Abstract
Congestion is a major reason for hospitalization in acute heart failure (HF). Therapeutic strategies to manage congestion include diuretics, vasodilators, ultrafiltration, vasopressin antagonists, mineralocorticoid receptor antagonists, and potentially also novel therapies such as gut sequesterants and serelaxin. Uncertainty exists with respect to the appropriate decongestion strategy for an individual patient. In this manuscript, we summarize the benefit and risk profiles for these decongestion strategies and provide guidance on selecting an appropriate approach for different patients. An evidence-based initial approach to congestion management involves high-dose intravenous diuretics with addition of vasodilators for dyspnea relief if blood pressure allows. To enhance diuresis or overcome diuretic resistance, options include dual nephron blockade with thiazide diuretics or natriuretic doses of mineralocorticoid receptor antagonists. Vasopressin antagonists may improve aquaresis and relieve dyspnea. If diuretic strategies are unsuccessful, then ultrafiltration may be considered. Ultrafiltration should be used with caution in the setting of worsening renal function. This review is based on discussions among scientists, clinical trialists and regulatory representatives at the 9th Global Cardio Vascular Clinical Trialists Forum in Paris, France, from November 30 to December 1, 2012.
Keywords: acute heart failure, decongestion, volume overload, strategies, outcomes
INTRODUCTION
Heart failure (HF) is a major and increasing public health problem worldwide(1–3). The primary reason for acute HF (AHF) hospitalization is congestion manifested by dyspnea, edema and fatigue due to elevated filling pressures(4–6). Despite inpatient treatment targeting decongestion with diuretics, many patients are discharged without weight loss and with persistent signs of congestion(7, 8). For instance, in an international AHF trial, persistent congestion was present at discharge in more than a quarter of patients(9). Baseline congestion and residual congestion at discharge are associated with increased rehospitalization and mortality, and successful decongestion is a major goal of AHF management(9–11).
Uncertainty exists with respect to the pathogenesis of congestion and how to best treat congestion prior to discharge(12, 13). In addition to diuretics, strategies to treat congestion include vasodilators, ultrafiltration, vasopressin antagonists, and mineralocorticoid receptor antagonists. Serelaxin and gut sequesterants may also be used for decongestion in the future. In this manuscript, we summarize the benefit and risk profiles for these therapies and provide guidance on selecting an appropriate approach for different patients. This review is based on discussions among scientists, clinical trialists, and regulatory representatives at the 9th Global CardioVascular Clinical Trialists Forum in Paris, France, from November 30 to December 1, 2012.
Pathophysiology of Congestion in Acute Heart Failure
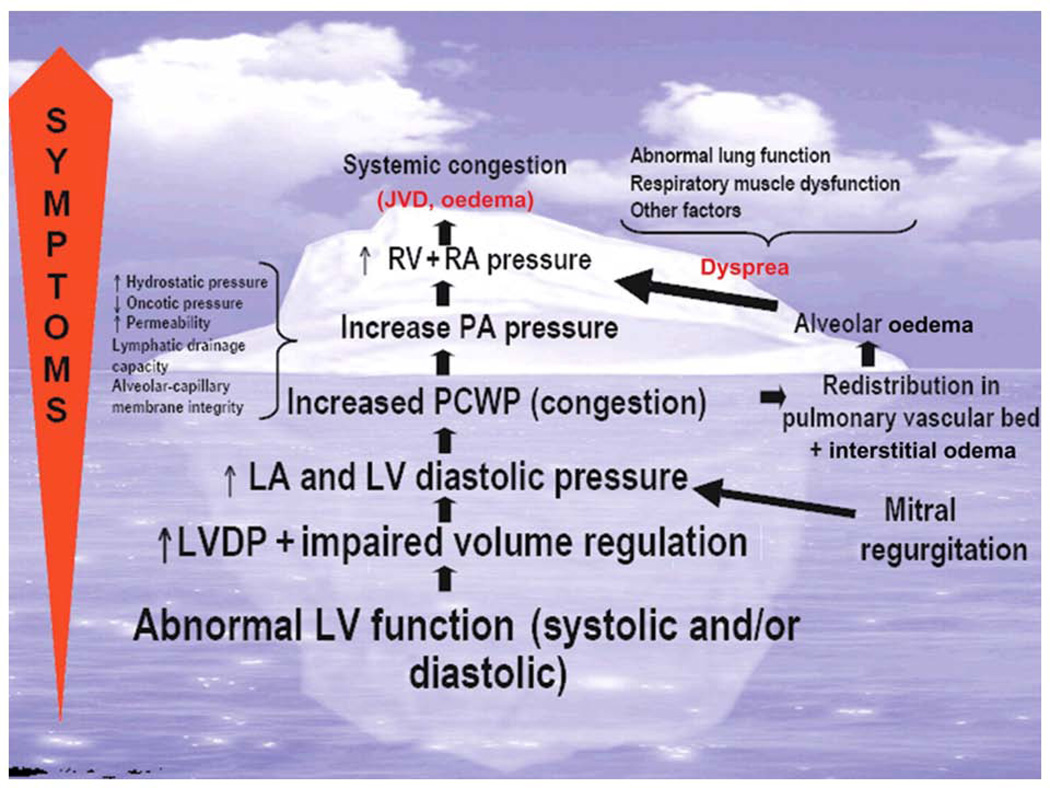
Congestion is defined as a high left ventricular (LV) end-diastolic pressure associated with signs and symptoms such as dyspnea, rales, and edema (Figure 1)(13). Recent data also demonstrate the importance of elevation in right-sided pressures as characterized by inferior vena cava dilation(14), which result in the characteristic signs and symptoms of hepatic and renal congestion.
Figure 1.
Pathophysiology of congestion
Abbreviations: RV=right ventricular, RA=right atrial, PA=pulmonary artery, PCWP=pulmonary capillary wedge pressure; LA, left atrial, LV=left ventricular, LVDP=left ventricular diastolic pressure, JVD=jugular venous distension.
Reproduced with permission from Gheorghiade M et al, Eur J Heart Fail 2010(13).
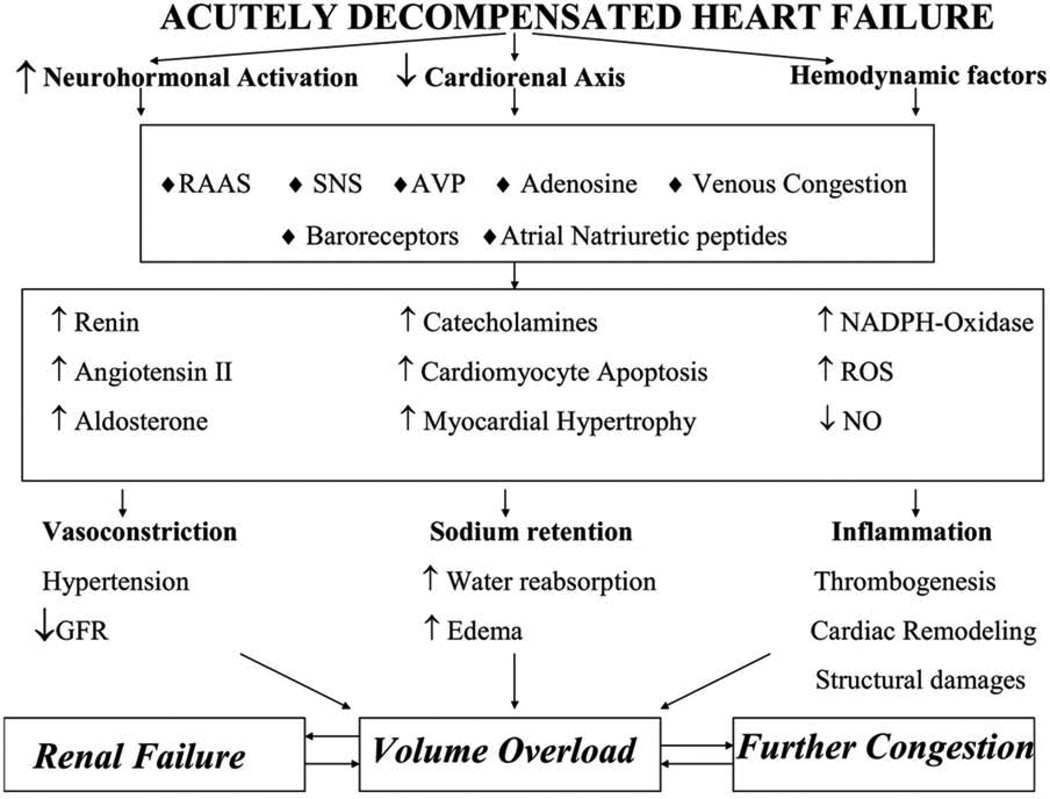
At present, the underlying mechanisms of congestion in AHF are poorly understood. The traditional paradigm assumes that hemodynamic abnormalities related to reduced cardiac output and activation of the renin-angiotensin-aldosterone system (RAAS) are the primary pathophysiologic drivers in AHF. Underlying cardiac dysfunction is exacerbated by coronary ischemia, hypertension, arrhythmia, infection or medical/dietary non-adherence with increased fluid retention. However, in many patients a specific precipitating factor cannot be identified and early symptoms of congestion occur without significant weight gain(15). Thus, there is increasing recognition that fluid redistribution may contribute to AHF. For instance, extracellular fluid volume can shift from the splanchnic veins into the effective circulating blood volume during AHF via autonomic mechanisms(16). Contemporary data also support a role for inflammation, endothelial cell activation, pro-thrombotic changes and abnormalities in arginine vasopressin (AVP) and adenosine signaling (Figure 2)(17). For instance, Colombo and colleagues recently demonstrated that peripheral venous congestion caused the release of inflammatory mediators and changes in endothelial cell response in an experimental model(18). The contribution of these mechanisms in different AHF patients varies(19). For instance, elderly females with preserved ejection fraction tend to more often present with rapidly progressive pulmonary edema in the setting of hypertension related to mechanisms of reduced arterial compliance and venous capacitance(20–22). Other patients present with a distinct phenotype characterized by the insidious onset of dyspnea, and peripheral edema with evidence of hepatic and renal dysfunction due, in part, to RAAS activation, inflammation and progressive cardiorenal syndrome(17, 23–25). Regardless of the specific underlying mechanisms for an individual patient, congestion contributes to HF progression through further neurohormonal activation, LV geometric changes, pulmonary hypertension, right ventricular (RV) dysfunction and adverse cardiorenal changes(26–28).
Figure 2.
The underlying pathophysiological mechanisms of volume overload in acutely decompensated heart failure
AVP, arginine vasopressin; GFR, glomerular filtration rate; NO, nitric oxide; RAAS, renin–angiotensin–aldosterone system; ROS, reactive oxygen species; SNS, sympathetic nervous system.
Reproduced with permission from Koniari K et al. Eur Heart J Acute Cardiovasc Care, 2013(17).
Assessment of Congestion and Decongestion
The pattern of congestion in AHF varies, but data suggest that 89% of patients present with dyspnea; rales and peripheral edema are present in 68% and 66%, respectively(4). While there is not currently a standardized definition of adequate decongestion, clinical trials have used the following criteria: jugular venous distension (JVD) < 8 cm of water, no more than trace peripheral edema, and the absence of orthopnea(29). These criteria have been simplified into an “orthoedema” congestion score based on the presence of orthopnea (≥2 pillow=2, <2 pillows=0) and peripheral edema (trace=0, moderate=1, severe=2) with the components added to classify congestion as mild (score 0–1), moderate (2) and severe (3–4)(30). A post-hoc analysis of the DOSE-HF and CARRESS-HF trials of AHF patients with congestion (and cardiorenal syndrome in the case of CARRESS-HF) found that baseline orthoedema was moderate in 22% of patients and severe in 62%(31). Following aggressive inpatient therapy targeting decongestion, more than one-third of patients (35%) had persistent moderate to severe congestion at discharge. Higher orthoedema scores at admission and discharge were both associated with increased risk for 60-day death or HF hospitalization.
These recent studies in combination with other data in AHF patients demonstrate that even with severe hemodynamic congestion, physical signs including rales, edema and JVD may have limited sensitivity and specificity(32). Moreover, markers of decongestion such as weight and fluid loss have a poor correlation with dyspnea relief(33). Given the difficulty of accurately assessing congestion by exam, biomarkers and other novel approaches may help clinician’s quantify congestion. For example, recent data highlight a role for the use of bioimpedence techniques in evaluating volume status(34). Natriuretic peptides are the most commonly used biomarker of volume status. Rather than using absolute thresholds of natriuretic peptides to signify congestion, the value of using “wet” and “optivolemic” values for individual patients has been demonstrated(35). In other words, clinicians may consider obtaining serial values (e.g., on admission for AHF with congestion, prior to discharge from AHF following decongestion, and during a period of euvolemia as an outpatient) in order to inform future clinical evaluation of an individual patient’s congestion status. Hemoconcentration during hospitalization, as characterized by increases in hemoglobin or hematocrit, albumin and total protein, represents another marker of decongestion. Hemoconcentration during AHF hospitalization has been associated with improved 180-day survival(36) despite an association with worsening renal function(37). Recent evidence has also highlighted the importance of the timing of hemoconcentration(38). Only hemoconcentration that occurs later during hospitalization is associated with improved outcomes, which suggests the importance of sustained decongestion. Altogether, the assessment of volume status is of paramount importance in order to tailor therapies to an individual patient’s needs. In contemporary clinical practice, the assessment of volume status is largely based on data from clinical examination and laboratory biomarker profiles. Future work will better define the role of novel techniques to monitor volume status such as bioimpedence and implantable monitors.
Decongestion Strategies
Loop Diuretics
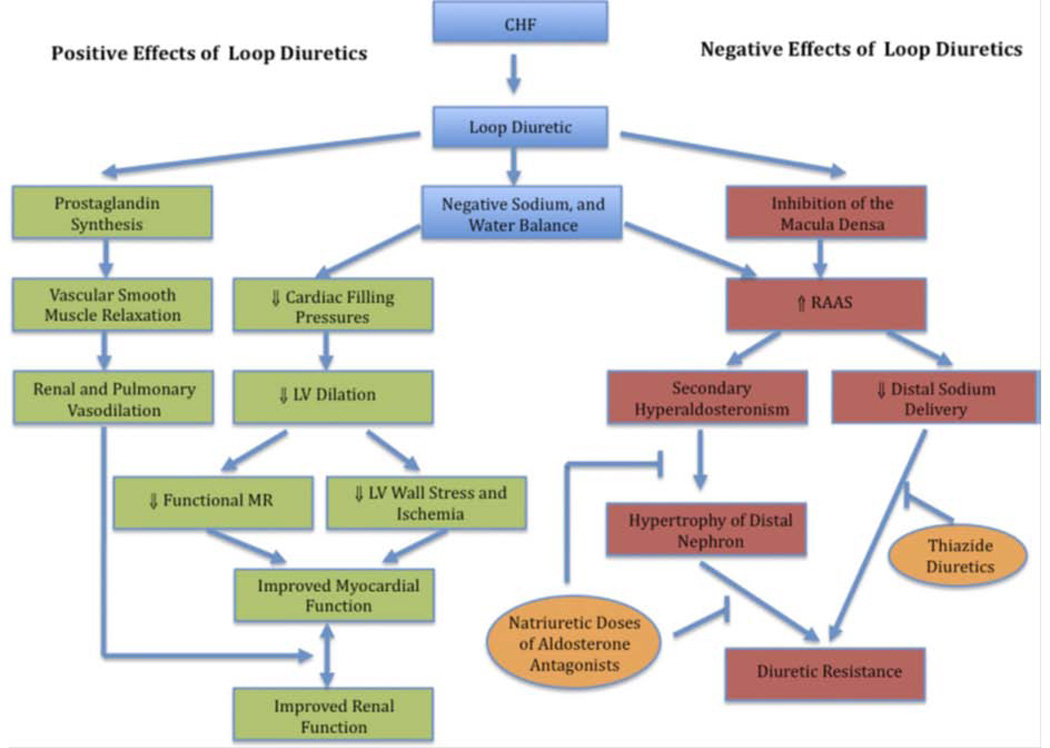
Historically, loop diuretics (e.g., furosemide, torsemide) have been the cornerstone of decongestive therapy(39). Loop diuretics inhibit the renal Na+/2Cl−/K+ cotransporter resulting in natriuresis and diuresis. In HF patients, the diuretic dose–response curve shifts downward and to the right, such that a higher dose is required to achieve the effect. Loop diuretics generally improve dyspnea and decrease ventricular filling pressures in AHF (Figure 3). Several small studies of torsemide vs. furosemide(40–42) and a recent meta-analysis(43) suggest a decrease in HF morbidity with torsemide compared to furosemide. However, a prospective large-scale trial is needed to support these findings.
Figure 3.
Diuretic Mechanisms. Proposed positive and negative effects of loop diuretics as well as sites of action for thiazide diuretics and natriuretic doses of aldosterone antagonists. CHF=congestive heart failure; LV=left ventricular; MR=mitral regurgitation; RAAS=renin-angiotensin-aldosterone system.
Reproduced, with permission, from Felker GM and Mentz RJ. J Am Coll Cardiol, 2012(39).
The DOSE trial (Diuretic Optimization Strategies Evaluation) is the largest randomized AHF trial to evaluate diuretic strategies(44). DOSE randomized 308 AHF patients to IV furosemide given as boluses or continuous infusion and to either a low dose (IV dose numerically equivalent to the patient’s oral dose) or a high dose (2.5 times oral dose given IV) strategy. There was no significant difference in the co-primary endpoints of global assessment of symptoms or change in serum creatinine over 72 h with any of these strategies. However, patients randomized to the higher dose strategy had more favorable outcomes for the secondary measures of dyspnea relief, change in weight, and fluid loss. However, as noted above, more than one-third of patients in a combined analysis of the DOSE and CARRESS trials had persistent congestion at discharge despite therapy targeting decongestion in the clinical trial setting(31). Thus, despite the efficacy of loop diuretics for dyspnea relief, data suggest limitations related to successful decongestion.
Loop diuretic use is also balanced by limitations of diuretic resistance, neurohormonal activation, and worsening renal function (WRF)(39). Diuretic resistance occurs when these agents fail to adequately control volume status despite appropriate dose escalation. Mechanisms of diuretic resistance include the “braking phenomenon”, “rebound” effect and hyperaldosteronism. The “braking phenomenon” occurs when long-term diuretic use results in a reduced natriuretic response due, in part, to nephron adaptations(45). The “rebound” effect involves post-diuretic sodium retention typically in the setting of inadequate dosing frequency and insufficient sodium restriction(46). Sequential nephron blockade with thiazide-type diuretics may be used in combination with loop diuretics to augment diuresis(47). However, their use has been associated with increased arrhythmia risk due to hypokalemia(48).
Observational studies have shown associations between high dose loop diuretics and adverse outcomes(49–51). However, these studies are confounded, since patients receiving higher doses of diuretics tend to have greater disease severity and/or comorbidity. Nonetheless, animal studies have shown that treatment with furosemide results in progression of LV systolic dysfunction(52). Potential mechanisms for worse outcomes with loop diuretics include RAAS activation, electrolyte disturbances, and WRF.
Loop diuretics may cause RAAS activation. Studies supporting this concept, however, generally predate contemporary HF pharmacotherapy. A retrospective study of the SOLVD trial demonstrated that plasma renin activity (PRA) was significantly elevated in HF patients receiving diuretics compared to those not receiving diuretics(53). In another study, acute dosing of IV furosemide resulted in rapid PRA elevation in HF patients treated chronically with digoxin, and this was associated with systemic vasoconstriction(54).
The association between higher diuretic dosing and WRF has been of particular interest given that WRF is associated with worse outcome(49, 55). However, recent data have suggested that transient WRF during AHF therapy may not affect post-discharge outcomes(37, 56). For instance, in the DOSE trial, higher dose diuretics were superior to lower dose diuretics for dyspnea relief and fluid loss at the cost of transient WRF that did not appear to have long-term consequences(44). Given that persistent congestion is associated with WRF(57) and adverse events, transient WRF may be a reasonable trade-off for decongestion. For instance, a recent analysis by Metra et al demonstrated that WRF was not associated with worse outcomes, but that WRF in the context of persistent congestion was an independent predictor of post-discharge morbidity and mortality(58).
Vasodilators
Current guidelines indicate that if symptomatic hypotension is absent during hospitalization for AHF, IV vasodilators may be considered as an adjuvant to diuretic therapy for dyspnea relief (1,3). There are currently no data to support improved outcomes in AHF patients treated with vasodilators. Overall, the data support modest beneficial effects on dyspnea relief and hemodynamics (e.g., central venous pressure and pulmonary capillary wedge pressure), but further details related to effects on peripheral edema, net fluid loss and weight loss are limited. Thus, further characterization of the utility of these agents to adequately decongest patients based on the metrics above is not possible.
IV nitroglycerin is primarily a venodilator that lowers preload and may help reduce pulmonary congestion(59). However, tachyphylaxis to nitroglycerin may develop within hours despite dose escalation(60). Nitroprusside is a balanced venodilator and arteriodilator with effects on the pulmonary vasculature(61). Efficacy data with nitroprusside in AHF patients are limited and largely confined to post-myocardial infarction LV dysfunction(62, 63). Nitroprusside use is typically confined to an intensive care setting with invasive hemodynamic monitoring due to the potential for marked hypotension(1).
Nesiritide reduces ventricular filling pressures, yet studies demonstrated variable effects on dyspnea relief. A randomized trial of nesiritide vs. placebo in 127 AHF patients demonstrated that nesiritide more rapidly reduced dyspnea compared to diuretics alone(64). The VMAC study investigated the use of nesiritide vs. nitroglycerin vs. placebo in 489 AHF patients(65). Nesiritide resulted in significant improvements in wedge pressure compared to nitroglycerin and placebo. However, there was no difference between nesiritide and nitroglycerin with respect to dyspnea benefits. ASCEND-HF demonstrated that nesiritide compared with placebo had a modest non-significant impact on dyspnea at the cost of more hypotension(66). Nesiritide did not affect post-discharge outcomes. A recent subgroup analysis also showed that nesiritide did not increase urine output compared to standard therapy(67). Thus, routine use of nesiritide has not been recommended in the broad AHF population.
Novel natriuretic peptides with vasodilatory effects such as ularitide are being investigated in acute HF patients. Two double-blind placebo-controlled proof-of-concept studies demonstrated hemodynamic and symptom benefits with ularitide(68). An ongoing phase III trial of ularitide is investigating a potential role for ularitide added to standard therapy in acute HF (NCT01661634).
Ultrafiltration
Ultrafiltration involves removal of plasma water across a semipermeable membrane in response to a transmembrane pressure gradient(39). If fluid removal does not exceed the interstitial fluid mobilization rate, then intravascular volume can be preserved, potentially avoiding RAAS activation and WRF(39). However, most studies investigating RAAS activation with UF pre-dated routine use of beta-blockers and ACE-inhibitors.
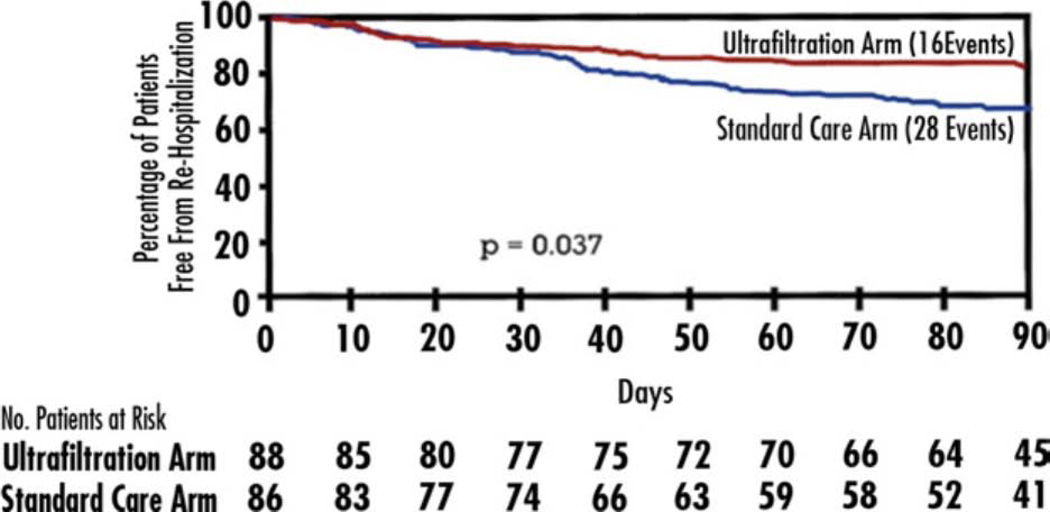
The UNLOAD trial was the first large scale trial of UF in AHF(69). This unblinded trial randomized 200 patients with AHF to either UF or loop diuretics as the primary decongestive therapy within 24 h of hospitalization. The duration and rate of UF were decided by the treating physicians. The co-primary endpoints were weight loss and dyspnea relief at 48 h. The UF group had greater weight loss (mean of 5.0 kg vs. 3.1 kg, P=0.001), but there was no difference in dyspnea relief. There was significantly less hypokalemia with UF, and other safety parameters (including serum creatinine change) were similar in the 2 study arms. Net fluid loss at 48 hours (a secondary endpoint) was also greater in the UF arm (mean of 4.6 L vs. 3.3 L, P=0.001). However, BNP improvements were similar with UF and usual care. There was a significant decrease in the secondary endpoint of rehospitalization for HF at 90 days with UF compared with diuretic therapy (Figure 4). These findings are hypothesis-generating given the small number of events, short follow-up and unblinded study design.
Figure 4.
Freedom From Heart Failure Rehospitalization. Kaplan-Meier estimate of freedom from rehospitalization for heart failure within 90 days after discharge in the ultrafiltration (red line) and standard care (blue line) groups.
Reprinted, with permission, Costanzo et al. J Am Coll Cardiol 2007(69).
CARRESS-HF was a randomized trial of UF vs. stepped pharmacologic therapy in 188 AHF patients with WRF and persistent volume overload(29). UF was performed at a fluid removal rate of 200mL/hr. Stepped pharmacologic therapy involved adjustments in loop diuretic doses as well as IV vasodilators and inotropic agents as a function of the signs and symptoms of congestion, urine output, blood pressure, ejection fraction (EF) and the presence or absence of RV failure at 48 hours. CARRESS-HF tested UF in a different patient population (i.e., cardiorenal syndrome type 1) compared with UNLOAD (i.e., routine AHF). UF was inferior for the primary endpoint of the bivariate change in the serum creatinine and body weight at 96 hours due to an increase in creatinine. Whether such a rise in serum creatinine represents desired effects of hemoconcentration or undesired worsening of renal function with subsequent outcome consequences is unknown. There was no significant between-group difference in weight loss or natriuretic peptides and a higher percentage of patients in the UF group had a serious adverse event. Overall, successful decongestion (JVD<8 cm of water, ≤peripheral edema, no orthopnea) occurred in only ~10% of patients at 96 hours in both treatment arms. These data further demonstrate that substantial weight and fluid loss are not adequate surrogates of decongestion.UF use must also be balanced by limitations related to the technology. UF involves a disposable, single-use extracorporeal blood circuit. A cost-consequences analysis found that despite a potential reduction in rehospitalization with UF, it was unlikely to result in costs savings from a societal level(70). Despite the current ability to perform UF through peripheral IV access, the lumens must provide 10 to 40 ml/min of blood flow such that patients are exposed to increased risk related to vascular access complications. Full anticoagulation is required. In CARRESS-HF, patients receiving UF had a high adverse event rate due to catheter or anticoagulation related complications (e.g., 2% catheter site hemorrhage, 9% sepsis, bacteremia or cellulitis, and 7% gastrointestinal hemorrhage). Provider experience and nursing support are additional concerns with the routine use of UF.
Current guidelines indicate that if diuretic strategies are unsuccessful, ultrafiltration may be considered(1, 3). The results of the ongoing Study of Heart Failure Hospitalizations After Aquapheresis Therapy Compared to Intravenous Diuretic Treatment (AVOID-HF, ClinicalTrials.gov Identifier: NCT01474200) are eagerly awaited. This trial is to enroll 810 patients admitted with AHF, and randomized to either UF or IV loop diuretics. The primary outcome is time to first HF event within 90 days after discharge from index hospitalization.
Vasopressin Antagonists
Inappropriate elevation of AVP in HF results in water retention with resultant congestive symptoms and electrolyte abnormalities(71). Vasopressin antagonists such as tolvaptan have been developed to block the action of AVP at the V2 receptor in renal tubules to promote aquaresis. V2 specific antagonists have the potential to increase AVP levels with consequent stimulation of V1 receptors involved in peripheral vasoconstriction.
EVEREST was a large trial comparing tolvaptan and placebo, which tested the hypothesis that adding an aquaretic agent to conventional diuretics in HF patients would improve symptoms and outcomes(72, 73). EVEREST enrolled 4133 patients admitted with AHF with EF<40% and ≥2 signs/symptoms of fluid overload. The primary short-term endpoint was a composite of patient-assessed global clinical status and weight at day 7 or discharge. The long-term co-primary endpoints were mortality and cardiovascular mortality/HF hospitalization. Tolvaptan showed greater improvement compared to placebo for weight change, but long-term outcomes were similar.
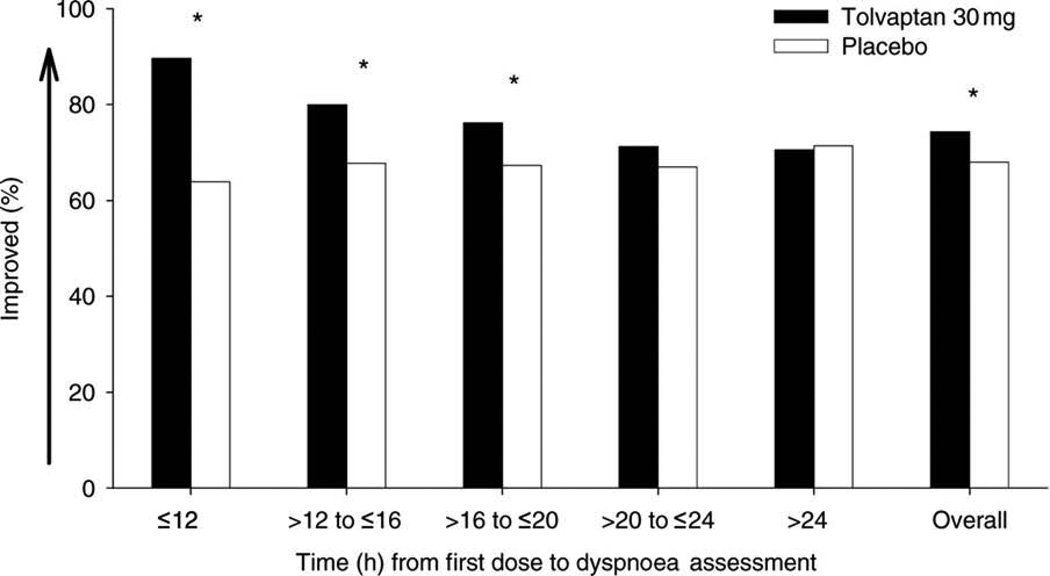
In EVEREST, tolvaptan in addition to standard diuretic therapy improved many, but not all, signs and symptoms related to congestion. Tolvaptan improved dyspnea, body weight, rales, JVD, and orthopnea over the first several days of hospitalization despite less use of diuretics. However, global clinical status, a component of the primary outcome, was not improved. The dyspnea benefits were greatest within 12 hours after the initial dose, and persisted up to 20 hours(74)(Figure 5). Tolvaptan’s benefits on dyspnea relief and weight reduction were more marked in those patients with hyponatremia(75). In the small cohort of patients with severe hyponatremia (sodium <130 mEq/L), tolvaptan was associated with reduced cardiovascular morbidity and mortality. Overall, serious adverse events including renal dysfunction, hypotension, and electrolyte abnormalities were similar with tolvaptan. These data have suggested that tolvaptan may be a useful adjunct to treating congestion early during hospitalization in patients with AHF. Tolvaptan is not specifically approved for treating congestion but rather is approved for the treatment of severe hyponatremia such as that seen in heart failure. At present, at least 2 placebo-controlled trials are exploring decongestion benefits with tolvaptan in AHF (NCT01644331, NCT01584557).
Figure 5.
Improvement in patient-assessed dyspnea with tolvaptan compared to placebo in EVEREST as a function of time from first dose of study drug.
Reproduced with permission from Pang et al. Eur Heart J 2009(74).
The FDA recently announced label changes for tolvaptan, which indicate that it should not be used for longer than 30 days and should not be used in patients with underlying liver disease. These changes were made after 3 cases of suspected liver injury with higher doses and prolonged use of tolvaptan were identified in the TEMPO study of patients with Polycystic Kidney Disease(76).
Natriuretic Doses of Mineralocorticoid Receptor Antagonists (MRAs)
Chronic therapy with a MRA, either spironolactone 25 mg daily or eplerenone 50 mg daily, is recommended in patients with NYHA class II-IV symptoms and an LVEF ≤35%(1). A post-hoc analysis of the EPHESUS study data suggested that eplerenone produced a mild short-term diuretic effect associated with better outcomes(77). However, the benefits at these doses seem to largely be due to cardioprotective effects rather than sodium handling(78). The current evidence-based MRA doses in HF are much lower than the natriuretic doses of up to 400mg/day used in cirrhotic patients(79). It has been suggested that natriuretic doses could provide additional decongestion benefits beyond loop diuretics in AHF(79).
In both cirrhosis and HF, hyperaldosteronism plays a major role in the development of congestive symptoms and contributes to diuretic resistance(39). The pathophysiology involves a failure to escape from the sodium-retaining effect of aldosterone. Hyperaldosteronism increases the major aldosterone-sensitive renal tubule channels(80). Therefore, sodium that is not reabsorbed in the loop of Henle due to the effects of loop diuretics may be reabsorbed in the distal nephron via aldosterone-related mechanisms. Natriuretic MRA doses in addition to loop diuretics may cause significant natriuresis even in the context of presumed diuretic resistance. To date, natriuretic MRA doses have not been empirically evaluated in an adequately powered HF study. However, several small studies have suggested potential benefits(81–84).
The use of natriuretic MRA doses must be balanced with concerns of hyperkalemia. A recent retrospective study examined the safety of natriuretic doses of spironolactone in HF patients(85). During a total of 738 patient-weeks, there was no significant increase in mean serum potassium or creatinine with natriuretic MRA doses. In contrast, a post-hoc analysis of the EPHESUS data suggested that eplerenone produced a mild short-term potassium-sparing effect, which was associated with better outcomes(77). Prospective, controlled studies are needed to explore the benefits related to decongestion.
Gut Sequesterants
Since many patients with AHF have chronic kidney disease (CKD) or WRF, it has been suggested that alternative, non-invasive strategies to remove fluid should be explored(86). In particular, agents are needed that assist with fluid removal without RAAS activation and hyperkalemia.
An acrylic polymer called cross-linked polyelectrolyte (CLP) removes sodium, potassium, and fluid from the GI tract with elimination in the feces. A double-blind randomized trial of CLP vs. placebo for 8 weeks was performed in 113 HF patients with CKD(87). There was no between-group difference in the primary endpoint of serum potassium over time, but the CLP treated patients had greater weight loss early in the study as well as improved symptoms and quality of life. There was a trend toward less dyspnea and lower natriuretic peptide levels with CLP. The primary concern was that 4 deaths occurred in the CLP arm. While this was a small study and investigators did not feel the deaths were attributable to study drug, these findings highlight the need for caution in subsequent studies. Future studies will need to explore whether these potential benefits in weight loss, dyspnea relief and natriuretic peptide levels translate into improve decongestion in the setting of larger, controlled clinical trials.
Serelaxin
Serelaxin is recombinant human relaxin-2 peptide, which regulates maternal adaptations in pregnancy(88). Serelaxin has potential benefits for decongestion given effects on arterial compliance, cardiac output and renal blood flow.
The RELAX-AHF trial was a phase III placebo-controlled trial of serelaxin in 1161 AHF patients with co-primary endpoints of dyspnea improvement(89). A 48-hour infusion of serelaxin resulted in an improvement in the visual analogue scale area under the curve from baseline to 5 days, but not dyspnea improvement by Likert scale. There were no between-group differences for secondary composite endpoints including death or hospitalization. However, all-cause mortality and cardiovascular mortality at 180 days were significantly lower with serelaxin (HR 0.63; 95% CI, 0.43–0.93 and HR 0.63; 95% CI, 0.41–0.96, respectively). Benefits with serelaxin were also observed for rates of worsening HF, length of stay and prognostic biomarkers(90). Serelaxin may offer benefits on congestion as evidence by effects on dyspnea relief and natriuretic peptides as well as the prevention of worsening HF, but further study is required. The ongoing RELAX-2 study is powered (target N=6375) for the primary endpoint of cardiovascular mortality through 180 days (ClinicalTrials.gov Identifier: NCT01870778). A recent randomized study investigated the renal hemodynamic effects of a 24-hour infusion of serelaxin vs. placebo in 65 patients with chronic HF(91). Serelaxin treated patients demonstrated a rise in renal plasma flow during the infusion, but there was no between-group difference in glomerular filtration rate. There were also no significant differences for changes in systolic blood pressure (SBP) or sodium excretion. Thus, ongoing study is required to investigate the mechanisms by which serelaxin may improve symptoms and outcomes in AHF.
Other Novel Agents Under Investigation
In addition to gut sequesterants, serelaxin and novel natriuretic peptides such as ularitide, there are a number of additional AHF therapeutic agents with potential decongestion benefits that are currently under investigation. For instance, these agents include luso-inotropic agents (istraoxime), cardiac myosin activators (omecamtiv mecarbil) and oral soluble guanylate cyclase stimulators, which have recently been reviewed(17, 68). Further studies will be needed to clarify the utility of these and other agents with regard to decongestion.
Selecting an Appropriate Decongestion Approach for Different Patients
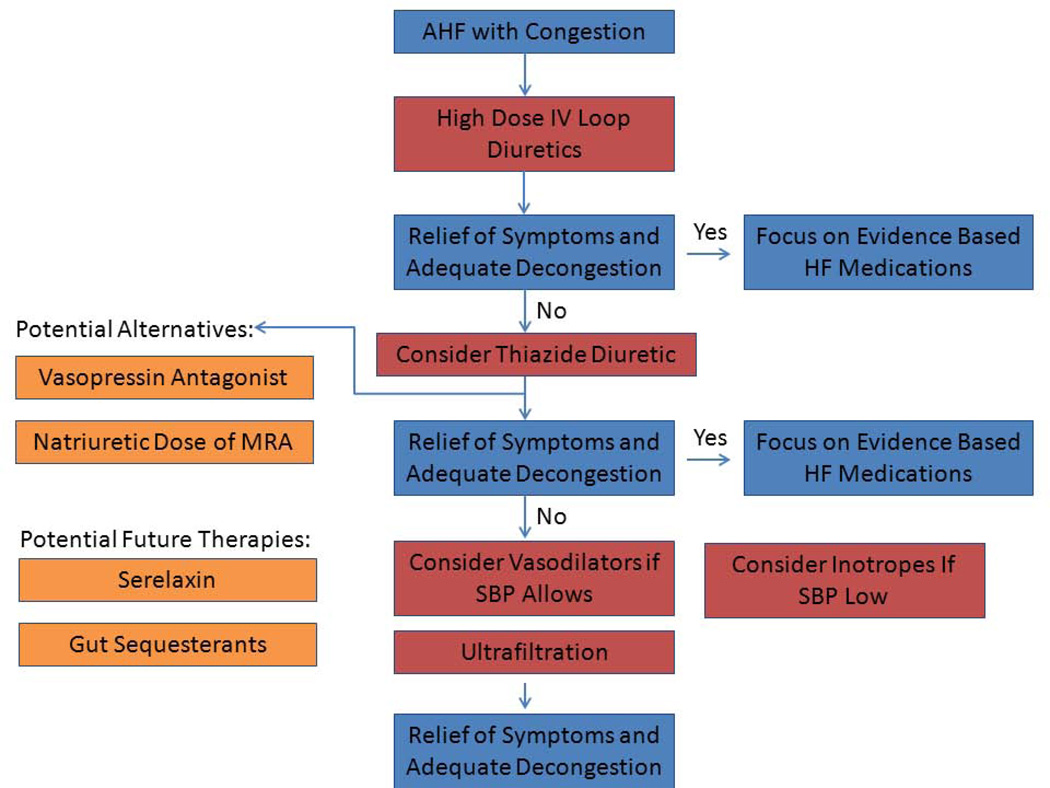
Figure 6 outlines an approach to managing congestion in AHF patients. An evidence-based initial approach to congestion management involves high-dose IV diuretics by either IV bolus or continuous infusion(44). Current guidelines indicate that the optimal dose of IV furosemide is uncertain(3), but based on DOSE-AHF, an initial IV furosemide dose of 2.5 times the patient’s home oral diuretic dose generally results in substantial diuresis and dyspnea relief. Diuretic dosing can be repeated as needed. Baseline electrolyte abnormalities or renal dysfunction should lead to consideration of other therapies including hemodiafiltration. In the subgroup of AHF patients with elevation in SBP, the use of vasodilators may be added for dyspnea relief. In order to augment diuresis or to overcome issues related to diuretic resistance, natriuretic MRA doses may be used as long as the patient does not have significant renal dysfunction or hyperkalemia. Alternative strategies to augment decongestion include the use of thiazide-type diuretics(39) or potentially also vasopressin antagonists (particularly in the setting of hyponatremia).
Figure 6.
An approach to managing congestion in acute HF patients.
Abbreviations: IV=intravenous, HF=heart failure, SBP=systolic blood pressure, MRA=mineralocorticoid receptor antagonist.
In the setting of worsening renal function, the CARRESS trial provided hypothesis-generating evidence to support stepped pharmacologic therapy to treat congestion(29). For the first 2 days, IV diuretics were adjusted (+/− a thiazide diuretic) to manage congestion and maintain a urine output of 3–5 L/day. If after 48 hours, urine output was inadequate, the use of IV vasodilators or inotropic agents was considered. The specific protocols (29) were dependent upon patient’s SBP, LVEF, and the presence or absence of RV failure. For instance, if SBP was <110 mmHg and the patient had a reduced LVEF or RV failure, then dopamine or dobutamine could be considered. If the patient’s SBP was >120 mmHg and he/she had severe symptoms, then nitroglycerin or nesiritide was considered. If urine output was still inadequate after 72 hours, then there was consideration for hemodynamic-guided IV therapy, crossover to UF or dialysis. Importantly, this stepped pharmacologic care algorithm has only been compared with UF and potential advantages over usual care have not been empirically demonstrated.
If all diuretic strategies including consideration of stepped pharmacologic are unsuccessful, then UF may be considered. However, the results of CARRESS indicate that UF should be used with caution in the setting of WRF. Alternatively, in AHF due to atrial fibrillation and when IV inotropes are not feasible digoxin may be considered. In the future, options for decongestion therapy might also include serelaxin and/or gut sequesterants.
Conclusion
Diuretics have been the mainstay of decongestion therapy. However, the approach to decongestion can be individualized based on a patient’s initial diuretic response, comorbidity burden, electrolytes abnormalities and hemodynamic profile. Different options may include natriuretic MRA doses, vasodilators and/or a stepped pharmacologic approach of diuretic uptitration and inotropes. At present time, the use of UF should be confined to patients that fail to respond to diuretic-based strategies. In the future, gut sequesterants and serelaxin may serve as additional or alternative therapies. Although many unanswered questions remain about the best approach for using these therapies, ongoing trials will inform the future treatment of congestion.
Table 1.
Strengths and limitations of different decongestion strategies.
| Strategy | Strengths | Limitations | Key References |
|---|---|---|---|
| Strategies routinely used for decongestion | |||
| Loop diuretics |
|
|
DOSE-AHF(44) Felker GM and Mentz RJ, 2012(39) Hasselblad V, et al. 2007(51) Metra M, et al. 2012(58) |
| Intravenous vasodilators |
|
|
VMAC(65) ASCEND(66) |
| Ultrafiltration |
|
|
UNLOAD(69) CARRESS(29) |
| Strategies in development or under investigation for decongestion | |||
| Vasopressin antagonists |
|
|
Konstam M, et al. 2007(72) Pang P, et al. 2009(74) |
| Mineralocorticoid receptor antagonists (natriuretic doses) |
|
|
Bansal S, et al. 2009(79) |
| Gut sequesterants |
|
|
Costanzo MR, et al. 2012(87) |
| Serelaxin |
|
|
Teerlink, JR et al. 2013(89) Metra M, et al. 2013(90) |
Acknowledgments
Funding
R.J.M. is supported by Grant Number T32GM086330 from the National Institute of General Medical Sciences. A.A.V. is supported by a grant from the European Commission: FP7-242209-BIOSTAT-CHF.
Footnotes
Disclosures
A.A.V. received consultancy fees and/or research grants from: Alere; Amgen; Anexon; Bayer; Boehringer Ingelheim; Cardio3Biosciences; Celladon; Merck; Novartis; Servier; Torrent; Vifor Pharma. G.F.C. served on advisory boards for MSD and Bayer. S.D.A is a consultant for Vifor International, Amgen, Bayer, Bosch GmbH, PsiOxus Therapeutics, Professional Dietetics, and Novartis; research support from Vifor International, PsiOxus Therapeutics. M.G. is a consultant for Abbott Labs, Astellas, AstraZeneca, Bayer Schering PharmaAG, CorThera Inc., Cytokinetics Inc., DebioPharm S.A., Errekappa Terapeutici (Milan, Italy), Glaxo Smith Kline, JNJ, Medtronic, Novartis Pharma AG, Otsuka, Sigma Tau, Solvay Pharmaceuticals, and Pericor Therapeutics. P.R has received honoraria from AstraZeneca, Baxter-Gambro, Daiichi-Sankyo, Fresenius Medical Care, Novartis Pharmaceuticals, Relypsa, and Servier and is a shareholder of CardioRenal diagnostics. F.Z. has received grants from Roche Diagnostics; has served on steering committees for Bayer, Boston Scientific, Gambro, Janssen, Novartis, Pfizer, Resmed, and Takeda; an event committee for Biotronik; and is a consultant/scientific advisory board member for Novartis, Servier, St Jude. B.P. is a consultant to Pfizer, Merck, Novartis, Takeda, Astra Zeneca, Bayer, Lilly, BMS, Cytopherx, Amorcyte, Relypsa, BG-Medicine, Aurasense, and GE-Health Care; stocks options in Relypsa, BG-Medicine, and Aurasense; and grants from Novartis, Forrest Laboratories, and Medtronic. C.M.O. is a consultant to Merck and Amgen.
The other authors declare no conflict of interest.
References
- 1.Yancy CW, Jessup M, Bozkurt B, Masoudi FA, Butler J, McBride PE, Casey DE, Jr, McMurray JJ, Drazner MH, Mitchell JE, Fonarow GC, Peterson PN, Geraci SA, Horwich T, Januzzi JL, Johnson MR, Kasper EK, Levy WC, Riegel B, Sam F, Stevenson LW, Tang WH, Tsai EJ, Wilkoff BL. 2013 ACCF/AHA Guideline for the Management of Heart Failure: A Report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines. J Am Coll Cardiol. 2013;62(16):e147–e239. doi: 10.1016/j.jacc.2013.05.019. [DOI] [PubMed] [Google Scholar]
- 2.Fang J, Mensah GA, Croft JB, Keenan NL. Heart failure-related hospitalization in the U.S., 1979 to 2004. J Am Coll Cardiol. 2008;52(6):428–434. doi: 10.1016/j.jacc.2008.03.061. [DOI] [PubMed] [Google Scholar]
- 3.McMurray JJ, Adamopoulos S, Anker SD, Auricchio A, Bohm M, Dickstein K, Falk V, Filippatos G, Fonseca C, Gomez-Sanchez MA, Jaarsma T, Kober L, Lip GY, Maggioni AP, Parkhomenko A, Pieske BM, Popescu BA, Ronnevik PK, Rutten FH, Schwitter J, Seferovic P, Stepinska J, Trindade PT, Voors AA, Zannad F, Zeiher A, Bax JJ, Baumgartner H, Ceconi C, Dean V, Deaton C, Fagard R, Funck-Brentano C, Hasdai D, Hoes A, Kirchhof P, Knuuti J, Kolh P, McDonagh T, Moulin C, Popescu BA, Reiner Z, Sechtem U, Sirnes PA, Tendera M, Torbicki A, Vahanian A, Windecker S, McDonagh T, Sechtem U, Bonet LA, Avraamides P, Ben Lamin HA, Brignole M, Coca A, Cowburn P, Dargie H, Elliott P, Flachskampf FA, Guida GF, Hardman S, Iung B, Merkely B, Mueller C, Nanas JN, Nielsen OW, Orn S, Parissis JT, Ponikowski P ESC guidelines for the diagnosis and treatment of acute and chronic heart failure 2012: The Task Force for the Diagnosis and Treatment of Acute and Chronic Heart Failure 2012 of the European Society of Cardiology. Developed in collaboration with the Heart Failure Association (HFA) of the ESC. Eur J Heart Fail. 2012;14(8):803–869. doi: 10.1093/eurjhf/hfs105. [DOI] [PubMed] [Google Scholar]
- 4.Adams KF, Jr, Fonarow GC, Emerman CL, LeJemtel TH, Costanzo MR, Abraham WT, Berkowitz RL, Galvao M, Horton DP. Characteristics and outcomes of patients hospitalized for heart failure in the United States: rationale, design, and preliminary observations from the first 100,000 cases in the Acute Decompensated Heart Failure National Registry (ADHERE) Am Heart J. 2005;149(2):209–216. doi: 10.1016/j.ahj.2004.08.005. [DOI] [PubMed] [Google Scholar]
- 5.Cleland JG, Swedberg K, Follath F, Komajda M, Cohen-Solal A, Aguilar JC, Dietz R, Gavazzi A, Hobbs R, Korewicki J, Madeira HC, Moiseyev VS, Preda I, van Gilst WH, Widimsky J, Freemantle N, Eastaugh J, Mason J. The EuroHeart Failure survey programme-- a survey on the quality of care among patients with heart failure in Europe Part 1: patient characteristics and diagnosis. Eur Heart J. 2003;24(5):442–463. doi: 10.1016/s0195-668x(02)00823-0. [DOI] [PubMed] [Google Scholar]
- 6.O’Connor CM, Stough WG, Gallup DS, Hasselblad V, Gheorghiade M. Demographics, clinical characteristics, and outcomes of patients hospitalized for decompensated heart failure: observations from the IMPACT-HF registry. J Card Fail. 2005;11(3):200–205. doi: 10.1016/j.cardfail.2004.08.160. [DOI] [PubMed] [Google Scholar]
- 7.Gheorghiade M, Filippatos G, De Luca L, Burnett J. Congestion in acute heart failure syndromes: an essential target of evaluation and treatment. Am J Med. 2006;119(12 Suppl 1):S3–s10. doi: 10.1016/j.amjmed.2006.09.011. [DOI] [PubMed] [Google Scholar]
- 8.Fonarow GC. The Acute Decompensated Heart Failure National Registry (ADHERE): opportunities to improve care of patients hospitalized with acute decompensated heart failure. Rev Cardiovasc Med. 2003;4(Suppl 7):S21–S30. [PubMed] [Google Scholar]
- 9.Ambrosy AP, Pang PS, Khan S, Konstam MA, Fonarow GC, Traver B, Maggioni AP, Cook T, Swedberg K, Burnett JC, Jr, Grinfeld L, Udelson JE, Zannad F, Gheorghiade M. Clinical course and predictive value of congestion during hospitalization in patients admitted for worsening signs and symptoms of heart failure with reduced ejection fraction: findings from the EVEREST trial. Eur Heart J. 2013;34(11):835–843. doi: 10.1093/eurheartj/ehs444. [DOI] [PubMed] [Google Scholar]
- 10.Drazner MH, Rame JE, Stevenson LW, Dries DL. Prognostic importance of elevated jugular venous pressure and a third heart sound in patients with heart failure. N Engl J Med. 2001;345(8):574–581. doi: 10.1056/NEJMoa010641. [DOI] [PubMed] [Google Scholar]
- 11.Lucas C, Johnson W, Hamilton MA, Fonarow GC, Woo MA, Flavell CM, Creaser JA, Stevenson LW. Freedom from congestion predicts good survival despite previous class IV symptoms of heart failure. Am Heart J. 2000;140(6):840–847. doi: 10.1067/mhj.2000.110933. [DOI] [PubMed] [Google Scholar]
- 12.Butler J, Kalogeropoulos A. Worsening heart failure hospitalization epidemic we do not know how to prevent and we do not know how to treat! J Am Coll Cardiol. 2008;52(6):435–437. doi: 10.1016/j.jacc.2008.04.037. [DOI] [PubMed] [Google Scholar]
- 13.Gheorghiade M, Follath F, Ponikowski P, Barsuk JH, Blair JE, Cleland JG, Dickstein K, Drazner MH, Fonarow GC, Jaarsma T, Jondeau G, Sendon JL, Mebazaa A, Metra M, Nieminen M, Pang PS, Seferovic P, Stevenson LW, van Veldhuisen DJ, Zannad F, Anker SD, Rhodes A, McMurray JJ, Filippatos G. Assessing and grading congestion in acute heart failure: a scientific statement from the acute heart failure committee of the heart failure association of the European Society of Cardiology and endorsed by the European Society of Intensive Care Medicine. Eur J Heart Fail. 2010;12(5):423–433. doi: 10.1093/eurjhf/hfq045. [DOI] [PubMed] [Google Scholar]
- 14.Pellicori P, Carubelli V, Zhang J, Castiello T, Sherwi N, Clark AL, Cleland JG. IVC diameter in patients with chronic heart failure: relationships and prognostic significance. JACC Cardiovasc Imaging. 2013;6(1):16–28. doi: 10.1016/j.jcmg.2012.08.012. [DOI] [PubMed] [Google Scholar]
- 15.Schiff GD, Fung S, Speroff T, McNutt RA. Decompensated heart failure: symptoms, patterns of onset, and contributing factors. Am J Med. 2003;114(8):625–630. doi: 10.1016/s0002-9343(03)00132-3. [DOI] [PubMed] [Google Scholar]
- 16.Fallick C, Sobotka PA, Dunlap ME. Sympathetically mediated changes in capacitance: redistribution of the venous reservoir as a cause of decompensation. Circ Heart Fail. 2011;4(5):669–675. doi: 10.1161/CIRCHEARTFAILURE.111.961789. [DOI] [PubMed] [Google Scholar]
- 17.Koniari K, Parissis J, Paraskevaidis I, Anastasiou-Nana M. Treating volume overload in acutely decompensated heart failure: established and novel therapeutic approaches. Eur Heart J Acute Cardiovasc Care. 2012;1(3):256–268. doi: 10.1177/2048872612457044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Colombo PC, Onat D, Harxhi A, Demmer RT, Hayashi Y, Jelic S, LeJemtel TH, Bucciarelli L, Kebschull M, Papapanou P, Uriel N, Schmidt AM, Sabbah HN, Jorde UP. Peripheral venous congestion causes inflammation, neurohormonal, and endothelial cell activation. Eur Heart J. 2013 Nov 20; doi: 10.1093/eurheartj/eht456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Cotter G, Metra M, Milo-Cotter O, Dittrich HC, Gheorghiade M. Fluid overload in acute heart failure — Re-distribution and other mechanisms beyond fluid accumulation. Eur J Heart Fail. 2008;10(2):165–169. doi: 10.1016/j.ejheart.2008.01.007. [DOI] [PubMed] [Google Scholar]
- 20.Milo-Cotter O, Adams KF, O’Connor CM, Uriel N, Kaluski E, Felker GM, Weatherley B, Vered Z, Cotter G. Acute heart failure associated with high admission blood pressure — A distinct vascular disorder? Eur J Heart Fail. 2007;9(2):178–183. doi: 10.1016/j.ejheart.2006.06.004. [DOI] [PubMed] [Google Scholar]
- 21.Balmain S, Padmanabhan N, Ferrell WR, Morton JJ, McMurray JJV. Differences in arterial compliance, microvascular function and venous capacitance between patients with heart failure and either preserved or reduced left ventricular systolic function. Eur J Heart Fail. 2007;9(9):865–871. doi: 10.1016/j.ejheart.2007.06.003. [DOI] [PubMed] [Google Scholar]
- 22.Kawaguchi M, Hay I, Fetics B, Kass DA. Combined Ventricular Systolic and Arterial Stiffening in Patients With Heart Failure and Preserved Ejection Fraction: Implications for Systolic and Diastolic Reserve Limitations. Circulation. 2003;107(5):714–720. doi: 10.1161/01.cir.0000048123.22359.a0. [DOI] [PubMed] [Google Scholar]
- 23.Milo O, Cotter G, Kaluski E, Brill A, Blatt A, Krakover R, Vered Z, Hershkoviz R. Comparison of inflammatory and neurohormonal activation in cardiogenic pulmonary edema secondary to ischemic versus nonischemic causes. Am J Cardiol. 2003;92(2):222–226. doi: 10.1016/s0002-9149(03)00545-9. [DOI] [PubMed] [Google Scholar]
- 24.Colombo PC, Banchs JE, Celaj S, Talreja A, Lachmann J, Malla S, DuBois NB, Ashton AW, Latif F, Jorde UP, Ware JA, LeJemtel TH. Endothelial Cell Activation in Patients With Decompensated Heart Failure. Circulation. 2005;111(1):58–62. doi: 10.1161/01.CIR.0000151611.89232.3B. [DOI] [PubMed] [Google Scholar]
- 25.Maxwell AP, Ong HY, Nicholls DP. Influence of progressive renal dysfunction in chronic heart failure. Eur J Heart Fail. 2002;4(2):125–130. doi: 10.1016/s1388-9842(01)00238-0. [DOI] [PubMed] [Google Scholar]
- 26.Firth JD, Raine AE, Ledingham JG. Raised venous pressure: a direct cause of renal sodium retention in oedema? Lancet. 1988;1(8593):1033–1035. doi: 10.1016/s0140-6736(88)91851-x. [DOI] [PubMed] [Google Scholar]
- 27.Schrier RW, Abraham WT. Hormones and hemodynamics in heart failure. N Engl J Med. 1999;341(8):577–585. doi: 10.1056/NEJM199908193410806. [DOI] [PubMed] [Google Scholar]
- 28.Nohria A, Hasselblad V, Stebbins A, Pauly DF, Fonarow GC, Shah M, Yancy CW, Califf RM, Stevenson LW, Hill JA. Cardiorenal interactions: insights from the ESCAPE trial. J Am Coll Cardiol. 2008;51(13):1268–1274. doi: 10.1016/j.jacc.2007.08.072. [DOI] [PubMed] [Google Scholar]
- 29.Bart BA, Goldsmith SR, Lee KL, Givertz MM, O’Connor CM, Bull DA, Redfield MM, Deswal A, Rouleau JL, LeWinter MM, Ofili EO, Stevenson LW, Semigran MJ, Felker GM, Chen HH, Hernandez AF, Anstrom KJ, McNulty SE, Velazquez EJ, Ibarra JC, Mascette AM, Braunwald E. Ultrafiltration in Decompensated Heart Failure with Cardiorenal Syndrome. N Engl J Med. 2012;367(24):2296–2304. doi: 10.1056/NEJMoa1210357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lala A, Dunlay S, Vader J, Zakeri R, Ravichandran A, AbouEzzadine O, Khazanie P, McNulty S. The Tides of Congestion during and after Hospitalization for Acute Decompensated Heart Failure. J Card Fail. 2013;19(8) Supplement S81. [Google Scholar]
- 31.Lala A, Dunlay S, Vader J, Zakeri R, Ravichandran A, AbouEzzadine O, Khazanie P, McNulty S. A Two-Symptom Congestion Score In Relation to Outcomes after Discharge with Acute Decompensated Heart Failure. J Card Fail. 2013;19(8) Supplement S39. [Google Scholar]
- 32.Stevenson LW, Perloff JK. The limited reliability of physical signs for estimating hemodynamics in chronic heart failure. JAMA. 1989;261(6):884–888. [PubMed] [Google Scholar]
- 33.Kociol RD, McNulty SE, Hernandez AF, Lee KL, Redfield MM, Tracy RP, Braunwald E, O’Connor CM, Felker GM. Markers of decongestion, dyspnea relief, and clinical outcomes among patients hospitalized with acute heart failure. Circ Heart Fail. 2013;6(2):240–245. doi: 10.1161/CIRCHEARTFAILURE.112.969246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Di Somma S, De Berardinis B, Bongiovanni C, Marino R, Ferri E, Alfei B. Use of BNP and bioimpedance to drive therapy in heart failure patients. Congest Heart Fail. 2010;16(Suppl 1):S56–S61. doi: 10.1111/j.1751-7133.2010.00162.x. [DOI] [PubMed] [Google Scholar]
- 35.Maisel A, Mueller C, Adams K, Jr, Anker SD, Aspromonte N, Cleland JG, Cohen-Solal A, Dahlstrom U, DeMaria A, Di Somma S, Filippatos GS, Fonarow GC, Jourdain P, Komajda M, Liu PP, McDonagh T, McDonald K, Mebazaa A, Nieminen MS, Peacock WF, Tubaro M, Valle R, Vanderhyden M, Yancy CW, Zannad F, Braunwald E. State of the art: using natriuretic peptide levels in clinical practice. Eur J Heart Fail. 2008;10(9):824–839. doi: 10.1016/j.ejheart.2008.07.014. [DOI] [PubMed] [Google Scholar]
- 36.van der Meer P, Postmus D, Ponikowski P, Cleland JG, O’Connor CM, Cotter G, Metra M, Davison BA, Givertz MM, Mansoor GA, Teerlink JR, Massie BM, Hillege HL, Voors AA. The predictive value of short-term changes in hemoglobin concentration in patients presenting with acute decompensated heart failure. J Am Coll Cardiol. 2013;61(19):1973–1981. doi: 10.1016/j.jacc.2012.12.050. [DOI] [PubMed] [Google Scholar]
- 37.Testani JM, Chen J, McCauley BD, Kimmel SE, Shannon RP. Potential effects of aggressive decongestion during the treatment of decompensated heart failure on renal function and survival. Circulation. 2010;122(3):265–272. doi: 10.1161/CIRCULATIONAHA.109.933275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Testani JM, Brisco MA, Chen J, McCauley BD, Parikh CR, Tang WHW. Timing of Hemoconcentration During Treatment of Acute Decompensated Heart Failure and Subsequent Survival: Importance of Sustained Decongestion. J Am Coll Cardiol. 2013;62(6):516–524. doi: 10.1016/j.jacc.2013.05.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Felker GM, Mentz RJ. Diuretics and ultrafiltration in acute decompensated heart failure. J Am Coll Cardiol. 2012;59(24):2145–2153. doi: 10.1016/j.jacc.2011.10.910. [DOI] [PubMed] [Google Scholar]
- 40.Murray MD, Deer MM, Ferguson JA, Dexter PR, Bennett SJ, Perkins SM, Smith FE, Lane KA, Adams LD, Tierney WM, Brater DC. Open-label randomized trial of torsemide compared with furosemide therapy for patients with heart failure. Am J Med. 2001;111(7):513–520. doi: 10.1016/s0002-9343(01)00903-2. [DOI] [PubMed] [Google Scholar]
- 41.Cosin J, Diez J. Torasemide in chronic heart failure: results of the TORIC study. Eur J Heart Fail. 2002;4(4):507–513. doi: 10.1016/s1388-9842(02)00122-8. [DOI] [PubMed] [Google Scholar]
- 42.Muller K, Gamba G, Jaquet F, Hess B, Torasemide VS. furosemide in primary care patients with chronic heart failure NYHA II to IV--efficacy and quality of life. Eur J Heart Fail. 2003;5(6):793–801. doi: 10.1016/s1388-9842(03)00150-8. [DOI] [PubMed] [Google Scholar]
- 43.Dinicolantonio JJ. Should torsemide be the loop diuretic of choice in systolic heart failure? Future Cardiol. 2012;8(5):707–728. doi: 10.2217/fca.12.54. [DOI] [PubMed] [Google Scholar]
- 44.Felker GM, Lee KL, Bull DA, Redfield MM, Stevenson LW, Goldsmith SR, LeWinter MM, Deswal A, Rouleau JL, Ofili EO, Anstrom KJ, Hernandez AF, McNulty SE, Velazquez EJ, Kfoury AG, Chen HH, Givertz MM, Semigran MJ, Bart BA, Mascette AM, Braunwald E, O’Connor CM. Diuretic strategies in patients with acute decompensated heart failure. N Engl J Med. 2011;364(9):797–805. doi: 10.1056/NEJMoa1005419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Kaissling B, Bachmann S, Kriz W. Structural adaptation of the distal convoluted tubule to prolonged furosemide treatment. Am J Physiol. 1985;248(3 Pt 2):F374–F381. doi: 10.1152/ajprenal.1985.248.3.F374. [DOI] [PubMed] [Google Scholar]
- 46.Ellison DH. Diuretic therapy and resistance in congestive heart failure. Cardiology. 2001;96(3–4):132–143. doi: 10.1159/000047397. [DOI] [PubMed] [Google Scholar]
- 47.Jentzer JC, DeWald TA, Hernandez AF. Combination of loop diuretics with thiazide-type diuretics in heart failure. J Am Coll Cardiol. 2010;56(19):1527–1534. doi: 10.1016/j.jacc.2010.06.034. [DOI] [PubMed] [Google Scholar]
- 48.Goyal A, Spertus JA, Gosch K, Venkitachalam L, Jones PG, Van den Berghe G, Kosiborod M. Serum potassium levels and mortality in acute myocardial infarction. JAMA. 2012;307(2):157–164. doi: 10.1001/jama.2011.1967. [DOI] [PubMed] [Google Scholar]
- 49.Schrier RW. Role of diminished renal function in cardiovascular mortality: marker or pathogenetic factor? J Am Coll Cardiol. 2006;47(1):1–8. doi: 10.1016/j.jacc.2005.07.067. [DOI] [PubMed] [Google Scholar]
- 50.Domanski M, Norman J, Pitt B, Haigney M, Hanlon S, Peyster E. Diuretic use, progressive heart failure, and death in patients in the Studies Of Left Ventricular Dysfunction (SOLVD) J Am Coll Cardiol. 2003;42(4):705–708. doi: 10.1016/s0735-1097(03)00765-4. [DOI] [PubMed] [Google Scholar]
- 51.Hasselblad V, Gattis Stough W, Shah MR, Lokhnygina Y, O’Connor CM, Califf RM, Adams KF., Jr Relation between dose of loop diuretics and outcomes in a heart failure population: results of the ESCAPE trial. Eur J Heart Fail. 2007;9(10):1064–1069. doi: 10.1016/j.ejheart.2007.07.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.McCurley JM, Hanlon SU, Wei SK, Wedam EF, Michalski M, Haigney MC. Furosemide and the progression of left ventricular dysfunction in experimental heart failure. J Am Coll Cardiol. 2004;44(6):1301–1307. doi: 10.1016/j.jacc.2004.04.059. [DOI] [PubMed] [Google Scholar]
- 53.Francis GS, Benedict C, Johnstone DE, Kirlin PC, Nicklas J, Liang CS, Kubo SH, Rudin-Toretsky E, Yusuf S. Comparison of neuroendocrine activation in patients with left ventricular dysfunction with and without congestive heart failure A substudy of the Studies of Left Ventricular Dysfunction (SOLVD) Circulation. 1990;82(5):1724–1729. doi: 10.1161/01.cir.82.5.1724. [DOI] [PubMed] [Google Scholar]
- 54.Francis GS, Siegel RM, Goldsmith SR, Olivari MT, Levine TB, Cohn JN. Acute vasoconstrictor response to intravenous furosemide in patients with chronic congestive heart failure Activation of the neurohumoral axis. Ann Intern Med. 1985;103(1):1–6. doi: 10.7326/0003-4819-103-1-1. [DOI] [PubMed] [Google Scholar]
- 55.Heywood JT, Fonarow GC, Costanzo MR, Mathur VS, Wigneswaran JR, Wynne J. High prevalence of renal dysfunction and its impact on outcome in 118,465 patients hospitalized with acute decompensated heart failure: a report from the ADHERE database. J Card Fail. 2007;13(6):422–430. doi: 10.1016/j.cardfail.2007.03.011. [DOI] [PubMed] [Google Scholar]
- 56.Aronson D, Burger AJ. The relationship between transient and persistent worsening renal function and mortality in patients with acute decompensated heart failure. J Card Fail. 2010;16(7):541–547. doi: 10.1016/j.cardfail.2010.02.001. [DOI] [PubMed] [Google Scholar]
- 57.Mullens W, Abrahams Z, Francis GS, Sokos G, Taylor DO, Starling RC, Young JB, Tang WH. Importance of venous congestion for worsening of renal function in advanced decompensated heart failure. J Am Coll Cardiol. 2009;53(7):589–596. doi: 10.1016/j.jacc.2008.05.068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Metra M, Davison B, Bettari L, Sun H, Edwards C, Lazzarini V, Piovanelli B, Carubelli V, Bugatti S, Lombardi C, Cotter G, Dei Cas L. Is Worsening Renal Function an Ominous Prognostic Sign in Patients With Acute Heart Failure?: The Role of Congestion and Its Interaction With Renal Function. Circ Heart Fail. 2012;5(1):54–62. doi: 10.1161/CIRCHEARTFAILURE.111.963413. [DOI] [PubMed] [Google Scholar]
- 59.Elkayam U, Akhter MW, Singh H, Khan S, Usman A. Comparison of effects on left ventricular filling pressure of intravenous nesiritide and high-dose nitroglycerin in patients with decompensated heart failure. Am J Cardiol. 2004;93(2):237–240. doi: 10.1016/j.amjcard.2003.09.051. [DOI] [PubMed] [Google Scholar]
- 60.Elkayam U, Kulick D, McIntosh N, Roth A, Hsueh W, Rahimtoola SH. Incidence of early tolerance to hemodynamic effects of continuous infusion of nitroglycerin in patients with coronary artery disease and heart failure. Circulation. 1987;76(3):577–584. doi: 10.1161/01.cir.76.3.577. [DOI] [PubMed] [Google Scholar]
- 61.Mullens W, Abrahams Z, Francis GS, Skouri HN, Starling RC, Young JB, Taylor DO, Tang WH. Sodium nitroprusside for advanced low-output heart failure. J Am Coll Cardiol. 2008;52(3):200–207. doi: 10.1016/j.jacc.2008.02.083. [DOI] [PubMed] [Google Scholar]
- 62.Hockings BE, Cope GD, Clarke GM, Taylor RR. Randomized controlled trial of vasodilator therapy after myocardial infarction. Am J Cardiol. 1981;48(2):345–352. doi: 10.1016/0002-9149(81)90618-4. [DOI] [PubMed] [Google Scholar]
- 63.Cohn JN, Franciosa JA, Francis GS, Archibald D, Tristani F, Fletcher R, Montero A, Cintron G, Clarke J, Hager D, Saunders R, Cobb F, Smith R, Loeb H, Settle H. Effect of short-term infusion of sodium nitroprusside on mortality rate in acute myocardial infarction complicated by left ventricular failure: results of a Veterans Administration cooperative study. N Engl J Med. 1982;306(19):1129–1135. doi: 10.1056/NEJM198205133061902. [DOI] [PubMed] [Google Scholar]
- 64.Colucci WS, Elkayam U, Horton DP, Abraham WT, Bourge RC, Johnson AD, Wagoner LE, Givertz MM, Liang CS, Neibaur M, Haught WH, LeJemtel TH. Intravenous nesiritide a natriuretic peptide in the treatment of decompensated congestive heart failure Nesiritide Study Group. N Engl J Med. 2000;343(4):246–253. doi: 10.1056/NEJM200007273430403. [DOI] [PubMed] [Google Scholar]
- 65.Intravenous nesiritide vs nitroglycerin for treatment of decompensated congestive heart failure: a randomized controlled trial. JAMA. 2002;287(12):1531–1540. doi: 10.1001/jama.287.12.1531. [DOI] [PubMed] [Google Scholar]
- 66.O’Connor CM, Starling RC, Hernandez AF, Armstrong PW, Dickstein K, Hasselblad V, Heizer GM, Komajda M, Massie BM, McMurray JJ, Nieminen MS, Reist CJ, Rouleau JL, Swedberg K, Adams KF, Jr, Anker SD, Atar D, Battler A, Botero R, Bohidar NR, Butler J, Clausell N, Corbalan R, Costanzo MR, Dahlstrom U, Deckelbaum LI, Diaz R, Dunlap ME, Ezekowitz JA, Feldman D, Felker GM, Fonarow GC, Gennevois D, Gottlieb SS, Hill JA, Hollander JE, Howlett JG, Hudson MP, Kociol RD, Krum H, Laucevicius A, Levy WC, Mendez GF, Metra M, Mittal S, Oh BH, Pereira NL, Ponikowski P, Tang WH, Tanomsup S, Teerlink JR, Triposkiadis F, Troughton RW, Voors AA, Whellan DJ, Zannad F, Califf RM. Effect of nesiritide in patients with acute decompensated heart failure. N Engl J Med. 2011;365(1):32–43. doi: 10.1056/NEJMoa1100171. [DOI] [PubMed] [Google Scholar]
- 67.Gottlieb SS, Stebbins A, Voors AA, Hasselblad V, Ezekowitz JA, Califf RM, O’Connor CM, Starling RC, Hernandez AF. Effects of Nesiritide and Predictors of Urine Output in Acute Decompensated Heart Failure: Results From ASCEND-HF (Acute Study of Clinical Effectiveness of Nesiritide and Decompensated Heart Failure) J Am Coll Cardiol. 2013;62(13):1177–1183. doi: 10.1016/j.jacc.2013.04.073. [DOI] [PubMed] [Google Scholar]
- 68.Givertz MM, Teerlink JR, Albert NM, Westlake Canary CA, Collins SP, Colvin-Adams M, Ezekowitz JA, Fang JC, Hernandez AF, Katz SD, Krishnamani R, Stough WG, Walsh MN, Butler J, Carson PE, Dimarco JP, Hershberger RE, Rogers JG, Spertus JA, Stevenson WG, Sweitzer NK, Tang WH, Starling RC. Acute decompensated heart failure: update on new and emerging evidence and directions for future research. J Card Fail. 2013;19(6):371–389. doi: 10.1016/j.cardfail.2013.04.002. [DOI] [PubMed] [Google Scholar]
- 69.Costanzo MR, Guglin ME, Saltzberg MT, Jessup ML, Bart BA, Teerlink JR, Jaski BE, Fang JC, Feller ED, Haas GJ, Anderson AS, Schollmeyer MP, Sobotka PA. Ultrafiltration versus intravenous diuretics for patients hospitalized for acute decompensated heart failure. J Am Coll Cardiol. 2007;49(6):675–683. doi: 10.1016/j.jacc.2006.07.073. [DOI] [PubMed] [Google Scholar]
- 70.Bradley SM, Levy WC, Veenstra DL. Cost-consequences of ultrafiltration for acute heart failure: a decision model analysis. Circ Cardiovasc Qual Outcomes. 2009;2(6):566–573. doi: 10.1161/CIRCOUTCOMES.109.853556. [DOI] [PubMed] [Google Scholar]
- 71.Goldsmith SR, Gheorghiade M. Vasopressin antagonism in heart failure. J Am Coll Cardiol. 2005;46(10):1785–1791. doi: 10.1016/j.jacc.2005.02.095. [DOI] [PubMed] [Google Scholar]
- 72.Konstam MA, Gheorghiade M, Burnett JC, Jr, Grinfeld L, Maggioni AP, Swedberg K, Udelson JE, Zannad F, Cook T, Ouyang J, Zimmer C, Orlandi C. Effects of oral tolvaptan in patients hospitalized for worsening heart failure: the EVEREST Outcome Trial. JAMA. 2007;297(12):1319–1331. doi: 10.1001/jama.297.12.1319. [DOI] [PubMed] [Google Scholar]
- 73.Gheorghiade M, Konstam MA, Burnett JC, Jr, Grinfeld L, Maggioni AP, Swedberg K, Udelson JE, Zannad F, Cook T, Ouyang J, Zimmer C, Orlandi C. Short-term clinical effects of tolvaptan, an oral vasopressin antagonist, in patients hospitalized for heart failure: the EVEREST Clinical Status Trials. JAMA. 2007;297(12):1332–1343. doi: 10.1001/jama.297.12.1332. [DOI] [PubMed] [Google Scholar]
- 74.Pang PS, Konstam MA, Krasa HB, Swedberg K, Zannad F, Blair JE, Zimmer C, Teerlink JR, Maggioni AP, Burnett JC, Jr, Grinfeld L, Ouyang J, Udelson JE, Gheorghiade M. Effects of tolvaptan on dyspnoea relief from the EVEREST trials. Eur Heart J. 2009;30(18):2233–2240. doi: 10.1093/eurheartj/ehp253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Hauptman PJ, Burnett J, Gheorghiade M, Grinfeld L, Konstam MA, Kostic D, Krasa HB, Maggioni A, Ouyang J, Swedberg K, Zannad F, Zimmer C, Udelson JE. Clinical course of patients with hyponatremia and decompensated systolic heart failure and the effect of vasopressin receptor antagonism with tolvaptan. J Card Fail. 2013;19(6):390–397. doi: 10.1016/j.cardfail.2013.04.001. [DOI] [PubMed] [Google Scholar]
- 76.Torres VE, Chapman AB, Devuyst O, Gansevoort RT, Grantham JJ, Higashihara E, Perrone RD, Krasa HB, Ouyang J, Czerwiec FS. Tolvaptan in patients with autosomal dominant polycystic kidney disease. N Engl J Med. 2012;367(25):2407–2418. doi: 10.1056/NEJMoa1205511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Rossignol P, Ménard J, Fay R, Gustafsson F, Pitt B, Zannad F. Eplerenone survival benefits in heart failure patients post-myocardial infarction are independent from its diuretic and potassium-sparing effects Insights from an EPHESUS (Eplerenone Post-Acute Myocardial Infarction Heart Failure Efficacy and Survival Study) substudy. JAMA. 2011;58(19):1958–1966. doi: 10.1016/j.jacc.2011.04.049. [DOI] [PubMed] [Google Scholar]
- 78.Effectiveness of spironolactone added to an angiotensin-converting enzyme inhibitor and a loop diuretic for severe chronic congestive heart failure (the Randomized Aldactone Evaluation Study [RALES]) Am J Cardiol. 1996;78(8):902–907. doi: 10.1016/s0002-9149(96)00465-1. [DOI] [PubMed] [Google Scholar]
- 79.Bansal S, Lindenfeld J, Schrier RW. Sodium retention in heart failure and cirrhosis: potential role of natriuretic doses of mineralocorticoid antagonist? Circ Heart Fail. 2009;2(4):370–376. doi: 10.1161/CIRCHEARTFAILURE.108.821199. [DOI] [PubMed] [Google Scholar]
- 80.Fernandez-Llama P, Ageloff S, Fernandez-Varo G, Ros J, Wang X, Garra N, Esteva-Font C, Ballarin J, Barcelo P, Arroyo V, Stokes JB, Knepper MA, Jimenez W. Sodium retention in cirrhotic rats is associated with increased renal abundance of sodium transporter proteins. Kidney Int. 2005;67(2):622–630. doi: 10.1111/j.1523-1755.2005.67118.x. [DOI] [PubMed] [Google Scholar]
- 81.Perez-Ayuso RM, Arroyo V, Planas R, Gaya J, Bory F, Rimola A, Rivera F, Rodes J Randomized comparative study of efficacy of furosemide versus spironolactone in nonazotemic cirrhosis with ascites. Relationship between the diuretic response and the activity of the renin-aldosterone system. Gastroenterology. 1983;84(5 Pt 1):961–968. [PubMed] [Google Scholar]
- 82.van Vliet AA, Donker AJ, Nauta JJ, Verheugt FW. Spironolactone in congestive heart failure refractory to high-dose loop diuretic and low-dose angiotensin-converting enzyme inhibitor. The Am J Cardiol. 1993;71(3):21a–28a. doi: 10.1016/0002-9149(93)90241-4. [DOI] [PubMed] [Google Scholar]
- 83.Hensen J, Abraham WT, Durr JA, Schrier RW. Aldosterone in congestive heart failure: analysis of determinants and role in sodium retention. Am J Nephrol. 1991;11(6):441–446. doi: 10.1159/000168356. [DOI] [PubMed] [Google Scholar]
- 84.Braunwald E, Plauth WH, Jr, Morrow AG. A method for the detection and quantification of impaired sodium excretion. Circulation. 1965;32:223–231. doi: 10.1161/01.cir.32.2.223. [DOI] [PubMed] [Google Scholar]
- 85.Shchekochikhin D, Lindenfeld J, Schrier R. Increased Spironolactone in Advanced Heart Failure: Effect of Doses Greater than 25 mg/Day on Plasma Potassium Concentration. Cardiorenal Med. 2013;3(1):1–6. doi: 10.1159/000346447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Zachariah D, Kalra PR. Managing patients with cardiorenal syndrome: time to look to the gut? Eur J Heart Fail. 2012;14(8):870–872. doi: 10.1093/eurjhf/hfs098. [DOI] [PubMed] [Google Scholar]
- 87.Costanzo MR, Heywood JT, Massie BM, Iwashita J, Henderson L, Mamatsashvili M, Sisakian H, Hayrapetyan H, Sager P, van Veldhuisen DJ, Albrecht D. A double-blind, randomized, parallel, placebo-controlled study examining the effect of cross-linked polyelectrolyte in heart failure patients with chronic kidney disease. Eur J Heart Fail. 2012;14(8):922–930. doi: 10.1093/eurjhf/hfs074. [DOI] [PubMed] [Google Scholar]
- 88.Conrad KP. Maternal vasodilation in pregnancy: the emerging role of relaxin. Am J Physiol Regul Integr Comp Physiol. 2011;301(2):R267–R275. doi: 10.1152/ajpregu.00156.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Teerlink JR, Cotter G, Davison BA, Felker GM, Filippatos G, Greenberg BH, Ponikowski P, Unemori E, Voors AA, Adams KF, Jr, Dorobantu MI, Grinfeld LR, Jondeau G, Marmor A, Masip J, Pang PS, Werdan K, Teichman SL, Trapani A, Bush CA, Saini R, Schumacher C, Severin TM, Metra M. Serelaxin, recombinant human relaxin-2, for treatment of acute heart failure (RELAX-AHF): a randomised, placebo-controlled trial. Lancet. 2013;381(9860):29–39. doi: 10.1016/S0140-6736(12)61855-8. [DOI] [PubMed] [Google Scholar]
- 90.Metra M, Cotter G, Davison BA, Felker GM, Filippatos G, Greenberg BH, Ponikowski P, Unemori E, Voors AA, Adams KF, Jr, Dorobantu MI, Grinfeld L, Jondeau G, Marmor A, Masip J, Pang PS, Werdan K, Prescott MF, Edwards C, Teichman SL, Trapani A, Bush CA, Saini R, Schumacher C, Severin T, Teerlink JR. Effect of serelaxin on cardiac, renal, and hepatic biomarkers in the Relaxin in Acute Heart Failure (RELAX-AHF) development program: correlation with outcomes. J Am Coll Cardiol. 2013;61(2):196–206. doi: 10.1016/j.jacc.2012.11.005. [DOI] [PubMed] [Google Scholar]
- 91.Voors A. HFSA Scientific Meeting; Late-breaking clinical trials. Orlando, FL: 2013. Sep 23, Renal hemodynamic effects of serelaxin in patients with chronic heart failure: main results of a randomized, placebo-controlled, multi-center study. [Google Scholar]