC free article] [PubMed] [Google Scholar]