ABSTRACT
The ileal lesions of Crohn’s disease (CD) patients are colonized by adherent-invasive Escherichia coli (AIEC) bacteria. These bacteria adhere to mannose residues expressed by CEACAM6 on host cells in a type 1 pilus-dependent manner. In this study, we investigated different antagonists of FimH, the adhesin of type 1 pili, for their ability to block AIEC adhesion to intestinal epithelial cells (IEC). Monovalent and multivalent derivatives of n-heptyl α-d-mannoside (HM), a nanomolar antagonist of FimH, were tested in vitro in IEC infected with the AIEC LF82 strain and in vivo by oral administration to CEACAM6-expressing mice infected with LF82 bacteria. In vitro, multivalent derivatives were more potent than the monovalent derivatives, with a gain of efficacy superior to their valencies, probably owing to their ability to form bacterial aggregates. Of note, HM and the multi-HM glycoconjugates exhibited lower efficacy in vivo in decreasing LF82 gut colonization. Interestingly, HM analogues functionalized with an isopropylamide (1A-HM) or β-cyclodextrin pharmacophore at the end of the heptyl tail (1CD-HM) exerted beneficial effects in vivo. These two compounds strongly decreased the amount of LF82 bacteria in the feces of mice and that of bacteria associated with the gut mucosa when administered orally at a dose of 10 mg/kg of body weight after infection. Importantly, signs of colitis and intestinal inflammation induced by LF82 infection were also prevented. These results highlight the potential of the antiadhesive compounds to treat CD patients abnormally colonized by AIEC bacteria and point to an alternative to the current approach focusing on blocking proinflammatory mediators.
IMPORTANCE
Current treatments for Crohn’s disease (CD), including immunosuppressive agents, anti-tumor necrosis factor alpha (anti-TNF-α) and anti-integrin antibodies, focus on the symptoms but not on the cause of the disease. Adherent-invasive Escherichia coli (AIEC) bacteria abnormally colonize the ileal mucosa of CD patients via the interaction of the mannose-specific adhesin FimH of type 1 pili with CEACAM6 mannosylated proteins expressed on the epithelial cell surface. Thus, we decided to develop an antiadhesive strategy based on synthetic FimH antagonists specifically targeting AIEC bacteria that would decrease intestinal inflammation. Heptylmannoside (HM)-based glycocompounds strongly inhibit AIEC adhesion to intestinal epithelial cells in vitro. The antiadhesive effect of two of these compounds of relatively simple chemical structure was also observed in vivo in AIEC-infected CEACAM6-expressing mice and was associated with a reduction in the signs of colitis. These results suggest a new therapeutic approach for CD patients colonized by AIEC bacteria, based on the development of synthetic FimH antagonists.
INTRODUCTION
Crohn’s disease (CD) is a chronic and commonly disabling inflammatory disorder of the intestine in which dysfunction of the immune response to gut microbiota occurs in the context of the host genetic predisposition. An altered gut microbiota has long been suspected to play an important part in the pathogenesis of CD. The evidence that enteric bacterial antigens continuously drive chronic, immune-mediated colitis and ileitis is provided by rodent models of spontaneous or induced intestinal inflammation (1).
A specific pathogenic group of Escherichia coli, called adherent-invasive E. coli (AIEC), has been extensively implicated in CD. AIEC bacteria strongly adhere to and invade intestinal epithelial cells (IEC), inducing inflammatory cytokine secretion (2). AIEC bacteria survive and replicate inside macrophages, induce extensive secretion of tumor necrosis factor alpha (TNF-α), and promote granuloma formation in vitro (3–5). AIEC bacteria express type 1 pili that can bind to host adhesion receptor CEACAM6 (carcinoembryonic antigen-related cell adhesion molecule 6) (6). The FimH adhesin located at the tip of type 1 fimbriae binds to oligomannosides displayed on this glycoprotein. CEACAM6 has been shown to be overexpressed in ileal tissue from CD patients than in ileal tissue from healthy controls, and the level of expression increased after gamma interferon (IFN-γ) or TNF-α stimulation and was upregulated by AIEC themselves (6). In transgenic CEABAC10 mice expressing human CEACAMs, an in vivo model reproducing the high expression of CEACAM6 reported in CD patients, the AIEC reference strain LF82 induced the development of severe clinical symptoms of colitis in a type 1 pili-dependent manner (7, 8). Analysis of the AIEC genome revealed the presence of pathoadaptive mutations in some genes or bacterial DNA sequences that could participate in AIEC pathogenicity in a susceptible host (9, 10). Recently acquired nonsynonymous substitutions have been reported in FimH expressed by AIEC strains, conferring on them greater adhesion ability (11).
Therapeutic strategies to impair AIEC adhesion to the gut mucosa, based on the development of FimH antagonists, should be considered for CD treatment. Synthetic mannosides have been developed for the treatment of urinary tract infections, with promising antiadhesive properties (12–17). One of the most potent antagonists of the FimH adhesin is the n-heptyl α-d-mannoside (HM) (18), which reduced the bacterial load in vivo in a murine cystitis model (19). Interestingly, compounds harboring multiple copies of HM exhibited stronger inhibitory properties than expected according to their valency, when assessed against the uropathogenic E. coli strain UTI89 (20, 21). Multivalent HM-based polymers of high valencies also exhibited excellent antiadhesive potencies against AIEC bacteria in vitro and ex vivo (22). This multivalency effect could be explained by the potency of the compounds to form bacterial aggregates (21).
Here we investigated the ability of monovalent HM or HM grafted on multi- and polymeric structures to inhibit AIEC LF82 adhesion to IEC and to decrease LF82 colonization in the CEABAC10 transgenic mouse model. HM was selected as the FimH binding motif because of its nanomolar affinity for the adhesin and its relatively simple chemical structure compared to previously described FimH antagonists. To evaluate possible multivalent effects in vivo, we selected our previously reported β-cyclodextrin-based 7-valent HM (named 7CD-HM [23]) and the polymeric 188-valent HM (named 188P-HM [22]) for the study. To accurately assess the potential effect of multivalency, 1CD-HM and 1A-HM, the two monovalent analogues of 7CD-HM and 188P-HM, were included in the assay. We demonstrated that HM derivatives are effective in inhibiting LF82 adhesion and that multivalency improved these inhibition properties in vitro. Interestingly, the monovalent mannosides 1A-HM and 1CD-HM exerted beneficial antiadhesive effects in vivo in the CEABAC10 mouse model, and AIEC elimination of the gut was accompanied by a decrease in intestinal inflammation. To eliminate AIEC bacteria from the gut and to decrease and/or prevent intestinal inflammation, optimized mannosides selected from this study represent promising lead candidates for the development of an antiadhesive strategy in CD patients.
RESULTS
Monovalent heptylmannosides and multivalent heptylmannosides exhibited strong antiadhesive effects on AIEC LF82 adhesion to intestinal epithelial cells.
HM, the monovalent derivative named 1A-HM, the β-cyclodextrin grafted with a single copy of HM named 1CD-HM and two multivalent derivatives named 7CD-HM, a β-cyclodextrin grafted with seven copies of HM, and 188P-HM, a polymethacrylamide having (on average) 188 pendant HM ligands, were assessed for their ability to inhibit AIEC LF82 adhesion to IEC T84 cells, expressing a high level of CEACAM6. In the preincubation protocol to estimate their antiadhesive potential in a prophylactic approach, the mannosides and LF82 bacteria were incubated together, before infection of IEC (Fig. 1A). In the postincubation protocol to assess their potential to disrupt a preestablished bacterial adhesion, LF82 bacteria were first incubated with T84 cells for a 3-h period before treatment with compounds (Fig. 1B). HM and 1A-HM exerted dose-dependent inhibitory effects on the ability of LF82 bacteria to adhere to T84 cells in both pre- and postincubation experiments (Fig. 1A and B). The postincubation assays showed that HM and 1A-HM have the ability to disrupt a preestablished adhesion of bacteria to their host receptor from the concentration of 10 µM. A similar decrease in bacterial adhesion was obtained with the monovalent β-cyclodextrin-based compound 1CD-HM at the higher concentration of 100 µM. Thus, 1CD-HM was 10-fold less potent in inhibiting bacterial adhesion than HM and 1A-HM. We next determined the β factor, a value frequently used to quantify a multivalent effect in glycocluster-lectin interactions. This value can be directly obtained by dividing the HM ligand concentration in solution from the multivalent structure of interest by the concentration of the monovalent HM reference required to give a similar inhibitory effect. As multivalent 7CD-HM and polyvalent 188P-HM were tested at equivalent molarity (Fig. 1), the valencies had to be corrected by a factor of 7 for 7CD-HM and by 188 for 188P-HM to determine the β factor. Multivalent derivative 7CD-HM significantly decreased AIEC LF82 adhesion at a concentration 100-fold lower than that of the monovalent analogue 1CD-HM, in the pre- and postincubation protocols, corresponding to a β factor of 14. Finally, polymer 188P-HM decreased bacterial adhesion down to 22% at the very low concentration of 1 nM in the preincubation assay, corresponding to a gain of 10,000-fold in comparison with the residual adhesion of 35.3% in the presence of a 10 µM concentration of 1A-HM compound (β factor of 53). This multivalent inhibitory effect was also observed in postincubation experiments, since 188P-HM was able to detach 80% of the adherent bacteria at a concentration of 100 nM. In contrast, a 1,000-fold-higher concentration of 1A-HM is required to obtain a similar effect (β factor of 5). Thus, these results confirmed that clustering HM in multiple copies to a synthetic scaffold significantly enhances the in vitro antiadhesive potential of the FimH antagonists.
FIG 1 .
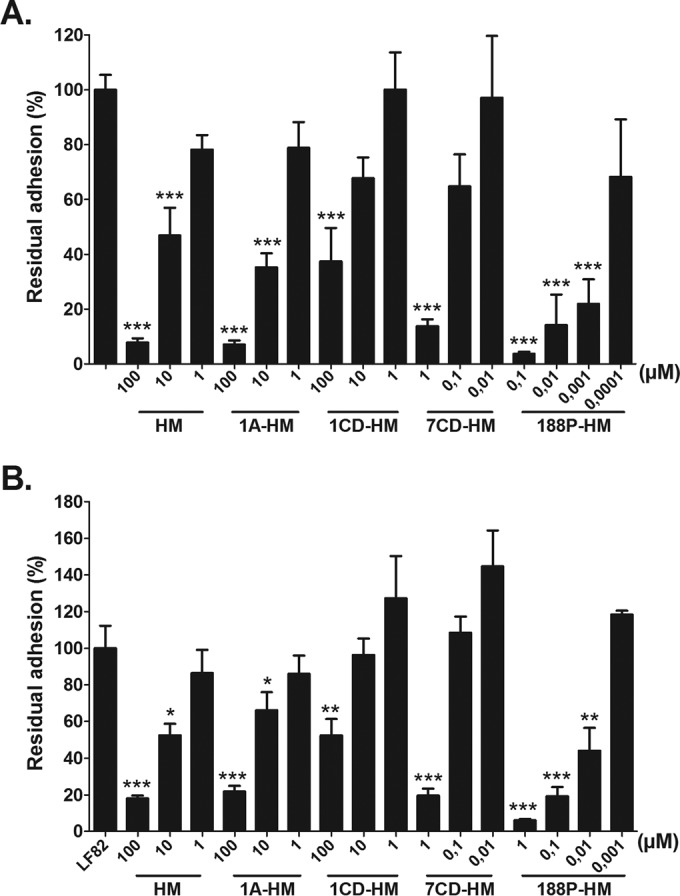
Effects of the monovalent and multivalent HM-based glycocompounds on the ability of the LF82 strain to adhere to intestinal epithelial cells T84. Cells were infected at a multiplicity of infection of 10 bacteria per cell for a 3-h period. Monovalent compounds HM, 1A-HM, and 1CD-HM and multivalent compounds 7CD-HM and 188P-HM were tested at concentrations ranging from 0.001 to 100 µM. (A) Preincubation experiment. The inhibitors were coincubated with AIEC LF82 strain 1 h before bacterial infection of T84 cells. (B) Postincubation experiment. The cells were infected with LF82 bacteria for 3 h, and then the inhibitors were added. Results are expressed in percentages of associated bacteria to the cells. The values in both panels are means plus SEM (error bars) of four to six experiments. Values that are significantly different from the control value (100%) are indicated by asterisks as follows: *, P < 0.05; **, P < 0.01; ***, P < 0.001. LF82 infection in the absence of treatment was normalized at 100%.
HM failed to improve the symptoms of colitis in CEACAM6-expressing mice infected with LF82 bacteria.
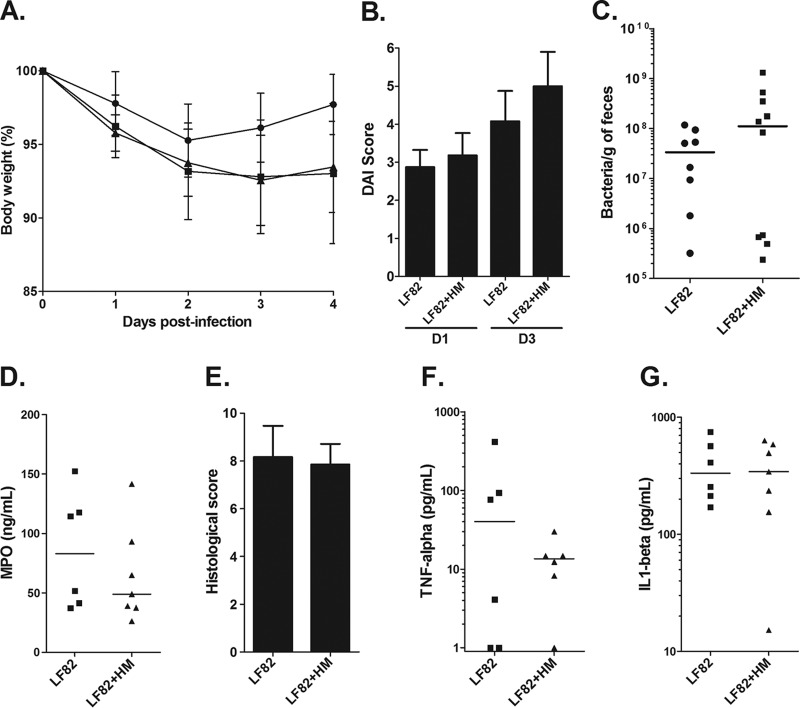
To mimic a curative treatment, effect of the reference FimH antagonist HM was evaluated in vivo in CEABAC10 transgenic mice by oral administration 2 h and 18 h after LF82 infection of mice at a dose of 10 mg/kg. The body weight and disease activity index (DAI) score of mice treated with HM were similar to those of the nontreated infected mice (Fig. 2A and B). LF82 bacterial clearance from the gastrointestinal tracts of mice was investigated by enumerating bacteria in the feces. In this experimental condition, HM treatment failed to prevent AIEC LF82 colonization (Fig. 2C). At day 4 postinfection, the colons were removed from the mice to assess mucosal inflammation and damage. HM treatment did not modulate the myeloperoxidase (MPO) concentration in the intestinal mucosa, did not ameliorate the histological score, and did not decrease production of proinflammatory cytokines (TNF-α and interleukin 1β [IL-1β]) in comparison with nontreated mice, which means that intestinal inflammation was not prevented by the mannoside administration (Fig. 2D to aG). The absence of an effect of HM on the symptoms of colitis can be correlated with the lack of antiadhesive effect of this compound in vivo. Thus, we decided to investigate the ability of monovalent and multivalent HM analogues to prevent AIEC colonization in vivo.
FIG 2 .
Effects of HM oral treatment in CEABAC10 mice on LF82 gut colonization and on the severity of colitis induced by LF82 bacteria. CEABAC10 mice were infected on day 0 with 3 × 109 LF82 bacteria and treated with mannosides (10 mg/kg) 2 h and 18 h after LF82 infection. (A) Change in body weight as a percentage (mean ± SEM). (B) Assessment of the disease activity index (DAI) score on days 1 (D1) and 3 (D3) postinfection (mean plus SEM). (C) Quantification of LF82 bacteria in the feces of LF82-infected CEABAC10 mice on day 4 postinfection. Each symbol represents the value for an individual mouse. Each horizontal bar represents the median value for a group of mice (seven or eight mice in a group). (D) MPO concentration in colonic tissues was determined by an ELISA. (E) Histological score (mean plus SEM) of colonic mucosa. (F and G) TNF-α and IL-1β cytokines released by proximal colon specimens were measured by ELISAs. Horizontal bars represent medians (seven or eight mice in a group).
Monovalent heptylmannosides 1A-HM and 1CD-HM exhibit strong antiadhesive properties in LF82-infected CEABAC10 mice.
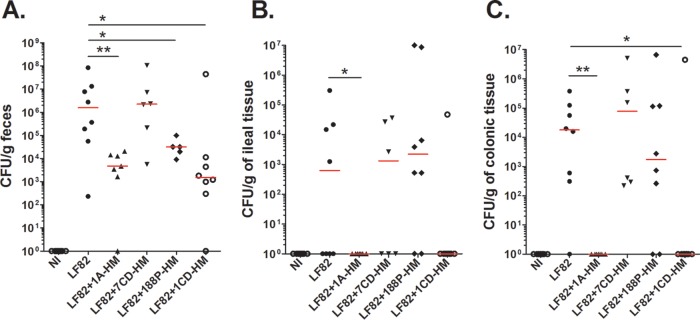
The bacterial antiadhesive potential of the monovalent mannoside 1A-HM, of the HM-grafted cyclodextrins 1CD-HM and 7CD-HM, and of the HM-grafted polymer 188P-HM, was assessed in AIEC LF82-infected CEABAC10 mice, according to an experimental design similar to that previously used for HM treatment. The monovalent derivatives 1A-HM and 1CD-HM were particularly effective in eliminating AIEC bacteria from the gut, as quantified in the feces and in the ileal and colonic tissues (Fig. 3). Indeed, a significant decrease (around 3 log units) in the amount of LF82 bacteria in the feces was observed at 4 days postinfection in groups of mice treated with the two monovalent compounds 1A-HM and 1CD-HM (Fig. 3A). In these two groups, the number of bacteria associated with the intestinal mucosa was below the detection level, indicating that 1A-HM and 1CD-HM were able to effectively eradicate bacteria from the gut at day 4 postinfection (Fig. 3B and C). 188P-HM treatment also decreased the bacterial load in the feces, but the antiadhesive properties of the polymer were less effective against AIEC LF82 bacteria associated with the intestinal tissues. Finally, no effect was observed on LF82 colonization with the heptavalent cyclodextrin 7CD-HM. Although multivalency gave a strong advantage to HM-based ligands in vitro, in vivo experiments revealed that multivalent compounds were less potent than their monovalent reference compounds in eliminating LF82 bacteria from the guts of CEABAC10 mice in these experimental conditions.
FIG 3 .
Effects of HM-based analogues (oral treatment) in CEABAC10 mice on LF82 gut colonization. CEABAC10 mice were infected on day 0 with 3 × 109 LF82 bacteria and treated with mannosides (10 mg/kg) 2 h and 18 h after LF82 infection. (A to C) Quantification of LF82 bacteria in the feces (A), in the ileal mucosa (B), and in the colonic mucosa (C) of LF82-infected CEABAC10 mice on day 4 postinfection. Each value represents the value for an individual mouse. Each horizontal bar represents the median for a group of mice (seven or eight mice in each group). Values that are significantly different are indicated by asterisks and bar as follows: *, P < 0.05; **, P < 0.01. NI, not infected (control).
Synthetic heptylmannosides decreased the severity of colitis in LF82-infected CEABAC10 mice.
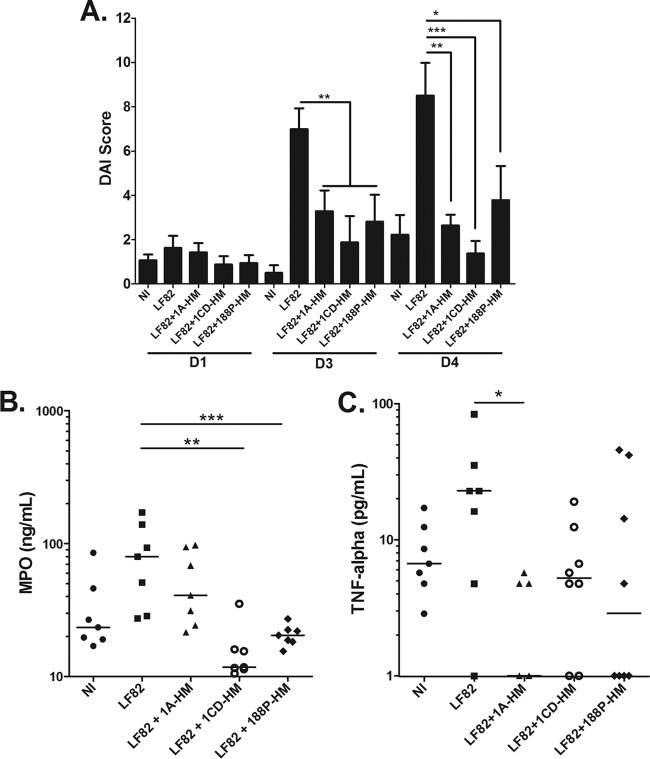
The effect of the oral treatment of LF82-infected CEABAC10 mice with the three glycocompounds showing in vivo antiadhesive properties (1A-HM, 1CD-HM, and 188P-HM) was then assessed on the symptoms of colitis. The DAI scores indicated that the three HM conjugates tested were effective in decreasing the signs of colitis from day 3 postinfection (Fig. 4A). These mannosides decreased neutrophil infiltration in the colon, as demonstrated by MPO quantification in the colonic tissues at day 4 postinfection (Fig. 4B). With regard to proinflammatory cytokines, all compounds succeeded in inhibiting TNF-α secretion induced by AIEC LF82 bacterial infection, with 1A-HM treatment being the most potent (Fig. 4C).
FIG 4 .
Effects of HM-based analogues (oral treatment) in CEABAC10 mice on the severity of colitis and intestinal inflammation. (A) Assessment of the DAI score on days 1, 3, and 4 postinfection (mean plus SEM). (B) MPO concentration in colonic tissues determined by an ELISA. (C) TNF-α cytokines released by proximal colon specimens measured by an ELISA. In panels B and C, each value represents the value for an individual mouse, and each horizontal bar represents the median for a group of mice (seven or eight mice in each group). Values that are significantly different are indicated by asterisks and bar as follows: *, P < 0.05; **, P < 0.01; ***, P < 0,001). NI, not infected (control).
DISCUSSION
Mannose-binding type 1 pili are crucial virulence factors for the establishment of AIEC colonization in the guts of patients with CD (23). To select molecules with the best antiadhesive properties, we have used two complementary approaches, in vitro assay of AIEC LF82 infection using IEC and in vivo LF82 infection in CEABAC10 transgenic mice. This method led to the characterization of two candidates of interest, 1A-HM and 1CD-HM, for an antiadhesive therapy in CD patients abnormally colonized by AIEC bacteria.
To date, HM is one of the best monomeric mannose-based inhibitors developed against FimH and has a nanomolar dissociation equilibrium constant for this target (18). The heptyl chain of HM strongly enhances FimH affinity compared to d-mannose, likely due to hydrophobic interactions with two tyrosines and one isoleucine at the entrance of the FimH binding site, the so-called tyrosine gate (18). HM was shown to prevent uropathogenic Escherichia coli adhesion to human bladder cells in culture at submicromolar concentrations. However, high concentrations (5 mM) of this compound were needed to reduce in vivo adhesion of these bacteria 10-fold in a model of murine cystitis (19). In the present study, we showed that HM effectively prevented AIEC LF82 adhesion and detached adherent LF82 bacteria from T84 cells at low concentrations of 1 and 10 µM, respectively, but failed to achieve any real effect in vivo in the CEABAC10 mouse model. The lack of antiadhesive effect of HM in vivo could be due to an inadequate pharmacodistribution of the compound, which may not have access to mucosa-associated bacteria in the gut. We hypothesize that the aliphatic chain of HM produces nonspecific interaction in the gut in vivo, thereby impairing its potential beneficial effect. Indeed, alkyl compounds can be used to increase absorption of therapeutic molecules (24) and can increase paracellular permeability of monolayers of Caco-2 cells in vitro (25–27), which suggests that they compromise intestinal barrier integrity.
Hence, HM monovalent or multivalent analogues represent an interesting strategy with the shielding of the end of the 7-carbon alkyl tail either with an isopropylamide function or with a common scaffold such as a cyclodextrin or linear polymer. Capping with polar groups could prevent nonspecific hydrophobic interactions with cell membranes while retaining a similar affinity for FimH (28). Furthermore, cyclodextrins are hydrophilic molecules of high molecular weight, and it is unlikely that they are absorbed in the intestine (29). Thus, their use as the organic scaffold for antiadhesive presentation and delivery should represent an advantage for treatment at an ileal site of action, which is the preferential site of AIEC colonization in CD. High synergistic effects were observed in vitro when HM ligands were multi- or polymerized on organic scaffolds. Indeed, HM ligands in 7CD-HM and 188P-HM are more effective than the corresponding monovalent reference compounds 1CD-HM and 1A-HM to prevent and disrupt AIEC bacterial adhesion to IEC in culture. This is in full accordance with our previous in vitro and in vivo results, which showed the greater potential of the multi-HM in disrupting adhesion of the uropathogenic E. coli strain UTI89 to bladder cells (20, 21, 30). This enhancing effect could be due either to a stronger affinity of the polyvalent multi-HM for the FimH protein or to a superior avidity of these inhibitors to multiple type 1 pili on the same bacterial cell, or it may reflect the potency of the compounds to form bacterial aggregates, as previously shown by dynamic light scattering and microscopy with E. coli strain UTI89 (22, 30). Bacterial clusters are formed by the simultaneous interaction of several HM ligands from the compound with FimH adhesins displayed by different bacteria, which allows the formation of a cross-linked network between the bacteria and ligands. Of note, monovalent cyclodextrin 1CD-HM was 10-fold less effective than HM and 1A-HM in inhibiting LF82 adhesion. This loss of inhibitory activity could be explained by the lower intrinsic affinity of 1CD-HM for FimH, as previously shown by microcalorimetry measurements (30).
The host/bacteria cross talk in host susceptibility to CD can be mimicked using CEABAC10 transgenic mice expressing human CEACAM6 receptor (8). In this CEABAC10 mouse model, AIEC bacteria colonize the gut, inducing colitis in a type 1 pilus-dependent manner (7). Previously, we demonstrated that a probiotic treatment, based on the use of Saccharomyces cerevisiae yeast in a prophylactic protocol, accelerated AIEC LF82 bacterial eradication from the guts of CEABAC10 mice, preventing the subsequent disruption of intestinal barrier function and colitis (31). As synthetic mannosides have the ability to efficiently disrupt preestablished AIEC LF82 adhesion to T84 cells, we assessed whether mannosides administered orally to AIEC LF82-infected CEABAC10 mice caused mucosa-associated AIEC to become detached. Bacterial colonization and the severity of colitis were decreased by 188P-HM multimeric mannosides, but to a lower extent than with the monovalent molecules 1A-HM and 1CD-HM. The fact that 7CD-HM failed to demonstrate any antiadhesive effect in this study is striking, considering that this compound was shown to be 100-fold more potent than 1CD-HM in reducing adhesion and invasion levels of the uropathogenic E. coli UTI89 strain in the bladder in a murine cystitis model (30). This discrepancy could be explained by differences in the mode of administration. In the cystitis model, 7CD-HM was coinstilled with the bacteria into the bladder, whereas in the present study, the compound was orally administered. This clearly illustrates that both the mode of administration and the bioavailability of the compounds are important factors to consider when setting up an antiadhesive therapy. Finally, 1A-HM and 1CD-HM treatments exerted the strongest beneficial effects on AIEC-infected mice in decreasing LF82 bacterial gut colonization, colitis severity and intestinal inflammation.
To conclude, this study led to the characterization of two candidates of therapeutic interest, 1A-HM and 1CD-HM, and revealed the potential of the FimH antagonists to reverse a preestablished colonization of AIEC bacteria in the gut of a mouse model mimicking CD susceptibility. Importantly, bacterial clearance led to a decrease in the inflammatory response. Our study opens new avenues for personalized therapies in which FimH antagonists could be a valuable alternative for CD patients abnormally colonized by AIEC bacteria.
MATERIALS AND METHODS
Synthesis of compounds HM (18), 1CD-HM and 7CD-HM (30), and 188P-HM (22) was previously described.
Chemistry. (i) Chemical synthesis of 1A-HM.
1A-HM was prepared from the previously described 1-azidoheptyl-mannoside, compound 1 (32), by a one-pot Staudinger-amide coupling with isobutyric acid to form acetyl-protected compound 2. Methanolysis of the acetate groups under Zemplén conditions led to 1A-HM (Fig. 5), according to the following experimental protocols.
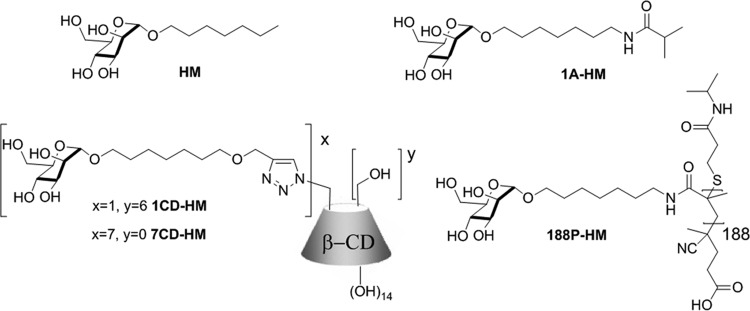
FIG 5 .
Structures of HM-based mannosides assessed for antiadhesive properties against the AIEC reference strain LF82. HM, 1A-HM (this study), 1CD-HM and 7CD-HM, and 188P-HM.
(ii) Compound 2.
Compound 1 (50 mg, 0.099 mmol) and isobutyric acid (17 µl, 0.178 mmol, 1.8 equivalents), were combined with hydroxybenzotriazole (HOBt) (24 mg, 0.178 mmol, 1.8 equivalents) in a flask and dried for 1 h in vacuo. This mixture was dissolved in dry tetrahydrofuran (THF) (2.5 ml) under nitrogen and cooled to 0°C. Then, 1,3-diisopropylcarbodiimide (DIC) (28 µl, 0.178 mmol, 1.8 equivalents) was then added, and the solution was stirred for 10 min, followed by the addition of triphenylphosphine (Ph3P) (47 mg, 0.178 mmol, 1.8 equivalents). The mixture was stirred for 1 h at 0°C, then stirred overnight at room temperature, diluted with water, and extracted twice with ethyl acetate (EtOAc). The combined organic phases were washed with brine, dried with MgSO4, filtered, and concentrated under reduced pressure. The crude product was purified by silica gel column chromatography (EtOAc/petroleum ether, 70:30 → EtOAc as eluents) to obtain amide 2 (42 mg, 0.079 mmol, 80%) as an oil.
[α]D = +71 (c = 0.81 in CHCl3); 1H nuclear magnetic resonance (NMR) (300 MHz, CDCl3): δ = 1.12 (6H, d, J = 6.9 Hz, 2 × CH3-isobutyric), 1.28 to 1.59 (1H, m), 1.97 (3H, s, acetoxy group [AcO]), 2.02 (3H, s, AcO), 2.08 (3H, s, AcO), 2.13 (3H, s, AcO), 2.31 (1H, m, CH, isobutyric), 3.21 (2H, m, H-7′), 3.41 (1H, m, H-1′a), 3.65 (1H, m, H-1′b), 3.95 (1H, ddd, J5,4 = 9.5 Hz, J5,6b = 5.3 Hz, J5,6a = 2.5 Hz, H-5), 4.10 (1H, dd, J6a,6b = 12.2 Hz, J6a,5 = 2.5 Hz, H-6a), 4.25 (1H, dd, J6b,6a = 12.2 Hz, J6b,5 = 5.3 Hz, H-6a), 4.77 (1H, d, J1,2 = 1.7 Hz, H-1), 5.20 (1H, dd, J2,3 = 3.3 Hz, J2,1 = 1.7 Hz, H-2), 5.24 (1H, dd, J4,3 = 10.1 Hz, J4,5 = 9.6 Hz, H-4), 5.32 (1H, dd, J3,4 = 10.1 Hz, J3,2 = 3.3 Hz, H-3), 5.60 (1H, bs, NH); 13C NMR (100.6 MHz, CDCl3): δ = 19.6 (2 × CH3, isobutyric), 20.6 (2 × CH3, 2 × AcO), 20.7 (CH3, AcO), 20.8 (CH3, AcO), 25.8 (CH2), 26.6 (CH2), 28.8 (CH2), 29.0 (CH2), 29.5 (CH2), 35.6 (CH, isobutyric), 39.2 (CH2, C-7′), 62.5 (CH, C-6), 66.2 (CH), 68.3 (CH, CH2, C-5, C-1′), 69.1 (CH), 69.6 (CH, C-2), 97.5 (CH, C-1), 169.7 (C, AcO), 169.9 (C, AcO), 170.1 (C, AcO), 170.6 (C, AcO), 176.8 (C, amide); high-resolution mass spectrometry (HRMS) (matrix-assisted laser desorption ionization [MALDI], 2,5-dihydrobenzoic acid [DHB]): m/z calculated for C25H42O11N [M + H]+: 532.2752, found: 532.2743.
(iii) Compound 1A-HM (Fig. 1).
The protected glycosyl amide 2 (81 mg, 0.128 mmol) was dissolved in dry methanol (MeOH) (30 ml) after which sodium methoxide (20 µl, 1 M solution in MeOH) was added. The mixture was stirred for 4 h, neutralized with Amberlite IR120 (H) and filtered, and the solvents were evaporated to dryness. 1A-HM was obtained after lyophilization (45 mg, 0.124 mmol, 97%) as an amorphous white solid.
[α]D = +48.1 (c = 1.32 in MeOH); 1H NMR (300 MHz, deuterated methanol [CD3OD]): δ = 1.10 (6H, d, J = 6.9 Hz, 2 × CH3-isobutyric), 1.29-1.64 (10 H, m), 2.42 (1H, m, CH-isobutyric), 3.15 (2H, t, J = 6.9 Hz, C-7′), 3.41 (1H, m, H-1a′), 3.52 (1H, ddd, J5,4 = 9.2 Hz, J5,6b = 5.6 Hz, J5,6a = 2.4 Hz, H-5), 3.61 (1H, dd, J4,3 = 9.4 Hz, J4,5 = 9.2 Hz, H-4), 3.67-3.75 (3H, m), 3.78 (1H, dd, J2,3 = 3.3 Hz, J2,1 = 1.7 Hz, H-2), 3.82 (1H, dd, J6b,6a = 11.9 Hz, J6b,5 = 2.5 Hz, H-6b), 4.73 (1H, d, J1,2 = 1.7 Hz, H-1), 13C NMR (100.6 MHz, MeOD): δ = 20.0 (2 × CH3, 2 × CH3-isobutyric), 27.2 (CH2), 27.8 (CH2), 30.1 (CH2), 30.4 (CH2), 30.5 (CH2), 36.3 (CH, CH-isobutyric), 40.2 (CH2, C-7′), 62.9 (CH, C-6), 68.5 (CH), 68.6 (CH), 72.2 (CH, C-5), 72.6 (CH, C-1′), 74.6 (CH, C-2), 101.5 (CH, C-1), 180.0 (C, amide); HRMS (MALDI, DHB): m/z calculated for C17H33N1O7Na [M + Na]+: 386.2155, found: 386.2155.
The other derivatives HM, 7CD-HM, 1CD-HM, and 188P-HM were prepared as previously described (18, 22, 30, 33, 34).
Bacterial strain and intestinal epithelial cells.
The ampicillin-erythromycin-resistant adherent-invasive E. coli (AIEC) strain LF82 isolated from an ileal biopsy specimen of a CD patient was used as the AIEC reference strain (23). Bacteria were routinely grown at 37°C in Luria-Bertani (LB) broth or on LB agar overnight.
Human intestinal epithelial cell line T84 was obtained from the American Type Culture Collection (ATCC) and was maintained in an atmosphere containing 5% CO2 at 37°C in the culture medium recommended by ATCC.
Adhesion assays of AIEC LF82 strain on intestinal epithelial cells T84 in the presence of synthetic mannosides.
Intestinal epithelial cells T84 were seeded in 48-well tissue culture plates at a density of 1.5 × 105 cells/well and incubated at 37°C for 48 h. For the preincubation protocol, AIEC LF82 bacteria were incubated for 1 h with HM derivatives at final concentrations within the cells of between 0.0001 and 100 µM, depending on the compounds. The cells were then infected with the bacterium/mannoside mixture at a multiplicity of infection (MOI) of 10 bacteria per cell for 3 h. The monolayers were washed three times with phosphate-buffered saline (PBS) and lysed with 1% Triton X-100 (Sigma) in deionized water. The samples were diluted and plated onto LB agar plates to determine the number of CFU. For the postincubation protocol, cells were infected with AIEC LF82 bacteria at an MOI of 10 for 3 h, then monolayers were extensively washed with PBS, and inhibitory compounds were added onto the cells for an additional 3-h period at final concentrations between 0.001 and 100 µM, depending on the compounds.
Mouse infection experiments and ethics statement.
Mice were housed in specific-pathogen-free conditions in the animal care facility of the University of Auvergne (Clermont-Ferrand, France). FVB/N wild-type mice, provided by Charles Rivers Laboratories France, and FVB/N CEABAC10 transgenic mice (heterozygote [8]) mated in our animal care facility. The animal protocols used in this study were approved by the CEMEA Auvergne committee for ethical issues (00730.02).
The infection protocol of CEABAC10 mice with AIEC LF82 bacteria for assessment of colonization and signs of colitis was performed as previously described with some modifications (7). Briefly, 7- to 8-week-old FVB/N CEABAC10 transgenic mice were given dextran sulfate sodium (DSS) (molecular mass of 36,000 to 50,000 Da; MP Biomedicals) at 0.5% in drinking water, starting 3 days before infection. Twenty-four hours before infection, mice were orally given 5 mg of the broad-spectrum antibiotic streptomycin (Euromedex) to disrupt normal resident bacterial flora in the intestinal tract. The mice were orally challenged with 3 × 109 CFU LF82 bacteria (day 0 of the experiment), 2 h after oral administration of cimetidine at 50 mg/kg of body weight to ablate gastric secretion. Mannosides were orally administered in a volume of 0.2 ml of PBS, 2 h and 18 h after LF82 infection at a dose of 10 mg/kg.
Colonization was assessed in fresh fecal samples collected at day 4 postinfection and suspended in sterile saline solution (NaCl [9‰]). Samples were diluted and plated onto LB agar containing 100 µg/ml ampicillin and 20 µg/ml erythromycin to select and enumerate LF82 bacteria. The bacterial colonization was expressed in the number of CFU per gram of feces. The severity of colitis was assessed by the disease activity index (DAI) score, which ranges from 0 (healthy) to 12 (high activity of colitis) (see Table S1 in the supplemental material).
Mice were anesthetized with isofluorane and then euthanized by cervical dislocation. LF82 bacteria associated with the colonic or ileal tissues were quantified on a 0.5-cm sample of tissue. Intestinal tissue was cut longitudinally, then washed in PBS, and homogenized in 1 ml of PBS. Samples were diluted and plated onto LB agar containing 100 µg/ml ampicillin and 20 µg/ml erythromycin. The amount of bacteria adherent to the intestine was expressed in the number of CFU per gram of tissue. For histological examinations, 4-µm sections of paraffin-embedded samples of colonic tissues were stained with hematoxylin-eosin-safranin (HES). Mucosal injuries were graded for the extent and depth of inflammation and the extent of crypt damage. A histological score was determined by veterinary histopathologists (ONIRIS, Nantes, France) in a blind manner (see Table S2 in the supplemental material). Assessment of neutrophil influx was performed on 0.5-cm samples of proximal colon tissues (50 mg/ml), thoroughly washed in PBS, and homogenized in 0.5% hexadecyltrimethylammonium bromide (Sigma). Myeloperoxidase (MPO) concentration was assayed in the supernatant by an enzyme-linked immunosorbent assay (ELISA) (R&D Systems). Quantification of the proinflammatory cytokines released (TNF-α and IL-1β) by the colonic mucosa was performed by ELISAs (R&D Systems).
Statistical analysis.
Data are expressed as means ± standard errors of the means (SEM) or as medians. Data were compared by Student’s t test analysis or nonparametric one-way analysis of variance Mann-Whitney test, when appropriate. Differences were significant when the P value was <0.05. Statistical analyses were performed with GraphPad Prism 6.0 (GraphPad Software, San Diego, CA) software package for personal computers (PC).
SUPPLEMENTAL MATERIAL
Disease activity index (DAI) score.
Histological grading of intestinal inflammation.
ACKNOWLEDGMENTS
We thank Abdelkrim Alloui for animal care (animal facilities, Clermont-Ferrand, France) and the CICS platform (Université Auvergne) for technical assistance with tissue preparation for microscopy analysis. We thank Laëtitia Dorso from LUNAM University, Oniris, PASAP (Plateforme d’Analyses et de Services en Anatomie Pathologique), CRIP (Centre de Recherche et d’Investigation Préclinique), Nantes, France for histological examinations. We thank Jeffrey Watts for help revising the English in the manuscript.
This study was supported by the Ministère de la Recherche et de la Technologie, INSERM (UMR INSERM Université d’Auvergne U1071), INRA (USC-2018), and by the French Agence Nationale de la Recherche (ANR Program Blanc STARLET, ANR-12-BSV5-0016-01).
Footnotes
Citation Sivignon A, Yan X, Alvarez Dorta D, Bonnet R, Bouckaert J, Fleury E, Bernard J, Gouin SG, Darfeuille-Michaud A, Barnich N. 2015. Development of heptylmannoside-based glycoconjugate antiadhesive compounds against adherent-invasive Escherichia coli bacteria associated with Crohn’s disease. mBio 6(6):e01298-15. doi:10.1128/mBio.01298-15.
REFERENCES
- 1.Kaser A, Zeissig S, Blumberg RS. 2010. Inflammatory bowel disease. Annu Rev Immunol 28:573–621. doi: 10.1146/annurev-immunol-030409-101225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Eaves-Pyles T, Allen CA, Taormina J, Swidsinski A, Tutt CB, Jezek GE, Islas-Islas M, Torres AG. 2008. Escherichia coli isolated from a Crohn’s disease patient adheres, invades, and induces inflammatory responses in polarized intestinal epithelial cells. Int J Med Microbiol 298:397–409. doi: 10.1016/j.ijmm.2007.05.011. [DOI] [PubMed] [Google Scholar]
- 3.Glasser AL, Boudeau J, Barnich N, Perruchot MH, Colombel JF, Darfeuille-Michaud A. 2001. Adherent invasive Escherichia coli strains from patients with Crohn’s disease survive and replicate within macrophages without inducing host cell death. Infect Immun 69:5529–5537. doi: 10.1128/IAI.69.9.5529-5537.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bringer MA, Glasser AL, Tung CH, Meresse S, Darfeuille-Michaud A. 2006. The Crohn’s disease-associated adherent-invasive Escherichia coli strain LF82 replicates in mature phagolysosomes within J774 macrophages. Cell Microbiol 8:471–484. doi: 10.1111/j.1462-5822.2005.00639.x. [DOI] [PubMed] [Google Scholar]
- 5.Meconi S, Vercellone A, Levillain F, Payré B, Al Saati T, Capilla F, Desreumaux P, Darfeuille-Michaud A, Altare F. 2007. Adherent-invasive Escherichia coli isolated from Crohn’s disease patients induce granulomas in vitro. Cell Microbiol 9:1252–1261. doi: 10.1111/j.1462-5822.2006.00868.x. [DOI] [PubMed] [Google Scholar]
- 6.Barnich N, Carvalho FA, Glasser AL, Darcha C, Jantscheff P, Allez M, Peeters H, Bommelaer G, Desreumaux P, Colombel JF, Darfeuille-Michaud A. 2007. CEACAM6 acts as a receptor for adherent-invasive E. coli, supporting ileal mucosa colonization in Crohn disease. J Clin Invest 117:1566–1574. doi: 10.1172/JCI30504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Carvalho FA, Barnich N, Sivignon A, Darcha C, Chan CHF, Stanners CP, Darfeuille-Michaud A. 2009. Crohn’s disease adherent-invasive Escherichia coli colonize and induce strong gut inflammation in transgenic mice expressing human CEACAM. J Exp Med 206:2179–2189. doi: 10.1084/jem.20090741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chan CH, Stanners CP. 2004. Novel mouse model for carcinoembryonic antigen-based therapy. Mol Ther 9:775–785. doi: 10.1016/j.ymthe.2004.03.009. [DOI] [PubMed] [Google Scholar]
- 9.Miquel S, Peyretaillade E, Claret L, de Vallée A, Dossat C, Vacherie B, Zineb EH, Segurens B, Barbe V, Sauvanet P, Neut C, Colombel JF, Medigue C, Mojica FJM, Peyret P, Bonnet R, Darfeuille-Michaud A. 2010. Complete genome sequence of Crohn’s disease-associated adherent-invasive E. coli strain LF82. PLoS One 5:pii:e1274. doi: 10.1371/journal.pone.0012714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Nash JH, Villegas A, Kropinski AM, Aguilar-Valenzuela R, Konczy P, Mascarenhas M, Ziebell K, Torres AG, Karmali MA, Coombes BK. 2010. Genome sequence of adherent-invasive Escherichia coli and comparative genomic analysis with other E. coli pathotypes. BMC Genomics 11:667. doi: 10.1186/1471-2164-11-667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Dreux N, Denizot J, Martinez-Medina M, Mellmann A, Billig M, Kisiela D, Chattopadhyay S, Sokurenko E, Neut C, Gower-Rousseau C, Colombel JF, Bonnet R, Darfeuille-Michaud A, Barnich N. 2013. Point mutations in FimH adhesin of Crohn’s disease-associated adherent-invasive Escherichia coli enhance intestinal inflammatory response. PLoS Pathog 9:e1003141. doi: 10.1371/journal.ppat.1003141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gouin SG, Roos G, Bouckaert J. 2014. Discovery and application of FimH antagonists, p 123–168. In Seeberger PH, Rademacher C (ed), Carbohydrates as drugs. Springer International, Cham, Switzerland. [Google Scholar]
- 13.Hartmann M, Papavlassopoulos H, Chandrasekaran V, Grabosch C, Beiroth F, Lindhorst TK, Röhl C. 2012. Inhibition of bacterial adhesion to live human cells: activity and cytotoxicity of synthetic mannosides. FEBS Lett 586:1459–1465. doi: 10.1016/j.febslet.2012.03.059. [DOI] [PubMed] [Google Scholar]
- 14.Han Z, Pinkner JS, Ford B, Obermann R, Nolan W, Wildman SA, Hobbs D, Ellenberger T, Cusumano CK, Hultgren SJ, Janetka JW. 2010. Structure-based drug design and optimization of mannoside bacterial FimH antagonists. J Med Chem 53:4779–4792. doi: 10.1021/jm100438s. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Han Z, Pinkner JS, Ford B, Chorell E, Crowley JM, Cusumano CK, Campbell S, Henderson JP, Hultgren SJ, Janetka JW. 2012. Lead optimization studies on FimH antagonists: discovery of potent and orally bioavailable ortho-substituted biphenyl mannosides. J Med Chem 55:3945–3959. doi: 10.1021/jm300165m. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Klein T, Abgottspon D, Wittwer M, Rabbani S, Herold J, Jiang X, Kleeb S, Lüthi C, Scharenberg M, Bezençon J, Gubler E, Pang L, Smiesko M, Cutting B, Schwardt O, Ernst B. 2010. FimH antagonists for the oral treatment of urinary tract infections: from design and synthesis to in vitro and in vivo evaluation. J Med Chem 53:8627–8641. doi: 10.1021/jm101011y. [DOI] [PubMed] [Google Scholar]
- 17.Cusumano CK, Pinkner JS, Han Z, Greene SE, Ford BA, Crowley JR, Henderson JP, Janetka JW, Hultgren SJ. 2011. Treatment and prevention of urinary tract infection with orally active FimH inhibitors. Sci Transl Med 3:109ra115. doi: 10.1126/scitranslmed.3003021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bouckaert J, Berglund J, Schembri M, De Genst E, Cools L, Wuhrer M, Hung CS, Pinkner J, Slättegård R, Zavialov A, Choudhury D, Langermann S, Hultgren SJ, Wyns L, Klemm P, Oscarson S, Knight SD, De Greve H. 2005. Receptor binding studies disclose a novel class of high-affinity inhibitors of the Escherichia coli FimH adhesin. Mol Microbiol 55:441–455. doi: 10.1111/j.1365-2958.2004.04415.x. [DOI] [PubMed] [Google Scholar]
- 19.Wellens A, Garofalo C, Nguyen H, Van Gerven N, Slättegård R, Hernalsteens JP, Wyns L, Oscarson S, De Greve H, Hultgren S, Bouckaert J. 2008. Intervening with urinary tract infections using anti-adhesives based on the crystal structure of the FimH-oligomannose-3 complex. PLoS One 3:e2040. doi: 10.1371/journal.pone.0002040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gouin SG, Wellens A, Bouckaert J, Kovensky J. 2009. Synthetic multimeric heptyl mannosides as potent antiadhesives of uropathogenic Escherichia coli. ChemMedChem 4:749–755. doi: 10.1002/cmdc.200900034. [DOI] [PubMed] [Google Scholar]
- 21.Almant M, Moreau V, Kovensky J, Bouckaert J, Gouin SG. 2011. Clustering of Escherichia coli type-1 fimbrial adhesins by using multimeric heptyl alpha-d-mannoside probes with a carbohydrate core. Chem Eur J 17:10029–10038. doi: 10.1002/chem.201100515. [DOI] [PubMed] [Google Scholar]
- 22.Yan X, Sivignon A, Yamakawa N, Crepet A, Travelet C, Borsali R, Dumych T, Li Z, Bilyy R, Deniaud D, Fleury E, Barnich N, Darfeuille-Michaud A, Gouin SG, Bouckaert J, Bernard J. 2015. Glycopolymers as anti-adhesives of E. coli-strains inducing inflammatory bowel diseases. Biomacromolecules 16:1827–1836. doi: 10.1021/acs.biomac.5b00413. [DOI] [PubMed] [Google Scholar]
- 23.Boudeau J, Glasser AL, Masseret E, Joly B, Darfeuille-Michaud A. 1999. Invasive ability of an Escherichia coli strain isolated from the ileal mucosa of a patient with Crohn’s disease. Infect Immun 67:4499–4509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Pillion DJ, Ahsan F, Arnold JJ, Balusubramanian BM, Piraner O, Meezan E. 2002. Synthetic long-chain alkyl maltosides and alkyl sucrose esters as enhancers of nasal insulin absorption. J Pharm Sci 91:1456–1462. doi: 10.1002/jps.10150. [DOI] [PubMed] [Google Scholar]
- 25.Eley JG, Triumalashetty P. 2001. In vitro assessment of alkylglycosides as permeability enhancers. AAPS Pharm Sci Tech 2:E19. doi: 10.1208/pt020319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Liu DZ, Morris-Natschke SL, Kucera LS, Ishaq KS, Thakker DR. 1999. Structure-activity relationships for enhancement of paracellular permeability by 2-alkoxy-3-alkylamidopropylphosphocholines across Caco-2 cell monolayers. J Pharm Sci 88:1169–1174. doi: 10.1021/js9900957. [DOI] [PubMed] [Google Scholar]
- 27.Liu DZ, LeCluyse EL, Thakker DR. 1999. Dodecylphosphocholine-mediated enhancement of paracellular permeability and cytotoxicity in Caco-2 cell monolayers. J Pharm Sci 88:1161–1168. doi: 10.1021/js990094e. [DOI] [PubMed] [Google Scholar]
- 28.Wellens A, Lahmann M, Touaibia M, Vaucher J, Oscarson S, Roy R, Remaut H, Bouckaert J. 2012. The tyrosine gate as a potential entropic lever in the receptor-binding site of the bacterial adhesin FimH. Biochemistry 51:4790–4799. doi: 10.1021/bi300251r. [DOI] [PubMed] [Google Scholar]
- 29.Winiwarter S, Bonham NM, Ax F, Hallberg A, Lennernäs H, Karlén A. 1998. Correlation of human jejunal permeability (in vivo) of drugs with experimentally and theoretically derived parameters. A multivariate data analysis approach. J Med Chem 41:4939–4949. doi: 10.1021/jm9810102. [DOI] [PubMed] [Google Scholar]
- 30.Bouckaert J, Li Z, Xavier C, Almant M, Caveliers V, Lahoutte T, Weeks SD, Kovensky J, Gouin SG. 2013. Heptyl alpha-d-mannosides grafted on a beta-cyclodextrin core to interfere with Escherichia coli adhesion: an in vivo multivalent effect. Chem Eur J 19:7847–7855. doi: 10.1002/chem.201204015. [DOI] [PubMed] [Google Scholar]
- 31.Sivignon A, de Vallée A, Barnich N, Denizot J, Darcha C, Pignède G, Vandekerckove P, Darfeuille-Michaud A. 2015. Saccharomyces cerevisiae CNCM I-3856 prevents colitis induced by AIEC bacteria in the transgenic mouse model mimicking Crohn’s disease. Inflamm Bowel Dis 21:276–286. doi: 10.1097/MIB.0000000000000280. [DOI] [PubMed] [Google Scholar]
- 32.Yan X, Delgado M, Fu A, Alcouffe P, Gouin SG, Fleury E, Katz JL, Ganachaud F, Bernard J. 2014. Simple but precise engineering of functional nanocapsules through nanoprecipitation. Angew Chem Int Ed Engl 53:6910–6913. doi: 10.1002/anie.201402825. [DOI] [PubMed] [Google Scholar]
- 33.Almant M, Mastouri A, Gallego-Yerga L, GarcíaFernandez JM, OrtizMellet C, Kovensky J, Morandat S, ElKira K, Gouin SG. 2013. Probing the nature of the cluster effect observed with synthetic multivalent galactosides and peanut agglutinin lectin. Chem Eur J 19:729–738. doi: 10.1002/chem.201202319. [DOI] [PubMed] [Google Scholar]
- 34.Brissonnet Y, Ortiz Mellet C, Morandat S, Garcia Moreno MI, Deniaud D, Matthews SE, Vidal S, Šesták S, El Kirat K, Gouin SG. 2013. Topological effects and binding modes operating with multivalent iminosugar-based glycoclusters and mannosidases. J Am Chem Soc 135:18427–18435. doi: 10.1021/ja406931w. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Disease activity index (DAI) score.
Histological grading of intestinal inflammation.