Abstract
Amyotrophic lateral sclerosis (ALS) is a progressive neurodegenerative disease that is usually fatal within 2–5 years. Unfortunately, the only treatment currently available is riluzole, which has a limited efficacy. As a redress, there is an expanding literature focusing on other potential treatments. One such potential treatment option utilizes the vascular endothelial growth factor (VEGF) family, which includes factors that are primarily associated with angiogenesis but are now increasingly recognized to have neurotrophic effects. Reduced expression of a member of this family, VEGF-A, in mice results in neurodegeneration similar to that of ALS, while treatment of animal models of ALS with either VEGF-A gene therapy or VEGF-A protein has yielded positive therapeutic outcomes. These basic research findings raise the potential for a VEGF therapy to be translated to the clinic for the treatment of ALS. This review covers the VEGF family, its receptors and neurotrophic effects as well as VEGF therapy in animal models of ALS and advances towards clinical trials.
Keywords: ALS, VEGF, Gene therapy, Protein therapy, Clinical trials
1. Introduction
1.1. Amyotrophic lateral sclerosis and ALS animal models
Amyotrophic lateral sclerosis (ALS), also known as Lou Gehrig's disease, is a progressive neurodegenerative condition that results in loss of both upper and lower motor neurons in the cerebral cortex, brainstem and spinal cord. The progression of the disease results in weakness, spasticity, muscle wasting, and, eventually, respiratory failure. It is usually fatal within 2–5 years of diagnosis. The incidence of ALS is ~1 in 50,000 (The ALS Association, www.alsa.org). ~90% of these cases are sporadic with the remaining 10% familial. Currently, the only therapy approved for the treatment of ALS is riluzole, which slows the loss of strength in limb muscles and extends survival by only a few months (Lacomblez et al., 1996).
Insights into the potential pathophysiology of the disease have implicated a number of gene mutations. One of the earliest ALS associated genes discovered was SOD1, coding for Cu/Zn superoxide dismutase 1 (Deng et al., 1993; Rosen et al., 1993). Within the familial cases of ALS, 20% are due to mutations in the Cu/Zn superoxide dismutase 1 (SOD1) gene (Howland et al., 2002). In addition to SOD1, a number of other genes have also been associated with ALS. The most recently reported mutation is an expansion of a non-coding hexanucleotide repeat in the C9ORF72 gene linked to both familial and sporadic ALS and the associated autosomal dominant frontotemporal dementia (FTD). An estimated 87% of all familial ALS cases in Finland are linked to either C9ORF72 or SOD1 mutations (DeJesus-Hernandez et al., 2011; Renton et al., 2011). Additional genes linked to familial ALS include ubiquitin-like protein ubiquilin 2 (UBQLN2) (Deng et al., 2011), fused in sarcoma (FUS) (Kwiatkowski et al., 2009; Vance et al., 2009), TAR DNA-binding domain protein (TDP43) (Sreedharan et al., 2008), polyphosphoinositide phosphatase (FIG4) (Chow et al., 2009), alsin (Yang et al., 2001), senataxin (SETX) (Y. Z. Chen et al., 2004), dynactin (LaMonte et al., 2002) and angiogenin (Greenway et al., 2006). For a comprehensive review of the current standing of ALS research see Turner et al. (2013).
Given the early discovery of the SOD1 mutation and the relatively large fraction of ALS familial cases associated with it, a number of animal models expressing a mutated form of the SOD1 gene have been developed. These SOD1 mutants have served as both a model of disease progression and as a platform to test potential therapies for ALS (Gurney et al., 1994; Howland et al., 2002). For example, rat and mouse models have been developed that overexpress the human SOD1G93A mutation, which displays many of the features found with ALS. In particular, these models display axonal and mitochondrial dysfunctions, progressive neuromuscular dysfunction, gliosis and motor neuron loss (Gurney et al., 1994; Howland et al., 2002). A large portion of this review will focus on these SOD1 models in the context of their utility to test experimental therapeutics that have the potential to ameliorate the ALS pathology. However, it should be noted that there are other emerging models based on the additional aforementioned genes associated with sporadic and familial ALS (Wegorzewska et al., 2009; Pelletier et al., 2012; Mitchell et al., 2013). The next section will provide a review of the VEGF proteins with an emphasis on their neurotrophic effects.
2. Vascular endothelial growth factor proteins and their neurotrophic effects
The vascular endothelial growth factor (VEGF) family is composed of multiple cell signaling proteins with known involvement in angiogenesis and lymphangiogenesis. The first identified protein was linked to vascular permeability induced by tumor cells (Senger et al., 1983) (originally the Vascular Permeability Factor, VPF), which was later shown to match the vascular endothelial growth factor protein discovered in 1989 (Ferrara & Henzel, 1989; Keck et al., 1989). Since the discovery of the first member, now known as VEGF-A, the family has grown to include several members including VEGF-A, VEGF-B (Grimmond et al., 1996; Olofsson et al., 1996), VEGF-C (V Joukov et al., 1996; Lee et al., 1996), VEGF-D (Orlandini et al., 1996; Yamada et al., 1997), VEGF-E (Ogawa et al., 1998), VEGF-F (Yamazaki et al., 2003) and Placental Growth Factor (PlGF) (Maglione et al., 1991). These disulfide linked, dimeric glycoproteins all fall into the joint platelet derived growth factor (PDGF)/VEGF factor protein family based on similar molecular structure. Table 1 and Fig. 1 outline the characteristics of the VEGF family and the current understanding of their roles.
Table 1.
Characteristics of the VEGF family.
| Protein | Number of isoforms | Known role | Knockout status | Key references |
|---|---|---|---|---|
| VEGF-A | Multiple isoforms | Angiogenesis; neuronal development | Knockout mice are embryonic lethal | (Carmeliet et al., 1996; Ferrara et al., 1996; Ruhrberg, 2003) |
| VEGF-B | At least two known isoforms | Role not clearly understood; expressed in heart, skeletal muscle and nervous system | Knockout mice not embryonic lethal – some cardiac and vascular dysfunction | (Olofsson et al., 1998; Bellomo et al., 2000; Aase et al., 2001) |
| VEGF-C | Multiple isoforms | Lymphangiogenesis; expressed in heart, lung and kidney | Knockout mice are embryonic lethal | (V Joukov et al., 1996; Kukk et al.,1996; Karkkainen et al., 2004; Wang et al., 2010) |
| VEGF-D | Two known isoforms | Lymphangiogenesis; expression found in heart, kidney, lung, skeletal muscle | Knockout mice not embryonic lethal; not essential for lymphangiogenesis in mice | (Avantaggiato, Orlandini, Acampora, Oliviero, & Simeone, 1998; Koch et al., 2009; Baldwin et al., 2001) |
| VEGF-E | Discovered in a dsDNA parapox virus | Causes angiogenesis when injected in Matrigel into the flanks of mice | Mice overexpressing VEGF-E had significant increase in vascularization in subcutaneous tissue | (Ogawa et al., 1998; Kiba et al., 2003) |
| VEGF-F | Isolated from snake venom | Increases vascular permeability and induces hypotension | N/A | (Yamazaki et al., 2003; Matsunaga et al., 2009) |
| PlGF | Four isoforms identified | Expressed in the placenta, heart, lung, thyroid, skeletal muscle, adipose tissue | Knockout mice have altered adipose dynamics & hyperinsulinemic | |
| Impaired angiogenesis and poor wound healing | (Carmeliet et al.,2001; Hemmeryckx et al., 2008; De Falco, 2012) |
Fig. 1.
Diagrammatic representation of the roles of the VEGF family. The diagram shows the major effects that VEGF proteins have across the cardiovascular, lymphatic and nervous system. (Sondell, Lundborg, & Kanje, 1999) (Hayakawa et al., 2011) (Forstreuter, Lucius, & Mentlein, 2002) (Stone et al., 1995) (Alon et al., 1995) (C. Ruiz de Almodovar et al., 2011a,b; Sondell, Sundler, & Kanje, 2000) (Meissirel, et al., 2011) (Schwarz, et al., 2004) (Witte, et al., 1998) (Ferrell, et al., 1998) (Jeltsch, et al., 1997; Oh, et al., 1997) (Shen, et al., 1993) (Clauss, et al., 1990) (Leung, Cachianes, Kuang, Goeddel, & Ferrara, 1989) (Connolly, et al., 1989).
2.1. Neurotrophic effects of vascular endothelial growth factor family
Interestingly, in addition to the classical roles of the VEGF protein family in angiogenesis and lymphangiogenesis, research over the last decade has suggested that they also have prominent neurotropic effects. The following section will review the individual family members and the studies focused on elucidating their role within the nervous system.
2.1.1. Vascular endothelial growth factor-A
VEGF-A has been shown to stimulate neurogenesis both in vivo and in vitro (Jin et al., 2002; Schänzer et al., 2004; Hashimoto et al., 2006). It has also been found to play a role in nerve migration. Schwartz et al. (2012) were able to show that VEGF-A is responsible for the migration of facial nerve soma, but is not necessarily responsible for axon guidance. However, studies in mice have identified a role for VEGF-A in axonal guidance. In one study, VEGF-A (with neuropilin 1, NPR-1) was found to be directly responsible for the guidance of retinal ganglion cell axons when deciding whether or not to cross the optic chiasm (Erskine et al., 2011). In a similar cross over scenario, another study showed that axons crossing the spinal cord utilize VEGF Receptor 2 (VEGFR2, discussed further below) and VEGF-A (Ruiz de Almodovar et al., 2011a,b).
VEGF-A also functions as a survival or protection factor. In the case of primary cortical neurons, VEGF-A treatment resulted in an increase in both the cell body diameter and the number of emerging neurites (Rosenstein et al., 2003). The survival of dopaminergic neurons in midbrain explants is also enhanced with VEGF-A application (Silverman et al., 1999). Several other studies have evaluated the effect of VEGF-A during challenges, all of which showed a protective effect. Such challenges include hypoxia (Jin et al., 2000; Oosthuyse et al., 2001), serum deprivation (Jin et al., 2000), excitotoxicity (Matsuzaki et al., 2001; Svensson et al., 2002), and mechanical trauma (Ma et al., 2011).
2.1.2. Vascular endothelial growth factor-B
Evidence suggests that VEGF-B can also serve as a survival factor for retinal and cortical neurons. In particular, Li et al. (2008) showed that treating mice with human-recombinant VEGF-B167 (e.g. the VEGF-B isoform with 167 amino acids) after optic nerve crush injury ameliorates the number of retinal ganglion cells undergoing apoptosis. Furthermore, they provided evidence of increased cortical neuron survival with VEGF-B167 treatment in the border zone of a stroke model using middle cerebral artery occlusion in mice (Y. Li et al., 2008). These results complement the work of Sun et al. (2004) who showed that the volume of cerebral infarct is larger in VEGF-B deficient mice when compared to controls (Sun et al., 2004).
2.1.3. Vascular endothelial growth factor-C
A small body of literature focuses on the role of VEGF-C in the development of the nervous system. Le Bras et al. (2006) showed the expression of VEGF Receptor 3 (VEGFR3, discussed further below) and VEGF-C in the mouse olfactory bulbs, suprachiasmatic area (ventricular and subventricular cells), and the optic nerve (particularly in the oligodendrocyte precursor cells). Additionally, they provided evidence that VEGF-C triggers a proliferative response in cultured optic nerve oligodendrocyte precursor cells. These results were corroborated by their observations with Vegfc+/− and Vegfcc−/− mouse embryos, both of which demonstrated reductions in the number of oligodendrocyte precursor cells compared to wild-type animals (Le Bras et al., 2006). The role of VEGF-C in the central nervous system is further expanded by Shin et al. (2008). Their findings suggest that there is a weak baseline expression of VEGF-C and VEGFR3 in the pyramidal and granule cell layers of the hippocampus. Furthermore, they showed an increased expression of VEGF-C and VEGFR-3 following transient ischemia, which localized to GFAP immunoreactive astrocytes and Iba1+/Ed1+ amoeboid shaped macrophages (Shin et al., 2008). Notably, VEGF-C has been shown by Piltonen et al. to promote the survival of dopaminergic cells and have a protective effect against 6-hydroxydopamine (6-OHDA) lesions in rats (to simulate Parkinson's disease). This study also showed that introduction of VEGF-C into the striatum results in proliferation and activation of astroglia and microglia and perturbation of the blood–brain barrier (Piltonen et al., 2011). The work of Piltonen et al. also reiterated the results of (Kranich et al., 2009), which showed that VEGFR3 is robustly expressed in murine glial precursors. Furthermore, Kranich et al. showed proliferation of glial precursor cells when stimulated with either VEGF-C or VEGF-D (Kranich et al., 2009).
2.1.4. Vascular endothelial growth factor-D
Although a limited number of studies have focused on the role of VEGF-D as a survival or protective factor for neurons, a recent report highlighted important results. Mauceri et al. (2011) showed that VEGF-D mRNA is expressed in cultured hippocampal neurons and that VEGF-D mRNA and protein levels are detectable at different developmental time points in the mouse cortex and hippocampus. More interestingly, they showed that RNA interference of VEGF-D expression in cultured hippocampal cells resulted in a reduction of dendritic length and complexity. They also determined that the VEGFR3 receptor with the p38 MAPK cascade is likely responsible for the effects on dendritic morphology (Mauceri et al., 2011). From a developmental standpoint, Schwartz et al. (2012) showed that VEGF-D, among other factors, contributes to the differentiation of human embryonic stem cells into dopaminergic neurons when co-cultured with stromal cell line PA6 (Schwartz et al., 2012).
2.1.5. Placental growth factor (PlGF)
There is limited literature implicating PlGF as a neurotrophic/neuroprotective factor. Two groups using the middle cerebral artery occlusion model showed increased levels of PlGF-2 in both neurons surrounding the core of the stroke (Beck et al., 2002) and in microvessels and interstitial spaces of the infarcted region (Du et al., 2010). Du et al. also found that cultured brain derived endothelial cells release PlGF upon glucose and oxygen deprivation. Finally, they showed that introduction of PlGF into a culture of neurons deprived of oxygen and glucose had a protective effect when compared to controls. A study on the role of PlGF in Wallerian degeneration showed that PlGF is found in the sciatic nerve, predominantly in the periphery of axons, in fibroblasts of the endoneurium, in the dorsal root ganglion neuron cell bodies, and in Schwann cells (though only after injury to the sciatic nerve with transection). Investigation of sciatic injury in PlGF knockout mice revealed a decrease in the number of Schwann cells proliferating around the injured nerve to form Bungner's bands, a delayed response of macrophage arrival and myelin sheath clearance, and a transient delay in axon regeneration and functional impairments in movement (Chaballe et al., 2011).
2.2. Current knowledge about vascular endothelial growth factor proteins and limitations
Several studies over the last decade have strongly established a role for the VEGF proteins in the nervous system. The evidence emerging suggests that VEGF proteins have diverse effects, including neuroprotection, axonal guidance, dendritic growth regulation, neuronal differentiation, and glial regulation. What is currently unclear in the literature is a coherent understanding of direct and indirect roles of VEGF proteins. Therefore, continued investigations of each of these roles will be essential to parse out the mechanisms of action versus downstream effects (e.g. is VEGF purely a vascular and/or glial regulator with positive outcomes for neurons?). An additional concern, as noted above, is the limited literature focusing on the neurotrophic effects of VEGF-C, VEGF-D, and PlGF. An increased focus on defining the effects of these factors is imperative to understand the global role of these proteins and their potential in therapeutic applications. Finally, there is a conspicuous lack of research focused on the neurotrophic roles of the VEGF-E and VEGF-F proteins.
3. Vascular endothelial growth factor receptors
Currently, there are three known receptors for the VEGF protein family. These receptors act through tyrosine kinase mechanisms with some specific effects noted for each receptor (e.g. VEGFR1, VEGFR2, and VEGFR3). In addition to these receptors, certain co-receptors or binding domains impact how VEGF proteins generate an effect. Included within this subset of modulators are neuropilins 1 and 2, which are noted receptors for semaphorins. Semaphorins are proteins that have a known role as axon guidance molecules. Furthermore, there is a Heparin binding domain in VEGF that interacts with Heparan sulfate glycosaminoglycans (HSPG), which also may serve as co-receptors. Table 2 and Fig. 2 outline some of the characteristics of the VEGF receptors. For a comprehensive review of VEGFR signaling see (Koch et al., 2011).
Table 2.
Characteristics of the VEGF receptors.
| Receptor/co-receptor | Binding protein | Expression patterns | Key references |
|---|---|---|---|
| VEGFR-1 | Binds VEGF-A, VEGF-B and PlGF | Developmental expression throughout the embryo | (de Vries et al., 1992; Fong et al., 1995; Olofsson et al., 1998) |
| VEGFR-2 | Binds VEGF-A, VEGF-C, VEGF-D and VEGF-E | Predominantly expressed in endothelial cells during embryogenesis | (Achen et al., 1998; V Joukov et al., 1996; Meyer et al., 1999; Millauer et al., 1993; Terman et al., 1992) |
| VEGFR-3 | Binds VEGF-C and VEGF-D | Expressed in endothelial cells of veins during embryogenesis | (V. Joukov et al., 1997; Kaipainen et al., 1995; Stacker et al., 1999) |
| Heparan sulfate glycosaminoglycans | Co-receptors, modulates receptor activity | Mice lacking heparin binding have perturbed blood vessel development and organization | (Houck et al., 1992; Stalmans et al., 2002) |
| Neuropilins 1 and 2 | Complexes with VEGF and heparan sulfate | Modulates VEGF activity | (Chen et al., 1997; Favier et al., 2006) |
Fig. 2.
VEGF receptors and their ligands. The three main VEGF receptors, the neuropilins (NRP), and the heparan sulfate proteoglycan (HPSG) are shown along with the VEGF family members that bind to each. VEGF receptor domains are indicated including the binding domain (BD), the transmembrane domain (TMD), the juxtamembrane domain (JMD), the ATP binding domain (TKD1), the kinase insert domain (KID), the phosphotransferase domain (TKD2), and the carboxy-terminal domain (CTD).
4. Evidence for the role of vascular endothelial growth factor in amyotrophic lateral sclerosis
The potential for a role for VEGF in ALS has been illustrated in a number of studies utilizing several variants of SOD1 mutation models (e.g. cell culture models, knock-in and knock-out models). The majority of these studies have focused on the VEGF-A and VEGF-B isoforms given the larger literature establishing their neurotrophic role.
4.1. Vascular endothelial growth factor-A and amyotrophic lateral sclerosis
In a seminal work that linked VEGF-A with ALS, Oosthuyse et al. (2001) showed that homozygous deletion of the hypoxia response element (HRE) of the VEGF-A promoter (so called VEGF-Aδ/δ) in mice resulted in motor neuron degeneration similar to ALS. ~60% of these mice died before or at birth due to vascular abnormalities in the lung. The remaining 40% showed symptoms of motor neuron degeneration at around 5 months of age. By 17 months, these mice had ~30% loss of motor neurons in the ventral horns of the spinal cord. There was also evidence for fewer Nissl bodies, abnormal mitochondria, irregular nuclei, peripheral aggregates of chromatin and reactive astrogliosis in the later stages (Oosthuyse et al., 2001). These changes in motor neuron morphology are analogous to those found in rodent models of ALS, which involves overexpression of a human pathogenic G93A mutation in the SOD1 gene (SOD1G93A). Follow-up research has since focused predominantly on the role of VEGF-A protein in treating SOD1 mutant mice and rats (details of some studies are presented below, others involving gene therapy approaches are presented in the next section). The earliest evidence for a protective role for VEGF-A is from a motor neuron-like cell culture model that was infected with an adenovirus vector containing the SOD1G93A mutation. When treated with VEGF-A, these cells demonstrated a dose dependent resistance to damage with noted activation of both MAPK and PI3-K pathways. Further analysis revealed that the protective effects of VEGF-A were exerted through the PI3k-Akt pathway (Li et al., 2003). Motor neuron culture studies have also shown that VEGF-A does indeed enhance survival of neurons, including under conditions of hypoxia, hypoglycemia, and serum deprivation. Interestingly, however, it did not appear to protect neurons from excitotoxicity (Van Den Bosch et al., 2004a,b).
Reports are conflicting about the relative levels of VEGF-A in SOD1G93A mice. Van Den Bosch et al. reported no differences in VEGF-A levels in the spinal cords of SOD1G93A mice when compared to wild-type controls (Van Den Bosch et al., 2004a,b). In a different study, Murakami et al. found the expression level of VEGF-A in the spinal cords of SOD1G93A mice to be higher at baseline when compared to wild-type counterparts (Murakami et al., 2003). Additionally, while Murakami et al. found limited VEGF-A induction with hypoxia in SOD1G93A mice, Van den Bosch et al. showed significant upregulation of VEGF-A in SOD1G93A mice exposed to hypoxic conditions (Murakami et al., 2003; Van Den Bosch et al., 2004a,b). The reason for this difference is unclear, and further analysis will be necessary to clarify the discrepancy. Using an in vitro model, Lu et al. showed that glial cells expressing SOD1G93A had downregulation of VEGF-A expression levels. The finding was linked to a novel gain of function of the mutated SOD1, which led to disruption in the VEGF 3′ untranslated region (UTR) ribonucleoprotein complex (Liang Lu et al., 2007) and consequently reduction in VEGF expression (studied more extensively by (X. Li et al., 2009; L. Lu et al., 2009; Mali & Zisapel, 2010)). Attempts to manipulate VEGF-A levels in follow-up work utilized mice that were a cross breed of SOD1G93A VEGF-Aδ/δ strains which resulted in mice with an earlier onset of motor neuron disease and earlier death (Lambrechts et al., 2003). In a different approach, Wang et al. cross bred SOD1G93A mice with mice overexpressing VEGF-A resulting in significant delays in motor neuron loss with concordant delays in motor deficits and an overall higher survival (Y. Wang et al., 2007).
In the clinical research field, a number of studies have looked at VEGF-A polymorphisms and their link to ALS. Results from these studies have been conflicting with limited reproducibility of the data, likely due to small and constrained sample sizes (e.g. most studies were <1000 ALS patients and matched controls from one geographical region) (Lambrechts et al., 2003; Terry et al., 2004). However, the initial research conducted in Swedish, English, Belgian, Russian and American populations suggested that certain single nucleotide polymorphisms (e.g. −2,578C/A, −1154 G/A, −634G/C) of the VEGF-A gene, particularly in the upstream promoter/leaser sequence, are associated with ALS risk (Lambrechts et al., 2003; Terry et al., 2004; Lysogorskaia et al., 2012). In contrast, Van Vught et al. found no such associations in a Danish population (Van Vught et al., 2005). Further studies showing no association between these polymorphisms and sporadic ALS included those looking at Chinese, Italian, and North American populations (W. Chen et al., 2006; Del Bo et al., 2008; Zhang et al., 2006). In an attempt to readdress the disparity between the findings, Lambrechts et al. conducted a large scale meta-analysis (7000+ patients) of the three VEGF polymorphisms in European and American populations and found that there was only a significant association of the −2578AA genotype with increased susceptibility to ALS in males (Lambrechts et al., 2009).
4.2. Vascular endothelial growth factor-B and amyotrophic lateral sclerosis
Given the rather minimal angiogenic and lymphangiogenic properties of VEGF-B, and the previous evidence showing protective effects for neurons, Poesen and colleagues explored the role of VEGF-B in ALS. In a rather extensive paper that is briefly reviewed below, they used multiple techniques including knockout mice, transgenic rat and mouse models, cultured neurons, histology, and behavioral testing to study the therapeutic potential of VEGF-B. In particular, their main focus was the use of the VEGF-B186 isoform (186 amino acids; alternative splicing isoform is VEGF-B167) as a neuroprotective factor in motor neuron degeneration models of ALS, both in vitro and in vivo (Poesen et al., 2008). Initially, Poesen et al. explored the expression of VEGF-B167 and VEGF-B186, which were detected in the large motor neurons of the ventral horn in wild-type mice. Second, their analysis of motor neuron development and total motor neuron counts in Vegfb−/− mice showed no difference from wild-type controls. Further, these knockout mice have normal longevity and do not display abnormal signs of motor function, muscle weakness or paralysis. Crossing SOD1G93A mice with Vegfb−/− mice resulted in two strains of interest, SOD1G93A × Vegfb+/− and SOD1G93A × Vegfb−/− which were also compared to littermate controls, SOD1G93A × Vegfb+/+. The results show that SOD1G93A mice deficient in VEGF-B (Vegfb−/−) were unable to stay on the rotarod as long as SOD1G93A mice with normal VEGF-B expression (Vegfb+/+) or heterozygous for VEGF-B (Vegfb+/−) mice. Additionally, SOD1G93A × Vegfb−/− mice died much earlier than their counterparts with at least one copy of the Vegfb gene. The results of the histological analysis of these strains revealed a greater loss of motor neurons in the SOD1G93A × Vegfb−/− group when compared to the SOD1G93A × Vegfb+/+ mice. Switching to primary rat motor neuron cultures, they then analyzed if there was any protective effects if the cultures were deprived of growth factor supplements. Using this model they showed that VEGF-B is neuroprotective in a dose dependent manner. Finally, moving beyond cultures, Poesen et al., showed that intracerebroventricular delivery of VEGF-B186 into SOD1G93A rats drastically increased their length of survival and the total motor neuron count, while also reducing the motor degeneration phenotype when compared to mice receiving only cerebrospinal fluid.
4.3. Perspectives on the relationship of vascular endothelial growth factor (VEGF-A, VEGF-B) and amyotrophic lateral sclerosis
Converging evidence from both small animal and human studies implicate a relationship between VEGF-A and ALS. Based on the recapitulation of ALS-like signs and symptoms in mice with a mutation in the VEGF-A promoter region and the association of a specific polymorphism with the disease in male patients, VEGF-A appears to have a role in the disorder. Given this evidence, further animal studies and future clinical studies are looking at therapeutic strategies to test the utility of VEGF-A. These studies are discussed further below.
With regard to VEGF-B and its association with ALS, the current state of the literature is sparse and the relationship is less clear. Perhaps most notably, a deficiency of VEGF-B in the context of normal SOD1 did not appear to recapitulate the ALS-like phenotype. However, in both rat and mouse ALS models with SOD1 mutation, deficiencies in VEGF-B did exacerbate the ALS-like phenotype. These results suggest that other forms of VEGF may compensate for a loss of VEGF-B, but in cases of additional stress like SOD1 mutation, the loss of VEGF-B may be critical. Clearly, if VEGF-B can ameliorate the rapid deterioration of ALS it would certainly provide utility as a therapeutic agent, especially considering that there is minimal angiogenic and lymphangiogenic activity as compared to VEGF-A. Given that there is to date one main but promising study on VEGF-B and ALS, future investigations are needed to determine the role of VEGF-B and its potential as a treatment option for ALS.
5. Therapeutic possibilities for vascular endothelial growth factor in amyotrophic lateral sclerosis
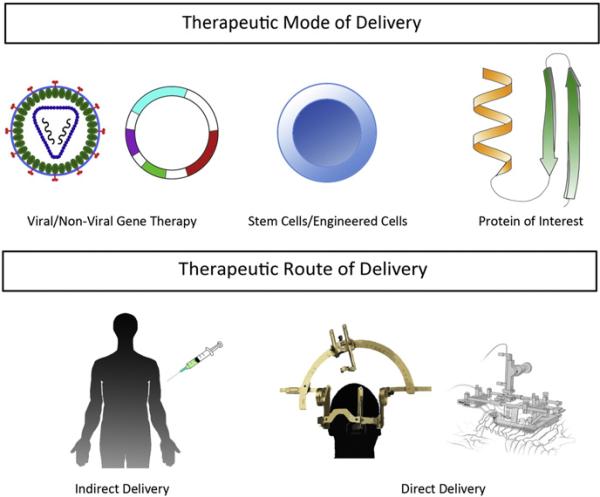
Given the association of VEGF-A and ALS, it is not surprising that it is considered an attractive potential therapy for ALS. Thus, the bulk of research studies exploring the therapeutic possibilities of VEGF proteins in ALS focus on VEGF-A. When contemplating a new therapy, the mode of delivery and the route of administration are vital (see Fig. 3). There are three possible modes of delivery for VEGF-A. The first is through the use of gene therapy by a viral or non-viral vector to produce VEGF-A (e.g. Vegfa) or another protein that would increase endogenous VEGF-A expression (e.g. transcription factors). The second is delivering cells that have been engineered to produce and secrete VEGF-A. The third is the direct delivery of the VEGF-A protein. Once the mode of delivery has been selected, determining the route of administration is the next step. There are two possibilities for the route. The first is remote delivery of the VEGF-A agent, which will be transported secondarily into the CNS. The second is direct delivery into the CNS. Direct delivery offers the advantage of access to the targeted area, which reduces the complication of off-target effects and requires a lower therapeutic dose. The disadvantage of direct delivery, especially in the CNS, is that delivery is targeted to an extremely sensitive area. Remote delivery has the advantage of allowing for a less invasive procedure to deliver the therapeutic. However, it does require a larger dose and has the additional issue of off-target effects especially considering the expression of receptors throughout the body. Additionally, when considering remote delivery to the CNS, the therapeutic should either be able to cross the blood–brain barrier, pial barrier, or be retrogradely transported to the CNS through axons extending into the periphery. The following section will explore each of the three modes of delivery and the studies utilizing them to evaluate VEGF-A gene therapy potential for ALS.
Fig. 3.
Potential modes and routes of VEGF therapy. Three general modes have been explored in animal models of ALS; these include gene therapy (e.g. viral and non-viral vectors), stem cell/engineered cells, and protein delivery. There are two routes used including an indirect approach (e.g. retrovirus injections into the musculature) and direct approaches (e.g. stereotactic injections into the brain and spinal cord or into the intrathecal space).
5.1. Vascular endothelial growth factor gene therapy and amyotrophic lateral sclerosis
A number of different studies have been conducted in animal models of ALS demonstrating that VEGF-A gene therapy has the potential to slow the progression of the disease. Here the discussion will focus on the use of viral gene therapy to deliver VEGF-A to SOD1G93A models. In one of the earliest studies to employ gene therapy, Azzouz et al. adopted a lentiviral vector pseudotyped with a Rabies-G envelope protein, which has enhanced retrograde delivery to motor neurons. Through this approach it was possible to increase VEGF-A levels in the motor neurons of SOD1G93A mouse model. Administration of this lentiviral vector via bilateral injections into the gastrocnemius muscle, diaphragm, intercostal, facial and tongue muscles before disease onset (at 21 days) resulted in prolonged survival (17–30% over controls), improved motor function, and delayed onset of ALS-like phenotype. In addition when the lentivirus-VEGF-A treated animals reached end stage of the disease they were found to have significantly higher levels of VEGF-A in their spinal cords when compared to control animals. This therapy was then initiated in SOD1G93A mice when ~50% of the motor neurons had already died (90 days). Treated animals had significantly improved motor function and extension of life span compared to control animals. As a control, the researchers used Lenti-glial cell derived neurotrophic factor (GDNF) administration at 90 days, which revealed only a modest increase in survival. Taken together, these results indicated that VEGF-A treatment can benefit SOD1G93A mice relative to both their natural disease course and above the effect achieved by GDNF (Azzouz et al., 2004). Another study by Dodge et al. employed the same SOD1G93A model of ALS but used either insulin-like growth factor 1 (IGF-1) or VEGF-A gene delivery. These researches used Adeno-associated virus-4 (AAV-4) as the delivery vector. AAV-4 was selected since it was found to target ependymal cells in both the brain and spinal cord after intracerebroventricuar (ICV) delivery (Dodge et al., 2010). ICV delivery of either AAV-4 insulin like growth factor 1 (IGF-1) or AAV-4 VEGF-A resulted in transgene expression in the brain and spinal cord with the highest levels in the cerebellum. Delivery of either AAV-4 IGF-1 or AAV-4 VEGF to SOD1G93A mice at 85 days of age increased survival and improved motor function. However, there were some differences in the outcome for mice treated with AAV-4 IGF-1 compared to those treated with AAV-4 VEGF-A, although these differences were not statistically significant. To determine if a combination therapy of AAV-4 IGF-1 and AAV-4 VEGF-A would have a greater effect, both vectors were simultaneously administered together in another group of SOD1G93A mice. Co-administration of both factors was not found to affect where they were expressed or the level of their expression in the brain and spinal cord, and no additional benefit was observed in either survival or motor function when compared to individual administration of either factor. The fact that there was no synergistic effect from co-administration likely indicates that IGF-1 and VEGF are acting on similar signaling pathways to slow progression of ALS (Dodge et al., 2010).
It is also possible to upregulate endogenous VEGF-A expression indirectly. One way of achieving this is to use a zinc finger protein specially designed for this purpose. The Cys2-His2 DNA binding motif of zinc finger transcription factors can be designed to bind to almost any DNA sequence. This allows the engineering of artificial transcription factors that have the ability to regulate endogenous chromosomal genes (Klug, 2010). The advantage of using a zinc finger transcription factor is that upregulation of chromosomal VEGF-A results in increased expression of all isoforms of VEGF-A. Since there are numerous isoforms, increasing the expression of all isoforms can potentially increase the impact of this approach. A study conducted by Sakowiski et al. used an engineered zinc finger protein delivered in an adenoviral vector (Adp65) to upregulate endogenous VEGF-A expression in a laryngeal nerve crush injury model. Transduction of primary rat motor neurons with Ad-p65 resulted in a significant increase in axon outgrowth. In the in vivo rat model, injection of Ad-p65 after the crush injury resulted in a reduction in the time it took for the animals to recover along with a faster recovery of nerve-endplate contacts (Sakowski et al., 2009). This study illustrated that upregulating endogenous VEGF-A via a zinc finger protein had a positive therapeutic outcome in laryngeal nerve injury model. Our group has also used a zinc finger transcription factor to up-regulate VEGF-A expression in the SOD1G93A rat model of ALS. The VEGF zinc finger transcription factor consisted of three zinc finger binding domains fused with the p65 activation domain of the nuclear factor kappa B (NFκB) which binds to the target sequence of VEGF-A. Plasmid DNA encoding a zinc finger transcription factor was injected into the hindlimb unilaterally, directed at the medial and lateral gastrocnemius muscle, on a weekly basis for six weeks. These animals performed better in behavioral tests, including improved grip strength and rotarod performance, compared to controls. However, the number of innervated neuromuscular junctions was found to be similar in both treated and control groups. Because this therapy was only delivered to a single muscle, there were no differences in lifespan or weight between control and treated animals (Kliem et al., 2011).
5.2. Stem cells, vascular endothelial growth factor and amyotrophic lateral sclerosis
Experimental attempts have been made to use engineered stem cells to treat ALS. One such study transplanted human umbilical cord blood cells that had been transfected with VEGF-A (using a non-viral plasmid construct) into SOD1G93A mice via retro-orbital injection. Transplanted cells differentiated into vascular endothelial cells, and the treated mice had a significant extension of life span (Rizvanov et al., 2008). Another study used intrathecal delivery of human neural stem cells engineered to overexpress VEGF-A as a therapeutic intervention with SOD1G93A mice. Mice treated before disease onset had a significant improvement in motor function and prolonged survival compared to control animals. The transplanted cells were found in the anterior horn of the spinal cord gray matter and differentiated into motor neurons. They did not proliferate once engrafted nor did they cause adverse effects such as tumor formation. Transplantation of these VEGF-A expressing neural stem cells was found to exert a neuroprotective effect by downregulating pro-apoptotic genes, caspase-3 and Bax, and upregulating anti-apoptotic genes, Bcl-2 and Bcl-XL (Hwang et al., 2009).
5.3. Delivery of vascular endothelial growth factor protein to the central nervous system (CNS) as a therapy for amyotrophic lateral sclerosis
Another option for using VEGF-A as a therapeutic agent in ALS is to deliver VEGF-A protein to the central nervous system (CNS). The Robberecht and Carmeliet groups have been collaborating on the direct protein delivery approach (Storkebaum et al., 2005a,b). The work began by studying the effect of VEGF-A on motor neuron degeneration in SOD1G93A mice (Van Den Bosch et al., 2004a,b). They showed that VEGF-A administered to motor neurons in vitro increased cell survival. Additionally, comparison of VEGF-A levels in the plasma, CSF and spinal cord between wild-type and SOD1G93A mice found that levels in CSF and plasma were below the limit of detection for both groups of animals. There was no difference in VEGF levels in the spinal cord between the two groups. They then showed that exposing SOD1G93A mice to hypoxic conditions induced endogenous VEGF expression, with the effect being most pronounced at the initial phase of hypoxia. However, this had no significant impact on survival (Van Den Bosch et al., 2004a,b). In the next series of studies, Storkebaum et al. attempted both a systemic and a direct delivery approach of VEGF-A protein in the SOD1G93A rat model. The initial attempt via systemic delivery was unsuccessful due to an elicited immune response in these animals, sequestering of VEGF-A by soluble Flt 1 (VEGFR 1 extracellular binding domain), and problems with VEGF-A not crossing the blood–brain barrier. Therefore, VEGF-A was delivered directly into the CNS by intracerebroventricular infusion. Examining the distribution of injected VEGF revealed that by 24 h it had completely cleared from the CSF and was found mostly in the brain (50%), spinal cord (12%) and peripheral organs (38%). ICV delivery of VEGF-A was found to delay onset of paralysis, improve motor function, and significantly prolong survival in these animals. It also delayed motor neuron degeneration in the brain stem, cervical and lumbar spinal cord. Continuing the study, they attempted to further clarify the neuroprotective effect of VEGF-A by studying the effect of overexpressing VEGF receptor 2 (Flk1). Transgenic mice were engineered to overexpress Flk1 in postnatal neurons and were then crossed with SOD1G93A mice. Neuronal overexpression of Flk1 was found to delay initiation of motor impairment and prolong survival. Motor neuron degeneration was also delayed in these mice. These effects of Flk1 overexpression in neurons were achieved by the transmission of survival signals from VEGF-A. Anterograde transport of VEGF-A was also demonstrated, which was thought to help protect neuromuscular junctions in these animals (Storkebaum et al., 2005a,b). The findings from these two studies complement earlier research conducted by this group demonstrating that phospho-Akt, an anti-apoptotic factor, is lost from motor neurons in ALS patients, both sporadic and familial. p-Akt is also down-regulated in the ventral spinal cord of SOD1G93A mice and is selectively lost from motor neurons. Notably, activated Akt is lost in SOD1G93A mice before they manifest symptoms of the disease (Gurney et al., 1994). Since VEGF-A is a strong upstream activator of the PI-3K/Akt pathway, it was hypothesized that ICV infusion of VEGF-A protein would result in increased levels of phospho-Akt. Later work by the same group provided evidence for this hypothesis demonstrating that treating SOD1G93A animals with VEGF-A protein increases phosphor-akt, improves motor performance and prolongs survival (Dewil et al., 2007). Another possible mechanism through which VEGF-A protects motor neurons in ALS appears to be through glutamate receptors. Work by Bogaert et al. demonstrated that motor neurons treated in vitro with VEGF-A had higher levels of glutamate receptor 2 (GluR2) than untreated cells. This effect of VEGF-A was found to be mediated via VEGFR2 present on motor neurons and was as a consequence of stimulation of GluR2 transcription. Furthermore, ICV delivery of VEGF-A in rats was also found to induce GluR2 expression, and this induction contributed to protection of motor neurons in these animals (Bogaert et al., 2010).
Intraperitoneal delivery of VEGF-A protein to SOD1G93A mice starting at 78 days of age given three times/week resulted in a slight but insignificant increase in motor neuron numbers. A significant increase in astrocyte number was noted in these animals compared to control mice. Given that astroglia have diverse roles (e.g. supporting, repairing, and nourishing neurons), this finding highlights one potential mechanism for the neuroprotective effects of VEGF-A. Additionally, while this study did not detect a significant difference in motor neurons, it did find a significant increase in Gap-43 positive neuromuscular junctions (NMJ) in treated mice compared to controls. However, these NMJ results should be interpreted in the context of no significant improvements with either motor function or length of survival (Zheng et al., 2007). In a similar study, excitotoxic spinal cord neurodegeneration was induced by administration of a glutamate receptor agonist either via chronic or acute infusion. This insult resulted in ~90% loss of lumbar motor neurons by 24 h, while administration of VEGF-A protein delayed this damage (Tovar et al., 2007). This delay in motor neuron loss was attributed to VEGF-A directly activating VEGFR2 expressed in motor neurons (Tovar & Tapia, 2010). Further research compared VEGF-A administration before or after the induction of spinal cord neurodegeneration. The latter condition is a clinically more relevant treatment paradigm as patients are often diagnosed and receive treatment after they manifest symptoms of ALS. The researchers also examined whether chronic infusion or a single injection of VEGF-A was more effective therapeutically. VEGF was delivered either via pump into the lumbar cord or by ICV infusion. Chronic infusion of VEGF-A into the lumbar cord before symptom onset protected motor neurons and halted progression of the disease. Animals that received VEGF-A after symptom onset still lost motor neurons and had an intense astrocytic inflammatory response compared to the pre-symptomatic delivery group. The effect and timing of a single injection of VEGF was also assessed. A single ICV injection of VEGF-A 30 min before induction of spinal neurodegeneration resulted in protection from paralysis, no motor impairment, and preservation of motor neurons compared to vehicle treated animals. Administration of a single injection of VEGF-A 1 h after induction of spinal neurodegeneration resulted in loss of ~25% of motor neurons (compared to ~90% with no treatment) when compared to the pre-treated group. Importantly, 24 h post-treatment, the residual motor neurons in the post-symptomatic group were sufficient to maintain motor function. On the other hand, animals that received ICV delivery of VEGF-A 2 h after induction of spinal neurodegeneration had a much greater motor neuron loss (~75%) at 24 h post-surgery when compared to pre-treated animals. The 2 hour delayed group also developed hind-limb deficits that were analogous to those seen in the vehicle treated group with 90% motor neuron loss (Tovar-y-Romo & Tapia, 2012).
5.4. Perspectives on the use of vascular endothelial growth factor as a treatment for amyotrophic lateral sclerosis
The overwhelming consensus from several preclinical VEGF-A treatment experiments in ALS models is that it has great potential to translate to clinical practice. Given the efficacy of earlier delivery of VEGF-A in models of ALS, advances towards both refined treatment approaches and earlier markers for the disease are essential. As whole, the literature supports the exploration of VEGF-A treatment through an FDA approved clinical trial (discussed below).
Despite the strength of the evidence across studies, an important caveat of the findings is that a predominant number of these studies utilized the same SOD1G93A model, which does not reflect the entire pathogenic mechanism of ALS. Therefore, while SOD1G93A is a useful model, further studies using other emerging ALS models (see Section 1.1) are necessary to understand the potential of VEGF-A to treat different subtypes of ALS. A second important caveat is the exclusive use of VEGF-A as a potential therapeutic agent with a notable absence of exploring the effects of other family members. The single but compelling study by Poesen et al. relating VEGF-B to ALS models suggests that there is a tremendous therapeutic potential for the VEGF family beyond VEGF-A; however there is currently no follow-up work (Poesen et al., 2008).
6. Progress to clinical trials
Given the successful treatment of ALS animal models with VEGF-A protein, there is now an effort to translate the findings from the bench to beside. Currently, there is one ongoing clinical trial using VEGF-A protein as a treatment for ALS. The approach is ICV administration of VEGF-A165 (165 amino acid length isoform) using an infusion pump. This trial is an open-label Phase 1 and 2 trials (NCT01384162) and is a continuation of a previous trial (NCT0080051). The goal of this continued study is to determine the ongoing safety and tolerability of VEGF-A165 and to determine if it improves motor function and prolongs survival in a cohort of 18 ALS patients. Patients will be monitored over 2.5 years for safety outcomes, disease activity as measured by the ALS functional rating scale, VEGF concentration in the CSF and for the presence of VEGF antibodies in plasma. To date no data have been published from this trial.
7. Conclusions
Based on observations in animal models of ALS, there is a clear potential for VEGF therapy that has led to one current clinical trial. The results from this trial will determine if administration of VEGF-A165 protein is tolerated and safe and if there are positive outcomes such as delayed loss of motor function and improved quality of life. Regardless of the outcome of this initial trial, there are also numerous other VEGF based treatment strategies that have strong evidence for possible efficacy in delaying or mitigating the symptoms of ALS. VEGF-A therapies such as AAV-VEGF-A or even a zinc finger protein expression to upregulate endogenous VEGF-A expression are translatable to the clinic. Additionally, there are numerous other members of the VEGF family besides VEGF-A that may also be potential therapeutic options with further research. In terms of delivery, advances include the development by our laboratory of technology that safely injects therapeutics into the spinal cord and, therefore, will allow for a broader range of treatment options. Our device consists of an injection platform that is mounted to the patient allowing the injection device to move with the patient's breathing and helps to minimize damage to the cord. The device is FDA approved for use in a clinical trial injecting stem cells into the spinal cord of ALS patients (Riley et al., 2008; Federici et al., 2009; Riley et al., 2011; Glass et al., 2012). The same device can be used for direct injection of a viral vector expressing VEGF-A targeting the motor neurons of the ventral horn. It also affords for intrathecal delivery of a VEGF-A gene therapy using the AAV-9 vector, which has the ability to cross the blood–brain barrier and transduce neural cells (Duque et al., 2009; Foust et al., 2009). Gene therapy using either direct delivery into the spinal cord or intrathecal delivery has the potential to be effective. Additionally, a combination therapy that targets the motor neurons of the ventral horn via direct injection, as well as the brain and spinal cord via intrathecal delivery, holds the possibility of a greater therapeutic outcome. Gene therapy mediated via an AAV vector also holds the possibility of sustained expression of VEGF-A over a prolonged period of time. This would allow for a single delivery as opposed to continuous infusion, thus, reducing the risk of infection. These gene therapies merit further testing in animal models of ALS to determine their efficacy. Available evidence suggests that the potential to develop and safely deliver VEGF based therapies to ALS patients exists and can hopefully be realized in the future.
Abbreviations
- AAV
adeno-associated virus
- ALS
amyotrophic lateral sclerosis
- CNS
central nervous system
- FTD
frontotemporal dementia
- FUS
fused in sarcoma
- HRE
hypoxia response element
- IGF-1
insulin-like growth factor 1
- ICV
intracerebroventricular
- NRP
neuropilin
- NFκB
nuclear factor kappa-light-chain-enhancer of activated B cells
- PlGF
placental growth factor
- FIG4
polyphosphoinositide phosphatase
- SETX
senataxin
- SOD1
superoxide dismutase 1
- TDP43
TAR DNA-binding domain protein
- UBQLN2
ubiquitin-like protein ubiquilin 2
- UTR
untranslated region
- VEGF
vascular endothelial growth factor
- VEGFR
vascular endothelia growth factor receptor
Footnotes
Conflicts of interest
The authors declare that they have no conflicts of interest.
References
- Aase K, von Euler G, Li X, Pontén A, Thorén P, Cao R, et al. Vascular endothelial growth factor-B-deficient mice display an atrial conduction defect. Circulation. 2001;104:358–364. doi: 10.1161/01.cir.104.3.358. [DOI] [PubMed] [Google Scholar]
- Achen MG, Jeltsch M, Kukk E, Mäkinen T, Vitali A, Wilks AF, et al. Vascular endothelial growth factor D (VEGF-D) is a ligand for the tyrosine kinases VEGF receptor 2 (Flk1) and VEGF receptor 3 (Flt4). Proc Natl Acad Sci U S A. 1998;95:548–553. doi: 10.1073/pnas.95.2.548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alon T, Hemo I, Itin A, Pe'er J, Stone J, Keshet E. Vascular endothelial growth factor acts as a survival factor for newly formed retinal vessels and has implications for retinopathy of prematurity. Nat Med. 1995;1:1024–1028. doi: 10.1038/nm1095-1024. [DOI] [PubMed] [Google Scholar]
- Avantaggiato V, Orlandini M, Acampora D, Oliviero S, Simeone A. Embryonic expression pattern of the murine FIGF gene, a growth factor belonging to platelet-derived growth factor/vascular endothelial growth factor family. Mech Dev. 1998;73:221–224. doi: 10.1016/s0925-4773(98)00049-5. [DOI] [PubMed] [Google Scholar]
- Azzouz M, Ralph GS, Storkebaum E, Walmsley LE, Mitrophanous KA, Kingsman SM, et al. VEGF delivery with retrogradely transported lentivector prolongs survival in a mouse ALS model. Nature. 2004;429:413–417. doi: 10.1038/nature02544. [DOI] [PubMed] [Google Scholar]
- Baldwin ME, Roufail S, Halford MM, Alitalo K, Stacker SA, Achen MG. Multiple forms of mouse vascular endothelial growth factor-D are generated by RNA splicing and proteolysis. J Biol Chem. 2001;276:44307–44314. doi: 10.1074/jbc.M106188200. [DOI] [PubMed] [Google Scholar]
- Beck H, Acker T, Puschel AW, Fujisawa H, Carmeliet P, Plate KH. Cell type-specific expression of neuropilins in an MCA-occlusion model in mice suggests a potential role in post-ischemic brain remodeling. J Neuropathol Exp Neurol. 2002;61:339–350. doi: 10.1093/jnen/61.4.339. [DOI] [PubMed] [Google Scholar]
- Bellomo D, Headrick JP, Silins GU, Paterson CA, Thomas PS, Gartside M, et al. Mice lacking the vascular endothelial growth factor-B gene (Vegfb) have smaller hearts, dysfunctional coronary vasculature, and impaired recovery from cardiac ischemia. Circ Res. 2000;86:E29–E35. doi: 10.1161/01.res.86.2.e29. [DOI] [PubMed] [Google Scholar]
- Bogaert E, Van Damme P, Poesen K, Dhondt J, Hersmus N, Kiraly D, et al. VEGF protects motor neurons against excitotoxicity by upregulation of GluR2. Neurobiol Aging. 2010;31:2185–2191. doi: 10.1016/j.neurobiolaging.2008.12.007. [DOI] [PubMed] [Google Scholar]
- Carmeliet P, Ferreira V, Breier G, Pollefeyt S, Kieckens L, Gertsenstein M, et al. Abnormal blood vessel development and lethality in embryos lacking a single VEGF allele. Nature. 1996;380:435–439. doi: 10.1038/380435a0. [DOI] [PubMed] [Google Scholar]
- Carmeliet P, Moons L, Luttun A, Vincenti V, Compernolle V, De Mol M, et al. Synergism between vascular endothelial growth factor and placental growth factor contributes to angiogenesis and plasma extravasation in pathological conditions. Nat Med. 2001;7:575–583. doi: 10.1038/87904. [DOI] [PubMed] [Google Scholar]
- Chaballe L, Close P, Sempels M, Delstanche S, Fanielle J, Moons L, et al. Involvement of placental growth factor in Wallerian degeneration. Glia. 2011;59:379–396. doi: 10.1002/glia.21108. [DOI] [PubMed] [Google Scholar]
- Chen H, Chédotal A, He Z, Goodman CS, Tessier-Lavigne M. Neuropilin-2, a novel member of the neuropilin family, is a high affinity receptor for the semaphorins Sema E and Sema IV but not Sema III. Neuron. 1997;19:547–559. doi: 10.1016/s0896-6273(00)80371-2. [DOI] [PubMed] [Google Scholar]
- Chen W, Saeed M, Mao H, Siddique N, Dellefave L, Hung W-Y, et al. Lack of association of VEGF promoter polymorphisms with sporadic ALS. Neurology. 2006;67:508–510. doi: 10.1212/01.wnl.0000227926.42370.04. [DOI] [PubMed] [Google Scholar]
- Chen YZ, Bennett CL, Huynh HM, Blair IP, Puls I, Irobi J, et al. DNA/RNA helicase gene mutations in a form of juvenile amyotrophic lateral sclerosis (ALS4). Am J Hum Genet. 2004;74:1128–1135. doi: 10.1086/421054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chow CY, Landers JE, Bergren SK, Sapp PC, Grant AE, Jones JM, et al. Deleterious variants of FIG4, a phosphoinositide phosphatase, in patients with ALS. Am J Hum Genet. 2009;84:85–88. doi: 10.1016/j.ajhg.2008.12.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clauss M, Gerlach M, Gerlach H, Brett J, Wang F, Familletti PC, et al. Vascular permeability factor: a tumor-derived polypeptide that induces endothelial cell and monocyte procoagulant activity, and promotes monocyte migration. J Exp Med. 1990;172:1535–1545. doi: 10.1084/jem.172.6.1535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Connolly DT, Heuvelman DM, Nelson R, Olander JV, Eppley BL, Delfino JJ, et al. Tumor vascular permeability factor stimulates endothelial cell growth and angiogenesis. J Clin Invest. 1989;84:1470–1478. doi: 10.1172/JCI114322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Falco S. The discovery of placenta growth factor and its biological activity. Exp Mol Med. 2012;44:1–9. doi: 10.3858/emm.2012.44.1.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Vries C, Escobedo JA, Ueno H, Houck K, Ferrara N, Williams LT. The fms-like tyrosine kinase, a receptor for vascular endothelial growth factor. Science. 1992;255:989–991. doi: 10.1126/science.1312256. [DOI] [PubMed] [Google Scholar]
- DeJesus-Hernandez M, Mackenzie IR, Boeve BF, Boxer AL, Baker M, Rutherford NJ, et al. Expanded GGGGCC hexanucleotide repeat in non-coding region of C9ORF72 causes chromosome 9p-linked FTD and ALS. Neuron. 2011;72:245–256. doi: 10.1016/j.neuron.2011.09.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Del Bo R, Scarlato M, Ghezzi S, Martinelli-Boneschi F, Corti S, Locatelli F, et al. Absence of angiogenic genes modification in Italian ALS patients. Neurobiol Aging. 2008;29:314–316. doi: 10.1016/j.neurobiolaging.2006.10.008. [DOI] [PubMed] [Google Scholar]
- Deng HX, Chen W, Hong ST, Boycott KM, Gorrie GH, Siddique N, et al. Mutations in UBQLN2 cause dominant X-linked juvenile and adult-onset ALS and ALS/dementia. Nature. 2011;477:211–215. doi: 10.1038/nature10353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deng HX, Hentati A, Tainer JA, Iqbal Z, Cayabyab A, Hung WY, et al. Amyotrophic lateral sclerosis and structural defects in Cu, Zn superoxide dismutase. Science. 1993;261:1047–1051. doi: 10.1126/science.8351519. [DOI] [PubMed] [Google Scholar]
- Dewil M, Lambrechts D, Sciot R, Shaw PJ, Ince PG, Robberecht W, et al. Vascular endothelial growth factor counteracts the loss of phospho-Akt preceding motor neurone degeneration in amyotrophic lateral sclerosis. Neuropathol Appl Neurobiol. 2007;33:499–509. doi: 10.1111/j.1365-2990.2007.00850.x. [DOI] [PubMed] [Google Scholar]
- Dodge JC, Treleaven CM, Fidler JA, Hester M, Haidet A, Handy C, et al. AAV4-mediated expression of IGF-1 and VEGF within cellular components of the ventricular system improves survival outcome in familial ALS mice. Mol Ther. 2010;18:2075–2084. doi: 10.1038/mt.2010.206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Du H, Li P, Pan Y, Li W, Hou J, Chen H, et al. Vascular endothelial growth factor signaling implicated in neuroprotective effects of placental growth factor in an in vitro ischemic model. Brain Res. 2010;1357:1–8. doi: 10.1016/j.brainres.2010.07.015. [DOI] [PubMed] [Google Scholar]
- Duque S, Joussemet B, Riviere C, Marais T, Dubreil L, Douar AM, et al. Intravenous administration of self-complementary AAV9 enables transgene delivery to adult motor neurons. Mol Ther. 2009;17:1187–1196. doi: 10.1038/mt.2009.71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erskine L, Reijntjes S, Pratt T, Denti L, Schwarz Q, Vieira JM, et al. VEGF signaling through neuropilin 1 guides commissural axon crossing at the optic chiasm. Neuron. 2011;70:951–965. doi: 10.1016/j.neuron.2011.02.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Favier B, Alam A, Barron P, Bonnin J, Laboudie P, Fons P, et al. Neuropilin-2 interacts with VEGFR-2 and VEGFR-3 and promotes human endothelial cell survival and migration. Blood. 2006;108:1243–1250. doi: 10.1182/blood-2005-11-4447. [DOI] [PubMed] [Google Scholar]
- Federici T, Riley J, Park J, Bain M, Boulis N. Preclinical safety validation of a stabilized viral vector direct injection approach to the cervical spinal cord. Clin Transl Sci. 2009;2:165–167. doi: 10.1111/j.1752-8062.2008.00084.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferrara N, Carver-Moore K, Chen H, Dowd M, Lu L, O'Shea KS, et al. Heterozygous embryonic lethality induced by targeted inactivation of the VEGF gene. Nature. 1996;380:439–442. doi: 10.1038/380439a0. [DOI] [PubMed] [Google Scholar]
- Ferrara N, Henzel WJ. Pituitary follicular cells secrete a novel heparin-binding growth factor specific for vascular endothelial cells. Biochem Biophys Res Commun. 1989;161:851–858. doi: 10.1016/0006-291x(89)92678-8. [DOI] [PubMed] [Google Scholar]
- Ferrell RE, Levinson KL, Esman JH, Kimak MA, Lawrence EC, Barmada MM, et al. Hereditary lymphedema: evidence for linkage and genetic heterogeneity. Hum Mol Genet. 1998;7:2073–2078. doi: 10.1093/hmg/7.13.2073. [DOI] [PubMed] [Google Scholar]
- Fong GH, Rossant J, Gertsenstein M, Breitman ML. Role of the Flt-1 receptor tyrosine kinase in regulating the assembly of vascular endothelium. Nature. 1995;376:66–70. doi: 10.1038/376066a0. [DOI] [PubMed] [Google Scholar]
- Forstreuter F, Lucius R, Mentlein R. Vascular endothelial growth factor induces chemotaxis and proliferation of microglial cells. J Neuroimmunol. 2002;132:93–98. doi: 10.1016/s0165-5728(02)00315-6. [DOI] [PubMed] [Google Scholar]
- Foust KD, Nurre E, Montgomery CL, Hernandez A, Chan CM, Kaspar BK. Intravascular AAV9 preferentially targets neonatal neurons and adult astrocytes. Nat Biotechnol. 2009;27:59–65. doi: 10.1038/nbt.1515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glass JD, Boulis NM, Johe K, Rutkove SB, Federici T, Polak M, et al. Lumbar intraspinal injection of neural stem cells in patients with ALS: results of a phase I trial in 12 patients. Stem Cells. 2012;30(6):1144–1151. doi: 10.1002/stem.1079. [DOI] [PubMed] [Google Scholar]
- Greenway MJ, Andersen PM, Russ C, Ennis S, Cashman S, Donaghy C, et al. ANG mutations segregate with familial and ‘sporadic’ amyotrophic lateral sclerosis. Nat Genet. 2006;38:411–413. doi: 10.1038/ng1742. [DOI] [PubMed] [Google Scholar]
- Grimmond S, Lagercrantz J, Drinkwater C, Silins G, Townson S, Pollock P, et al. Cloning and characterization of a novel human gene related to vascular endothelial growth factor. Genome Res. 1996;6:124–131. doi: 10.1101/gr.6.2.124. [DOI] [PubMed] [Google Scholar]
- Gurney ME, Pu H, Chiu AY, Dal Canto MC, Polchow CY, Alexander DD, et al. Motor neuron degeneration in mice that express a human Cu, Zn superoxide dismutase mutation. Science. 1994;264:1772–1775. doi: 10.1126/science.8209258. [DOI] [PubMed] [Google Scholar]
- Hashimoto T, Zhang X-M, Chen B. Y.-k, Yang X-J. VEGF activates divergent intracellular signaling components to regulate retinal progenitor cell proliferation and neuronal differentiation. Development. 2006;133:2201–2210. doi: 10.1242/dev.02385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayakawa K, Pham LD, Som AT, Lee BJ, Guo S, Lo EH, et al. Vascular endothelial growth factor regulates the migration of oligodendrocyte precursor cells. J Neurosci. 2011;31:10666–10670. doi: 10.1523/JNEUROSCI.1944-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hemmeryckx B, van Bree R, Van Hoef B, Vercruysse L, Lijnen HR, Verhaeghe J. Adverse adipose phenotype and hyperinsulinemia in gravid mice deficient in placental growth factor. Endocrinology. 2008;149:2176–2183. doi: 10.1210/en.2007-1272. [DOI] [PubMed] [Google Scholar]
- Houck KA, Leung DW, Rowland AM, Winer J, Ferrara N. Dual regulation of vascular endothelial growth factor bioavailability by genetic and proteolytic mechanisms. J Biol Chem. 1992;267:26031–26037. [PubMed] [Google Scholar]
- Howland DS, Liu J, She Y, Goad B, Maragakis NJ, Kim B, et al. Focal loss of the glutamate transporter EAAT2 in a transgenic rat model of SOD1 mutant-mediated amyotrophic lateral sclerosis (ALS). Proc Natl Acad Sci U S A. 2002;99:1604–1609. doi: 10.1073/pnas.032539299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hwang DH, Lee HJ, Park IH, Seok JI, Kim BG, Joo IS, et al. Intrathecal transplantation of human neural stem cells overexpressing VEGF provide behavioral improvement, disease onset delay and survival extension in transgenic ALS mice. Gene Ther. 2009;16:1234–1244. doi: 10.1038/gt.2009.80. [DOI] [PubMed] [Google Scholar]
- Jeltsch M, Kaipainen A, Joukov V, Meng X, Lakso M, Rauvala H, et al. Hyperplasia of lymphatic vessels in VEGF-C transgenic mice. Science. 1997;276:1423–1425. doi: 10.1126/science.276.5317.1423. [DOI] [PubMed] [Google Scholar]
- Jin K, Zhu Y, Sun Y, Mao XO, Xie L, Greenberg DA. Vascular endothelial growth factor (VEGF) stimulates neurogenesis in vitro and in vivo. Proc Natl Acad Sci U S A. 2002;99:11946–11950. doi: 10.1073/pnas.182296499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jin KL, Mao XO, Greenberg DA. Vascular endothelial growth factor: direct neuroprotective effect in in vitro ischemia. Proc Natl Acad Sci U S A. 2000;97:10242–10247. doi: 10.1073/pnas.97.18.10242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joukov V, Pajusola K, Kaipainen A, Chilov D, Lahtinen I, Kukk E, et al. A novel vascular endothelial growth factor, VEGF-C, is a ligand for the Flt4 (VEGFR-3) and KDR (VEGFR-2) receptor tyrosine kinases. EMBO J. 1996;15:290–298. [PMC free article] [PubMed] [Google Scholar]
- Joukov V, Sorsa T, Kumar V, Jeltsch M, Claesson-Welsh L, Cao Y, et al. Proteolytic processing regulates receptor specificity and activity of VEGF-C. EMBO J. 1997;16:3898–3911. doi: 10.1093/emboj/16.13.3898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaipainen A, Korhonen J, Mustonen T, van Hinsbergh VW, Fang GH, Dumont D, et al. Expression of the fms-like tyrosine kinase 4 gene becomes restricted to lymphatic endothelium during development. Proc Natl Acad Sci U S A. 1995;92:3566–3570. doi: 10.1073/pnas.92.8.3566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karkkainen MJ, Haiko P, Sainio K, Partanen J, Taipale J, Petrova TV, et al. Vascular endothelial growth factor C is required for sprouting of the first lymphatic vessels from embryonic veins. Nat Immunol. 2004;5:74–80. doi: 10.1038/ni1013. [DOI] [PubMed] [Google Scholar]
- Keck PJ, Hauser SD, Krivi G, Sanzo K, Warren T, Feder J, et al. Vascular permeability factor, an endothelial cell mitogen related to PDGF. Science. 1989;246:1309–1312. doi: 10.1126/science.2479987. [DOI] [PubMed] [Google Scholar]
- Kiba A, Sagara H, Hara T, Shibuya M. VEGFR-2-specific ligand VEGF-E induces non-edematous hyper-vascularization in mice. Biochem Biophys Res Commun. 2003;301:371–377. doi: 10.1016/s0006-291x(02)03033-4. [DOI] [PubMed] [Google Scholar]
- Kliem MA, Heeke BL, Franz CK, Radovitskiy I, Raore B, Barrow E, et al. Intramuscular administration of a VEGF zinc finger transcription factor activator (VEGF-ZFP-TF) improves functional outcomes in SOD1 rats. Amyotroph Lateral Scler. 2011;12:331–339. doi: 10.3109/17482968.2011.574142. [DOI] [PubMed] [Google Scholar]
- Klug A. The discovery of zinc fingers and their applications in gene regulation and genome manipulation. Annu Rev Biochem. 2010;79:213–231. doi: 10.1146/annurev-biochem-010909-095056. [DOI] [PubMed] [Google Scholar]
- Koch M, Dettori D, Van Nuffelen A, Souffreau J, Marconcini L, Wallays G, et al. VEGF-D deficiency in mice does not affect embryonic or postnatal lymphangiogenesis but reduces lymphatic metastasis. J Pathol. 2009;219:356–364. doi: 10.1002/path.2605. [DOI] [PubMed] [Google Scholar]
- Koch S, Tugues S, Li X, Gualandi L, Claesson-Welsh L. Signal transduction by vascular endothelial growth factor receptors. Biochem J. 2011;437:169–183. doi: 10.1042/BJ20110301. [DOI] [PubMed] [Google Scholar]
- Kranich S, Hattermann K, Specht A, Lucius R, Mentlein R. VEGFR-3/Flt-4 mediates proliferation and chemotaxis in glial precursor cells. Neurochem Int. 2009;55:747–753. doi: 10.1016/j.neuint.2009.07.007. [DOI] [PubMed] [Google Scholar]
- Kukk E, Lymboussaki A, Taira S, Kaipainen A, Jeltsch M, Joukov V, et al. VEGF-C receptor binding and pattern of expression with VEGFR-3 suggests a role in lymphatic vascular development. Development. 1996;122:3829–3837. doi: 10.1242/dev.122.12.3829. [DOI] [PubMed] [Google Scholar]
- Kwiatkowski TJ, Jr., Bosco DA, Leclerc AL, Tamrazian E, Vanderburg CR, Russ C, et al. Mutations in the FUS/TLS gene on chromosome 16 cause familial amyotrophic lateral sclerosis. Science. 2009;323:1205–1208. doi: 10.1126/science.1166066. [DOI] [PubMed] [Google Scholar]
- Lacomblez L, Bensimon G, Leigh PN, Guillet P, Meininger V. Dose-ranging study of riluzole in amyotrophic lateral sclerosis. amyotrophic lateral sclerosis/riluzole study group II. Lancet. 1996;347:1425–1431. doi: 10.1016/s0140-6736(96)91680-3. [DOI] [PubMed] [Google Scholar]
- Lambrechts D, Poesen K, Fernandez-Santiago R, Al-Chalabi A, Del Bo R, Van Vught PW, et al. Meta-analysis of vascular endothelial growth factor variations in amyotrophic lateral sclerosis: increased susceptibility in male carriers of the −2578AA genotype. J Med Genet. 2009;46:840–846. doi: 10.1136/jmg.2008.058222. [DOI] [PubMed] [Google Scholar]
- Lambrechts D, Storkebaum E, Morimoto M, Del-Favero J, Desmet F, Marklund SL, et al. VEGF is a modifier of amyotrophic lateral sclerosis in mice and humans and protects motoneurons against ischemic death. Nat Genet. 2003;34:383–394. doi: 10.1038/ng1211. [DOI] [PubMed] [Google Scholar]
- LaMonte BH, Wallace KE, Holloway BA, Shelly SS, Ascano J, Tokito M, et al. Disruption of dynein/dynactin inhibits axonal transport in motor neurons causing late-onset progressive degeneration. Neuron. 2002;34:715–727. doi: 10.1016/s0896-6273(02)00696-7. [DOI] [PubMed] [Google Scholar]
- Le Bras B, Barallobre M-J, Homman-Ludiye J, Ny A, Wyns S, Tammela T, et al. VEGF-C is a trophic factor for neural progenitors in the vertebrate embryonic brain. Nat Neurosci. 2006;9:340–348. doi: 10.1038/nn1646. [DOI] [PubMed] [Google Scholar]
- Lee J, Gray A, Yuan J, Luoh SM, Avraham H, Wood WI. Vascular endothelial growth factor-related protein: a ligand and specific activator of the tyrosine kinase receptor Flt4. Proc Natl Acad Sci U S A. 1996;93:1988–1992. doi: 10.1073/pnas.93.5.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leung DW, Cachianes G, Kuang WJ, Goeddel DV, Ferrara N. Vascular endothelial growth factor is a secreted angiogenic mitogen. Science. 1989;246:1306–1309. doi: 10.1126/science.2479986. [DOI] [PubMed] [Google Scholar]
- Li B, Xu W, Luo C, Gozal D, Liu R. VEGF-induced activation of the PI3-K/Akt pathway reduces mutant SOD1-mediated motor neuron cell death. Brain Res Mol Brain Res. 2003;111:155–164. doi: 10.1016/s0169-328x(03)00025-1. [DOI] [PubMed] [Google Scholar]
- Li X, Lu L, Bush DJ, Zhang X, Zheng L, Suswam EA, et al. Mutant copper–zinc superoxide dismutase associated with amyotrophic lateral sclerosis binds to adenine/uridine-rich stability elements in the vascular endothelial growth factor 3′-untranslated region. J Neurochem. 2009;108:1032–1044. doi: 10.1111/j.1471-4159.2008.05856.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Y, Zhang F, Nagai N, Tang Z, Zhang S, Scotney P, et al. VEGF-B inhibits apoptosis via VEGFR-1-mediated suppression of the expression of BH3-only protein genes in mice and rats. J Clin Invest. 2008;118:913–923. doi: 10.1172/JCI33673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu L, Wang S, Zheng L, Li X, Suswam EA, Zhang X, et al. Amyotrophic lateral sclerosis-linked mutant SOD1 sequesters Hu antigen R (HuR) and TIA-1-related protein (TIAR): implications for impaired post-transcriptional regulation of vascular endothelial growth factor. J Biol Chem. 2009;284:33989–33998. doi: 10.1074/jbc.M109.067918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu L, Zheng L, Viera L, Suswam E, Li Y, Li X, et al. Mutant Cu/Zn-superoxide dismutase associated with amyotrophic lateral sclerosis destabilizes vascular endothelial growth factor mRNA and downregulates its expression. J Neurosci. 2007;27:7929–7938. doi: 10.1523/JNEUROSCI.1877-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lysogorskaia EV, Abramycheva N, Zakharova MN, Illarioshkin SN. Association between the VEGF-2578 capital ES, cyrillic/A polymorphism and amyotrophic lateral sclerosis in a Russian population. Zh Nevrol Psikhiatr Im S S Korsakova. 2012;112:42–45. [PubMed] [Google Scholar]
- Ma Y, Liu W, Wang Y, Chao X, Qu Y, Wang K, et al. VEGF protects rat cortical neurons from mechanical trauma injury induced apoptosis via the MEK/ERK pathway. Brain Res Bull. 2011;86:441–446. doi: 10.1016/j.brainresbull.2011.07.007. [DOI] [PubMed] [Google Scholar]
- Maglione D, Guerriero V, Viglietto G, Delli-Bovi P, Persico MG. Isolation of a human placenta cDNA coding for a protein related to the vascular permeability factor. Proc Natl Acad Sci U S A. 1991;88:9267–9271. doi: 10.1073/pnas.88.20.9267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mali Y, Zisapel N. VEGF up-regulation by G93A superoxide dismutase and the role of malate-aspartate shuttle inhibition. Neurobiol Dis. 2010;37:673–681. doi: 10.1016/j.nbd.2009.12.005. [DOI] [PubMed] [Google Scholar]
- Matsunaga Y, Yamazaki Y, Suzuki H, Morita T. VEGF-A and VEGF-F evoke distinct changes in vascular ultrastructure. Biochem Biophys Res Commun. 2009;379:872–875. doi: 10.1016/j.bbrc.2008.12.129. [DOI] [PubMed] [Google Scholar]
- Matsuzaki H, Tamatani M, Yamaguchi A, Namikawa K, Kiyama H, Vitek MP, et al. Vascular endothelial growth factor rescues hippocampal neurons from glutamate-induced toxicity: signal transduction cascades. FASEB J. 2001;15:1218–1220. [PubMed] [Google Scholar]
- Mauceri D, Freitag HE, Oliveira AMM, Bengtson CP, Bading H. Nuclear calcium-VEGFD signaling controls maintenance of dendrite arborization necessary for memory formation. Neuron. 2011;71:117–130. doi: 10.1016/j.neuron.2011.04.022. [DOI] [PubMed] [Google Scholar]
- Meissirel C, Ruiz de Almodovar C, Knevels E, Coulon C, Chounlamountri N, Segura I, et al. VEGF modulates NMDA receptors activity in cerebellar granule cells through Src-family kinases before synapse formation. Proc Natl Acad Sci U S A. 2011;108:13782–13787. doi: 10.1073/pnas.1100341108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyer M, Clauss M, Lepple-Wienhues A, Waltenberger J, Augustin HG, Ziche M, et al. A novel vascular endothelial growth factor encoded by Orf virus, VEGF-E, mediates angiogenesis via signalling through VEGFR-2 (KDR) but not VEGFR-1 (Flt-1) receptor tyrosine kinases. EMBO J. 1999;18:363–374. doi: 10.1093/emboj/18.2.363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Millauer B, Wizigmann-Voos S, Schnürch H, Martinez R, Møller NP, Risau W, et al. High affinity VEGF binding and developmental expression suggest Flk-1 as a major regulator of vasculogenesis and angiogenesis. Cell. 1993;72:835–846. doi: 10.1016/0092-8674(93)90573-9. [DOI] [PubMed] [Google Scholar]
- Mitchell JC, McGoldrick P, Vance C, Hortobagyi T, Sreedharan J, Rogelj B, et al. Overexpression of human wild-type FUS causes progressive motor neuron degeneration in an age- and dose-dependent fashion. Acta Neuropathol. 2013;125:273–288. doi: 10.1007/s00401-012-1043-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murakami T, Ilieva H, Shiote M, Nagata T, Nagano I, Shoji M, et al. Hypoxic induction of vascular endothelial growth factor is selectively impaired in mice carrying the mutant SOD1 gene. Brain Res. 2003;989:231–237. doi: 10.1016/s0006-8993(03)03374-2. [DOI] [PubMed] [Google Scholar]
- Ogawa S, Oku A, Sawano A, Yamaguchi S, Yazaki Y, Shibuya M. A novel type of vascular endothelial growth factor, VEGF-E (NZ-7 VEGF), preferentially utilizes KDR/Flk-1 receptor and carries a potent mitotic activity without heparin-binding domain. J Biol Chem. 1998;273:31273–31282. doi: 10.1074/jbc.273.47.31273. [DOI] [PubMed] [Google Scholar]
- Oh SJ, Jeltsch MM, Birkenhager R, McCarthy JE, Weich HA, Christ B, et al. VEGF and VEGF-C: specific induction of angiogenesis and lymphangiogenesis in the differentiated avian chorioallantoic membrane. Dev Biol. 1997;188:96–109. doi: 10.1006/dbio.1997.8639. [DOI] [PubMed] [Google Scholar]
- Olofsson B, Korpelainen E, Pepper MS, Mandriota SJ, Aase K, Kumar V, et al. Vascular endothelial growth factor B (VEGF-B) binds to VEGF receptor-1 and regulates plasminogen activator activity in endothelial cells. Proc Natl Acad Sci U S A. 1998;95:11709–11714. doi: 10.1073/pnas.95.20.11709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olofsson B, Pajusola K, Kaipainen A, von Euler G, Joukov V, Saksela O, et al. Vascular endothelial growth factor B, a novel growth factor for endothelial cells. Proc Natl Acad Sci U S A. 1996;93:2576–2581. doi: 10.1073/pnas.93.6.2576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oosthuyse B, Moons L, Storkebaum E, Beck H, Nuyens D, Brusselmans K, et al. Deletion of the hypoxia-response element in the vascular endothelial growth factor promoter causes motor neuron degeneration. Nat Genet. 2001;28:131–138. doi: 10.1038/88842. [DOI] [PubMed] [Google Scholar]
- Orlandini M, Marconcini L, Ferruzzi R, Oliviero S. Identification of a c-fos-induced gene that is related to the platelet-derived growth factor/vascular endothelial growth factor family. Proc Natl Acad Sci U S A. 1996;93:11675–11680. doi: 10.1073/pnas.93.21.11675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pelletier S, Gingras S, Howell S, Vogel P, Ihle JN. An early onset progressive motor neuron disorder in Scyl1-deficient mice is associated with mislocalization of TDP-43. J Neurosci. 2012;32:16560–16573. doi: 10.1523/JNEUROSCI.1787-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piltonen M, Planken A, Leskelä O, Myöhänen TT, Hänninen A-L, Auvinen P, et al. Vascular endothelial growth factor C acts as a neurotrophic factor for dopa-mine neurons in vitro and in vivo. Neuroscience. 2011;192:550–563. doi: 10.1016/j.neuroscience.2011.06.084. [DOI] [PubMed] [Google Scholar]
- Poesen K, Lambrechts D, Van Damme P, Dhondt J, Bender F, Frank N, et al. Novel role for vascular endothelial growth factor (VEGF) receptor-1 and its ligand VEGF-B in motor neuron degeneration. J Neurosci. 2008;28:10451–10459. doi: 10.1523/JNEUROSCI.1092-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Renton AE, Majounie E, Waite A, Simon-Sanchez J, Rollinson S, Gibbs JR, et al. A hexanucleotide repeat expansion in C9ORF72 is the cause of chromosome 9p21-linked ALS-FTD. Neuron. 2011;72:257–268. doi: 10.1016/j.neuron.2011.09.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riley J, Butler J, Baker KB, McClelland S, 3rd, Teng Q, Yang J, et al. Targeted spinal cord therapeutics delivery: stabilized platform and microelectrode recording guidance validation. Stereotact Funct Neurosurg. 2008;86:67–74. doi: 10.1159/000112426. [DOI] [PubMed] [Google Scholar]
- Riley JP, Raore B, Taub JS, Federici T, Boulis NM. Platform and cannula design improvements for spinal cord therapeutics delivery. Neurosurgery. 2011;69(Suppl Operative):ons147–ons154. doi: 10.1227/NEU.0b013e3182195680. [DOI] [PubMed] [Google Scholar]
- Rizvanov AA, Kiyasov AP, Gaziziov IM, Yilmaz TS, Kaligin MS, Andreeva DI, et al. Human umbilical cord blood cells transfected with VEGF and L(1) CAM do not differentiate into neurons but transform into vascular endothelial cells and secrete neuro-trophic factors to support neuro-genesis — a novel approach in stem cell therapy. Neurochem Int. 2008;53:389–394. doi: 10.1016/j.neuint.2008.09.011. [DOI] [PubMed] [Google Scholar]
- Rosen DR, Siddique T, Patterson D, Figlewicz DA, Sapp P, Hentati A, et al. Mutations in Cu/Zn superoxide dismutase gene are associated with familial amyotrophic lateral sclerosis. Nature. 1993;362:59–62. doi: 10.1038/362059a0. [DOI] [PubMed] [Google Scholar]
- Rosenstein JM, Mani N, Khaibullina A, Krum JM. Neurotrophic effects of vascular endothelial growth factor on organotypic cortical explants and primary cortical neurons. J Neurosci. 2003;23:11036–11044. doi: 10.1523/JNEUROSCI.23-35-11036.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruhrberg C. Growing and shaping the vascular tree: multiple roles for VEGF. Bioessays. 2003;25:1052–1060. doi: 10.1002/bies.10351. [DOI] [PubMed] [Google Scholar]
- Ruiz de Almodovar C, Fabre PJ, Knevels E, Coulon C, Segura I, Haddick PC, et al. VEGF mediates commissural axon chemoattraction through its receptor Flk1. Neuron. 2011a;70:966–978. doi: 10.1016/j.neuron.2011.04.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruiz de Almodovar C, Fabre PJ, Knevels E, Coulon C, Segura I, Haddick PCG, et al. VEGF mediates commissural axon chemoattraction through its receptor Flk1. Neuron. 2011b;70:966–978. doi: 10.1016/j.neuron.2011.04.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakowski SA, Heavener SB, Lunn JS, Fung K, Oh SS, Spratt SK, et al. Neuroprotection using gene therapy to induce vascular endothelial growth factor-A expression. Gene Ther. 2009;16:1292–1299. doi: 10.1038/gt.2009.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schänzer A, Wachs F-P, Wilhelm D, Acker T, Cooper-Kuhn C, Beck H, et al. Direct stimulation of adult neural stem cells in vitro and neurogenesis in vivo by vascular endothelial growth factor. Brain Pathol. 2004;14:237–248. doi: 10.1111/j.1750-3639.2004.tb00060.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwartz CM, Tavakoli T, Jamias C, Park S-S, Maudsley S, Martin B, et al. Stromal factors SDF1α, sFRP1, and VEGFD induce dopaminergic neuron differentiation of human pluripotent stem cells. J Neurosci Res. 2012;90:1367–1381. doi: 10.1002/jnr.23064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwarz Q, Gu C, Fujisawa H, Sabelko K, Gertsenstein M, Nagy A, et al. Vascular endothelial growth factor controls neuronal migration and cooperates with Sema3A to pattern distinct compartments of the facial nerve. Genes Dev. 2004;18:2822–2834. doi: 10.1101/gad.322904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Senger DR, Galli SJ, Dvorak AM, Perruzzi CA, Harvey VS, Dvorak HF. Tumor cells secrete a vascular permeability factor that promotes accumulation of as-cites fluid. Science. 1983;219:983–985. doi: 10.1126/science.6823562. [DOI] [PubMed] [Google Scholar]
- Shen H, Clauss M, Ryan J, Schmidt AM, Tijburg P, Borden L, et al. Characterization of vascular permeability factor/vascular endothelial growth factor receptors on mononuclear phagocytes. Blood. 1993;81:2767–2773. [PubMed] [Google Scholar]
- Shin Y-J, Choi J-S, Lee J-Y, Choi J-Y, Cha J-H, Chun M-H, et al. Differential regulation of vascular endothelial growth factor-C and its receptor in the rat hippocampus following transient forebrain ischemia. Acta Neuropathol. 2008;116:517–527. doi: 10.1007/s00401-008-0423-x. [DOI] [PubMed] [Google Scholar]
- Silverman WF, Krum JM, Mani N, Rosenstein JM. Vascular, glial and neuronal effects of vascular endothelial growth factor in mesencephalic explant cultures. Neuroscience. 1999;90:1529–1541. doi: 10.1016/s0306-4522(98)00540-5. [DOI] [PubMed] [Google Scholar]
- Sondell M, Lundborg G, Kanje M. Vascular endothelial growth factor stimulates Schwann cell invasion and neovascularization of acellular nerve grafts. Brain Res. 1999;846:219–228. doi: 10.1016/s0006-8993(99)02056-9. [DOI] [PubMed] [Google Scholar]
- Sondell M, Sundler F, Kanje M. Vascular endothelial growth factor is a neurotrophic factor which stimulates axonal outgrowth through the flk-1 receptor. Eur J Neurosci. 2000;12:4243–4254. doi: 10.1046/j.0953-816x.2000.01326.x. [DOI] [PubMed] [Google Scholar]
- Sreedharan J, Blair IP, Tripathi VB, Hu X, Vance C, Rogelj B, et al. TDP-43 mutations in familial and sporadic amyotrophic lateral sclerosis. Science. 2008;319:1668–1672. doi: 10.1126/science.1154584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stacker SA, Stenvers K, Caesar C, Vitali A, Domagala T, Nice E, et al. Biosyn-thesis of vascular endothelial growth factor-D involves proteolytic processing which generates non-covalent homodimers. J Biol Chem. 1999;274:32127–32136. doi: 10.1074/jbc.274.45.32127. [DOI] [PubMed] [Google Scholar]
- Stalmans I, Ng Y-S, Rohan R, Fruttiger M, Bouché A, Yuce A, et al. Arteriolar and venular patterning in retinas of mice selectively expressing VEGF isoforms. J Clin Invest. 2002;109:327–336. doi: 10.1172/JCI14362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stone J, Itin A, Alon T, Pe'er J, Gnessin H, Chan-Ling T, et al. Development of retinal vasculature is mediated by hypoxia-induced vascular endothelial growth factor (VEGF) expression by neuroglia. J Neurosci. 1995;15:4738–4747. doi: 10.1523/JNEUROSCI.15-07-04738.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Storkebaum E, Lambrechts D, Dewerchin M, Moreno-Murciano M-P, Appelmans S, Oh H, et al. Treatment of motoneuron degeneration by intracerebroventricular delivery of VEGF in a rat model of ALS. Nat Neurosci. 2005a;8:85–92. doi: 10.1038/nn1360. [DOI] [PubMed] [Google Scholar]
- Storkebaum E, Lambrechts D, Dewerchin M, Moreno-Murciano MP, Appelmans S, Oh H, et al. Treatment of motoneuron degeneration by intracerebroventricular delivery of VEGF in a rat model of ALS. Nat Neurosci. 2005b;8:85–92. doi: 10.1038/nn1360. [DOI] [PubMed] [Google Scholar]
- Sun Y, Jin K, Childs JT, Xie L, Mao XO, Greenberg DA. Increased severity of cerebral ischemic injury in vascular endothelial growth factor-B-deficient mice. J Cereb Blood Flow Metab. 2004;24:1146–1152. doi: 10.1097/01.WCB.0000134477.38980.38. [DOI] [PubMed] [Google Scholar]
- Svensson B, Peters M, König H-G, Poppe M, Levkau B, Rothermundt M, et al. Vascular endothelial growth factor protects cultured rat hippocampal neurons against hypoxic injury via an antiexcitotoxic, caspase-independent mechanism. J Cereb Blood Flow Metab. 2002;22:1170–1175. doi: 10.1097/01.wcb.0000037988.07114.98. [DOI] [PubMed] [Google Scholar]
- Terman BI, Dougher-Vermazen M, Carrion ME, Dimitrov D, Armellino DC, Gospodarowicz D, et al. Identification of the KDR tyrosine kinase as a receptor for vascular endothelial cell growth factor. Biochem Biophys Res Commun. 1992;187:1579–1586. doi: 10.1016/0006-291x(92)90483-2. [DOI] [PubMed] [Google Scholar]
- Terry PD, Kamel F, Umbach DM, Lehman TA, Hu H, Sandler DP, et al. VEGF promoter haplotype and amyotrophic lateral sclerosis (ALS). J Neurogenet. 2004;18:429–434. doi: 10.1080/01677060490894450. [DOI] [PubMed] [Google Scholar]
- Tovar-y-Romo LB, Tapia R. Delayed administration of VEGF rescues spinal motor neurons from death with a short effective time frame in excitotoxic experimental models in vivo. ASN Neuro. 2012;4 doi: 10.1042/AN20110057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tovar YRLB, Tapia R. VEGF protects spinal motor neurons against chronic excitotoxic degeneration in vivo by activation of PI3-K pathway and inhibition of p38MAPK. J Neurochem. 2010;115:1090–1101. doi: 10.1111/j.1471-4159.2010.06766.x. [DOI] [PubMed] [Google Scholar]
- Tovar YRLB, Zepeda A, Tapia R. Vascular endothelial growth factor prevents paralysis and motoneuron death in a rat model of excitotoxic spinal cord neurodegeneration. J Neuropathol Exp Neurol. 2007;66:913–922. doi: 10.1097/nen.0b013e3181567c16. [DOI] [PubMed] [Google Scholar]
- Turner MR, Hardiman O, Benatar M, Brooks BR, Chio A, de Carvalho M, et al. Controversies and priorities in amyotrophic lateral sclerosis. Lancet Neurol. 2013;12:310–322. doi: 10.1016/S1474-4422(13)70036-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Den Bosch L, Storkebaum E, Vleminckx V, Moons L, Vanopdenbosch L, Scheveneels W, et al. Effects of vascular endothelial growth factor (VEGF) on motor neuron degeneration. Neurobiol Dis. 2004a;17:21–28. doi: 10.1016/j.nbd.2004.06.004. [DOI] [PubMed] [Google Scholar]
- Van Den Bosch L, Storkebaum E, Vleminckx V, Moons L, Vanopdenbosch L, Scheveneels W, et al. Effects of vascular endothelial growth factor (VEGF) on motor neuron degeneration. Neurobiol Dis. 2004b;17:21–28. doi: 10.1016/j.nbd.2004.06.004. [DOI] [PubMed] [Google Scholar]
- Van Vught PWJ, Sutedja NA, Veldink JH, Koeleman BPC, Groeneveld GJ, Wijmenga C, et al. Lack of association between VEGF polymorphisms and ALS in a Dutch population. Neurology. 2005;65:1643–1645. doi: 10.1212/01.wnl.0000184514.39853.56. [DOI] [PubMed] [Google Scholar]
- Vance C, Rogelj B, Hortobagyi T, De Vos KJ, Nishimura AL, Sreedharan J, et al. Mutations in FUS, an RNA processing protein, cause familial amyotrophic lateral sclerosis type 6. Science. 2009;323:1208–1211. doi: 10.1126/science.1165942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y, Mao XO, Xie L, Banwait S, Marti HH, Greenberg DA, et al. Vascular endothelial growth factor overexpression delays neurodegeneration and prolongs survival in amyotrophic lateral sclerosis mice. J Neurosci. 2007;27:304–307. doi: 10.1523/JNEUROSCI.4433-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang ZG, Puri Tipu S., Quigg Richard J. Characterization of novel VEGF (vascular endothelial growth factor)-C splicing isoforms from mouse. Biochem J. 2010;428:347–354. doi: 10.1042/BJ20100379. [DOI] [PubMed] [Google Scholar]
- Wegorzewska I, Bell S, Cairns NJ, Miller TM, Baloh RH. TDP-43 mutant transgenic mice develop features of ALS and frontotemporal lobar degeneration. Proc Natl Acad Sci U S A. 2009;106:18809–18814. doi: 10.1073/pnas.0908767106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Witte MH, Erickson R, Bernas M, Andrade M, Reiser F, Conlon W, et al. Phenotypic and genotypic heterogeneity in familial Milroy lymphedema. Lymphology. 1998;31:145–155. [PubMed] [Google Scholar]
- Yamada Y, Nezu J, Shimane M, Hirata Y. Molecular cloning of a novel vascular endothelial growth factor, VEGF-D. Genomics. 1997;42:483–488. doi: 10.1006/geno.1997.4774. [DOI] [PubMed] [Google Scholar]
- Yamazaki Y, Takani K, Atoda H, Morita T. Snake venom vascular endothelial growth factors (VEGFs) exhibit potent activity through their specific recognition of KDR (VEGF receptor 2). J Biol Chem. 2003;278:51985–51988. doi: 10.1074/jbc.C300454200. [DOI] [PubMed] [Google Scholar]
- Yang Y, Hentati A, Deng HX, Dabbagh O, Sasaki T, Hirano M, et al. The gene encoding alsin, a protein with three guanine-nucleotide exchange factor domains, is mutated in a form of recessive amyotrophic lateral sclerosis. Nat Genet. 2001;29:160–165. doi: 10.1038/ng1001-160. [DOI] [PubMed] [Google Scholar]
- Zhang Y, Zhang H, Fu Y, Song H, Wang L, Zhang J, et al. VEGF C2578A polymorphism does not contribute to amyotrophic lateral sclerosis susceptibility in sporadic Chinese patients. Amyotroph Lateral Scler. 2006;7:119–122. doi: 10.1080/14660820600600657. [DOI] [PubMed] [Google Scholar]
- Zheng C, Skold MK, Li J, Nennesmo I, Fadeel B, Henter JI. VEGF reduces astrogliosis and preserves neuromuscular junctions in ALS transgenic mice. Biochem Biophys Res Commun. 2007;363:989–993. doi: 10.1016/j.bbrc.2007.09.088. [DOI] [PubMed] [Google Scholar]