Abstract
Purpose
The purpose of this study was to compare the biomechanics of the effortful pitch glide (EPG) with swallowing using dynamic MRI. The EPG is a combination of a pitch glide and a pharyngeal squeeze maneuver for targeting laryngeal and pharyngeal muscles. The authors hypothesized that the EPG would elicit significantly greater structural excursions of anterior hyoid, superior hyoid, hyolaryngeal approximation, laryngeal elevation, and lateral pharyngeal wall medialization compared with swallowing.
Method
Eleven healthy, young subjects with a mean age of 25 were recruited. The EPG was first taught and verified via laryngoscopy. Then 2-planar (coronal and sagittal) dynamic MRI acquisitions captured 10 repeated swallows and 3 EPGs. Kinematic analyses of minimum and maximum excursion of anatomical landmarks were calculated.
Results
Results showed a nonsignificant difference between the 2 tasks for range of excursion with all measured biomechanics except for superior hyoid, where the swallow showed significantly greater excursion. This indicated that swallowing and EPG biomechanics were comparable, lending support for the potential use of the EPG as another nonswallowing exercise.
Conclusion
Findings suggest EPG may be an effective exercise to target several important swallowing muscles, especially the long pharyngeal muscles that elevate the larynx and shorten the pharynx in swallowing.
Keywords: swallow, exercise, dynamic MRI, pitch glide, pharyngeal squeeze maneuver
Introduction
Rehabilitation of the swallow using exercises is frequently implemented to improve deglutitive function. However, the underlying mechanics of many of these exercises have not been fully explored. Two examples of these exercises are the falsetto and the pharyngeal squeeze maneuver (PSM). The falsetto, described by Logemann (1997), was suggested as an exercise to target laryngeal elevation because the “larynx elevates almost as much as it does during the swallow” (p. 210). The PSM is a technique used in the clinical setting with the goal of improving pharyngeal strength (Bastian, 1993; Fuller, Leonard, Aminpour, & Belafsky, 2009). In this study we proposed an exercise combining the pitch glide and PSM called the effortful pitch glide (EPG) and we compared the mechanics of the exercise with the mechanics of swallowing.
With the falsetto exercise, the patient is instructed to glide up in pitch, reaching a high, squeaky voice, then sustaining it for several seconds using effort (Logemann, 1997). In a study by Malandraki, Hind, Gangnon, Logemann, and Robbins (2011), the patients who were found to have more severe penetration-aspiration scores exhibited lower fundamental frequency and abnormal pitch elevation, judged acoustically and perceptually, respectively. These results highlight the relationship between vocal range and certain aspects of deglutitive function, specifically airway protection.
The PSM is another technique used in the clinical setting with the goal of improving pharyngeal strength. The PSM was developed by Bastian (1993) to evaluate contraction of the pharyngeal constrictors. Subjects are instructed to sustain a vowel at the highest intensity possible to engage the contraction of the pharyngeal constrictors by producing a forceful “ee” sound. With use of the PSM, an estimate of pharyngeal strength may be inferred by observing pharyngeal wall muscle contraction during the maneuver through a binary rating of normal or abnormal (Bastian, 1993; Fuller et al., 2009).
The PSM and falsetto are two examples of techniques that are considered nonswallow exercises. Although principles of strength training stress task specificity, that is, the exercise resembles the physiology of the swallow, there are several other exercises that have revealed improved function that are not considered task specific, including the Lee Silverman Voice Treatment and the Shaker exercise (Easterling, 2008; El Sharkawi et al., 2002). These studies demonstrated a principle of neural plasticity, known as transference, where improvements in function from one type of modality can also facilitate acquisition of similar functions (Kleim & Jones, 2008).
The EPG is a combination of a pitch glide and PSM, designed to target both the laryngeal and pharyngeal muscles. In a recent study by Pearson, Hindson, Langmore, and Zumwalt (2013), muscle function MRI (mfMRI) was used to determine that the suprahyoids and longitudinal pharyngeal muscles demonstrated significant changes during the EPG, as evidenced in T2 signal profiles. When a muscle is activated, changes occur in the intracellular water concentration by the metabolic by-products generated during the contraction and is measured by the T2 signal. The stronger the muscle activation, the more increased changes in the intracellular water concentration are seen. Thus, the EPG was shown to elicit significantly greater muscle activation in these muscle groups compared with swallowing. However, muscle activation differs from muscle “action.” Therefore, the purpose of this study was to determine whether the biomechanics, or muscle action, of the EPG relevant to swallowing (hyoid excursion, laryngeal elevation, and pharyngeal wall constriction) approximated the biomechanics of swallowing by using dynamic MRI.
Dynamic MRI has been used to evaluate laryngeal kinematics associated with pitch elevation (Ahmad, Dargaud, Morin, & Cotton, 2009; Echternach, Traser, Markl, & Richter, 2011; Miller et al., 2012). Echternach, Traser, Markl, and Richter (2011) used dynamic real-time MRI to analyze the vocal tract of male alto singers producing the /a/ sound transitioning from modal register to stage and naïve falsetto. Elevation of the larynx was shown to be highly associated with production of high pitch in the modal register. In another study, laryngeal elevation, approximation of the larynx to the hyoid, superior and anterior movement of the hyoid, and a shortening and widening of the supraglottic area were also observed during an ascending humming task examined by MRI (Miller et al., 2012). Laryngeal kinematics from these two studies suggest the production of high pitch causes an elevation of the larynx.
On the basis of these findings from the mfMRI and dynamic MRI studies, we hypothesized the biomechanics of the EPG would elicit significantly greater excursions relevant to swallow biomechanics, specifically in anterior hyoid, superior hyoid, hyolaryngeal approximation, laryngeal elevation, pharyngeal shortening, and lateral pharyngeal wall medialization. If greater or similar movements were recorded, this would suggest that the EPG has the potential to target similar biomechanics of the swallow.
Methods
Eleven healthy subjects (six men, five women) age 22–30 years (mean age = 25) participated in this study under a research protocol approved by the Boston Medical Campus Institutional Review Board. Each subject first participated in a laryngoscopic exam and then two-planar dynamic MRI. Subjects were first taught how to produce the /i/ sound starting at their modal pitch, gradually elevating to their highest pitch, and then exerting effort once their highest pitch was attained to produce a forceful “ee” sound, as described by Bastian (1993). When they were able to perform the task perceptually, they were prepared for the laryngoscopic exam using two sprays of a nasal decongestant (neosyenephrine) and two sprays (0.1 ml) of 4% Lidocaine delivered via an atomizer through one nare. Following this, a flexible laryngoscope with an outer diameter of 3.4 mm was passed through the anesthetized nare. The scope was positioned just above the tip of the epiglottis for viewing the hypopharynx. Subjects were again provided with instructions for performing the EPG during the exam. One to three attempts were needed to achieve an acceptable EPG, as judged endoscopically as well as perceptually (see Figure 1, Panels A and B). Each subject completed the exam first in seated and then supine position in order to emulate the required posture for the MRI.
Figure 1.
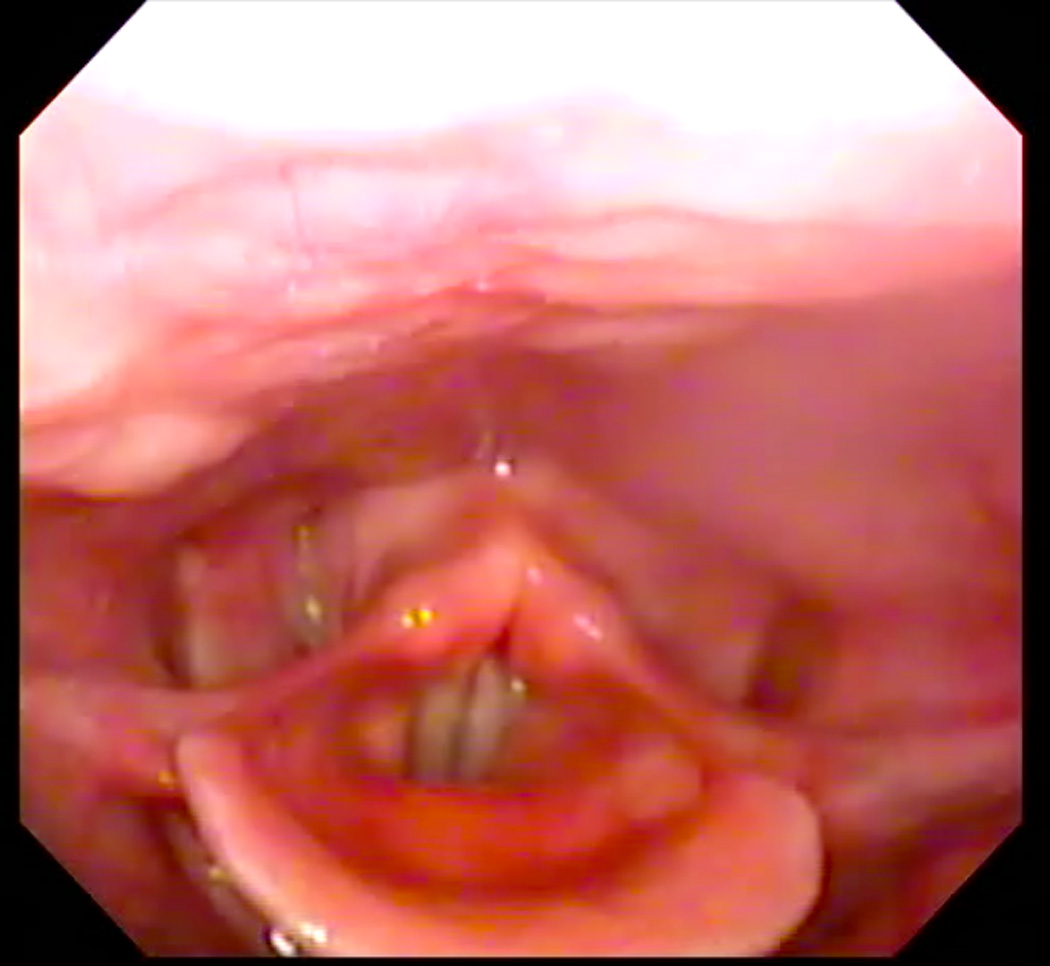
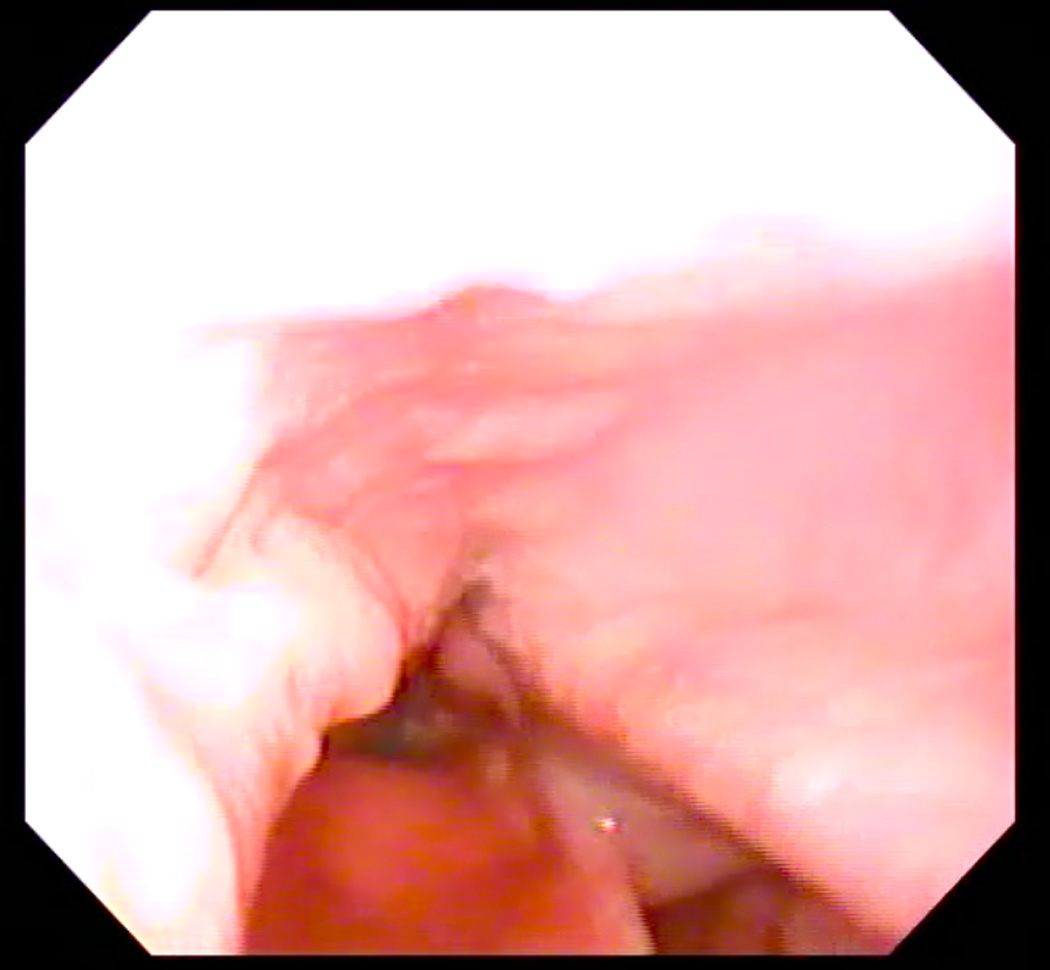
A: Endoscopic image of hypopharynx at rest. B: Endoscopic image of effortful pitch glide (EPG) illustrating medialization of pharyngeal walls.
Dynamic MRI
Within a week following the laryngoscopic exam, two-planar dynamic MRI scans were acquired from subjects during a repeated swallows task and EPG. A 3 Tesla Philips Achieva MRI scanner with 16-channel neurovascular coil was used to acquire a block of 10-mm slice images (sagittal alternating with coronal) from above the hard palate to below the cricoid cartilage at rest at 4.3 frames per second]fps] using a T1-weighted fast gradient echo sequence, with TE/ TR = 0.9/2.4 ms. A lower frame rate was chosen to ensure high-quality anatomic images for biomechanical analysis. Faster frame rates provide better temporal data, whereas lower frame rates provide better spatial data. To compensate for this trade-off, a sequential swallowing task was chosen to capture the dynamic range of movement in swallowing. Because MRI cannot capture all frames in one swallow, the swallows were repeated, or they were sequential, meaning a series of swallows were performed and analyzed. Subjects were provided with a visual image of PowerPoint slides guiding subjects through the protocol. Each acquisition included an “at rest” phase followed by either 10 sequential swallows or three EPGs. To facilitate the sequential swallows task, a magnesium-infused bolus, serving as a contrast for T1-weighted sequences, was delivered through a tube connected to a container of liquid.
Biomechanical Analysis
Osirix digital imaging and communication in medicine software was used to produce digital QuicktimeTM videos of dynamic MRI swallows and EPG (www.osirix-viewer.com). Image J image analysis software was used to collect coordinate data of anatomical landmarks from the digital video files (http://rsbweb.nih.gov/ij). From the sagittal image, coordinates of anatomical landmarks tracking the movement of the hyoid, larynx, and pharyngeal walls were collected at rest and at maximum excursion for both the repeated swallowing task and EPG. Minimum and maximum hyolaryngeal excursion among all dynamics (frames) were selected for measurement to ensure that the dynamic range of movement was characterized. Anatomical landmarks included the mandible (inferior mental spine), the posterior edge of the hard palate, the tubercle of the atlas (as a proxy for the styloid process in the lateral view), the anterior inferior edge of C2 and C4, the anterior superior edge of the hyoid, the anterior commissure of the vocal fold, the posterior attachment of the vocal fold, and the most inferior point of the air column in the hypopharynx proximal to the upper esophageal sphincter (UES; see Figure 2, Panels A–D). From coronal images, coordinates of anatomical landmarks mapping pharyngeal wall medialization were measured at the level of the arytenoids for both the repeated swallowing task and EPG (see Figures 3, Panels A–D). Coordinates mapping anatomical landmarks were used to calculate excursion measurements in centimeters for swallowing and EPG, including anterior and superior hyoid movement, hyolaryngeal approximation, laryngeal elevation, and pharyngeal shortening in the midsaggital plane and pharyngeal wall medialization in the coronal plane (see Figure 4). A speech-language pathologist and anatomist measured all subjects independently for interrater reliability.
Figure 2.


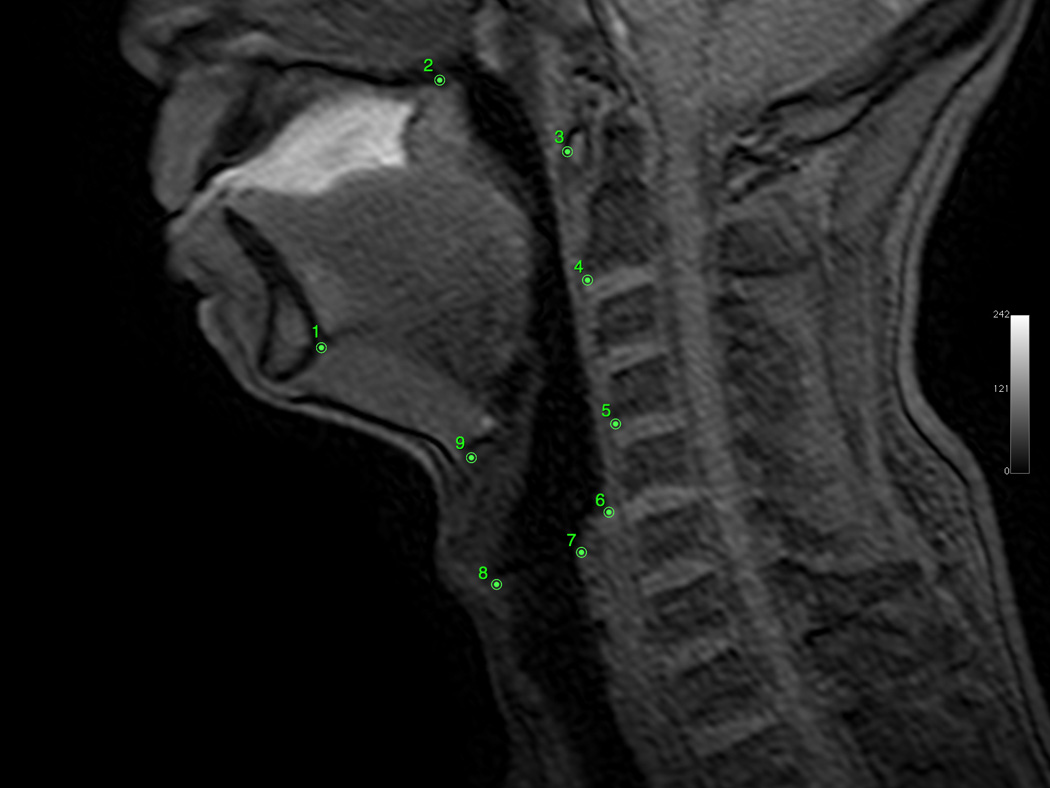

A: Sagittal view of minimum hyolaryngeal excursion during EPG. B: Sagittal view of maximum hyolaryngeal excursion during EPG. C: Sagittal view of minimum hyolaryngeal excursion during swallowing. D: Sagittal view of maximum hyolaryngeal excursion during swallowing.
Figure 3.


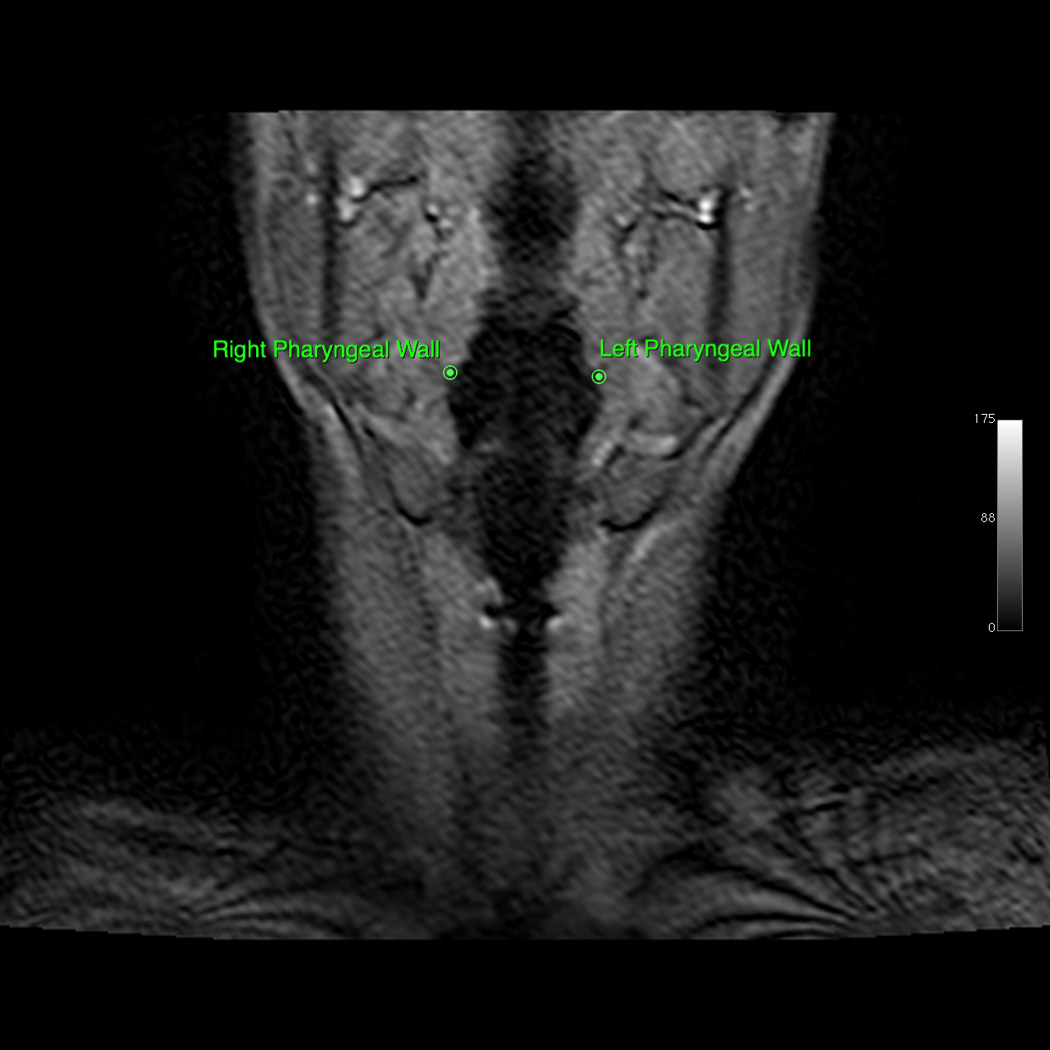

A: Coronal view of minimum hyolaryngeal excursion during EPG. B: Coronal view of maximum hyolaryngeal excursion during EPG. C: Coronal view of minimum hyolaryngeal excursion during swallowing. D: Coronal view of maximum hyolaryngeal excursion during swallowing. a. Coronal view of minimum hyolaryngeal excursion during effortful pitch glide
Figure 4.

Schematic of kinematic measurements: 1 = pharyngeal wall medialization; 2 = anterior hyoid movement; 3 = superior hyoid movement; 4 = hyolaryngeal approximation; 5 = laryngeal elevation; 6 = pharyngeal shortening.
Data Analysis
Interrater reliability of coordinates and final results were calculated using the Pearson product–moment correlation coefficient. The interrater reliability for measuring the anatomical landmarks obtained from MRI ranged from 0.76 to 0.97. A two-tailed paired t-test comparison was used to compare extent of movement with the swallow and EPG at rest and at maximum excursion. A Bonferroni correction was applied to the t tests to establish statistical significance with multiple comparisons (a = .05, p = .0083).
Results
None of the biomechanics measured from the EPG proved to have greater excursion than swallowing, contrary to the hypothesis. Five of six excursions measured demonstrated no statistically significant differences between the repeated swallows task and EPG, indicating that the biomechanics of the EPG were similar to the repeated swallows task for anterior hyoid movement, hyolaryngeal approximation, laryngeal elevation, pharyngeal shortening, and lateral pharyngeal wall medialization (see Figure 5). The remaining measurement, superior movement of the hyoid, was statistically greater in swallowing compared with the EPG. Means, standard deviations, and p values are reported in Table 1.
Figure 5.

Means (in cm) and 95% confidence intervals (error bars) for kinematic measurements of the EPG and swallowing task as represented by dynamic MRI: anterior hyoid movement (AntHy), superior hyoid movement (SupHy), hyolaryngeal approximation (HyLxApp), laryngeal elevation (LxEl), pharyngeal shortening (PhxShrt), and pharyngeal wall medialization (PhxMed).
Table 1.
Biomechanics of EPG and Swallow in cm
| EPG Rest to Max (cm) Mean ± SD |
Swallow Rest to Max (cm) Mean± SD |
p-values | |
|---|---|---|---|
| Anterior Hyoid Movement | 1.02±0.56 | 1.02±0.34 | 0.98 |
| Superior Hyoid Movement | 0.49±0.68 | 1.37±0.73 | 0.00* |
| Hyolaryngeal Approximation | 0.27±0.28 | −0.17±0.5 | 0.02 |
| Laryngeal Elevation | 1.4±0.65 | 1.61±0.64 | 0.3 |
| Pharyngeal Shortening | 1.3±0.69 | 0.96±0.67 | 0.2 |
| Lateral Pharyngeal Wall Approximation | 1.81±0.68 | 2.29±0.51 | 0.16 |
p value significant
Discussion
All biomechanical measurements of the EPG in this study, except for superior hyoid movement, were neither significantly greater than nor significantly less than the biomechanics of swallowing. These results suggest that kinematics of the EPG resembled the kinematics of swallowing.
In this study, the biomechanics of swallowing and the EPG were nearly identical (p = .98) for anterior hyoid movement. During the swallow, anterior motion of hyoid displacement contributes to laryngeal elevation and opening of the UES by contraction of the suprahyoid muscles, allowing the cricopharyngeus to be pulled open for the passage of a bolus (Sivarao & Goyal, 2000). Anterior hyoid movement has also been identified during rising pitch tasks (Miller et al., 2012; Sonninen, Hurme, & Laukkanen, 1999). Structural evidence suggests that geniohyoid is primarily responsible for the anterior movement of the hyoid in swallowing (Pearson, Langmore, & Zumwalt, 2011). The geniohyoid was shown to be active during the EPG, as measured by mfMRI with a medium effect size (d > .50) (Pearson et al., 2013). Of course, without neural inhibition of the UES, this sphincter did not open during the EPG. These data together indicate that the EPG might target the geniohyoid to facilitate anterior hyoid movement for UES opening during swallowing, when the UES is inhibited and able to be opened.
In the current study, the laryngeal elevation measurement approximated the actions of the stylopharyngeus (coordinate 7 represented the distal attachment, and coordinate 3 represented the proximal attachment), and the pharyngeal shortening measurement approximated actions of the palatopharyngeus (coordinate 6 represented the distal attachment, and coordinate 2 represented the proximal attachment). Both the stylopharyngeus and the palatopharyngeus, along with the salpingopharyngeus, are positioned to elevate the larynx and shorten the pharynx (Pearson et al., 2011). The EPG elicited slightly more pharyngeal shortening compared with swallowing, 1.3 cm compared with 0.96 cm, but this difference was not significant (p = .2).
Laryngeal elevation can also be achieved through the force translated from the hyoid and thyrohyoid membrane, applied by suprahyoid muscles, including the mylohyoid and digastric muscles. In this study, laryngeal elevation was measured at a mean of 1.4 cm for the EPG compared with 1.61 cm during the swallow, but again this was not significantly different (p = .3). This movement of the hyoid, along with the action of the thyrohyoid muscles, is commonly thought to elevate the larynx (Cook et al., 1989). However, in this study, the EPG produced laryngeal elevation with significantly reduced superior hyoid movement when compared with swallowing, with a mean excursion of only 1.37 cm compared with 0.49 cm for the EPG (p = .00). This suggests that the long pharyngeal muscles may contribute to laryngeal elevation in the EPG, distinct from the action of the suprahyoid muscles.
Taken together, this provides evidence that the EPG is especially effective in targeting the function of the long pharyngeal muscles. The palatopharyngeus is involved in velopharyngeal activity during speech tasks for sealing the nasal passage during the production of oral sounds (Perry, 2011). Palatopharyngeus activity has also been recorded during speech tasks consisting of phonemes and vowels (Bell-Berti, 1976), providing another possible explanation for pharyngeal shortening seen during the EPG. Interestingly, the /i/ sound has been documented to require more power during closure of the velopharynx compared with other vowels because of the manner with which it is produced (Kuehn & Moon, 2005). Additionally, the production of the /i/ sound has exhibited an increase in laryngeal elevation. Maximal laryngeal height was measured highest with the /i/ sound compared with the /a/, /u/, /e/, /o/, and /sh/ sounds during a sustained phonation task when examined with dynamic MRI (Ahmad et al., 2009; Demolin, Hassid, Metens, & Soquet, 2002). Thus, /i/ may be the best sound to use when performing the EPG.
The EPG elicited increased hyolaryngeal approximation (M = 0.27 ± 0.28 cm), whereas the swallow did not (M = −0.17 ± 0.5 cm, p = .02). The positive hyolaryngeal approximation measurement during the EPG indicated that the larynx outpaced the hyoid in hyolaryngeal excursion, and the negative hyolaryngeal approximation measurement during swallowing indicated that the hyoid outpaced the larynx during swallowing. A possible explanation of this finding may have been attributed to the experimental conditions with the subjects in supine position during the MRI, where hyoid movement needed to work against gravity.
Regarding medialization of the lateral pharyngeal walls, the EPG elicited a similar effect compared with the swallow. In this study, pharyngeal wall medialization moved a mean of 1.81 cm for the EPG compared with a mean of 2.29 cm during the swallow, which was not significantly different (p = .16). During deglutition, the contraction of the pharyngeal constrictors medialize the pharyngeal walls to propel the bolus through the pharynx (Langmore, 2001). Medialization of the lateral pharyngeal walls has been documented during the PSM (Bastian, 1993; Fuller et al., 2009). The upper lateral walls of the pharynx form portions of the superior pharyngeal constrictors that also contribute to closure of the velopharynx during phonation (Sumida, Yamashita, & Kitamura, 2012). In addition to the pharyngeal constrictors, the palatopharyngeus muscles contribute to the contraction of the lateral pharyngeal walls during phonation tasks (Sumida et al., 2012). The combined contraction of the pharyngeal constrictors and palatopharyngeus muscles may account for the similarities shown during the EPG and the swallow for medialization of the lateral pharyngeal walls.
Lastly, superior movement of the hyoid was much greater in swallowing than in the EPG. The mylohyoid and digastric have been shown to have a structural advantage for the superior elevation of the hyoid bone (Pearson et al., 2011). Interestingly, the superior hyoid movement in the current study was significantly less in the EPG than in the swallowing task, although the mfMRI data showed that muscle activation was greater in the mylohyoid and digastric during EPG than in the repeated swallows task (Pearson et al., 2013). This phenomenon may be attributable to the formation of the tongue required to make the /i/ sound. This increases the superior-to-inferior axis of the body of the tongue in the oral cavity, and the hyoid is the stabilizer, acting to support the actions of the tongue. Another possible explanation, as illustrated by the mfMRI and dynamic MRI evidence combined, suggests that the contraction of the mylohyoid during the EPG is more static than dynamic (i.e., an isometric contraction) compared with the dynamic action of the swallow.
There are several limitations of our study. Temporal resolution was sacrificed in order to gain anatomical detail in multiple planes within a large field of view. To compensate for a low frame rate (8.6 fps in two planes, or 4.3 fps in one plane), subjects swallowed 10 times and performed the EPG three times each to capture a full range of movement. Furthermore, humans swallow in an upright position, and not in the supine as necessitated by the experimental conditions of an MRI. It would also be useful to replicate this study with fluoroscopy in the upright position at a frame rate of 30 fps.
In the current study we did not obtain any acoustic or perceptual measures during the production of the EPG. These measures may be useful for the confirmation of EPG in a clinical setting where endoscopy may not be available for instruction. A recent article highlighted the clinical relevance of the current study. Malandraki et al. (2011) demonstrated an association between penetration-aspiration scores (Rosenbek, Robbins, Roecker, Coyle, & Woods, 1996) and reduced pitch elevation using perceptual and acoustical measurements in dysphagic patients. Subjects who were perceptually rated as having an abnormal, or reduced, pitch elevation and acoustically rated as lower maximum F0, or pitch level, exhibited worse penetration-aspiration scale scores. This study highlights the relationship between phonation and the swallow and also supports the importance of laryngeal elevation for preventing penetration and aspiration.
Instructions for teaching the EPG differed between the laryngoscopic exam and MRI. During the laryngoscopic exam, subjects were provided with verbal instructions and modeling of the task, whereas during the MRI, subjects were provided with the same printed instructions. The instructions and modeling were not standardized and thus could have affected performance, though none were observed in the dynamic MRI. Because subjects were instructed to produce a forceful “ee” sound, there is a concern of possible vocal fold hyperadduction if patients were to practice the EPG repeatedly, which could pose potential harm to the vocal folds.
The EPG, like the Shaker exercise, may prove to be a nonswallowing exercise that improves both swallowing and vocal function (Easterling, 2008). Although our study was done on young, healthy subjects who were able to perform the EPG, older patients with neurological and/or respiratory problems may not be able to produce the EPG adequately, though the exercise appeared to be relatively easy for subjects to learn and produce.
Lastly, our small sample size and subjects limit the generalizability to other populations. A future study is needed to evaluate the effects of the EPG in a larger sample with dysphagic patients.
Conclusion
In this study, we used a dynamic MRI sequence to document the relevance of the EPG to swallowing function. Although EPG biomechanics do not fully mimic swallowing mechanics, findings in this study show they do target several muscle functions relevant to swallowing, including laryngeal elevation, anterior hyoid excursion, and especially, the long pharyngeal muscles that elevate the larynx and shorten the pharynx in swallowing. Further studies are needed to examine the effects of the EPG in patients with dysphagia to determine the feasibility and potential therapeutic benefits of swallow rehabilitation.
Acknowledgements
Completion of this project was supported in part by Grant Number F31DC011705 from the National Institute On Deafness And Other Communication Disorders. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institute On Deafness And Other Communication Disorders or the National Institutes of Health.
References
- Ahmad M, Dargaud J, Morin A, Cotton F. Dynamic MRI of larynx and vocal fold vibrations in normal phonation. Journal of Voice. 2009;23:235–239. doi: 10.1016/j.jvoice.2007.08.008. [DOI] [PubMed] [Google Scholar]
- Bastian RW. The videoendoscopic swallowing study: An alternative and partner to the videofluoroscopic swallowing study. Dysphagia. 1993;8:359–367. doi: 10.1007/BF01321780. [DOI] [PubMed] [Google Scholar]
- Bell-Berti F. An electromyographic study of velopharyngeal function in speech. Journal of Speech and Hearing Research. 1976;19:225–240. doi: 10.1044/jshr.1902.225. [DOI] [PubMed] [Google Scholar]
- Cook IJ, Dodds WJ, Dantas RO, Massey B, Kern MK, Lang IM, Hogan WJ. Opening mechanisms of the human upper esophageal sphincter. The American Journal of Physiology. 1989;20:G748–G759. doi: 10.1152/ajpgi.1989.257.5.G748. [DOI] [PubMed] [Google Scholar]
- Demolin D, Hassid S, Metens T, Soquet A. Realtime MRI and articulatory coordination in speech. Comptes Rendus Biologies. 2002;325:547–556. doi: 10.1016/s1631-0691(02)01458-0. [DOI] [PubMed] [Google Scholar]
- Easterling C. Does an exercise aimed at improving swallow function have an effect on vocal function in the healthy elderly? Dysphagia. 2008;23:317–326. doi: 10.1007/s00455-008-9158-z. [DOI] [PubMed] [Google Scholar]
- Echternach M, Traser L, Markl M, Richter B. Vocal tract configurations in male alto register functions. Journal of Voice. 2011;25:670–677. doi: 10.1016/j.jvoice.2010.09.008. [DOI] [PubMed] [Google Scholar]
- El Sharkawi A, Ramig L, Logemann J, Pauloski B, Rademaker A, Smith C, Werner C. Swallowing and voice effects of Lee Silverman voice treatment (LSVT): A pilot study. Journal of Neurology, Neurosurgery & Psychiatry. 2002;72:31–36. doi: 10.1136/jnnp.72.1.31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fuller SC, Leonard R, Aminpour S, Belafsky PC. Validation of the pharyngeal squeeze maneuver. Otolaryngology—Head and Neck Surgery. 2009;140:391–394. doi: 10.1016/j.otohns.2008.12.015. [DOI] [PubMed] [Google Scholar]
- Kleim JA, Jones TA. Principles of experience-dependent neural plasticity: Implications for rehabilitation after brain damage. Journal of Speech, Language, and Hearing Research. 2008;51:S225–S239. doi: 10.1044/1092-4388(2008/018). [DOI] [PubMed] [Google Scholar]
- Kuehn DP, Moon JB. Histologic study of intravelar structures in normal human adult specimens. The Cleft Palate-Craniofacial Journal. 2005;42:481–489. doi: 10.1597/04-125r.1. [DOI] [PubMed] [Google Scholar]
- Langmore SE. Normal swallowing: The endoscopic perspective. In: Langmore SE, editor. Endoscopic evaluation and treatment of swallowing disorders. New York, NY: Thieme Medical Publishers; 2001. pp. 37–59. [Google Scholar]
- Logemann J. Evaluation and treament of swallowing disorders. 2nd ed. Austin, TX: Pro-Ed; 1997. [Google Scholar]
- Malandraki GA, Hind JA, Gangnon R, Logemann JA, Robbins J. The utility of pitch elevation in the evaluation of oropharyngeal dysphagia: Preliminary findings. American Journal of Speech-Language Pathology. 2011;20:262–268. doi: 10.1044/1058-0360(2011/10-0097). [DOI] [PubMed] [Google Scholar]
- Miller NA, Gregory JS, Semple SI, Aspden RM, Stollery PJ, Gilbert FJ. The effects of humming and pitch on craniofacial and craniocervical morphology measured using MRI. Journal of Voice. 2012;26:90–101. doi: 10.1016/j.jvoice.2010.10.017. [DOI] [PubMed] [Google Scholar]
- Pearson WG, Jr, Hindson DF, Langmore SE, Zumwalt AC. Evaluating swallowing muscles essential for hyolaryngeal elevation by using muscle functional magnetic resonance imaging. International Journal of Radiation Oncology, Biology, Physics. 2013;85:735–740. doi: 10.1016/j.ijrobp.2012.07.2370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pearson WG, Jr, Langmore SE, Zumwalt AC. Evaluating the structural properties of suprahyoid muscles and their potential for moving the hyoid. Dysphagia. 2011;26:345–351. doi: 10.1007/s00455-010-9315-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perry JL. Anatomy and physiology of the velopharyngeal mechanism. Seminars in Speech and Language. 2011;32:83–92. doi: 10.1055/s-0031-1277712. [DOI] [PubMed] [Google Scholar]
- Rosenbek JC, Robbins JA, Roecker EB, Coyle JL, Wood JL. A penetration-aspiration scale. Dysphagia. 1996;11(2):93–98. doi: 10.1007/BF00417897. [DOI] [PubMed] [Google Scholar]
- Sivarao DV, Goyal RK. Functional anatomy and physiology of the upper esophageal sphincter. American Journal of Medicine. 2000;108(Suppl. 1):27S–37S. doi: 10.1016/s0002-9343(99)00337-x. [DOI] [PubMed] [Google Scholar]
- Sonninen A, Hurme P, Laukkanen AM. The external frame function in the control of pitch, register, and singing mode: Radiographic observations of a female singer. Journal of Voice. 1999;13:319–340. doi: 10.1016/s0892-1997(99)80039-3. [DOI] [PubMed] [Google Scholar]
- Sumida K, Yamashita K, Kitamura S. Gross anatomical study of the human palatopharyngeus muscle throughout its entire course from origin to insertion. Clinical Anatomy. 2012;25:314–323. doi: 10.1002/ca.21233. [DOI] [PubMed] [Google Scholar]


