Abstract
Bamboo plants play a significant role in traditional Asian medicine, especially in China and Japan. Biomedical investigations on the health-benefiting effects as well as toxicity of different parts and species of bamboo have been carried out worldwide since the 1960s, and documented a wide range of protective effects of bamboo-derived products, such as protection against oxidative stress, inflammation, lipotoxicity, cancer, and cardiovascular disease. Some of these products may interfere with male and female reproductive function, thyroid hormone metabolism, and hepatic xenobiotransformation enzymes. The diversity of bamboo species, parts of the plants available for medicinal use, and different extraction methods suggest that bamboo has great potential for producing a range of extracts with functional utility in medicine.
Keywords: Bamboo, traditional medicine, natural product, antioxidant, inflammation, cancer, lipotoxicity, cardiovascular disease, toxicity, reproduction, thyroid hormone, phase I and phase II hepatic enzymes
1. Overview
Bamboo refers to any of a group of plants in the subfamily Bambusoideae, which is a part of the true grass family. This subfamily consists of more than 70 genera and about 1,450 species (Gratani et al., 2008), and members of this subfamily grow in diverse climates from subarctic to tropical regions. The versatile application of bamboo in people’s daily lives in Asia was vividly described by William Edgar Geil in his book A Yankee on The Yangtze: being a narrative of a journey from Shanghai through the central kingdom to Burma, which was first published in 1904 (Geil, 2010). He wrote, “A man can sit in a bamboo house under a bamboo roof, on a bamboo chair at a bamboo table, with a bamboo hat on his head and bamboo sandals on his feet. He can at the same time hold in one hand a bamboo bowl, in the other hand bamboo chopsticks and eat bamboo sprouts. When through with his meal, which has been cooked over a bamboo fire, the table may be washed with a bamboo cloth, and he can fan himself with a bamboo fan, take a siesta on a bamboo bed, lying on a bamboo mat with his head resting on a bamboo pillow…He might then take a walk over a bamboo suspension bridge, drink water from a bamboo ladle, and scrape himself with a bamboo scraper.”
In addition to the uses in daily life, different parts of bamboo have also been employed in traditional Asian medicine for a variety of purposes, as summarized by Dr. S. Dharmananda at the Institute for Traditional Medicine (Portland, OR) in his online article “Bamboo as Medicine” (http://www.itmonline.org/arts/bamboo.htm). In traditional Chinese medicine, bamboo is generally considered cooling, calming, and phlegm resolving, and is incorporated in many traditional formulas to treat lung and stomach heat, febrile disease, and correct up-flowing qi (qi is a fundamental concept in traditional Chinese medicine referring to the energy flow in a living being).
The earliest scientific documentation of potential medicinal use of bamboo was published in the early 1960s (Sakai et al., 1963), followed by a series of studies carried out by Shibata et al. in the 1970s (Okabe et al., 1975; Shibata et al., 1975; Shibata et al., 1976, 1978; Shibata et al., 1979). Bamboo, as a biomedical research topic was relatively silent during the 1980s and 1990s, but research interest increased worldwide since the beginning of this century (Panee, 2008). In this article, the author summarizes recent findings on both health benefits and toxicity of extracts derived from different parts and species of bamboo.
2. Bamboo Leaf Extracts
Traditional Chinese medicine distinguishes bamboo leaves into two kinds. (1) Kuzhuye (“Ku” means bitter, “Zhu” means bamboo, and “Ye” means leaf), dried leaves of Pleioblastus amarus (Keng) Keng f. “It is pungent, sweet, slightly bitter, and cold, and enters the heart and lung meridians. It is often used to treat febrile diseases when there is heat in the heart, lung, or chest” (Yang, 2002). (2) Danzhuye (“Dan” means bland), which are dried leaves and stem of Lophatherum gracile Brongn. “It is less strong in clearing heat than the bitter form, but is good at promoting urination, thereby leaching out the heat from the heart and the small intestine. In clinical practice, it is used to treat urinary dysfunction, which starts or worsens in stressful situations. It is also used to treat eczema due to damp heat” (Yang, 2002). According to Dr. S. Dharmananda, the leaves of the black bamboo [Phyllostachys nigra (Lodd.) Munro] are used similarly as “Danzhuye” in Japan.
In current biomedical research, the leaves of bamboo seem to be the part of the plant triggering the most intensive interest, possibly because the leaves comprise a significant portion of the total biomass of bamboo plants, are easy to harvest and process, and some can even be obtained as waste of bamboo timber industry. Extracts and compounds from the leaves of Sasa senanensis (Franch. & Sav.) Rehder, Phyllostachys nigra (Lodd.) Munro, and Phyllostachys edulis (Carrière) J.Houz have been the most commonly studied, although a few published studies have been on the leaves from other bamboo species, such as Indocalamus tessellatus (Munro) Keng f, Bambusa arundinacea Willd, and Sasa borealis (Hack.) Makino & Shibata (Chen et al., 1998; Muniappan and Sundararaj, 2003; Park et al., 2007).
2.1 Potential Health Benefits of Bamboo Leaf Extracts
2.1.1 Phyllostachys edulis (Carrière) J.Houz
This species of bamboo is known as “Maozhu” in Chinese or “Moso” in Japanese, and it is one of the largest and fastest-growing bamboo species in the world. Studies carried out in the author’s laboratory have shown that an ethanolic extract from the leaves of Phyllostachys edulis (Carrière) J.Houz contains high levels of polyphenols and flavonoids (Lin et al., 2008). This bamboo extract (termed as BEX) protects a variety of cells against lipotoxicity induced by high levels of palmitic acid (Panee et al., 2008). Specifically, BEX inhibits palmitic acid-induced overproduction of proinflammatory cytokines, such as interleukin 6 (IL-6) and monocyte chemoattractant protein 1(MCP-1), and this anti-inflammatory effect is partially mediated by flavonoids such as tricin and 7-O-methyltricin (Higa et al., 2012) through NFkappaB and AP-1 pathway inhibition (Higa and Panee, 2011). In addition, BEX also seems to affect the central nervous system and cancer development. For example, BEX as a dietary supplement ameliorated anxiety-like neurobehaviors in mice fed high fat diet (Del Rosario et al., 2012), and decreased the incidence of DMBA-induced mammary tumors in rats (Lin et al., 2008).
Further protective effects have been reported on 3-O-caffeoyl-one-methylquinic acid, an antioxidant isolated from the leaves of Phyllostachys edulis (Carrière) J.Houz. This compound was found to upregulate the expression of heme oxygenase-1 (HO-1) and other Nrf2- dependent phase II detoxifying genes, and protect against peroxide-induced cytotoxicity (Kweon et al., 2004; Kweon et al., 2006). This compound also prevents photocarcinogenesis via apoptotic elimination of tumor protein 53 (p53) mutant and DNA-repair defective cells (Kweon et al., 2007).
Furthermore, it has been documented that a butanol-soluble extract from the leaves of Phyllostachys edulis (Carrière) J.Houz contains derivatives of chlorogenic acid and has antioxidant activity (Kweon et al., 2001). Essential oils of Phyllostachys edulis (Carrière) J.Houz extracted by steam distillation contain cis-3- hexenol and have antioxidant and antimicrobial activities (Jin et al., 2011).
2.1.2 Phyllostachys nigra (Lodd.) Munro
Commonly known as “black bamboo”, this is another major species in the genus Phyllostachys. Flavonoids extracted from black bamboo leaves have potent antioxidant activities (Hu et al., 2000; Zhang et al., 2002). Among these flavonoids, flavone c-glucosides and p-coumaric acid reportedly have long retention time in the colon and may contribute to free radical scavenging (Zhang et al., 2007). Luteolin 6-C-(6″-O-trans-caffeoylglucoside), an antioxidant isolated from the leaves, has been reported to protect against oxidative stress-induced cytotoxicity in retinal ganglion cells (Lee et al., 2010). It also enhances the activity of aldose reductase and inhibits the formation of advanced glycation end products, and therefore may potentially have a role in the prevention of diabetic complications (Jung et al., 2007).
Orientin, another antioxidant obtained from the leaves of black bamboo, exerts vaso-relaxant activity through the inhibition of intracellular Ca2+ release and extracellular Ca2+ influx (Fu et al., 2005). Orientin also prevents apoptosis induced by hypoxia and reoxygenation in myocardium and cardiomyocytes. The mechanism for this effect is inhibition of the activation of mitochondrial apoptotic pathway (Fu et al., 2006). These studies suggest potential for cardio-protective effects.
The acetone fraction of the leaves of Phyllostachys nigra (Lodd.) Munro has been shown to enhance leukemia cell differentiation (Kim et al., 2007b), and inhibit interleukin-12 (IL-12) production in lipopolysaccharide-activated macrophages via decreasing NF-kappaB binding activity (Kim et al., 2007a). These preliminary findings suggest potential anti-leukemia and anti-inflammation effects of the acetone extract.
2.1.3 Sasa senanensis (Franch. & Sav.) Rehder
This species is known as Kumaizasa bamboo, found in Hokkaido, and is used in traditional medicine in Japan. Between 1998 and 2004 several articles documented a wide range of biological functions of an alkaline extract made from the leaves of this species of bamboo (the product was named “Sasa Health”). When the diet of mice was supplemented with this extract, the incidence and growth of mammary tumors in mice with spontaneous or Her2/NeuN-induced mammary tumorigenesis was inhibited (Ren et al., 2004; Tsunoda et al., 1998), spontaneous motor activity of both intact and gonadectomized mice was influenced (Nagasawa and Hattori, 2001), and some of the deleterious effects of restricted feeding were alleviated (Nagasawa et al., 2001).
Some anti-tumor effects of a new hot water extract from Sasa leaves were published in 2010. This extract inhibited the incidence and growth of 7,12 -dimethylbenz[α]anthracene (DMBA)-induced mammary tumors and the growth of inoculated sarcoma and melanoma cells (Seki and Maeda, 2010). The anti-tumor efficacy of the extract was attributed to immunopotentiation.1,3-β-glucan has been identified as the probable primary immunopotentiating factor in this extract (Seki et al., 2010).
2.2 Toxicity Evaluations of Bamboo Leaf Extracts
2.2.1 Ethanolic Extract from the Leaves of Phyllostachys edulis (Carrière) J.Houz
It has been shown in the author’s laboratory that the aforementioned ethanolic extract from the leaves of Phyllostachys edulis (Carrière) J.Houz (BEX) has regulatory effects on phase I and phase II enzymes in mouse liver. For example, it upregulates the activities of cytochromes P450 (CYP) enzymes CYP1A2 and CYP3A11, and uridine diphosphate glucuronosyltransferase (UGT), and slightly downregulates the activity of glutathione-S-transferase (GST). In Obese/diabetic mice the activities of CYP1A2 and CYP3A11 are upregulated, and supplementation with BEX further upregulates these phase I enzymes (Koide et al., 2011). Although these effects are not toxic per se, potential interactions between BEX and drug metabolism should be considered, especially in obese/diabetic subjects.
2.2.2 Antioxidant-rich Extract from Phyllostachys nigra (Lodd.) Munro
An antioxidant-rich extract prepared from the leaves of Phyllostachys nigra (Lodd.) Munro containing a high level of polyphenols has been reported to be non-toxic. Acute oral toxicity tests showed that the maximum tolerated dose of this product was greater than10 g/kg body weight in both rats and mice, without mutagenic effects. A subchronic administration of this product for up to 90 days resulted in a no-observed-adverse-effect level (NOAEL) at a dose of 4.30 g/kg per day (Lu et al., 2005). When pregnant rats were treated with this extract at the NOAEL dose, the pregnant rats did not show significant changes in fertility and gestation index, and there were no effects on embryo-fetal number, viability, sex ratio, and development observed (Lu et al., 2006).
2.2.3 Abortifacient Effect of the Leaves of Bambusa vulgaris Schrad
In contrast to the aforementioned antioxidant-rich extract from the leaves of the Phyllostachys nigra (Lodd.) Munro, an extract from the leaves of Bambusa vulgaris Schrad is used to induce abortion in Nigerian folk medicine. An aqueous extract of Bambusa vulgarisSchrad leaves, containing alkaloids, tannins, phenolics, glycosides, saponins, flavonoids and anthraquinones, was found to dramatically increase abortion frequency and decrease fetal survival rate in pregnant rabbits at doses of 250–500 mg/kg body weight per day (Yakubu and Bukoye, 2009). This dose was associated with increased resorption index and postimplantation loss, decreased serum progesterone, follicle-stimulating and luteinizing hormones, and decreased alkaline phosphatase activity and glucose concentration in the uterus (Yakubu and Bukoye, 2009; Yakubu et al., 2009). Interestingly, leaves of Bambusa vulgaris Schrad (boiled or raw) are also used to relieve labor pains or as a postpartum cleanser for live stock (Lans et al., 2007).
3. Bamboo Shoot Extracts
In traditional Chinese medicine, bamboo shoots are used to ease labor and the expulsion of the placenta by inducing uterine contractions. A poultice of the shoots is often used for cleaning wounds and healing infections. Bamboo shoot decoction taken along with honey is used to treat respiratory disorders. However, to most people bamboo shoots are best known as food. Fresh, dried, or fermented bamboo shoots are used in numerous Asian recipes. Nutrient analysis on freshly emerging juvenile bamboo shoots has shown high contents of amino acids, proteins, carbohydrates, vitamins, and minerals, and a low content of fat. As bamboo shoots age, the dietary fiber and moisture start to increase while vitamin and mineral contents decrease (Nirmala et al., 2007).
3.1 Potential Health Benefits of Bamboo Shoots
Consumption of bamboo shoots (360 g per day for 6 days as a dietary fiber, the species of bamboo used in this study was not disclosed by the authors) reportedly decreased serum total cholesterol, low-density lipoprotein (LDL), and the atherogenic index, and increased fecal volume and bowel movement frequency in healthy young women when compared with controls on a dietary fiber-free diet (Park and Jhon, 2009).
Bamboo shoot oil extracted from Phyllostachys edulis (Carrière) J.Houz (250–1000 mg/kg body weight per day) has been documented to decrease the same aforementioned serum parameters (total cholesterol and LDL), as well as serum triacylglycerol, phytosterol, and lipoprotein lipase. This same oil extract ameliorated fatty liver and increased the cholesterol content in feces in rats challenged with a high fat diet. These lipid-lowering effects have been attributed to inhibition of cholesterol absorption and increase of cholesterol excretion (Lu et al., 2010).
A high level of acetylcholine, an important neurotransmitter in the cholinergic nervous systems of vertebrates and insects, has been found in the upper portion of bamboo shoots (Horiuchi et al., 2003). Nutritional and clinical applications of bamboo shoots based on the presence of this compound have not been explored.
3.2 Toxicity Evaluations of Bamboo Shoot Extracts
3.2.1 Impact on Male Fertility
It has been reported that an ethanolic extract of the tender shoots of Bambusa arundinacea Willd (dose of 300 mg/kg body weight per day) changed the structural and functional integrity of the epididymis and reduced fertility of male rats. A 7-day treatment markedly reduced the count and motility of sperm (Manonayagi et al., 1989), and decreased the fertility index by 85 percent (Vanithakumari et al., 1989). Mating activity was significantly compromised after 4 days of treatment. After withdrawal of the treatment for approximately one week, although the mating activity completely recovered, the fertility index only increased by 8 percent (Vanithakumari et al., 1989). The serum profile of protein and oxaloacetic/pyruvic transaminase activity suggested that this extract had no systemic toxicity (Vanithakumari et al., 1989).
3.2.2 Potential Interference with Thyroid Function
Thyroid hormones, such as thyroxine (T4) and triiodothyronine (T3), have critical roles in regulating cortical cerebral neuronal migration during fetal brain development. It is postulated that maternal hypothyroxinemia, during the period of fetal neuronal cell migration (weeks 8–12 of pregnancy) may result in morphological changes in fetal brain leading to autism (Román, 2007). Thyroid peroxidase (TPO) is a thyroid enzyme liberating iodine for the production of T4 and T3. Both raw and cooked extracts from the shoots of Bambusa arundinacea Willd have been found to inhibit TPO activity in an in vitro system and to have hypothyroid-like effects in vivo (Chandra et al., 2004a; Chandra et al., 2004b), which is thought to be due to the high contents of anti-thyroidal substances, such as cyanogenic glucoside, thiocyanate, and glucosinolate, in bamboo shoots (Chandra et al., 2004a). Feeding bamboo shoots to rats for up to 3 months significantly increased thyroid weight and iodine excretion, and decreased TPO activity and levels of T3 and T4 (Chandra et al., 2004a; Chandra et al., 2004b). Iodide supplementation in these experiments reduced, but did not abolish the effects of bamboo shoots on thyroid function (Chandra et al., 2004a; Chandra et al., 2004b). These studies suggest that consumption of shoots of Bambusa arundinacea Willd during pregnancy may result in birth defect (such as autism).
3.2.3 Other Effects
Consumption of bamboo shoots was positively associated with hyperuricemia – a risk factor for cardiovascular disease – in an epidemiological study in Taiwan (Chuang et al., 2011). In a study done in three hospitals in Thailand, dietary intake of bamboo shoots was associated with aggravation of dyspepsia (Premgamone et al., 2010).
Although arsenic is predominantly found in inorganic forms in most terrestrial plants, a significant amount of organic arsenic was found in shoots of Phyllostachys edulis Carrière) J.Houz despite an absence of such compounds in the soil, suggesting a possible arsenic methylation process in the shoots, especially in the core section (Zhao et al., 2006). The toxicological aspect of this finding has yet to be further explored.
4. Bamboo Stem Products
In traditional Chinese medicine, bamboo stems can be processed into varied forms as summarized below. (1) Zhuru (bamboo shavings), dried, long and thin slices shaved from the intermediate layer of the bamboo stalk after the outer skin is removed. It is “sweet and slightly cold”, and used for stomach heat syndromes that produce incorrect flow of qi, commonly causing nausea, loss of appetite, hiccups or vomiting (Yang, 2002). (2) Tianzhuhuang, also known as tabashir, is a dried resinous secretion with very high silica content (up to 85 percent silica) from the knots of certain female species of bamboo (Jones et al., 1966). It is “sweet and cold”, clears heat, resolves phlegm, relieves convulsion, especially used in remedies for children’s feverish disorders and epilepsy (Yang, 2002). (3) Zhuli, is the liquid sap obtained from the ends of freshly cut bamboo pieces with outer surface removed and exposed to heat. Zhuli is “colder” than Zhuru and Tianzhuhuang, enters the heart, lung, and stomach meridians. It has a lubricating nature, strongly eliminates phlegm-heat, and is used to treat epilepsy, schizophrenia, hemiplegia, facial paralysis, and numbness and tingling or cramp of the limbs (Yang, 2002). (4) Bamboo vinegar, a liquid condensed from the vapor generated by heating bamboo at very high temperature in an airless vessel (a process to make bamboo charcoal). It has high content of acetic acid, accompanied by phenols. Bamboo vinegar is largely produced in Japan and is used to treat varied skin diseases. Recent biomedical research has been mainly focused on the biological function of bamboo shavings, bamboo vinegar, and ethanolic extracts of bamboo culms, as summarized below.
4.1 Potential Health Benefits of Products Derived from Bamboo Stems
4.1.1 Bamboo Shavings
Recent studies have been conducted on triterpenoid-rich extracts prepared using carbon dioxide supercritical fluid extraction from the shavings of different bamboo species.
An extract from the shavings of Phyllostachys nigra var. henonis (Mitford) Rendle (100–300 mg/kg body weight per day) decreased serum total cholesterol and total triglyceride levels in hyperlipidemic rats (Jiao et al., 2007). The same extract also reduced systolic pressure in rats with spontaneous hypertension, primarily by a vasodilatory effect (Jiao et al., 2007). In vitro results suggested that friedelin, a main triterpenoid compound in the extract, probably contributed to the vasodilatory effect (Jiao et al., 2007).
An extract from the shavings of Bambusa tuldoides Munro (40–250 mg/kg body weight/day) had an anti-fatigue effect in mice (i.e., it helped prolong the duration of weight-loaded swimming and climbing), also increased hepatic glycogen content, and decreased serum urea nitrogen and lactic acid levels (Zhang et al., 2006).
4.1.2 Bamboo Vinegar
Bamboo vinegar is known as “Chikusakueki” in Japan, “Zhook-Ryeok” in Korean, and “Zhucu” in Chinese. In most recent publications, bamboo vinegar has been studied under the name of Bambusae caulis in Liquamen (BCL).
Transdermal administration of BCL (produced from Phyllostachys bambusoides Siebold & Zucc, 100 μl to the entire dorsal skin) on hairless mice suppressed the development of 2,4-Dinitrochlorobenzene (DNCB)-induced atopic dermatitis-like skin lesions, and the proposed mechanisms included improved skin barrier function, suppression of the overproduction of serum IgE and leukocytes, and balancing the expression of Th1/Th2 cytokines in the spleen (Qi et al., 2009). Using an in vitro model (human keratinocyte cell line HaCaT) the same group reported that BCL suppressed of IFN-γ-induced expression of thymus and activation-regulated chemokine (TARC) and macrophage-derived chemokine (MDC), as well as NF-κB activation, at least in part due to its antioxidant capacities (Qi et al., 2012). The antioxidant activity of BCL was confirmed by another publication (Je et al., 2009), which also documented the inhibitory effects of BCL on oxidant-induced cell death and DNA damage. 1,2-dihydroxybenzene and 1,3-dihydroxybenzene have been isolated from BCL produced from Phyllostachys nigra var. henonis (Gramineae), both showed free radical scavenging activity, inhibited nitric oxide production in lipopolysaccharide-stimulated RAW 264.7 macrophage cells, reduced tyrosinase activity and melanin production in B16F10 melanoma cells, and therefore was considered to have anti-oxidative, anti-inflammatory and whitening properties (Park and Lee, 2012).
Due to the high temperature employed in the production, bamboo vinegar contains tar-derivatives that can be potentially carcinogenic. Using an in vitro transformation model, [BALB/c 3T3 A31-1-1 cells treated with 3-methylcholanthrene (3-MCA) and 2-O-tetradecanoylphorbol-13-acetate (TPA)], bamboo vinegar produced from Phyllostachys pubescens J. Houz was shown to be not carcinogenic nor co-carcinogenic (Kimura et al., 2002).
4.1.3 Bamboo Culm Extracts
An ethanolic extract from the culm of Phyllostachys bambusoides Siebold & Zucc has been reported to ameliorate risk factors of cardiovascular diseases in mice treated with a high cholesterol diet: Adding this extract to the diet at 1–3 percent (wet weight) of total dietary intake decreased plasma total cholesterol, and increased high-density lipoprotein (HDL) (Lee et al., 2008b). This extract also decreased hepatic lipid peroxidation, protein carbonylation, and NFkappaB activity, and increased hepatic antioxidant enzyme activities (Lee et al., 2008a; Lee et al., 2008b).
4.2 Toxicity Evaluations of Bamboo Stem Products
The only toxicity study conducted on bamboo stem products was on the aforementioned triterpenoid-rich extract from the shavings of Phyllostachys nigra var. henonis (Mitford) Rendle. This extract showed essentially no toxic effect. The oral maximum tolerated dose was over 10 g/kg body weight in both rats and mice. No mutagenicity was found by Ames, mouse bone marrow cell micronucleus, or mouse sperm abnormality tests. No abnormal symptoms, clinical signs or deaths were observed in rats during a 30-day subchronic feeding study using doses up to 830 mg/kg body weight per day. No abnormalities in organ development and hematological parameters were associated with feeding of this product (Zhang et al., 2004).
5. Concluding Remarks
This review documents the medicinal use of bamboo in traditional Asian medicine, and summarizes current scientific findings on the biological effects of bamboo-derived products. The potential health-benefiting effects are summarized in Table 1 (in vivo studies) and Table 2 (in vitro studies), the toxicity and side effects are listed in Table 3. Figure 1 shows that the overall balance favorites the positive function of bamboo-derived natural products. It is evident that the ethnopharmacological knowledge was the driving force behind some of the research, such as those on bamboo vinegar and skin diseases. While most of the studies have oriented toward “modern diseases”, such as heart diseases, obesity, and cancer. The difficulty of translation between the concepts in the traditional medicine (such as the “cool” nature of the bamboo-based medicine) and the conventional western knowledge system hinders full exploration of the ancient literature by biomedical researchers. Meanwhile this review also reveals the lack of scientific investigation on some of the well-documented bamboo medicine, such as Danzhuye, Kuzhuye, Tianzhuhuang, and Zhuli. Although the extracts from the leaves and stems of bamboo plants may have overlapping functions with these traditional ingredients in Chinese medicine, direct investigation on these materials may reveal valuable information that can enhance our understanding on the concepts of the traditional medicine.
Table 1.
Summary of reported health-benefiting effects of bamboo extracts from studies using in vivo models.
| Species | Plant part | Extraction method | Research model | Treatment | Dose, route, duration & control | Result | Reference |
|---|---|---|---|---|---|---|---|
| Sasa senanensis (Franch. & Sav.) Rehder | Leaf | Alkaline extraction | Her2/Neu mouse | Enhanced tumorigenesis | Chronic delivery in drinking water, 0.044%–0.088% Fe-Chlorophyllin Na (reference compound), extract-free as control. | Inhibited mammary tumor incidence | (Ren et al., 2004) |
| SHN mouse | Spontaneous tumorigenesis | (Tsunoda et al., 1998) | |||||
| ICR mouse | Gonadectomy | Normalized spontaneous motor activity to the baseline levels | (Nagasawa and Hattori, 2001) | ||||
| SHN mouse | Food restriction | (Nagasawa et al., 2001) | |||||
| Hot water extraction (100°C) | SD rat | DMBA treatment | 0.03–0.5% (w/w) lyophilized extract in diet, up to 7 wks for tumor inoculation, up to 26 wks for DMBA treatment, extract-free as control. | Inhibited mammary tumor incidence and growth | (Seki and Maeda, 2010) | ||
| BALB/c mouse | Fibrosarcoma and sarcoma inoculation | Inhibited tumor growth, prolonged survival time | |||||
| C57BL/6 mouse | Melanoma inoculation | ||||||
| Hot water extraction (up to 196°C) | ddY mouse | Sarcoma inoculation | 0.05–0.5% (w/w) extract in diet, up to 120 days, extract-free as control. | (Seki et al., 2010) | |||
| C57BL/6 mouse | Colon carcinoma Inoculation | ||||||
| Phyllostachys nigra (Lodd.) Munro | Leaf | Ethanolic extraction | SD rat | Absorption and excretion tests | Single gavage delivery, 1 g dry mass per kg body weight. | Long retention time of flavone C-glucosides in colon | (Zhang et al., 2007) |
| Orientin, purified | Wistar rat | Cardiac ischaemia/reperfusion | 0.5–2.0 mg/kg, 10 min before ischemia. Sham, orientin-free, and verapamil as controls. | Prevented apoptosis in cardiomyocytes | (Fu et al., 2006) | ||
| Shoots | Trimmed and boiled | Healthy young women | dietary supplementation | 360 g bamboo shoots/day, 6 days; diet containing 25 g cellulose as control. | Decreased atherogenic index, and increased fecal volume and bowel movement frequency | (Park and Jhon, 2009) | |
| Stem (Shaving) | CO2 supercritical fluid extraction | SD rat | Hyperlipidemic diet | Hyperlipidemia study: 0.04–0.25 g/ Kg, 2 wks, extract-free as control; hypertension study: 0.1–0.3 g/Kg, 1 wk, Tween80 as control. | Reduced cholesterol and triglyceride in serum | (Jiao et al., 2007) | |
| Spontaneous hypertensive rat | Reduced systolic pressure | ||||||
| Phyllostachys edulis (Carrière) J.Houz | Leaf | Ethanolic extraction | SD rat | DMBA treatment | 11 g dry mass per 4057 Kcal, ~1% w/w, mixed in diet, chronic treatment up to 6 months, extract-free experimental diet or normal diet as controls. | Inhibited mammary tumor incidence | (Lin et al., 2008) |
| C57BL/6J mouse | High fat diet | Decreased MCP-1 in serum | (Higa et al., 2011) | ||||
| Ameliorated anxiety-like neurobehaviors | (Del Rosario et al., 2012) | ||||||
| Shoots | CO2 supercritical fluid extraction | SD rats | High fat, high cholesterol diet | 0.25–1g/Kg in drinking water, 6 wks, extract-free and 0.25 g/kg beta-sitosterol as controls. | Hypolipidemic effect | (Lu et al., 2010) | |
| Bambusa tuldoides Munro | Stem (Shaving) | CO2 supercritical fluid extraction | BALB/c mice | Weight-loaded swimming test and climbing test | 0.04–0.25 g/kg, oral gavage, 30 days, extract-free as control. | Anti-fatigue activity | (Zhang et al., 2006) |
| Phyllostachys bambusoides Siebold & Zucc | Stem (Clum) | Ethanolic extraction | C57BL/6 mice | High-cholesterol diet | 1 or 3% (w/w) for 16 wks, extract-free experimental diet or normal diet as controls. | Decreased atherogenic index, increased hepatic antioxidant enzyme activities | (Lee et al., 2008a; Lee et al., 2008b) |
| Bambusa arundinacea Willd | Leaves | Methanol extraction | Albino rats | Carrageenin-induced paw oedema or immunologically induced inflammation | 50–200 mg/kg, intraperitoneal injection, vehicle or standard drug as controls. | Against carrageenin- and immunologically-induced paw oedema; antiulcer activity. | (Muniappan and Sundararaj, 2003) |
Table 2.
Health-benefiting effects of fractions and compounds from bamboo plants demonstrated in in vitro studies.
| Species | Compound/ fraction | Evaluation method | Function | Reference |
|---|---|---|---|---|
| Phyllostachys nigra (Lodd) Munro | Total flavonoids of leaves | Cell-free assay | Anti-free radical, IC50 for O2− was 11 μg/ml, IC50 for .OH was 5.3 μg/ml. | (Zhang et al., 2002) |
| luteolin 6-C-(6″-O-trans-caffeoylglucoside) | (1) Cell-free assay (2) RGC-5 cell survival rate |
(1) Antioxidative, inhibited aldose reductase activity (IC50 was 0.0134 μM); reduced advanced glycation endproducts formation. (2) Attenuated oxidative stress in retinal ganglion cells. |
(Jung et al., 2007) (Lee et al., 2010) |
|
| Orientin | (1) Thoracic aortic rings from rabbit (2) H9c2 cardiomytocytes ischemia/reperfusion (I/R) model |
(1) Vasodilatation, IC50 was 7.27 μM, inhibiting intracellular Ca2+ release and extracellular Ca2+ influx. (2) Against I/R-induced apoptosis, modulated mitochondrial permeability transition, regulated PI3K/Akt pathway. |
(Fu et al., 2006) (Fu et al., 2005) (Lu et al., 2011) |
|
| Phyllostachys nigra var. Henonis (Mitford) Rendle | Chlorogenic acid, caffeic acid, and luteolin 7-glucoside | Cell-free assay | Antioxidant; pro-oxidant at the presence of transitional metal ions. | (Hu et al., 2000) |
| Acetone fraction of leaves | Raw264.7 cells and mouse spleen cells, Lipopolysaccharides (LPS) stimulation | Reduced IL-12 production in LPS-activated macrophages via NF-kappaB inhibition. | (Kim et al., 2007a) | |
| Phyllostachys edulis (Carrière) J.Houz | 3-O-caffeoyl-one-methylquinic acid | (1) Bovine vascular endothelial cells exposed to peroxide. (2) UVA-mediated apoptosis in HaCaT keratinocytes (3) Exposing human umbilical vascular endothelial cells (HUVECs) to the compound |
(1) Heme oxygenase-1 induction and cytoprotection. (2) Prevented photocarcinogenesis via apoptotic elimination of p53 mutant and DNA-repair defective cells. (3) Induced Nrf2-dependent phase II detoxifying genes and altered intracellular glutathione redox. |
(Kweon et al., 2004) (Kweon et al., 2007) (Kweon et al., 2006) |
| Butanol fraction of leaves; chlorogenic acid derivatives |
Cell-free assay | Antioxidant activity | (Kweon et al., 2001) | |
| Ethanol fraction of leaves; tricin, 7-o-methyltricin | Muscle, liver, and fat cells treated with high levels of palmitic acid | Protected lipoapoptosis, inhibited cytokine overproduction and NFkappaB activation. | (Panee et al., 2008) (Higa and Panee, 2011) (Higa et al., 2012) |
|
| Essential oils; cis-3-Hexenol | (1) Cell-free assay (2) Bacterial assay |
(1) Antioxidant capacity. (2) Inhibited Staphylococcus epidermidis and E. coli |
(Jin et al., 2011) | |
| Sasa borealis (Hack.) Makino & Shibata | Butanol fraction of leaves; isoorientin; 2″-O-alpha-L-rhamnoside | (1) Cell-free assay (2) Oxidative damage to HepG2 cells |
For isoorientin, DPPH radical scavenging activity IC50 was 9.5 μM, cytoprotection against peroxide-induced damage in HepG2 cells IC50 was 1.1 μM; for 2-O-alpha-L-rhamnoside, the 2 values were 34.5 μM and 0.8 μM, respectively. | (Park et al., 2007) |
| Phyllostachys bambusoides Siebold & Zucc | Methanol extract of leaves; 201-hydroxypurpurin-7 δ-lactone esters | Apoptosis of human leukemia CMK-7 cells and colon adenocarcinoma Colo320 DM cells | Photodynamic induction of cell apoptosis. | (Kim et al., 2003) |
Table 3.
Summary of toxicity and side effects of bamboo extracts observed in in vivo studies.
| Species | Plant part | Extraction method | Research model | Treatment | Dose, route, duration & control | Result | Reference |
|---|---|---|---|---|---|---|---|
| Phyllostachys edulis (Carrière) J.Houz | Leaf | Ethanolic extraction | C57BL/6J mouse | High fat diet | 11 g dry mass per 4057 Kcal, ~1% w/w, 6 months, extract-free experimental diet or normal diet as controls. | Extract upregulated hepatic CYP1A2 and CYP3A11 activities, in synergy with obese/diabetic condition | (Koide et al., 2011) |
| Phyllostachys nigra (Lodd.) Munro | Leaf | Regurgitant boiling water extraction | SD rat | Oral acute and subchronic toxicity | Up to 4.3 g/kg for 90 days, extract-free as control. | Non-toxic | (Lu et al., 2005) |
| Kun-Ming mouse | Mutagenicity | ||||||
| Not disclosed | SD rat | Developmental toxicity | Non-toxic | (Lu et al., 2006) | |||
| Stem (Shaving) | CO2 supercritical extraction | SD rat | Oral acute toxicity | Oral gavage 1–10 g/kg, 30 days, extract-free as control. | Non-toxic | (Zhang et al., 2004) | |
| Kunming mouse | Mutagenicity | ||||||
| Bambusa vulgaris Schrad | Leaf | Cold water extraction | Dutch rabbit | Pregnant condition | 250 and 500 mg/kg in drinking water, days 1–9 or 18–20 of pregnancy, extract-free water as control. | Increased abortion frequency and decreased fetal survival rate | (Yakubu and Bukoye, 2009) |
| Compromised synthetic, secretory, reabsorptive and excretory functions of liver and kidney | (Yakubu et al., 2009) | ||||||
| Bambusa arundinaceae Willd | Shoots | Ethanolic extraction | Male rat | Fertility tests | 300 mg/kg, 7 days, extract-free as control. | Reduced fertility index and count/motility of sperm | (Manonayagi et al., 1989), (Vanithakumari et al., 1989) |
| Raw or boiled | Rat | Chronic oral delivery | 1/3 of the diet was replaced by bamboo shoots, up to 90 days, bamboo-free diet as control. | Hypothyroidism effects | (Chandra et al., 2004a) | ||
| Leaves | Methanol extraction | Rat | Acute toxicity study | Non-specific | The LD50 was 1812.5 mg/kg (i.p.) and 2552.2 mg/kg (p.o.). The animals were less active compared to the vehicle controls. | (Muniappan and Sundararaj, 2003) |
Figure 1.
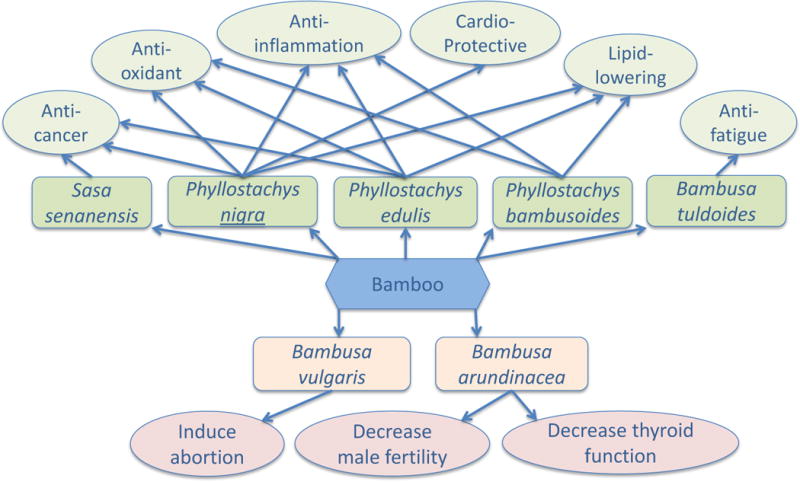
Outline of the health-benefiting and toxic effects of products derived from different species of bamboo plants.
Acknowledgments
This study was made possible by grant numbers R21 AT003874 (Panee) and R21 AT005139 (Panee) from NCCAM and ORWH, 8G12MD007601 from NIMHD, and 5G12RR003061 (RCMI/BRIDGES), U54RR022762 (RTRN Small Grant Program) and 5P20RR016467-11 and 8P20GMl03466-11 (INBRE II) from NCRR. Its contents are solely the responsibility of the author and do not necessarily represent the official views of the NCCAM, ORWH, NIMHD, NCRR, or the National Institute of Health (NIH).
Footnotes
POTENTIAL CONFLICTS OF INTEREST
The author declares that there are no conflicts of interest.
References
- Chandra AK, Ghosh D, Mukhopadhyay S, Tripathy S. Effect of bamboo shoot, Bambusa arundinacea (Retz) Willd on thyroid status under conditions of varying iodine intake in rats. Indian J Exp Biol. 2004;42:781–786. [PubMed] [Google Scholar]
- Chandra AK, Mukhopadhyay S, Lahari D, Tripathy S. Goitrogenic content of Indian cyanogenic plant foods & their in vitro anti-thyroidal activity. Indian J Med Res. 2004;119:180–185. [PubMed] [Google Scholar]
- Chen C, Huang X, Zhou J, Xu H, Jiang Y, Zhu G, Sun D. Sulfation of polysaccharides isolated from Indocalamus tesselatus and their anticytopathic effect on human immunodeficiency virus type I. Yao Xue Xue Bao. 1998;33:264–268. [PubMed] [Google Scholar]
- Chuang SY, Lee SC, Hsieh YT, Pan WH. Trends in hyperuricemia and gout prevalence: Nutrition and Health Survey in Taiwan from 1993–1996 to 2005–2008. Asia Pac J Clin Nutr. 2011;20:301–308. [PubMed] [Google Scholar]
- Del Rosario A, McDermott MM, Panee J. Effects of a high-fat diet and bamboo extract supplement on anxiety- and depression-like neurobehaviours in mice. Br J Nutr. 2012;108:1143–1149. doi: 10.1017/S0007114511006738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fu XC, Wang MW, Li SP, Zhang Y, Wang HL. Vasodilatation produced by orientin and its mechanism study. Biol Pharm Bull. 2005;28:37–41. doi: 10.1248/bpb.28.37. [DOI] [PubMed] [Google Scholar]
- Fu XC, Wang MW, Li SP, Wang HL. Anti-apoptotic effect and the mechanism of orientin on ischaemic/reperfused myocardium. J Asian Nat Prod Res. 2006;8:265–272. doi: 10.1080/10286020500207347. [DOI] [PubMed] [Google Scholar]
- Geil WE. A Yankee on the Yangtze: being a narrative of a journey from Shanghai through the central kingdom to Burma. Nabu Press; Charleston: 2010. [Google Scholar]
- Gratani L, Crescente MF, Varone L, Fabrini G, Digiulio E. Growth pattern and photosynthetic activity of different bamboo species growing in the Botanical Garden of Rome. Flora. 2008;203:77–84. [Google Scholar]
- Higa JK, Liu W, Berry MJ, Panee J. Supplement of bamboo extract lowers serum monocyte chemoattractant protein-1 concentration in mice fed a diet containing a high level of saturated fat. Br J Nutr. 2011;106:1810–1813. doi: 10.1017/S0007114511002157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Higa JK, Panee J. Bamboo extract reduces interleukin 6 (IL-6) overproduction under lipotoxic conditions through inhibiting the activation of NF-κB and AP-1 pathways. Cytokine. 2011;55:18–23. doi: 10.1016/j.cyto.2011.02.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Higa JK, Liang Z, Williams PG, Panee J. Phyllostachys edulis Compounds Inhibit Palmitic Acid-Induced Monocyte Chemoattractant Protein 1 (MCP-1) Production. PLoS One. 2012;7:e45082. doi: 10.1371/journal.pone.0045082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horiuchi Y, Kimura R, Kato N, Fujii T, Seki M, Endo T, Kato T, Kawashima K. Evolutional study on acetylcholine expression. Life Sci. 2003;72:1745–2556. doi: 10.1016/s0024-3205(02)02478-5. [DOI] [PubMed] [Google Scholar]
- Hu C, Zhang Y, Kitts DD. Evaluation of antioxidant and prooxidant activities of bamboo Phyllostachys nigra var. Henonis leaf extract in vitro. J Agric Food Chem. 2000;48:3170–3176. doi: 10.1021/jf0001637. [DOI] [PubMed] [Google Scholar]
- Je JY, Park PJ, Kim EK, Ahn CB. Antioxidant and angiotensin I converting enzyme inhibitory activity of Bambusae caulis in Liquamen. Food Chem. 2009;113:932–935. [Google Scholar]
- Jiao J, Zhang Y, Lou D, Wu X, Zhang Y. Antihyperlipidemic and antihypertensive effect of a triterpenoid-rich extract from bamboo shavings and vasodilator effect of friedelin on phenylephrine-induced vasoconstriction in thoracic aortas of rats. Phytother Res. 2007;21:1135–1141. doi: 10.1002/ptr.2223. [DOI] [PubMed] [Google Scholar]
- Jin YC, Yuan K, Zhang J. Chemical Composition, and Antioxidant and Antimicrobial Activities of Essential Oil of Phyllostachys heterocycla cv. Pubescens Varieties from China. Molecules. 2011;16:4318–4327. doi: 10.3390/molecules16054318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones LH, Milne AA, Sanders JV. Tabashir: an opal of plant origin. Science. 1966;151:464–466. doi: 10.1126/science.151.3709.464. [DOI] [PubMed] [Google Scholar]
- Jung SH, Lee JM, Lee HJ, Kim CY, Lee EH, Um BH. Aldose reductase and advanced glycation Phyllostachys nigra. Biol Pharm Bull. 2007;30:1569–1572. doi: 10.1248/bpb.30.1569. [DOI] [PubMed] [Google Scholar]
- Kim KK, Kawano Y, Yamazaki Y. A novel porphyrin photosensitizer from bamboo leaves that induces apoptosis in cancer cell lines. Anticancer Res. 2003;23:2355–2361. [PubMed] [Google Scholar]
- Kim SH, Kim TS, Kim SJ, Seong CN, Lee OH, Lee HJ, Yoo JC. Inhibition of interleukin-12 production in mouse macrophages via suppression of nuclear 1 factor-kappaB binding activity by Phyllostachys nigra var. henonis. Immunopharmacol Immunotoxicol. 2007;29:131–139. doi: 10.1080/08923970701283476. [DOI] [PubMed] [Google Scholar]
- Kim SH, Kim TS, Lee HJ, Yoo JC. Enhancement of 1,25-dihydroxyvitamin D3- and all-trans retinoic acid-induced differentiation of human leukemia HL-60 cells by Phyllostachys nigra var. henonis. Immunopharmacol Immunotoxicol. 2007;29:119–129. doi: 10.1080/08923970701283310. [DOI] [PubMed] [Google Scholar]
- Kimura Y, Suto S, Tatsuka M. Evaluation of carcinogenic/co-carcinogenic activity of chikusaku-eki, a bamboo charcoal by-product used as a folk remedy, in BALB/c 3T3 cells. Biol Pharm Bull. 2002;25:1026–1029. doi: 10.1248/bpb.25.1026. [DOI] [PubMed] [Google Scholar]
- Koide CL, Collier AC, Berry MJ, Panee J. The effect of bamboo extract on hepatic biotransforming enzymes–findings from an obese-diabetic mouse model. J Ethnopharmacol. 2011;133:37–45. doi: 10.1016/j.jep.2010.08.062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kweon MH, Hwang HJ, Sung HC. Identification and antioxidant activity of novel chlorogenic acid derivatives from bamboo (Phyllostachys edulis) J Agric Food Chem. 2001;49:4646–4655. doi: 10.1021/jf010514x. [DOI] [PubMed] [Google Scholar]
- Kweon MH, Jung MJ, Sung HC. Cytoprotective effects of heme oxygenase-1 induction by 3-O-caffeoyl-1-methylquinic acid. Free Radic Biol Med. 2004;36:40–52. doi: 10.1016/j.freeradbiomed.2003.09.023. [DOI] [PubMed] [Google Scholar]
- Kweon MH, In Park Y, Sung HC, Mukhtar H. The novel antioxidant 3-O-caffeoyl-1-methylquinic acid induces Nrf2- dependent phase II detoxifying genes and alters intracellular glutathione redox. Free Radic Biol Med. 2006;40:1349–1361. doi: 10.1016/j.freeradbiomed.2005.12.002. [DOI] [PubMed] [Google Scholar]
- Kweon MH, Afaq F, Bhat KM, Setaluri V, Mukhtar H. A novel antioxidant 3-O-Caffeoyl-1-methylquinic acid enhances ultraviolet A- mediated apoptosis in immortalized HaCaT keratinocytes via Sp1-dependent transcriptional activation of p21(WAF1/Cip1) Oncogene. 2007;26:3559–3571. doi: 10.1038/sj.onc.1210135. [DOI] [PubMed] [Google Scholar]
- Lans C, Georges K, Brown G. Non-experimental validation of ethnoveterinary plants and indigenous knowledge used for backyard pigs and chickens in Trinidad and Tobago. Trop Anim Health Prod. 2007;39:375–385. doi: 10.1007/s11250-007-9026-0. [DOI] [PubMed] [Google Scholar]
- Lee HJ, Kim KA, Kang KD, Lee EH, Kim CY, Um BH, Jung SH. The compound isolated from the leaves of Phyllostachys nigra protects oxidative stress-induced retinal ganglion cells death. Food Chem Toxicol. 2010;48:1721–1727. doi: 10.1016/j.fct.2010.03.052. [DOI] [PubMed] [Google Scholar]
- Lee MJ, Kim MJ, Song YS, Song YO, Moon GS. Bamboo culm extract supplementation elevates HDL-cholesterol and ameliorates oxidative stress in C57BL/6 mice fed atherogenic diet. J Med Food. 2008a;11:69–77. doi: 10.1089/jmf.2007.009. [DOI] [PubMed] [Google Scholar]
- Lee MJ, Park WH, Song YS, Lee YW, Song YO, Moon GS. Effect of bamboo culm extract on oxidative stress and genetic expression: bamboo culm extract ameliorates cell adhesion molecule expression and NFkappaB activity through the suppression of the oxidative stress. Clin Nutr. 2008b;27:755–763. doi: 10.1016/j.clnu.2008.06.002. [DOI] [PubMed] [Google Scholar]
- Lin Y, Collier AC, Liu W, Berry MJ, Panee J. The inhibitory effect of bamboo extract on the development of 7,12-dimethylbenz[a]anthracene (DMBA)-induced breast cancer. Phytother Res. 2008;22:1440–1445. doi: 10.1002/ptr.2439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu B, Wu X, Tie X, Zhang Y, Zhang Y. Toxicology and safety of anti-oxidant of bamboo leaves. Part 1: Acute and subchronic toxicity studies on anti-oxidant of bamboo leaves. Food Chem Toxicol. 2005;43:783–792. doi: 10.1016/j.fct.2005.01.019. [DOI] [PubMed] [Google Scholar]
- Lu B, Wu X, Shi J, Dong Y, Zhang Y. Toxicology and safety of antioxidant of bamboo leaves. Part 2: developmental toxicity test in rats with antioxidant of bamboo leaves. Food Chem Toxicol. 2006;44:1739–1743. doi: 10.1016/j.fct.2006.05.012. [DOI] [PubMed] [Google Scholar]
- Lu B, Xia D, Huang W, Wu X, Zhang Y, Yao Y. Hypolipidemic effect of bamboo shoot oil (P. pubescens) in Sprague-Dawley rats. J Food Sci. 2010;75:H205–H211. doi: 10.1111/j.1750-3841.2010.01716.x. [DOI] [PubMed] [Google Scholar]
- Manonayagi S, Vanithakumari G, Padma S, Malini T. Effects of bamboo buds: structural and functional changes in the epididymis of rats. J Ethnopharmacol. 1989;25:201–212. doi: 10.1016/0378-8741(89)90022-6. [DOI] [PubMed] [Google Scholar]
- Muniappan M, Sundararaj T. Antiinflammatory and antiulcer activities of Bambusa arundinacea. J Ethnopharmacol. 2003;88:161–167. doi: 10.1016/s0378-8741(03)00183-1. [DOI] [PubMed] [Google Scholar]
- Nagasawa H, Hattori A. Effects of gonadectomy at different ages and Sasa Health, bamboo grass leaf extract, on spontaneous motor activity in female and male mice. In Vivo. 2001;15:503–511. [PubMed] [Google Scholar]
- Nagasawa H, Murayama Y, Ishigame H. Food restriction and spontaneous motor activity in male mice: effects of feeding pattern, far-infrared ray and bamboo grass leaf extract. In Vivo. 2001;15:309–318. [PubMed] [Google Scholar]
- Nirmala C, David E, Sharma ML. Changes in nutrient components during ageing of emerging juvenile bamboo shoots. Int J Food Sci Nutr. 2007;58:612–618. doi: 10.1080/09637480701359529. [DOI] [PubMed] [Google Scholar]
- Okabe S, Takeuchi K, Takagi K, Shibata M. Stimulatory effect of the water extract of bamboo grass (Folin solution) on gastric acid secretion in pylorus-ligated rats. Jpn J Pharmacol. 1975;25:608–609. doi: 10.1254/jjp.25.608. [DOI] [PubMed] [Google Scholar]
- Panee J. Bamboo extract in the prevention of diabetes and breast cancer. In: Watson RR, editor. Complementary and alternative therapies and the aging population: an evidence-based approach. Elsevier; 2008. pp. 159–191. [Google Scholar]
- Panee J, Liu W, Lin Y, Gilman C, Berry MJ. A novel function of bamboo extract in relieving lipotoxicity. Phytother Res. 2008;22:675–680. doi: 10.1002/ptr.2395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park EJ, Jhon DY. Effects of bamboo shoot consumption on lipid profiles and bowel function in healthy young women. Nutrition. 2009;25:723–728. doi: 10.1016/j.nut.2009.01.007. [DOI] [PubMed] [Google Scholar]
- Park HS, Lim JH, Kim HJ, Choi HJ, Lee IS. Antioxidant flavone glycosides from the leaves of Sasa borealis. Arch Pharm Res. 2007;30:161–166. doi: 10.1007/BF02977689. [DOI] [PubMed] [Google Scholar]
- Park KH, Lee MW. Anti-oxidative, anti-inflammatory and whitening effects of phenolic compounds from Bambusae Caulis in Liquamen. Nat Prod Res. 2012;26:1687–1691. doi: 10.1080/14786419.2011.593517. [DOI] [PubMed] [Google Scholar]
- Premgamone A, Maskasem S, Thamrongwarangoon A, Ussavaphark W. Risks of repeated visits for uninvestigated dyspepsia in three community hospitals of Khon Kaen, Thailand. J Med Assoc Thai. 2010;93:S30–S37. [PubMed] [Google Scholar]
- Qi XF, Kim DH, Yoon YS, Li JH, Jin D, Deung YK, Lee KJ. Effects of Bambusae caulis in Liquamen on the development of atopic dermatitis-like skin lesions in hairless mice. J ethnopharmacol. 2009;123:195–200. doi: 10.1016/j.jep.2009.03.020. [DOI] [PubMed] [Google Scholar]
- Qi XF, Kim DH, Yoon YS, Song SB, Teng YC, Cai DQ, Lee KJ. Bambusae caulis in Liquamen Suppresses the Expression of Thymus and Activation-Regulated Chemokine and Macrophage-Derived Chemokine in Human Keratinocytes due to Antioxidant Effect. Evidence-based complementary and alternative medicine. 2012 doi: 10.1155/2012/617494. eCAM:617494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ren M, Reilly RT, Sacchi N. Sasa health exerts a protective effect on Her2/NeuN mammary tumorigenesis. Anticancer Res. 2004;24:2879–2884. [PubMed] [Google Scholar]
- Román GC. Autism: transient in utero hypothyroxinemia related to maternal flavonoid ingestion during pregnancy and to other environmental antithyroid agents. J Neurol Sci. 2007;262:15–26. doi: 10.1016/j.jns.2007.06.023. [DOI] [PubMed] [Google Scholar]
- Sakai S, Saito G, Sugayama J, Kamasuka T, Takada S, Takano T. On the Anticancer Action of Bamboo Extract. J Antibiot B. 1963;16:387–391. [PubMed] [Google Scholar]
- Seki T, Kida K, Maeda H. Immunostimulation-Mediated Anti-tumor Activity of Bamboo (Sasa senanensis) Leaf Extracts Obtained Under ‘Vigorous’ Condition. Evid Based Complement Alternat Med. 2010;7:447–457. doi: 10.1093/ecam/nen026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seki T, Maeda H. Cancer preventive effect of Kumaizasa bamboo leaf extracts administered prior to carcinogenesis or cancer inoculation. Anticancer Res. 2010;30:111–118. [PubMed] [Google Scholar]
- Shibata M, Yamatake Y, Sakamoto M, Kanamori M, Takagi K. Phamacological studies on bamboo grass (1). Acute toxicity and anti-inflammatory and antiulcerogenic activities of water-soluble fraction(Folin) extracted from Sasa albomarginata Makino et Shibata. Nippon Yakurigaku Zasshi. 1975;71:481–490. [PubMed] [Google Scholar]
- Shibata M, Kubo K, Onoda M. Pharmacological studies on bamboo grass. (2) Central depressant and antitoxic actions of a water-soluble fraction (folin) extracted from Sasa albomarginata Makino et Shibata. Nippon Yakurigaku Zasshi. 1976;72:531–541. doi: 10.1254/fpj.72.531. [DOI] [PubMed] [Google Scholar]
- Shibata M, Kubo K, Onoda M. Pharmacological studies on bamboo grass. III Effects on cardiovascular and isolated organs of water-soluble fraction extracted from Sasa albomarginata Makino et Shibata (Bambusaceae) (author’s transl) Yakugaku Zasshi. 1978;98:1436–1440. doi: 10.1248/yakushi1947.98.10_1436. [DOI] [PubMed] [Google Scholar]
- Shibata M, Fojii M, Yamaguchi R. Pharmacological studies on bamboo grass. IV Toxicological and pharmacological effects of the extract (FIII) obtained from Sasa albomarginata Makino et Shibata (author’s transl) Yakugaku Zasshi. 1979;99:663–668. doi: 10.1248/yakushi1947.99.6_663. [DOI] [PubMed] [Google Scholar]
- Tsunoda S, Yamamoto K, Sakamoto S, Inoue H, Nagasawa H. Effects of Sasa Health, extract of bamboo grass leaves, on spontaneous mammary tumourigenesis in SHN mice. Anticancer Res. 1998;18:153–158. [PubMed] [Google Scholar]
- Vanithakumari G, Manonayagi S, Padma S, Malini T. Antifertility effect of Bambusa arundinacea shoot extracts in male rats. J Ethnopharmacol. 1989;25:173–180. doi: 10.1016/0378-8741(89)90019-6. [DOI] [PubMed] [Google Scholar]
- Yakubu MT, Bukoye BB. Abortifacient potentials of the aqueous extract of Bambusa vulgaris leaves in pregnant Dutch rabbits. Contraception. 2009;80:308–313. doi: 10.1016/j.contraception.2009.03.003. [DOI] [PubMed] [Google Scholar]
- Yakubu MT, Bukoye BB, Oladiji AT, Akanji MA. Toxicological implications of aqueous extract of Bambusa vulgaris leaves in pregnant Dutch rabbits. Hum Exp Toxicol. 2009;28:591–598. doi: 10.1177/0960327109106975. [DOI] [PubMed] [Google Scholar]
- Yang Y. Chinese Herbal Medicines Comparisons and Characteristics. Churchill Livingstone; London: 2002. [Google Scholar]
- Zhang Y, Wu XQ, Yu ZY. Comparison study on total flavonoid content and anti-free redical activity of the leaves of bamboo, phyllostachys nigra, and Ginkgo bilabo. Zhongguo Zhong Yao Za Zhi. 2002;27:254–257. [PubMed] [Google Scholar]
- Zhang Y, Wu X, Ren Y, Fu J, Zhang Y. Safety evaluation of a triterpenoid-rich extract from bamboo shavings. Food Chem Toxicol. 2004;42:1867–1875. doi: 10.1016/j.fct.2004.07.005. [DOI] [PubMed] [Google Scholar]
- Zhang Y, Yao X, Bao B, Zhang Y. Anti-fatigue activity of a triterpenoid-rich extract from Chinese bamboo shavings (Caulis bamfusae in taeniam) Phytother Res. 2006;20:872–876. doi: 10.1002/ptr.1965. [DOI] [PubMed] [Google Scholar]
- Zhang Y, Tie X, Bao B, Wu X, Zhang Y. Metabolism of flavone C- glucosides and p-coumaric acid from antioxidant of bamboo leaves (AOB) in rats. Br J Nutr. 2007;97:484–494. doi: 10.1017/S0007114507336830. [DOI] [PubMed] [Google Scholar]
- Zhao R, Zhao M, Wang H, Taneike Y, Zhang X. Arsenic speciation in moso bamboo shoot–a terrestrial plant that contains organoarsenic species. Sci Total Environ. 2006;371:293–303. doi: 10.1016/j.scitotenv.2006.03.019. [DOI] [PubMed] [Google Scholar]


